Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive interstitial lung disease characterized by accumulation of extracellular matrix (ECM) and impaired gas exchange. The pathobiological mechanisms that account for disease progression are poorly understood but likely involve alterations in innate inflammatory cells, epithelial cells, and fibroblasts. Thus we seek to review the most recent literature highlighting the complex roles of neutrophils and macrophages as both promoters of fibrosis and defenders against infection. With respect to epithelial cells and fibroblasts, we review the data suggesting that defective autophagy promotes the fibrogenic potential of both cell types and discuss new evidence related to matrix metalloproteinases, growth factors, and cellular metabolism in the form of lactic acid generation that may have consequences for promoting fibrogenesis. We discuss potential cross talk between innate and structural cell types and also highlight literature that may help explain the limitations of current IPF therapies.
Keywords: epithelial cells, fibroblasts, fibrosis, innate immunity, lung
the pulmonary interstitium is a source of wonder in its structure, function, and ability to support human life through gas exchange. Understandably, disorders of the pulmonary interstitium often have severe consequences for affected individuals, but the causes and pathogenesis of many of these diseases have eluded modern science and medicine. While some disorders like hypersensitivity pneumonitis and asbestosis are associated with a known etiology, other forms of interstitial pneumonia are of unknown cause and thus are labeled idiopathic. The most common form of idiopathic interstitial pneumonia is idiopathic pulmonary fibrosis (IPF), and, given its invariably fatal clinical course, IPF has garnered the most attention within the research community (98). IPF has a median survival of 2 to 3 years and a steady incidence in older adults (97). IPF is a chronic and progressive lung disorder characterized by aberrant deposition of extracellular matrix (ECM) leading to extensive lung remodeling (52). Patients display significant heterogeneity in their clinical courses and outcomes (35, 109). The disease paradigm currently centers on repetitive injury to the alveolar epithelium with the release of molecules that result in proliferation of resident fibroblasts, myofibroblast differentiation, aberrantly regulated ECM deposition, and, in turn, dysfunctional repair and remodeling (52). However, given the natural history of IPF and the existence of different clinical phenotypes and genotypes (35, 95, 109), it has become clear that the molecular mechanisms promoting disease biology are also diffuse and heterogeneous and may involve an extensive array of different pathways and processes including apoptosis (126), oxidative stress (42), intra-alveolar coagulation (104), aberrant development (107), endoplasmic reticulum stress (62), and telomere shortening (4). Animal models have generally centered on the bleomycin model due to its clinical relevance, but there is also interest in asbestos-related fibrosis models because they develop characteristic pathological lesions of usual interstitial pneumonia seen in IPF (77a).
Recent studies have provided new insight into the roles that innate immune cells, particularly neutrophils and macrophages, may play in lung fibrosis. In addition, the role of infectious agents as drivers of fibrogenesis is gaining attention. As we learn more about how cells of the lung cope with cellular stress from infection or injury, studies have explored how autophagy may modulate fibrogenic responses. Finally, as fibroblasts and myofibroblasts are believed to be the main effector cells in fibrosis, understanding how these cells are activated and how their metabolic phenotypes may change also offers new targets for antifibrotic therapies. In this review we cover some of the recent advances in these areas and highlight potential interactions. Figure 1 summarizes how these factors contribute to lung fibrosis.
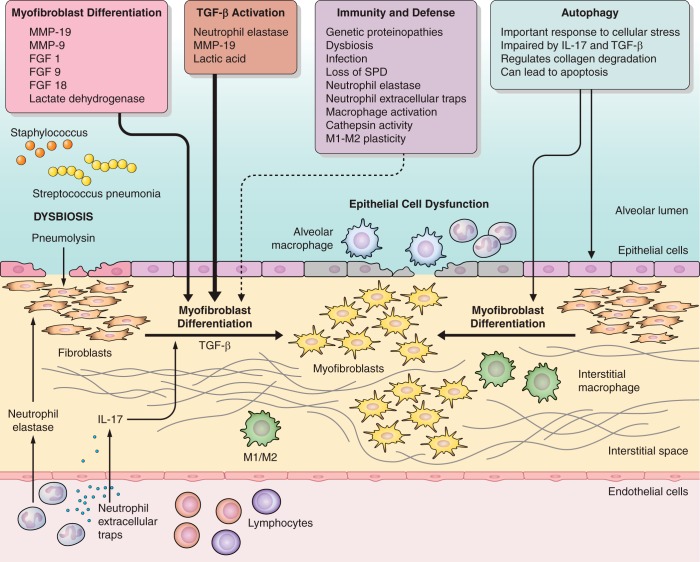
Fig. 1.
Schematic depicting recent insights into pathogenesis of pulmonary fibrosis. Pulmonary fibrosis is influenced by the orchestration of epithelial, mesenchymal, and immune interactions. Recent data highlight the important roles of innate immune cells, especially monocytes and neutrophils, in regulating TGFβ activation, fibroblast phenotypes, and epithelial injury. Additionally, immune dysfunction may lead to infections that can exacerbate lung fibrosis. Autophagy plays an important role in epithelial cell response to stressors and autophagy is impaired by profibrotic factors such as IL-17 and TGFβ that can be delivered by innate immune cells. Metabolic changes resulting in release of lactic acid are now appreciated to activate TGFβ to drive myofibroblast activation. Additionally, MMPs and fibroblast growth factors influence fibroblast phenotypes in lung fibrosis as well. The concepts highlighted in the boxes are the subject of this review of recent literature.
New Insights into Innate Immunity and Fibrosis
Neutrophils as mediators of fibrotic pathogenesis.
The role of inflammation in IPF and other forms of lung fibrosis has long been debated (17). Neutrophils are considered short-lived mediators of acute inflammation. Migration of neutrophils to a site of injury allows them to exhibit a range of functions, including the release of neutrophil elastase (NE) where they may then impact the fibrotic process (Table 1). NE promotes fibroblast proliferation and myofibroblast differentiation in vitro and mice deficient in NE are protected from asbestos-induced pulmonary fibrosis (37). Furthermore, mice treated with a NE antagonist after asbestos injury demonstrate reduced hydroxyproline content consistent with attenuated fibrosis (37). Interestingly, the mechanism of myofibroblast differentiation induced by NE was independent of transforming growth factor (TGF)-β, a classical inducer of myofibroblast differentiation. Chua et al. (24) reported an attenuated fibrotic response in NE−/− mice inoculated with bleomycin despite the fact that neutrophil influx in lavage fluid was comparable in wild-type (WT) and NE−/− mice. Interestingly, the bleomycin-treated NE−/− mice showed deficits of TGFβ in vivo. Similarly, Sivelestat, a NE inhibitor, mediates an attenuated fibrotic response to bleomycin in mice through impaired TGF-β activation and reduced pulmonary migration of inflammatory cells (124). Studies of bronchopulmonary dysplasia with hyperoxia-induced inflammation and fibrosis in rat models demonstrate that treatment with metformin (a common prescribed antihypoglycemic agent) results in decreased neutrophil and macrophage influx with associated lower levels of inflammation and fibrosis (21). In contrast, overactive NE can result in development of emphysema, especially when the protease/antiprotease balance is disrupted as occurs with α-1-antitrypsin deficiency or through mutation of this circulating proteinase inhibitor, and recent studies have shown that NE retains proteolytic activity even when complexed to circulating α-2-macroglobulin (113).
Table 1.
Neutrophil roles in lung fibrosis
| Activities That May Promote Fibrosis | Activities That May Limit Fibrosis |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
Neutrophil extracellular traps (NETs) are released by neutrophils and consist of filaments of decondensed chromatin and granular proteins. The release of NETs may cause local tissue damage and inflammation with already notable roles in asthma, cystic fibrosis, and acute viral-mediated lung injury (31, 71, 82). Neutrophils that release NETs are also capable of releasing the profibrotic cytokine IL-17 (64). NETs have been shown to promote human lung fibroblast proliferation and differentiation in vitro and NET release is associated with IL-17-fueled upregulation of connective tissue growth factor (CCN2, CTGF) and ECM generation (23). In human IPF patients, the presence of neutrophils is significant and a doubling of neutrophils, specifically in lavage fluid at baseline, is a predictor of early death (51). It is interesting that prostaglandin E2, the first mediator shown to be an endogenous inhibitor of neutrophil NETS (30), is also a molecule known to be deficient in IPF (68, 133), perhaps suggesting that the fibrotic lung milieu is prone to NET formation.
Moncytes/macrophages as drivers of lung fibrosis.
Macrophages reside in almost all tissue compartments and play crucial roles in development and homeostasis (32, 78). The ability to phenotype the many subpopulations of macrophages in the lungs of mice and humans by flow cytometry has recently been described (11, 13, 72, 142). Desai et al. (29), using mRNA sequencing of peripheral blood mononuclear cells from IPF patients, identified upregulated genes associated with monocyte movement, adhesion and macrophage activation. Mantovani et al. (70) have previously proposed a nomenclature for classically and alternatively activated macrophages as M1 and M2, respectively, and this has been broadly adopted. The M2 macrophage phenotype has well-established functions in wound healing and plays a role in aberrant fibroproliferation that occurs during pulmonary fibrosis (115, 122). However, recent work has prompted a reconsideration of simple macrophage phenotypes and polarization in fibrotic disease. Pulmonary macrophages may coexpress surface markers of both M1 and M2 activation. Anthony et al. (5) demonstrated that in human-derived monocytes stimulated with serum amyloid A, the subsequent macrophage phenotype is mixed with increased expression of proinflammatory genes (IL-6 and IL-1β) and coexpression of the M2-related CD163 marker. Lung-resident macrophages may display significant phenotypic plasticity that may be related to specific stimuli or a disease microenvironment. Thus these M1/M2 phenotypes may represent a transient spectrum of activation and may explain some of the biological heterogeneity seen in interstitial lung disease.
Recent work by Withana et al. (134) confirmed the presence of activated macrophages in areas of fibrotic lung in both human IPF patients and bleomycin-challenged mice using novel cysteine cathepsin-targeting imaging probes. The authors monitored the contribution of activated macrophages and demonstrated, for the first time, the accumulation of monocyte/macrophages expressing active cathepsins in the lungs of IPF patients. This evidence suggests that activated macrophages support IPF pathogenesis; however, other studies have indicated a protective role for spleen-derived macrophages (M2 phenotype) partially due to an overabundance of M1 macrophages in pulmonary fibrosis (130). The activation of macrophages occupies a pivotal role in the translation of injury to aberrant repair in IPF (Table 2), but the reasons for macrophage accumulation in lung fibrosis are not always clear. However, one possible driver of macrophage accumulation and activation may be loss of surfactant protein D (SPD) in the lungs.
Table 2.
Monocyte/macrophage roles in lung fibrosis
| Activities That May Promote Fibrosis | Activities That May Limit Fibrosis |
|---|---|
|
|
|
|
|
|
|
SPD has been strongly linked to lung fibrosis (6, 26, 36, 45, 59, 61, 84, 112, 123, 132), but results depend on where SPD is measured. Multiple studies have shown that SPD levels are elevated in circulation of IPF patients (26, 36, 45, 112, 123, 132) and that these levels predict disease severity or progression. In contrast, if measurements are made in the bronchoalveolar lavage (BAL) fluid (BALF), studies have shown that SPD levels are lower (45, 59, 61, 84). This is likely explained by the fact that SPD has high hydrophilicity, making it easy to move out of the lung and into the vasculature (84). Loss of SPD in the lung compartment predisposes to worse fibrosis. Aono et al. (6) used a triple transgenic inducible SPD murine model (doxycycline treatment generated SPD production) to demonstrate that absence of SPD production resulted in increased pulmonary macrophage infiltration, elevated profibrotic cytokines in the lungs, and increased numbers of fibrocytes, suggesting a crucial role for SPD in the regulation of both macrophage and fibrocyte recruitment in fibrosis. Once recruited, macrophages and fibrocytes are also capable of producing matricellular proteins like periostin (80) that have been shown to promote myofibroblast differentiation (9). These data highlight the fact that genetic alterations that lead to macrophage accumulation may promote fibrogenesis. It is also possible, however, that loss of the immune collectin function of SPD in the alveolar space leaves patients susceptible to infection while also increasing macrophage accumulation.
The evidence reviewed thus far supports a pivotal role for the influx of inflammatory cells, in particular neutrophils and macrophages, in activation of profibrotic mediators to regulate fibrogenesis. However, the categorical failure of anti-inflammatory and immunosuppression therapy in IPF has led many to believe inflammation is unimportant, or that the inflammatory phase of the disease is over by the time most patients develop symptoms. It is equally possible, however, that we do not yet fully understand the nature of how or which inflammatory immune cells may be contributing to pathogenesis. Leukocytes such as mast cells, eosinophils, innate lymphoid cells, and lymphocytes may also play roles in fibrogenesis. It is likely that the immunosuppressive agents we have used to date may not have effectively targeted the profibrotic actions of innate immune cells and in fact may have left patients more susceptible to infection-related exacerbations.
Infection and fibrosis.
IPF represents a complex interaction between host genetics and the environment. Recent genomic studies have expanded our knowledge of the potential genetic risk factors present in IPF patients. Several genes that play a role in innate immunity and its regulation within the lung have been identified, namely Toll interacting protein (TOLLIP), Toll-like receptor (TLR) 3, and MUC5B (15, 85, 86, 105, 118). These can have a profound effect on the risk of disease development and may alter clinical course. For instance, the rs5743890 TOLLIP variant is associated with increased susceptibility to disease, increased mortality, and reduced expression of the protein in IPF patients (85). MUC5B studies in IPF have identified protein overexpression in the lung and accumulation in terminal bronchioles (105). While the exact contribution of MUC5B to IPF pathogenesis remains unclear, authors central to the MUC5B discovery process have hypothesized that the abnormal accumulation of the protein may lead to impaired localized host defense and recurrent injury (139). Thus several of the major risk alleles for IPF susceptibility and disease progression are regulators of innate immunity and play a role in the response of the airways to injury [danger- and pathogen-associated molecular patterns (DAMPS and PAMPS, respectively)].
One of the features of the current paradigm involves injury to the alveolar epithelium. There is considerable biological plausibility for known and occult infection as a promoter of injury in this environment. Furthermore, modifiers of innate responses in the airway including several of the airway defensins have reported associations with IPF. The role of infection in IPF pathogenesis is evolving with several recent publications supporting a role for bacterial and viral pathogens. Knippenberg et al. (53) have reported that a streptococcus-produced toxin, pneumolysin, can promote the progression of fibrosis in several models of murine pulmonary fibrosis. Molyneaux et al. (75) have reported an association with disease progression and bacterial burden. Analysis of genomic DNA through amplification of the bacterial I6S rRNA gene led to quantification and identification of bacterial communities from BALF from IPF patients and controls (which included chronic obstructive pulmonary disease and healthy control patients). Disease progression in this cohort was associated with an increased bacterial burden, independent of the MUC5B promoter polymorphism. Streptococcus was one of the pathogens found at increased burden compared with controls (75). The authors postulated that trials of antimicrobial therapy may be merited to define whether bacterial burden is truly associated with the biology of disease progression in IPF. The case for bacterial influence on disease biology was further supported by trials of the antibiotic trimethoprim-sulfamethoxazole that demonstrated improved mortality and quality of life in a double-blind multicenter study in patients with fibrotic lung disease (111). The authors adjusted for concurrent immunosuppressant treatment and generated sensitivity testing for features of IPF within the patient cohort, concluding that antimicrobial prophylaxis required further study in IPF. The microbiome has been studied in chronic lung disease and alterations in community structure have been associated with exacerbations of lung disease (87). Analysis of the lung microbiome in the COMET cohort of IPF patients reported an association with changes in community structure, namely an increase in Streptococcal and Staphylococcal species, and IPF progression (40). Taken together, the data to implicate Streptococcus in IPF disease progression are especially strong; however, Pseudomonas aeruginosa, a common pathogen in chronic lung disease, has not yet been implicated in fibrogenesis. We have shown that bleomycin-treated mice demonstrate effective Pseudomonas clearance without enhanced ECM deposition (8). Conversely, we have previously demonstrated the capacity for latent viral infection, namely herpesvirus, to exacerbate murine pulmonary fibrosis models (129). Several candidate viruses have been identified in the BAL and lung tissue in pulmonary fibrosis patients and the potential role for viruses in IPF disease biology has been reviewed elsewhere (77); however, causality has not been established. The presence of virus may simply be secondary to IPF pathophysiology due to impaired host defense, architectural modifications, or the possible impact of immunosuppression. The identification of herpesvirus DNA in cell-free BAL of at-risk individuals (relatives of cases of familial interstitial pneumonia) and the increased expression of herpesvirus antigen in alveolar epithelial cells highlights the potential causal association with IPF and supports further mechanistic work (58). Finally, indirect support for a role for infectious pathogens is drawn from the increased risk of mortality and hospitalization with pharmaceutical immunosuppression in IPF (96). At face value, it is hard to reconcile data suggesting that pathogens drive IPF pathogenesis with the observations of increased innate immune cells in the fibrotic lung. These results could be interpreted either as IPF patients having impaired innate immune functions, allowing outgrowth of potential pathogens that cause lung injury, or could be viewed as an expected accumulation of immune cells in response to microbial stimulation. Future studies investigating antibiotics in IPF and specific immune cell functions in the fibrotic lung should help clarify these questions.
The most likely cell types to be targeted by injury or infection are the epithelial cells of the lung. The inability to effectively cope with damage or a failure to repair the epithelium can also exaggerate the fibrotic process. Thus the cellular process of autophagy, a process that has implications for host defense as well as cell survival, has garnered significant attention as a common pathophysiological mediator of fibrosis.
Autophagy as a Regulator of Lung Fibrosis
Autophagy is a cellular process activated by various physiological stresses than can be induced to promote cell survival. It involves the sequestration of dysfunctional cellular cytoplasmic components within double-walled membrane vesicles termed autophagosomes for delivery to the lysosome for destruction and recycling. Autophagy is classified as microautophagy, chaperone-mediated autophagy, and macroautophagy (144). Canonical macroautophagy (hereafter called autophagy) involves recruitment of a hierarchical set of autophagy-related (Atg) proteins to form the autophagosome (25), and this process is implicated in the pathogenesis of chronic pulmonary diseases (2). In addition, there are also noncanonical pathways to generate autophagosomes within cells (25). As mentioned, the process can serve important functions in host defense against internalized pathogens (81, 138) by delivering the autophagosomal contents to the lysosome for degradation. However, uncontrolled autophagy can lead to apoptosis. A process known as selective autophagy is employed to recycle particular organelles (e.g., mitochondria via mitophagy), in response to cellular stress (74). The process of autophagy is highly regulated via complex posttranslational modification of Atg proteins and has recently been reviewed (16). Given that IPF is a disease associated with aging, and that defective autophagy is also noted in aging (28, 116), it is not yet clear whether defective autophagy is causal or merely coincident with IPF. However, there is growing evidence that tissues from IPF patients are characterized by defective autophagy responses (92, 101). Additionally, recent proteomic analysis of IPF patient plasma revealed that low levels of legumain and cathepsin-S, two proteases putatively involved in autophagy (89, 91), predict poor outcomes in IPF (7). It is also interesting to speculate, however, that reduced levels of these proteases may allow for pathogens to accumulate and injure lung tissue. Recent studies are revealing the extent to which these pathways are altered in fibrotic lungs and are providing some clues to mechanisms (Table 3).
Table 3.
Consequences of reduced autophagy in IPF
|
|
|
|
|
|
|
Defective autophagy promotes epithelial cell dysfunction.
Epithelial cells serve important barrier functions in the lung, but they are also pivotal in the setting of lung fibrosis because they can secrete both antifibrotic/anti-inflammatory mediators like PGE2 (12, 117) as well as profibrotic factors (106, 140). The majority of studies related to autophagy and fibrosis have focused on epithelial cells due to a prevailing hypothesis that epithelial cell stress (e.g., infection) leads to chronic or repetitive lung injury which in turn promotes fibroproliferation. For instance, Liu et al. (65) demonstrated that treatment of lung epithelial cells with profibrotic IL-17, a cytokine known to be released by neutrophils that undergo NETosis (64), reduced the expression of several important genes within the autophagy pathway including beclin 1 and Atg14 (65). It is speculated that the attenuation of autophagy induced by IL-17 helps promote fibrogenesis and likely suppresses collagen degradation pathways. In addition to IL-17 inhibiting autophagy, bioactive lipids like sphingosine-1 phosphate and lysophosphatidic acid are also known to inhibit autophagy (46, 125) and to promote fibrogenesis (110).
One of the clearest examples of how defective autophagy may impact interstitial lung disease comes from the study of lung proteinopathies such as surfactant protein C (SPC) and A mutations or Hermansky-Pudlak syndrome (HPS) that cause accumulation of misfolded proteins within the epithelium leading to cell stress and eventual fibrosis (57). For example, the mutation of threonine for isoleucine at codon 73 in the human SPC gene accounts for a significant fraction of SPC mutation-associated interstitial lung disease. Cell lines engineered to stably express this mutation expressed elevated levels of Atg8/LC3, sequestosome (p62), and Rab 7 consistent with a block in the ability to form mature autophagosomes (41). Overexpression of this mutant protein caused a block in autophagic flux leading to decreased proteostasis and mitophagy, suggesting that patients harboring such mutations would have diminished ability to handle secondary insults, making them more susceptible to epithelial injury and development of lung fibrosis. Recently, a transgenic mouse created to express the common misfolded variant of α-1 anti-trypsin, the so-called PiZ mouse, was also shown to model the proteotoxicity of the misfolded proteins within lung epithelium and to develop leukocyte infiltration and spontaneous fibrosis. Interestingly, treatment with drugs to enhance autophagy improved outcomes (44). Impaired autophagy has also been noted in the epithelial cells of patients with Hermansky-Pudlak disease as well as in mice carrying genetic mutations in HPS genes (3). In vitro studies demonstrated knockdown of the HPS1 gene in A549 lung epithelial cells resulted in accumulation of lipidated LC3b and p62 as well as an increase in proapoptotic caspases; however, overexpression of LC3b restored the defects in autophagy and reduced p62 accumulation (3).
Genome-wide association studies identified the autophagy gene Cep55 as having a polymorphism significantly associated with susceptibility to bleomycin-induced lung fibrosis in mice (93). Similarly, MMP-19−/− mice, which develop an exaggerated form of bleomycin-induced lung fibrosis, show downregulation of the autophagy gene Atg4c, which is a peptidase necessary for expanding the autophagosomal membranes (48). When this work is taken in context with the HPS-1 knockdown studies above (3), it is interesting to speculate that some forms of lung fibrosis are characterized by defects in the ability to mobilize the membranes for formation of the autophagosomes. Undoubtedly, the situation is far more complicated than that, however, because some forms of lung injury (e.g., amiodarone challenge) result in accumulation of lamellar bodies leading to progressive hypertrophy of type II epithelial cells (14). With amiodarone challenge, autophagosomes accumulate in direct connection with, and likely originating from, the limiting membrane of the lamellar body. Yet, in this setting, accumulation of SPC was associated with formation of more, rather than fewer, autophagosomes and eventual apoptosis of the epithelial cells correlating with development of interstitial fibrosis (67).
Other studies focused on epithelial cells have shown that Atg4b-deficient mice have a greater inflammatory response 7 days postbleomycin and augmented apoptosis of alveolar and bronchial epithelial cells leading to more extensive fibrosis and collagen accumulation (18). Similarly, conditional knockdown of the tuberous sclerosis-1 (Tsc1) gene in epithelial cells causes mice to be more susceptible to bleomycin-induced fibrosis, a condition that was reversible when rapamycin or chloroquine was administered to stimulate autophagy (38).
Autophagy influences on myofibroblast activation.
The pathological deposition of ECM in lung fibrosis is believed to be accomplished by α-smooth muscle actin (α-SMA)-expressing myofibroblasts. Thus studies of autophagy have also focused on this cell type. TGFβ1-stimulation is known to inhibit autophagic flux in fibroblasts (116). Activation of mammalian target of rapamycin (mTOR) complex 1 via treatment with rapamycin in vitro can limit expression of the myofibroblast proteins α-SMA and fibronectin (92). Acquisition of the pathological myofibroblast phenotype changes the way fibroblasts respond to the stiffening ECM within the lung during fibrogenesis. For instance, when normal fibroblasts encounter stiffened polymerized collagen, autophagy pathways are activated in response to the external stress; however, myofibroblasts derived from IPF patients do not activate this stress response and instead show low levels of autophagy induction on polymerized collagen (83). This is driven by the aberrant PTEN/Akt/mTOR signaling pathway that allows the fibrotic myofibroblasts to survive in the face of accumulating matrix stiffness (83). The molecular pathway that accounts for this was recently worked out. FoxO3a is a downstream target of Akt and is implicated in the transcriptional activation of autophagy. FoxO3a mRNA and protein levels are low in IPF fibroblasts, and this accounts for the impaired induction of LC3b mRNA expression that occurs on polymerized collagen (47). Because IPF fibroblasts already have low basal levels of autophagy, disruption of autophagosomes by 3-methyladenine or chloroquine only modestly increased death of IPF fibroblasts on polymerized collagen (83). In endothelial cells, loss of ATG7 function resulted in upregulation of profibrotic TGFβ and promotion of endothelial-mesenchymal transition, features that were associated with development of more severe pulmonary fibrosis in response to bleomycin administration (114).
Therapeutic implications related to autophagy.
Looking at data summarized above from both epithelial cells as well as fibroblasts, it is generally true that both epithelial dysfunction and myofibroblast survival are associated with deficient autophagy. Thus several studies have examined modulation of autophagy pathways in the setting of lung fibrosis. One of the two currently available therapeutics to treat IPF, nintedanib induces noncanonical autophagy (beclin 1-dependent, but Atg7-independent) coincident with its ability to downregulate ECM production in IPF fibroblasts (101). Berberine was also recently shown to enhance autophagy and suppress bleomycin-induced fibrosis (22). Interestingly, studies showed that giving rapamycin as a pretreatment to augment autophagy could limit bleomycin-induced fibrosis in wild-type mice (38), but treatment with rapamycin or its analog sirolimus had no effect if initiated 8 or 9 days after bleomycin injection (38, 127). It is interesting to speculate that early enhancement of autophagy may protect epithelium, but once myofibroblast differentiation has occurred and the matrix starts to stiffen, myofibroblasts may be relatively insensitive to autophagy manipulation. This may also explain the failure of the mTOR inhibitor everolimus, which would be expected to induce autophagy to improve outcomes in IPF (69). In fact, everolimus use was associated with more rapid disease progression. The reasons for this are still not clear and highlight the fact that more research is needed to understand how autophagy regulates the responses of multiple cell types, including myeloid cells (1, 20), in the setting of lung fibrosis before we can intelligently target this pathway for clinical therapy. It is also possible that pharmacological strategies to induce autophagy must be tightly controlled lest the overactivation of these pathways lead to epithelial apoptosis.
As mentioned initially, the current paradigm for the pathogenesis of lung fibrosis suggests that loss of the lung epithelium, as may occur in the setting of defective autophagy, results in activation of fibroblasts, differentiation of myofibroblasts, and deposition of ECM. These fibroblasts and myofibroblasts can then remodel the lung architecture via matrix and mediator production as well as via the secretion of metalloproteinases. The next section will review the recent insights into the regulation of myofibroblasts and will highlight new data suggesting that metabolic reprogramming of the fibroblasts may perpetuate lung injury and fibrogenesis.
New Insights into Myofibroblast Biology
Myofibroblasts are characterized, in part, by the presence of α-SMA and their ability to activate TGFβ and produce ECM proteins including type I collagen. Lung tissue from patients with pulmonary fibrosis contains myofibroblasts (60, 73, 90), and these have been thought to be responsible for the deposition of collagen and other ECM components during development and progression of pulmonary fibrosis (94). However, new evidence using reporter mice for collagen 1 and α-SMA has suggested that the majority of the collagen-producing fibroblasts in the lung are actually α-SMA negative, as opposed to the liver where they are generally α-SMA positive (121). In the lung, it appears that α-SMA-negative fibroblasts retain the capacity to activate TGFβ and promote fibrogenesis (121). Regardless, myofibroblasts do accumulate in lung fibrosis and many recent studies of their activation and phenotypes have focused on matrix metalloproteinases (MMPs), especially MMP19 and 9; fibroblast growth factor receptors (FGFRs); and lactate dehydrogenase (LDH).
Opposing functions of MMP 19 and 9 in lung fibrosis.
MMPs are metal-requiring catalytic enzymes capable of degrading ECM (79). Initially, MMPs were envisioned as being antifibrotic due to their ability to degrade matrix, but they also regulate cellular proliferation, migration, and activation, suggesting that they could play multiple and complex roles in the regulation of fibrosis (27). Recently, new studies have looked at effects of MMP19 and MMP9, which appear to have opposing roles. MMP19 was found to be highly expressed in hyperplastic epithelium of IPF patients (141), where it is believed to play a protective role via regulation of cyclooxygenase-2 expression and therefore prostaglandin synthesis. Given the importance of prostaglandins in limiting myofibroblast differentiation (55), it is perhaps not surprising that MMP19−/− mice develop more severe lung fibrosis in response to bleomycin (141). In fact, overexpression of MMP19 in lung epithelial cells can limit fibroblast proliferation in coculture (135). When considered along with data presented above, it is interesting to speculate that MMP19 overexpression from epithelial cells promotes the ability of the fibroblasts to form autophagosomes (48) as an additional mechanism to limit fibroproliferation. Not surprisingly, gene array analysis showed that MMP19−/− lung fibroblasts showed increased collagen mRNA and protein production and increased α-SMA expression (48). Likewise, MMP19-deficient lung fibroblasts showed a significant increase in proliferation (48).
In contrast to the protective role of MMP19 in lung fibrosis, MMP9, also known as gelatinase-B, is increased in the lungs of IPF patients (43, 63, 119) but may promote pathogenesis in a cell type- and context-dependent manner (reviewed in Ref. 27). MMP9 is expressed by immune cells (macrophages and neutrophils) as well as structural cells such as fibroblasts and alveolar epithelial cells (43, 63, 108, 119). MMP9 is also highly expressed in IPF fibroblastic foci (108) and in the BALF of bleomycin-treated mice (33). In a study assessing the role of endogenous MMP9 in modulating fibroblast-mediated contraction of 3D collagen gels, lung fibroblasts from MMP9−/− mice showed a significant decrease in contractility compared with WT controls (54). The same result was seen in human lung fibroblasts treated with the pan-MMP inhibitor GM-6001 or fibroblasts with expression of MMP9 knocked down using siRNA. The overall loss of MMP9 resulted in decreased TGFβ1 activity in both murine and human lung fibroblast cultures (54). Additionally, TGFβ has been shown to modulate the release of MMP9 (143) and Thy-1 (CD90)-deficient lung fibroblasts stimulated with TGFβ1 expressed MMP9 through the activation of ERK1/2 signaling pathways, while Thy-1-positive cells did not (100). These findings suggest that TGFβ induction of MMP9 in lung fibroblasts could be a positive feedback loop that promotes fibroblast activation during lung fibrosis. When considering cross talk between immune cells and structural cells, it is interesting to speculate that the TGFβ signal that initiates MMP9 expression may come either from damaged epithelium as a result of defective autophagy, or possibly from accumulation of innate immune cells. In turn, the immune cells may be activated to secrete more MMP9, potentiating the lung damage. Fibroblast activation would also be exacerbated by the loss of protective MMP19 from injured epithelium as well.
FGFRs and lung fibrosis.
FGFRs regulate a number of biological functions in both embryonic and adult stages of development by binding to the necessary receptors and activating downstream signaling pathways. FGF1/FGFR is highly expressed in pathogenic regions of IPF lung tissue as well as whole lung homogenates, suggesting a role in IPF pathogenesis (66). Nintedanib, which is currently being used in IPF clinics, is a multitarget tyrosine kinase inhibitor that also targets FGFRs and showed significant efficacy in phase III studies (103). Thus a prevailing notion is that FGFR signaling is detrimental in the setting of lung fibrosis. Recent results, however, suggest that this interpretation may be too simplistic.
In a bleomycin-induced model of fibrosis, FGFR2b- signaling to alveolar epithelial cells was shown to cause increased survival and decreased lung fibrosis (39, 120). Joannes et al. (49) assessed the expression of FGF9, FGF18, and FGFRs in lung tissues from IPF patients and controls. They demonstrated that FGF9, FGF18, and all FGFRs were present in the remodeled alveolar epithelium close to the fibroblastic foci (49) and they tested the effects of these growth factors on lung fibroblasts. These mediators affected the overall biology of lung fibroblasts through multiple mechanisms, some of which were antifibrotic. For instance, FGF9 downregulated collagen-I and α-SMA expression in IPF fibroblasts compared with controls, while FGF18 had no effect on TGFβ1-induced myofibroblast differentiation (49). Regarding proliferation, FGF18 inhibited cell growth in control fibroblasts but not IPF fibroblasts whereas FGF9 and FGF1 had no effect on fibroblast proliferation. Thus these beneficial effects of FGFR signaling may actually be impaired with nintedanib.
In contrast, FGF9, FGF18, and FGF1 all enhanced the migratory effects of control and IPF lung fibroblasts. FGF1 was previously shown to promote migration in a number of cell types, such as rat fibroblasts, arterial smooth muscle cells and breast cancer cells (99). FGF18 drives migration in colorectal cancer cells and endothelial cells as well (34, 88). In terms of myofibroblast survival, however, both FGF9 and 18 decreased Fas ligand-induced apoptosis in control but not IPF fibroblasts. Thus FGFR signaling in fibroblasts is complex and the mixed responses may explain why current therapies are only partially effective.
Lactate dehydrogenase in myofibroblasts.
The mechanisms to explain cellular metabolism changes during IPF are still poorly understood. Lactic acid was recently identified as a metabolite that is elevated in the lung tissue of IPF patients (56), demonstrating a role for dysregulated glycolysis in promoting lung fibrosis. For example, lactic acid concentration was elevated in lung tissue from patients with IPF, and endogenous production of lactic acid was capable of activating latent TGFβ in cell culture in a pH-dependent manner (56). Additionally, overexpression of LDH induced myofibroblast differentiation in a pH-dependent and TGFβ-dependent manner. Inhibition of LDH using siRNA caused a significant decrease in TGFβ-induced myofibroblast differentiation (56).
Recently gossypol, a polyphenolic compound from cottonseed oil and an inhibitor of LDH, was used in preclinical fibrosis studies. This compound has numerous pharmacological properties including antifungal, anti-inflammatory, antitumor, and antifertility activity (76, 128). Additionally, it has been reported that gossypol exhibits immunosuppressive effects on mouse lymphocytes in vitro and suppresses delayed-type hypersensitivity in vivo in a mouse model, through a mechanism that was thought to involve lymphocyte proliferation and induction of cell death (137). Kottman et al. (56) demonstrated that gossypol inhibited TGFβ-induced myofibroblast differentiation in both control and fibrotic human lung fibroblasts. To identify a mechanism outside of what was previously known about gossypol, the authors incubated TGFβ-treated cell lysates with gossypol and assessed LDH activity to determine whether release of lactic acid could be activating latent TGFβ to induce myofibroblast differentiation (56). It is interesting to note that even though gossypol inhibited TGFβ-stimulated myofibroblast differentiation in control fibroblast the effect was less pronounced in IPF fibroblasts at lower concentrations. This may be due to a higher expression of the LDH gene (LDHA) in IPF fibroblasts and higher rates of extracellular acidification compared with control fibroblasts (56). Future studies are necessary to understand the antifibrotic and the likely anti-inflammatory effects of gossypol and how it could be used as a therapeutic. However, the data strongly suggest that LDH inhibition diminishes TGFβ-induced myofibroblast differentiation. A more recent study by Chen et al. (19) also showed a decrease in liver fibrosis in diabetic rats that were treated with gossypol. Type 2 diabetes was induced in rats by feeding with high-fat diet and injection of streptozocin and then treated with gossypol for 4 wk. After gossypol treatment there was a significant decrease in liver fibrosis as noted by a decrease in the mRNA levels of ECM proteins (Col I, Col 3, fibronectin), tissue inhibitor of matrix metalloproteases (Timp)-1 and 2, as well as glucose-6-phosphatase (19). These data provide evidence of a potential therapeutic role for gossypol in treating different fibrotic conditions including pulmonary fibrosis, Type 2 diabetes, and diabetes-related fibrosis. Glycolytic reprogramming as a regulatory step in the differentiation of fibroblasts to myofibroblasts is also supported by recent work showing inhibition of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) in human IPF fibroblasts in vitro attenuates fibroblast to myofibroblast differentiation and that targeting glycolysis with 3PO, a PFKFB3 inhibitor, reduces fibrosis in both a bleomycin model and TGF-β1-driven animal model of fibrosis (136).
LDH inhibition also seems to regulate radiation-induced fibrosis. For instance, LDHA was shown to be upregulated in radiation-induced fibrosis (50). This upregulation was seen both in lung tissue as well as in irradiated lung fibroblasts, confirming previous data that lactate is required for radiation-induced myofibroblast differentiation. Based on these data it is hypothesized that LDHA could be a possible therapeutic target for multiple forms of fibrosis. Exactly how acidification of the extracellular environment cross talks with innate immune cells, which are abundant sources of latent TGFβ to regulate fibrogenesis, is currently unknown but is certainly an area of study suggested by these recent observations.
Summary
As new data continue to accumulate, our understanding of the complexity of lung fibrosis also expands. Targeting any particular cell type (immune cells, epithelial cells, or fibroblasts) is challenging because each of these cell types participates in both pathological as well as homeostatic actions. We also know that innate immune cells have more opportunity to cross talk with structural cells of the lung than was previously appreciated. For example, release of IL-17 from neutrophils promotes fibroblast proliferation; release of MMP9 from innate immune cells may activate TGFβ to drive myofibroblast differentiation; PGE2 secretion from epithelial cells may limit NETosis and also inhibit fibroblast actions; glycolytic reprogramming of fibroblasts may acidify the extracellular environment leading to TGFβ activation from immune cells. We also reviewed literature suggesting that neutrophils can activate TGFβ via expression of NE. Once TGFβ is present, it can further promote fibrogenesis by blocking autophagy and increasing ECM deposition. We now know that α-SMA-negative fibroblasts are capable of inducing TGFβ as effectively as α-SMA-expressing myofibroblasts (121), prompting us to need to reevaluate many factors that may be pathological but overlooked due to their inability to regulate myofibroblast differentiation when tested. Additionally, we reviewed literature suggesting innate immune cells are also critical for host defense against infections and suggested that outgrowth of certain pathogens may potentiate IPF pathogenesis (e.g., Streptococcus spp and herpesviruses). Thus we will need to develop targeted therapies that can reduce fibrosis without compromising host defense. In this regard, strategies to enhance autophagy are particularly intriguing as they could help epithelial cells resist injury or cell stress while potentially improving antibacterial and antiviral functions as well (102, 131), provided that the activation of the autophagy pathway is not so extreme as to induce epithelial cell apoptosis. The more we learn, the more we start to understand how current therapies like nintedanib may be suboptimal because they block beneficial as well as pathological growth factor signaling. This underscores the need for additional preclinical research to develop more specific and effective therapies.
GRANTS
This research was supported by National Institutes of Health Grants HL115618, AI117229, HL127805, and HL119682 to B. B. Moore and AI007413 to S. L. Ashley as well as a scholarship from UNCF-Merck (S. L. Ashley).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.O., S.L.A., and B.B.M. analyzed data; D.O. prepared figures; D.O., S.L.A., and B.B.M. drafted manuscript; D.O., S.L.A., and B.B.M. edited and revised manuscript; D.O., S.L.A., and B.B.M. approved final version of manuscript.
REFERENCES
- 1.Abdel Fattah E, Bhattacharya A, Herron A, Safdar Z, Eissa NT. Critical role for IL-18 in spontaneous lung inflammation caused by autophagy deficiency. J Immunol 194: 5407–5416, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal S, Mannam P, Zhang J. Differential regulation of autophagy and mitophagy in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol 311: L433–L452, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahuja S, Knudsen L, Chillappagari S, Henneke I, Ruppert C, Korfei M, Gochuico BR, Bellusci S, Seeger W, Ochs M, Guenther A, Mahavadi P. MAP1LC3B overexpression protects against Hermansky-Pudlak syndrome type-1 induced defective autophagy in vitro. Am J Physiol Lung Cell Mol Physiol 310: L519–L531, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, Vulto I, Xie M, Qi X, Tuder RM, Phillips JA 3rd, Lansdorp PM, Loyd JE, Armanios MY. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA 105: 13051–13056, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anthony D, McQualter JL, Bishara M, Lim EX, Yatmaz S, Seow HJ, Hansen M, Thompson M, Hamilton JA, Irving LB, Levy BD, Vlahos R, Anderson GP, Bozinovski S. SAA drives proinflammatory heterotypic macrophage differentiation in the lung via CSF-1R-dependent signaling. FASEB J 28: 3867–3877, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aono Y, Ledford JG, Mukherjee S, Ogawa H, Nishioka Y, Sone S, Beers MF, Noble PW, Wright JR. Surfactant protein-D regulates effector cell function and fibrotic lung remodeling in response to bleomycin injury. Am J Respir Crit Care Med 185: 525–536, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashley SL, Xia M, Murray S, O'Dwyer DN, Grant E, White ES, Flaherty KJ, Martinez FJ, Moore BB. Six-SOMAmer index relating to immune, protease and angiogenic functions predicts progression in IPF. PLoS One 11: e0159878, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashley SL, Jegal Y, Moore TA, van Dyk LF, Laouar Y, Moore BB. γ-Herpes virus-68, but not Pseudomonas aeruginosa or influenza A (H1N1), exacerbates established murine lung fibrosis. Am J Physiol Lung Cell Mol Physiol 307: L219–L230, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashley SL, Wilke CA, Kim KK, Moore BB. Periostin regulates fibrocyte function to promote myofibroblast differentiation and lung fibrosis. Mucosal Immunol. 2016. July 20. doi: 10.1038/mi.2016.61 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballinger MN, Christman JW. Pulmonary macrophages: overlooked and underappreciated. Am J Respir Cell Mol Biol 54: 1–2, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Bauman KA, Wettlaufer SH, Okunishi K, Vannella KM, Stoolman JS, Huang SK, Courey AJ, White ES, Hogaboam CM, Simon RH, Toews GB, Sisson TH, Moore BB, Peters-Golden M. The antifibrotic effects of plasminogen activation occur via prostaglandin E2 synthesis in humans and mice. J Clin Invest 120: 1950–1960, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bharat A, Bhorade SM, Morales-Nebreda L, McQuattie-Pimentel AC, Soberanes S, Ridge K, DeCamp MM, Mestan KK, Perlman H, Budinger GR, Misharin AV. Flow cytometry reveals similarities between lung macrophages in humans and mice. Am J Respir Cell Mol Biol 54: 147–149, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birkelbach B, Lutz D, Ruppert C, Henneke I, Lopez-Rodriguez E, Gunther A, Ochs M, Mahavadi P, Knudsen L. Linking progression of fibrotic lung remodeling and ultrastructural alterations of alveolar epithelial type II cells in the amiodarone mouse model. Am J Physiol Lung Cell Mol Physiol 309: L63–L75, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Borie R, Crestani B, Dieude P, Nunes H, Allanore Y, Kannengiesser C, Airo P, Matucci-Cerinic M, Wallaert B, Israel-Biet D, Cadranel J, Cottin V, Gazal S, Peljto AL, Varga J, Schwartz DA, Valeyre D, Grandchamp B. The MUC5B variant is associated with idiopathic pulmonary fibrosis but not with systemic sclerosis interstitial lung disease in the European Caucasian population. PLoS One 8: e70621, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol 15: 713–720, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bringardner BD, Baran CP, Eubank TD, Marsh CB. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redox Signal 10: 287–301, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabrera S, Maciel M, Herrera I, Nava T, Vergara F, Gaxiola M, Lopez-Otin C, Selman M, Pardo A. Essential role for the ATG4B protease and autophagy in bleomycin-induced pulmonary fibrosis. Autophagy 11: 670–684, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen G, Wang R, Chen H, Wu L, Ge RS, Wang Y. Gossypol ameliorates liver fibrosis in diabetic rats induced by high-fat diet and streptozocin. Life Sci 149: 58–64, 2016. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Yuan J, Yao S, Jin Y, Chen G, Tian W, Xi J, Xu Z, Weng D, Chen J. Lipopolysaccharides may aggravate apoptosis through accumulation of autophagosomes in alveolar macrophages of human silicosis. Autophagy 11: 2346–2357, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Walther FJ, Sengers RM, Laghmani el H, Salam A, Folkerts G, Pera T, Wagenaar GT. Metformin attenuates hyperoxia-induced lung injury in neonatal rats by reducing the inflammatory response. Am J Physiol Lung Cell Mol Physiol 309: L262–L270, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chitra P, Saiprasad G, Manikandan R, Sudhandiran G. Berberine inhibits Smad and non-Smad signaling cascades and enhances autophagy against pulmonary fibrosis. J Mol Med 93: 1015–1031, 2015. [DOI] [PubMed] [Google Scholar]
- 23.Chrysanthopoulou A, Mitroulis I, Apostolidou E, Arelaki S, Mikroulis D, Konstantinidis T, Sivridis E, Koffa M, Giatromanolaki A, Boumpas DT, Ritis K, Kambas K. Neutrophil extracellular traps promote differentiation and function of fibroblasts. J Pathol 233: 294–307, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Chua F, Dunsmore SE, Clingen PH, Mutsaers SE, Shapiro SD, Segal AW, Roes J, Laurent GJ. Mice lacking neutrophil elastase are resistant to bleomycin-induced pulmonary fibrosis. Am J Pathol 170: 65–74, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Codogno P, Mehrpour M, Proikas-Cezanne T. Canonical and non-canonical autophagy: variations on a common theme of self-eating? Nat Rev Mol Cell Biol 13: 7–12, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Collard HR, Calfee CS, Wolters PJ, Song JW, Hong SB, Brady S, Ishizaka A, Jones KD, King TE Jr, Matthay MA, Kim DS. Plasma biomarker profiles in acute exacerbation of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 299: L3–L7, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craig VJ, Zhang L, Hagood JS, Owen CA. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 53: 585–600, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy 1: 131–140, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Desai B, Mattson J, Paintal H, Nathan M, Shen F, Beaumont M, Malinao MC, Li Y, Canfield J, Basham B, de Waal Malefyt R, McClanahan T, Krishna G, Fick R Jr. Differential expression of monocyte/macrophage- selective markers in human idiopathic pulmonary fibrosis. Exp Lung Res 37: 227–238, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Domingo-Gonzalez R, Martinez-Colon GJ, Smith AJ, Smith CK, Ballinger MN, Xia M, Murray S, Kaplan MJ, Yanik GA, Moore BB. Inhibition of neutrophil extracellular trap formation after stem cell transplant by prostaglandin E2. Am J Respir Crit Care Med 193: 186–197, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dworski R, Simon HU, Hoskins A, Yousefi S. Eosinophil and neutrophil extracellular DNA traps in human allergic asthmatic airways. J Allergy Clin Immunol 127: 1260–1266, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity 41: 21–35, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez IE, Amarie OV, Mutze K, Konigshoff M, Yildirim AO, Eickelberg O. Systematic phenotyping and correlation of biomarkers with lung function and histology in lung fibrosis. Am J Physiol Lung Cell Mol Physiol 310: L919–L927, 2016. [DOI] [PubMed] [Google Scholar]
- 34.Franco-Montoya ML, Boucherat O, Thibault C, Chailley-Heu B, Incitti R, Delacourt C, Bourbon JR. Profiling target genes of FGF18 in the postnatal mouse lung: possible relevance for alveolar development. Physiol Genomics 43: 1226–1240, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodwin AT, Jenkins G. Molecular endotyping of pulmonary fibrosis. Chest 149: 228–237, 2016. [DOI] [PubMed] [Google Scholar]
- 36.Greene KE, King TE Jr, Kuroki Y, Bucher-Bartelson B, Hunninghake GW, Newman LS, Nagae H, Mason RJ. Serum surfactant proteins-A and -D as biomarkers in idiopathic pulmonary fibrosis. Eur Respir J 19: 439–446, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Gregory AD, Kliment CR, Metz HE, Kim KH, Kargl J, Agostini BA, Crum LT, Oczypok EA, Oury TA, Houghton AM. Neutrophil elastase promotes myofibroblast differentiation in lung fibrosis. J Leukoc Biol 98: 143–152, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gui YS, Wang L, Tian X, Li X, Ma A, Zhou W, Zeng N, Zhang J, Cai B, Zhang H, Chen JY, Xu KF. mTOR overactivation and compromised autophagy in the pathogenesis of pulmonary fibrosis. PloS One 10: e0138625, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupte VV, Ramasamy SK, Reddy R, Lee J, Weinreb PH, Violette SM, Guenther A, Warburton D, Driscoll B, Minoo P, Bellusci S. Overexpression of fibroblast growth factor-10 during both inflammatory and fibrotic phases attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med 180: 424–436, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han MK, Zhou Y, Murray S, Tayob N, Noth I, Lama VN, Moore BB, White ES, Flaherty KR, Huffnagle GB, Martinez FJ COMET. Investigators. Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir Med 2: 548–556, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hawkins A, Guttentag SH, Deterding R, Funkhouser WK, Goralski JL, Chatterjee S, Mulugeta S, Beers MF. A non-BRICHOS SFTPC mutant (SP-CI73T) linked to interstitial lung disease promotes a late block in macroautophagy disrupting cellular proteostasis and mitophagy. Am J Physiol Lung Cell Mol Physiol 308: L33–L47, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15: 1077–1081, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henry MT, McMahon K, Mackarel AJ, Prikk K, Sorsa T, Maisi P, Sepper R, Fitzgerald MX, O'Connor CM. Matrix metalloproteinases and tissue inhibitor of metalloproteinase-1 in sarcoidosis and IPF. Eur Respir J 20: 1220–1227, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Hidvegi T, Stolz DB, Alcorn JF, Yousem SA, Wang J, Leme AS, Houghton AM, Hale P, Ewing M, Cai H, Garchar EA, Pastore N, Annunziata P, Kaminski N, Pilewski J, Shapiro SD, Pak SC, Silverman GA, Brunetti-Pierri N, Perlmutter DH. Enhancing autophagy with drugs or lung-directed gene therapy reverses the pathological effects of respiratory epithelial cell proteinopathy. J Biol Chem 290: 29742–29757, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Honda Y, Kuroki Y, Matsuura E, Nagae H, Takahashi H, Akino T, Abe S. Pulmonary surfactant protein D in sera and bronchoalveolar lavage fluids. Am J Respir Crit Care Med 152: 1860–1866, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Huang LS, Berdyshev EV, Tran JT, Xie L, Chen J, Ebenezer DL, Mathew B, Gorshkova I, Zhang W, Reddy SP, Harijith A, Wang G, Feghali-Bostwick C, Noth I, Ma SF, Zhou T, Ma W, Garcia JG, Natarajan V. Sphingosine-1-phosphate lyase is an endogenous suppressor of pulmonary fibrosis: role of S1P signalling and autophagy. Thorax 70: 1138–1148, 2015. [DOI] [PubMed] [Google Scholar]
- 47.Im J, Hergert P, Nho RS. Reduced FoxO3a expression causes low autophagy in idiopathic pulmonary fibrosis fibroblasts on collagen matrices. Am J Physiol Lung Cell Mol Physiol 309: L552–L561, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jara P, Calyeca J, Romero Y, Placido L, Yu G, Kaminski N, Maldonado V, Cisneros J, Selman M, Pardo A. Matrix metalloproteinase (MMP)-19-deficient fibroblasts display a profibrotic phenotype. Am J Physiol Lung Cell Mol Physiol 308: L511–L522, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joannes A, Brayer S, Besnard V, Marchal-Somme J, Jaillet M, Mordant P, Mal H, Borie R, Crestani B, Mailleux AA. FGF9 and FGF18 in idiopathic pulmonary fibrosis promote survival and migration and inhibit myofibroblast differentiation of human lung fibroblasts in vitro. Am J Physiol Lung Cell Mol Physiol 310: L615–L629, 2016. [DOI] [PubMed] [Google Scholar]
- 50.Judge JL, Owens KM, Pollock SJ, Woeller CF, Thatcher TH, Williams JP, Phipps RP, Sime PJ, Kottmann RM. Ionizing radiation induces myofibroblast differentiation via lactate dehydrogenase. Am J Physiol Lung Cell Mol Physiol 309: L879–L887, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kinder BW, Brown KK, Schwarz MI, Ix JH, Kervitsky A, King TE Jr. Baseline BAL neutrophilia predicts early mortality in idiopathic pulmonary fibrosis. Chest 133: 226–232, 2008. [DOI] [PubMed] [Google Scholar]
- 52.King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet 378: 1949–1961, 2011. [DOI] [PubMed] [Google Scholar]
- 53.Knippenberg S, Ueberberg B, Maus R, Bohling J, Ding N, Tort Tarres M, Hoymann HG, Jonigk D, Izykowski N, Paton JC, Ogunniyi AD, Lindig S, Bauer M, Welte T, Seeger W, Guenther A, Sisson TH, Gauldie J, Kolb M, Maus UA. Streptococcus pneumoniae triggers progression of pulmonary fibrosis through pneumolysin. Thorax 70: 636–646, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kobayashi T, Kim H, Liu X, Sugiura H, Kohyama T, Fang Q, Wen FQ, Abe S, Wang X, Atkinson JJ, Shipley JM, Senior RM, Rennard SI. Matrix metalloproteinase-9 activates TGF-β and stimulates fibroblast contraction of collagen gels. Am J Physiol Lung Cell Mol Physiol 306: L1006–L1015, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E. prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol 29: 537–544, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Kottmann RM, Trawick E, Judge JL, Wahl LA, Epa AP, Owens KM, Thatcher TH, Phipps RP, Sime PJ. Pharmacologic inhibition of lactate production prevents myofibroblast differentiation. Am J Physiol Lung Cell Mol Physiol 309: L1305–L1312, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kropski JA, Lawson WE, Young LR, Blackwell TS. Genetic studies provide clues on the pathogenesis of idiopathic pulmonary fibrosis. Dis Model Mech 6: 9–17, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kropski JA, Pritchett JM, Zoz DF, Crossno PF, Markin C, Garnett ET, Degryse AL, Mitchell DB, Polosukhin VV, Rickman OB, Choi L, Cheng DS, McConaha ME, Jones BR, Gleaves LA, McMahon FB, Worrell JA, Solus JF, Ware LB, Lee JW, Massion PP, Zaynagetdinov R, White ES, Kurtis JD, Johnson JE, Groshong SD, Lancaster LH, Young LR, Steele MP, Phillips JA III, Cogan JD, Loyd JE, Lawson WE, Blackwell TS. Extensive phenotyping of individuals at risk for familial interstitial pneumonia reveals clues to the pathogenesis of interstitial lung disease. Am J Respir Crit Care Med 191: 417–426, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kucejko W, Chyczewska E, Naumnik W, Ossolinska M. Concentration of surfactant protein D, Clara cell protein CC-16 and IL-10 in bronchoalveolar lavage (BAL) in patients with sarcoidosis, hypersensitivity pneumonitis and idiopathic pulmonary fibrosis. Folia Histochem Cytobiol 47: 225–230, 2009. [DOI] [PubMed] [Google Scholar]
- 60.Kuhn C, McDonald JA. The roles of the myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol 138: 1257–1265, 1991. [PMC free article] [PubMed] [Google Scholar]
- 61.Kuroki Y, Takahashi H, Chiba H, Akino T. Surfactant proteins A and D: disease markers. Biochim Biophys Acta 1408: 334–345, 1998. [DOI] [PubMed] [Google Scholar]
- 62.Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XC, Newcomb DC, Jones BR, Roldan J, Lane KB, Morrisey EE, Beers MF, Yull FE, Blackwell TS. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci USA 108: 10562–10567, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lemjabbar H, Gosset P, Lechapt-Zalcman E, Franco-Montoya ML, Wallaert B, Harf A, Lafuma C. Overexpression of alveolar macrophage gelatinase B (MMP-9) in patients with idiopathic pulmonary fibrosis: effects of steroid and immunosuppressive treatment. Am J Respir Cell Mol Biol 20: 903–913, 1999. [DOI] [PubMed] [Google Scholar]
- 64.Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, Villanueva EC, Shah P, Kaplan MJ, Bruce AT. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol 187: 490–500, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu H, Mi S, Li Z, Hua F, Hu ZW. Interleukin 17A inhibits autophagy through activation of PIK3CA to interrupt the GSK3B-mediated degradation of BCL2 in lung epithelial cells. Autophagy 9: 730–742, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.MacKenzie B, Korfei M, Henneke I, Sibinska Z, Tian X, Hezel S, Dilai S, Wasnick R, Schneider B, Wilhelm J, El Agha E, Klepetko W, Seeger W, Schermuly R, Gunther A, Bellusci S. Increased FGF1-FGFRc expression in idiopathic pulmonary fibrosis. Respir Res 16: 83, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mahavadi P, Knudsen L, SV, Henneke I, Hegermann J, Wrede C, Ochs M, Ahuja S, Chillappagari S, Ruppert C, Seeger W, Korfei M, Guenther A. Regulation of macroautophagy in amiodarone-induced pulmonary fibrosis. J Pathol Clin Res 1: 252–263, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maher TM, Evans IC, Bottoms SE, Mercer PF, Thorley AJ, Nicholson AG, Laurent GJ, Tetley TD, Chambers RC, McAnulty RJ. Diminished prostaglandin E2 contributes to the apoptosis paradox in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 182: 73–82, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malouf MA, Hopkins P, Snell G, Glanville AR. An investigator-driven study of everolimus in surgical lung biopsy confirmed idiopathic pulmonary fibrosis. Respirology 16: 776–783, 2011. [DOI] [PubMed] [Google Scholar]
- 70.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686, 2004. [DOI] [PubMed] [Google Scholar]
- 71.Manzenreiter R, Kienberger F, Marcos V, Schilcher K, Krautgartner WD, Obermayer A, Huml M, Stoiber W, Hector A, Griese M, Hannig M, Studnicka M, Vitkov L, Hartl D. Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J Cyst Fibros 11: 84–92, 2012. [DOI] [PubMed] [Google Scholar]
- 72.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol 49: 503–510, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mitchell J, Woodcock-Mitchell J, Reynolds S, Low R, Leslie K, Adler K, Gabbiani G, Skalli O. Alpha-smooth muscle actin in parenchymal cells of bleomycin-injured rat lung. Lab Invest 60: 643–650, 1989. [PubMed] [Google Scholar]
- 74.Mizumura K, Cloonan S, Choi ME, Hashimoto S, Nakahira K, Ryter SW, Choi AM. Autophagy: friend or foe in lung disease? Ann Am Thorac Soc 13, Suppl 1: S40–S47, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Molyneaux PL, Cox MJ, Willis-Owen SA, Mallia P, Russell KE, Russell AM, Murphy E, Johnston SL, Schwartz DA, Wells AU, Cookson WO, Maher TM, Moffatt MF. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 190: 906–913, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moon DO, Choi YH, Moon SK, Kim WJ, Kim GY. Gossypol decreases tumor necrosis factor-alpha-induced intercellular adhesion molecule-1 expression via suppression of NF-kappaB activity. Food Chem Toxicol 49: 999–1005, 2011. [DOI] [PubMed] [Google Scholar]
- 77.Moore BB, Moore TA. Viruses in idiopathic pulmonary fibrosis. Etiology and exacerbation. Ann Am Thorac Soc, 12 Suppl 2: S186–S192, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77a.Moore BB, Lawson WE, Oury TD, Sisson TH, Raghavendran K, Hogaboam CM. Animal models of fibrotic lung disease. Am J Respir Cell Mol Biol 49: 167–179, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morales-Nebreda L, Misharin AV, Perlman H, Budinger GR. The heterogeneity of lung macrophages in the susceptibility to disease. Eur Respir Rev 24: 505–509, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murphy G, Knauper V, Cowell S, Hembry R, Stanton H, Butler G, Freije J, Pendas AM, Lopez-Otin C. Evaluation of some newer matrix metalloproteinases. Ann NY Acad Sci 878: 25–39, 1999. [DOI] [PubMed] [Google Scholar]
- 80.Naik PK, Bozyk PD, Bentley JK, Popova AP, Birch CM, Wilke CA, Fry CD, White ES, Sisson TH, Tayob N, Carnemolla B, Orecchia P, Flaherty KR, Hershenson MB, Murray S, Martinez FJ, Moore BB. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 303: L1046–L1056, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakahira K, Cloonan SM, Mizumura K, Choi AM, Ryter SW. Autophagy: a crucial moderator of redox balance, inflammation, and apoptosis in lung disease. Antioxid Redox Signal 20: 474–494, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, Phoon MC, van Rooijen N, Chow VT. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol 179: 199–210, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nho RS, Hergert P. IPF fibroblasts are desensitized to type I collagen matrix-induced cell death by suppressing low autophagy via aberrant Akt/mTOR kinases. PLoS One 9: e94616, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nishikiori H, Chiba H, Ariki S, Kuronuma K, Otsuka M, Shiratori M, Ikeda K, Watanabe A, Kuroki Y, Takahashi H. Distinct compartmentalization of SP-A and SP-D in the vasculature and lungs of patients with idiopathic pulmonary fibrosis. BMC Pulm Med 14: 196, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Noth I, Zhang Y, Ma SF, Flores C, Barber M, Huang Y, Broderick SM, Wade MS, Hysi P, Scuirba J, Richards TJ, Juan-Guardela BM, Vij R, Han MK, Martinez FJ, Kossen K, Seiwert SD, Christie JD, Nicolae D, Kaminski N, Garcia JG. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med 1: 309–317, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O'Dwyer DN, Armstrong ME, Trujillo G, Cooke G, Keane MP, Fallon PG, Simpson AJ, Millar AB, McGrath EE, Whyte MK, Hirani N, Hogaboam CM, Donnelly SC. The Toll-like receptor 3 L412F polymorphism and disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 188: 1442–1450, 2013. [DOI] [PubMed] [Google Scholar]
- 87.O'Dwyer DN, Dickson RP, Moore BB. The lung microbiome, immunity, and the pathogenesis of chronic lung disease. J Immunol 196: 4839–4847, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Otte JM, Schmitz F, Kiehne K, Stechele HU, Banasiewicz T, Krokowicz P, Nakamura T, Folsch UR, Herzig K. Functional expression of HGF and its receptor in human colorectal cancer. Digestion 61: 237–246, 2000. [DOI] [PubMed] [Google Scholar]
- 89.Overbye A, Saetre F, Hagen LK, Johansen HT, Seglen PO. Autophagic activity measured in whole rat hepatocytes as the accumulation of a novel BHMT fragment (p10), generated in amphisomes by the asparaginyl proteinase, legumain. Autophagy 7: 1011–1027, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pache JC, Christakos PG, Gannon DE, Mitchell JJ, Low RB, Leslie KO. Myofibroblasts in diffuse alveolar damage of the lung. Mod Pathol 11: 1064–1070, 1998. [PubMed] [Google Scholar]
- 91.Pan L, Li Y, Jia L, Qin Y, Qi G, Cheng J, Qi Y, Li H, Du J. Cathepsin S deficiency results in abnormal accumulation of autophagosomes in macrophages and enhances Ang II-induced cardiac inflammation. PloS One 7: e35315, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patel AS, Lin L, Geyer A, Haspel JA, An CH, Cao J, Rosas IO, Morse D. Autophagy in idiopathic pulmonary fibrosis. PLoS One 7: e41394, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paun A, Lemay AM, Tomko TG, Haston CK. Association analysis reveals genetic variation altering bleomycin-induced pulmonary fibrosis in mice. Am J Respir Cell Mol Biol 48: 330–336, 2013. [DOI] [PubMed] [Google Scholar]
- 94.Phan SH. The myofibroblast in pulmonary fibrosis. Chest 122: 286S–289S, 2002. [DOI] [PubMed] [Google Scholar]
- 95.Putman RK, Rosas IO, Hunninghake GM. Genetics and early detection in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 189: 770–778, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Raghu G, Anstrom KJ, King TE Jr, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med 366: 1968–1977, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Raghu G, Chen SY, Hou Q, Yeh WS, Collard HR. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18–64 years old. Eur Respir J 48: 179–186, 2016. [DOI] [PubMed] [Google Scholar]
- 98.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ; ATS/ERS/JRS/ ALAT. Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raju R, Palapetta SM, Sandhya VK, Sahu A, Alipoor A, Balakrishnan L, Advani J, George B, Kini KR, Geetha NP, Prakash HS, Prasad TS, Chang YJ, Chen L, Pandey A, Gowda H. A network map of FGF-1/FGFR signaling system. J Signal Transduct 2014: 962962, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramirez G, Hagood JS, Sanders Y, Ramirez R, Becerril C, Segura L, Barrera L, Selman M, Pardo A. Absence of Thy-1 results in TGF-beta induced MMP-9 expression and confers a profibrotic phenotype to human lung fibroblasts. Lab Invest 91: 1206–1218, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rangarajan S, Kurundkar A, Kurundkar D, Bernard K, Sanders YY, Ding Q, Antony VB, Zhang J, Zmijewski J, Thannickal VJ. Novel mechanisms for the antifibrotic action of nintedanib. Am J Respir Cell Mol Biol 54: 51–59, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rey-Jurado E, Riedel CA, Gonzalez PA, Bueno SM, Kalergis AM. Contribution of autophagy to antiviral immunity. FEBS Lett 589: 3461–3470, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR INPULSIS. Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370: 2071–2082, 2014. [DOI] [PubMed] [Google Scholar]
- 104.Scotton CJ, Krupiczojc MA, Konigshoff M, Mercer PF, Lee YC, Kaminski N, Morser J, Post JM, Maher TM, Nicholson AG, Moffatt JD, Laurent GJ, Derian CK, Eickelberg O, Chambers RC. Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. J Clin Invest 119: 2550–2563, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, Evans CM, Garantziotis S, Adler KB, Dickey BF, du Bois RM, Yang IV, Herron A, Kervitsky D, Talbert JL, Markin C, Park J, Crews AL, Slifer SH, Auerbach S, Roy MG, Lin J, Hennessy CE, Schwarz MI, Schwartz DA. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 364: 1503–1512, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc 3: 364–372, 2006. [DOI] [PubMed] [Google Scholar]
- 107.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med 5: e62, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Selman M, Ruiz V, Cabrera S, Segura L, Ramirez R, Barrios R, Pardo A. TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis. A prevailing nondegradative lung microenvironment? Am J Physiol Lung Cell Mol Physiol 279: L562–L574, 2000. [DOI] [PubMed] [Google Scholar]
- 109.Sgalla G, Biffi A, Richeldi L. Idiopathic pulmonary fibrosis: diagnosis, epidemiology and natural history. Respirology 21: 427–437, 2016. [DOI] [PubMed] [Google Scholar]
- 110.Shea BS, Tager AM. Role of the lysophospholipid mediators lysophosphatidic acid and sphingosine 1-phosphate in lung fibrosis. Proc Am Thorac Soc 9: 102–110, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shulgina L, Cahn AP, Chilvers ER, Parfrey H, Clark AB, Wilson EC, Twentyman OP, Davison AG, Curtin JJ, Crawford MB, Wilson AM. Treating idiopathic pulmonary fibrosis with the addition of co-trimoxazole: a randomised controlled trial. Thorax 68: 155–162, 2013. [DOI] [PubMed] [Google Scholar]
- 112.Sims MW, Beers MF, Ahya VN, Kawut SM, Sims KD, Lederer DJ, Palmer SM, Wille K, Lama VN, Shah PD, Orens JB, Bhorade S, Crespo M, Weinacker A, Demissie E, Bellamy S, Christie JD, Ware LB. Effect of single vs bilateral lung transplantation on plasma surfactant protein D levels in idiopathic pulmonary fibrosis. Chest 140: 489–496, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sinden NJ, Baker MJ, Smith DJ, Kreft JU, Dafforn TR, Stockley RA. α-1-Antitrypsin variants and the proteinase/antiproteinase imbalance in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 308: L179–L190, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Singh KK, Lovren F, Pan Y, Quan A, Ramadan A, Matkar PN, Ehsan M, Sandhu P, Mantella LE, Gupta N, Teoh H, Parotto M, Tabuchi A, Kuebler WM, Al-Omran M, Finkel T, Verma S. The essential autophagy gene ATG7 modulates organ fibrosis via regulation of endothelial-to-mesenchymal transition. J Biol Chem 290: 2547–2559, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Song E, Ouyang N, Horbelt M, Antus B, Wang M, Exton MS. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell Immunol 204: 19–28, 2000. [DOI] [PubMed] [Google Scholar]
- 116.Sosulski ML, Gongora R, Danchuk S, Dong C, Luo F, Sanchez CG. Deregulation of selective autophagy during aging and pulmonary fibrosis: the role of TGFbeta1. Aging Cell 14: 774–783, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Speth JM, Bourdonnay E, Penke LR, Mancuso P, Moore BB, Weinberg JB, Peters-Golden M. Alveolar epithelial cell-derived prostaglandin E2 serves as a request signal for macrophage secretion of suppressor of cytokine signaling 3 during innate inflammation. J Immunol 196: 5112–5120, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stock CJ, Sato H, Fonseca C, Banya WA, Molyneaux PL, Adamali H, Russell AM, Denton CP, Abraham DJ, Hansell DM, Nicholson AG, Maher TM, Wells AU, Lindahl GE, Renzoni EA. Mucin 5B promoter polymorphism is associated with idiopathic pulmonary fibrosis but not with development of lung fibrosis in systemic sclerosis or sarcoidosis. Thorax 68: 436–441, 2013. [DOI] [PubMed] [Google Scholar]
- 119.Suga M, Iyonaga K, Okamoto T, Gushima Y, Miyakawa H, Akaike T, Ando M. Characteristic elevation of matrix metalloproteinase activity in idiopathic interstitial pneumonias. Am J Respir Crit Care Med 162: 1949–1956, 2000. [DOI] [PubMed] [Google Scholar]
- 120.Sugahara K, Iyama K, Kuroda MJ, Sano K. Double intratracheal instillation of keratinocyte growth factor prevents bleomycin-induced lung fibrosis in rats. J Pathol 186: 90–98, 1998. [DOI] [PubMed] [Google Scholar]
- 121.Sun KH, Chang Y, Reed NI, Sheppard D. α-Smooth muscle actin is an inconsistent marker of fibroblasts responsible for force-dependent TGFβ activation or collagen production across multiple models of organ fibrosis. Am J Physiol Lung Cell Mol Physiol 310: L824–L836, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun L, Louie MC, Vannella KM, Wilke CA, LeVine AM, Moore BB, Shanley TP. New concepts of IL-10-induced lung fibrosis: fibrocyte recruitment and M2 activation in a CCL2/CCR2 axis. Am J Physiol Lung Cell Mol Physiol 300: L341–L353, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Takahashi H, Shiratori M, Kanai A, Chiba H, Kuroki Y, Abe S. Monitoring markers of disease activity for interstitial lung diseases with serum surfactant proteins A and D. Respirology 11 Suppl: S51–S54, 2006. [DOI] [PubMed] [Google Scholar]
- 124.Takemasa A, Ishii Y, Fukuda T. A neutrophil elastase inhibitor prevents bleomycin-induced pulmonary fibrosis in mice. Eur Respir J 40: 1475–1482, 2012. [DOI] [PubMed] [Google Scholar]
- 125.Tatler AL, Jenkins G. Sphingosine-1-phosphate metabolism: can its enigmatic lyase promote the autophagy of fibrosis? Thorax 70: 1106–1107, 2015. [DOI] [PubMed] [Google Scholar]
- 126.Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc 3: 350–356, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tulek B, Kiyan E, Toy H, Kiyici A, Narin C, Suerdem M. Anti-inflammatory and anti-fibrotic effects of sirolimus on bleomycin-induced pulmonary fibrosis in rats. Clin Invest Med 34: E341, 2011. [DOI] [PubMed] [Google Scholar]
- 128.Turco E, Vizzuso C, Franceschini S, Ragazzi A, Stefanini FM. The in vitro effect of gossypol and its interaction with salts on conidial germination and viability of Fusarium oxysporum sp. vasinfectum isolates. J Appl Microbiol 103: 2370–2381, 2007. [DOI] [PubMed] [Google Scholar]
- 129.Vannella KM, Luckhardt TR, Wilke CA, van Dyk LF, Toews GB, Moore BB. Latent herpesvirus infection augments experimental pulmonary fibrosis. Am J Respir Crit Care Med 181: 465–477, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Venosa A, Malaviya R, Gow AJ, Hall L, Laskin JD, Laskin DL. Protective role of spleen-derived macrophages in lung inflammation, injury, and fibrosis induced by nitrogen mustard. Am J Physiol Lung Cell Mol Physiol 309: L1487–L1498, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weiss G, Schaible UE. Macrophage defense mechanisms against intracellular bacteria. Immunol Rev 264: 182–203, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.White ES, Xia M, Murray S, Dyal R, Flaherty CM, Flaherty KR, Moore BB, Cheng L, Doyle TJ, Villalba J, Dellaripa PF, Rosas IO, Kurtis JD, Martinez FJ. Plasma surfactant protein-D, matrix metalloproteinase-7, and osteopontin index distinguishes idiopathic pulmonary fibrosis from other idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2016. May 5 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wilborn J, Crofford LJ, Burdick MD, Kunkel SL, Strieter RM, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest 95: 1861–1868, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Withana NP, Ma X, McGuire HM, Verdoes M, van der Linden WA, Ofori LO, Zhang R, Li H, Sanman LE, Wei K, Yao S, Wu P, Li F, Huang H, Xu Z, Wolters PJ, Rosen GD, Collard HR, Zhu Z, Cheng Z, Bogyo M. Non-invasive imaging of idiopathic pulmonary fibrosis using cathepsin protease probes. Sci Rep 6: 19755, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu X, Qi H, Yang Y, Yin Y, Ma D, Li H, Qu Y. Downregulation of matrix metalloproteinase19 induced by respiratory syncytial viral infection affects the interaction between epithelial cells and fibroblasts. Mol Med Rep 13: 167–173, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xie N, Tan Z, Banerjee S, Cui H, Ge J, Liu RM, Bernard K, Thannickal VJ, Liu G. Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am J Respir Crit Care Med 192: 1462–1474, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu WB, Xu LH, Lu HS, Ou-Yang DY, Shi HJ, Di JF, He XH. The immunosuppressive effect of gossypol in mice is mediated by inhibition of lymphocyte proliferation and by induction of cell apoptosis. Acta Pharmacol Sin 30: 597–604, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xu Y, Eissa NT. Autophagy in innate and adaptive immunity. Proc Am Thorac Soc 7: 22–28, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang IV, Fingerlin TE, Evans CM, Schwarz MI, Schwartz DA. MUC5B and idiopathic pulmonary fibrosis. Ann Am Thorac Soc 12, Suppl 2: S193–S199, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang J, Velikoff M, Canalis E, Horowitz JC, Kim KK. Activated alveolar epithelial cells initiate fibrosis through autocrine and paracrine secretion of connective tissue growth factor. Am J Physiol Lung Cell Mol Physiol 306: L786–L796, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yu G, Kovkarova-Naumovski E, Jara P, Parwani A, Kass D, Ruiz V, Lopez-Otin C, Rosas IO, Gibson KF, Cabrera S, Ramirez R, Yousem SA, Richards TJ, Chensny LJ, Selman M, Kaminski N, Pardo A. Matrix metalloproteinase-19 is a key regulator of lung fibrosis in mice and humans. Am J Respir Crit Care Med 186: 752–762, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yu YR, Hotten DF, Malakhau Y, Volker E, Ghio AJ, Noble PW, Kraft M, Hollingsworth JW, Gunn MD, Tighe RM. Flow cytometric analysis of myeloid cells in human blood, bronchoalveolar lavage, and lung tissues. Am J Respir Cell Mol Biol 54: 13–24, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao H, Dong Y, Tian X, Tan TK, Liu Z, Zhao Y, Zhang Y, Harris D, Zheng G. Matrix metalloproteinases contribute to kidney fibrosis in chronic kidney diseases. World J Nephrol 2: 84–89, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhong Z, Sanchez-Lopez E, Karin M. Autophagy, inflammation, and immunity: a troika governing cancer and its treatment. Cell 166: 288–298, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]