Abstract
CCR2-expressing leukocytes are required for the progression of fibrosis in models of induced lung injury as well as models of bone marrow transplant (BMT)-related idiopathic pneumonia syndrome. Infection with murid γ-herpesvirus-68 (γHV-68) results in severe pneumonitis and pulmonary fibrosis following syngeneic BMT; however, the roles that various proinflammatory leukocyte populations play in this process remain unclear. Deletion of CCR2 in both non-BMT and BMT mice increased early lytic viral replication and resulted in a reduction in the numbers of lung-infiltrating GR1+,F4/80+ and CXCR1+ cells, while maintaining robust neutrophil infiltration. Similarly, in γHV-68-infected CCR2−/− BMT mice, recruitment of monocytes and lymphocytes were reduced whereas neutrophil recruitment was increased compared with wild-type (WT) BMT mice. Interestingly, levels of profibrotic IL-17 were increased in infected CCR2 BMT mice compared with WT BMT. Furthermore, an increase in lung-associated collagen was detected even though there was an overall decrease in the number of profibrotic CCR2+ fibrocytes detected in the lungs of CCR2−/− BMT mice. These data indicate that, contrary to most models of fibrosis, deletion of CCR2 offers no protection from γ-herpesvirus-induced pneumonitis and fibrosis, and, indeed, CCR2+ cells play a suppressive role during the development of pulmonary fibrosis following γ-herpesvirus infection post-BMT by limiting IL-7 and collagen production.
Keywords: BMT, CCR2, fibrosis, herpesvirus, IL17
pulmonary fibrosis is characterized by accumulation of activated fibroblasts/myofibroblasts and excessive deposition of extracellular matrix (ECM) proteins, which can result in severe lung dysfunction and death. Pulmonary fibrosis may result from exposure to environmental irritants such as silica or asbestos, from infection, or from chemotherapy exposure, or it may be idiopathic; however, lung epithelial damage, inflammation, and an abnormal wound healing response are thought to be commonalities (12, 13).
Pulmonary complications, including fibrosis, are also common after hematopoietic stem cell transplantation (HSCT) occurring in 30–60% of patients (2, 64). Idiopathic pneumonia syndrome (IPS) and bronchiolitis obliterans syndrome (BOS) both arise as initial pneumonitis that can lead to pulmonary fibrosis, lung dysfunction, and death. Traditionally, IPS and BOS have been considered noninfectious (41, 49, 51); however, these diseases may arise in the context of occult infection or may result from immune processes that happen after resolution of an initial infection (39, 48, 61). Herpesviruses are widely distributed and establish life-long infections with the capacity to reactivate. Herpesvirus DNA has been detected with high frequency in lungs of patients suffering from pulmonary complications following HSCT (48, 61). In addition, cells harboring latent viral genomes can express profibrotic cytokines and murine gamma herpesvirus (γHV-68) has demonstrated fibrogenic capacity in various mouse models (4, 16, 29, 36, 53, 56, 58). Thus herpesviruses represent a potential bridge between infection and the development of “sterile” diseases such as IPS and BOS following HSCT.
We recently characterized a murine model of pulmonary fibrosis arising from γHV-68 infection following syngeneic bone marrow transplant (BMT) that mimics many clinical manifestations of IPS with fibrosis seen in humans (14, 65). Mice infected with γHV-68, a virus similar to Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated virus (KSHV), respond by producing TH1 cytokines, which are important in limiting and maintaining long term control of viral replication (45, 47, 52). However, mice challenged with γHV-68 post-BMT produce much less interferon-γ (IFNγ) and instead skew CD4+ T cells toward a TH17 phenotype (15, 65). While eventual viral clearance was not affected, BMT mice developed severe pneumonitis and pulmonary fibrosis 3 wk postinfection in the absence of viral replication. Importantly, high levels of IL-17 and IL-6 in the absence of IFNγ were shown to be associated with development and severity of IPS in humans following allogeneic HSCT as well (59).
We have found CD11c+ antigen-presenting cells (APCs) produced high levels of TH17 skewing cytokines TGFβ, IL-6, IL-1β, and IL-23 following syngeneic BMT to promote IL-17-dependent fibrosis (65). Additionally, dendritic cells (DCs) and CCR2+ monocytes/macrophages were recruited to the lungs of mice in experimental models of IPS following allogeneic BMT (21, 28). Furthermore, in the allogeneic BMT models, transplantation with CCR2−/− bone marrow or neutralization of monocyte chemoattractant protein 1 (MCP-1/CCL2) abrogated disease (21).
Likewise, administration of bleomycin (either to the lungs or systemically) or fluorescein isothiocyanate (FITC) to the lungs in mice has also demonstrated the importance of recruited CCR2+ leukocytes for fibrotic disease progression (7, 18, 34) as CCR2−/− mice were protected from collagen deposition and development of fibrotic lung pathology to either agent (32, 34). Furthermore, CD45+ Col I+ fibrocytes are believed to contribute to fibrotic pathogenesis (19, 43) and we have shown these cells can be recruited to the lungs in a CCL12/CCR2-dependent fashion after bleomycin or FITC administration in nontransplanted mice (33) and that recruitment of fibrocytes is enhanced in the setting of γHV-68 infection (29).
Thus, given the pathogenic role of CCR2+ leukocytes in both transplant- and non-transplant-related models of pulmonary inflammation and fibrosis, we sought to characterize the role of CCR2+ cells in a model of γ-herpesvirus-induced pneumonitis and fibrosis following syngeneic BMT. In contrast to essentially all previously published work, no ameliorative effect was observed after γHV-68 challenge in the context of CCR2−/− BMT. Thus this study suggests γ-herpesvirus-induced fibrogenesis post-BMT is independent of CCR2, whereas models of sterile fibrogenesis are not.
MATERIALS AND METHODS
Mouse strains, cell and virus lines.
Male 6- to 8-wk-old C57Bl/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). CCR2−/− mice have been previously described (26); mice were bred in the animal facility of the University of Michigan, Ann Arbor, MI and were used for experiments at 6–8 wk of age. To prevent nonspecific germline mutations, CCR2−/− mice were backcrossed to a WT C57BL/6J background every 15 generations. All animals were housed in specific pathogen-free conditions. Experiments were approved by the University of Michigan Committee on the Use and Care of Animals. γHV-68 (strain WUMS VR-1465) was purchased from the American Type Culture Collection (ATCC, Manassas, VA) and was propagated and titered on 3T12 fibroblast monolayers also purchased from ATCC.
Bone marrow transplantation and viral infections.
Bone marrow was harvested from either C57BL/6J or CCR2−/− donor mice by flushing femurs with complete DMEM supplemented with 10% FBS. Bone marrow cells (5 × 106) were injected intravenously into the tail veins of mice that had been lethally irradiated with 13 Gy from a 137Cs irradiator (two split doses of 650 Gy, 3 h apart). Mice were given acid water (pH 3.3) for the first 3 wk after BMT then switched to plain water for an additional 2 wk. Viral infections were carried out by diluting stocks of γHV-68 to a concentration of 5,000 plaque-forming units (pfu) in 20 μl of PBS per mouse. Mice were anesthetized with ketamine and xylazine and infected intranasally (i.n.).
Lung collagenase digestion and differential staining of leukocytes.
Mice were euthanized by CO2 asphyxiation, the chest cavity opened and perfusion through the right ventricle of the heart was performed with 3–5 ml of saline. Lungs were resected and minced with scissors, after which digestion was carried out with 15 mg/ml collagenase A (Roche, Indianapolis, IN) and 250 units of DNase I/ml (Sigma-Aldrich, St. Louis, MO) in complete DMEM supplemented with 10% FBS for 30 min at 37°C. Single cell suspensions were obtained by disrupting cells through a syringe and filtering through sterile 100 μM Nytex mesh. Leukocytes were further purified by centrifugation through 28.5% Percoll in serum-free media. Cells were enumerated and 5×104 cells per sample were deposited onto glass slides using a cytospin 3 (Shandon; Astmoor, UK) and processed by differential staining for identification of various leukocyte populations.
Histopathological analysis.
Formaldehyde-fixed paraffin-embedded (FFPE) lung sections were prepared as follows: Mice were euthanized at the indicated times after infection and perfusion was performed through the right ventricle of the heart with 3–5 ml PBS. Lungs were inflated with 10% buffered formalin and fixed for 24 h. Lungs were subsequently dehydrated in ethanol before being embedded in paraffin, sectioned to a 3 μM thickness, and stained with either hematoxylin and eosin (H&E) or Masson's trichrome stain. All microscopic images were obtained on an Olympus BX-51 microscope (Olympus Scientific, Waltham, MA).
Flow cytometry.
For flow cytometric analysis, 1×106 cells per collagenase-digested lung sample were first incubated with Fc block anti-CD16/32 antibody (BD Bioscience, San Jose, CA) for 30 min on ice before being stained with appropriately diluted fluorophore-conjugated antibodies. Antibodies used in this study are as follows: anti-CD45, CD11b, GR-1, CD4, TCRβ, TCRγδ, CD8, NK1.1, CD19, CD25, and IL-17 (BD Bioscience, San Jose, CA); F4/80, IFNγ (eBioscience, San Diego, CA); CX3CR1 (R&D Systems, Minneapolis, MN). For intracellular cytokine analysis 10×106 cells per mouse were first incubated at 37°C for 4 h with 10 ng/ml PMA plus 10 μM ionomycin with the addition of GolgiStop reagent (BD Bioscience) before permeabilization and staining with appropriately diluted conjugated antibodies.
Quantitative real-time RT-PCR.
Total RNA was extracted from either whole lung tissue or cell culture with Ambion TRIzol reagent (ThermoFisher, Waltham, MA) following the manufacturer's directions. RNA quantity was assessed by analyzing 260 nm/280 nm ratios on a NanoDrop Lite spectrophotometer (ThermoFisher). RNA from individual samples was adjusted to equal concentrations before being analyzed for transcript expression using an RNA-to-Ct 1-Step qRT-PCR kit (ThermoFisher) and an ABI StepOnePlus real-time thermocycler (ThermoFisher) by using the protocol supplied with the kit. Sequences for TaqMan primers and probes for all transcripts analyzed in this study are detailed in Table 1.
Table 1.
Primers and probes used for qRT-PCR
| Gene Name | Primer Sequence |
|---|---|
| CXCL1 | Forward 5′-GCGCCTATCGCCAATGAG-3′ |
| Reverse 5′-GCAACACCTTCAAGCTCTGGAT-3′ | |
| Probe 5′-TGCCTGCAGACCATGGCTGGG-3′ | |
| CXCL2 | Forward 5′-CCTGCCAAGGGTTGACTTCA-3′ |
| Reverse 5′-CCTTGAGAGTGGCTATGACTTCTG-3′ | |
| Probe 5′-CGCCCCCAGGACCCCACTG-3′ | |
| CCL2 | Forward 5′-GGCTCAGCCAGATGCAGTTAAC-3′ |
| Reverse 5′-CCTACTCATTGGGATCATCTTGCT-3′ | |
| Probe 5′-CCCCACTCACCTGCTGCTACTCAT-3′ | |
| CCL5 | Forward 5′-GCAAAAATTCCTCACCGCTG-3′ |
| Reverse 5′-GGCGTGACTGTTTCACATTCC-3′ | |
| Probe 5′-TGTGTTGATCACGGTCCTAAG-3′ | |
| CCL12 | Forward 5′-TGGCTGGACCAGATGCG-3′ |
| Reverse 5′-GACGTGAATCTTCTGCTTAACAACA-3′ | |
| Probe 5′-GCACCCCAGTCACGTGCTGTT-3′ | |
| GM-CSF | Forward 5′-TCTGCCTTAAAGGGACCAAGAG-3′ |
| Reverse 5′-GTCCAAGCTGAGTCAGCGTTT-3′ | |
| Probe 5′-GGCACAGCCACAGTTGGAGGGC-3′ | |
| TNFα | Forward 5′-CCAGACCCTCACACTCAGATCA-3′ |
| Reverse 5′-CCTCCACTTGGTGGTTTGCT-3′ | |
| Probe 5′-TCGAGTGACAAGCCTGTAGCCCACG-3′ | |
| IL-17A | Forward 5′-CCGCAATGAAGACCCTGATAG-3′ |
| Reverse 5′-GCTTTCCCTCCGCATTGA-3′ | |
| Probe 5′-GGGAAGCTCAGTGCCGCCAG-3′ | |
| iNOS | Forward 5′-ACATCAGGTCGGCCATCACT-3′ |
| Reverse 5′-CGTACCGGATGAGCTGTGAAT-3′ | |
| Probe 5′-CCCCAGCGGAGTGACGGCA-3′ | |
| Arg1 | Forward 5′-ACCACAGTCTGGCAGTTGGAA-3′ |
| Reverse 5′-GCATCCACCCAAATGACACA-3′ | |
| Probe 5′-CTGGCCACGCCAGGGTCCAC-3′ |
Collagen quantitation by hydroxyproline assay.
Lungs were homogenized in PBS containing a complete protease inhibitor cocktail (Roche). Equal parts of the lung homogenate and 12 N HCl were incubated overnight at 120°C, after which samples were reacted with chloramine T solution for 20 min followed by development with Erlich's reagent at 65°C for 15 min as previously described (55). Collagen was quantified against a standard of hydroxyproline by measuring absorbance at 550 nm.
Statistical analysis.
All statistical analysis was conducted with GraphPad Prism v6.01 (GraphPad Software, La Jolla, CA). Figures that compare only two means to each other were analyzed by Student's t-test. Figures that compare three or more means together in the same group were analyzed by one-way ANOVA. Figures that compared three or more means in two or more groups, i.e., experiments that looked at the effect of antibody treatment and the effect of BMT, were analyzed by two-way ANOVA; n values and other pertinent statistical information for representative experiments are given in individual figure legends.
RESULTS
γHV-68 early replication is increased in the lungs of nontransplanted CCR2−/− mice.
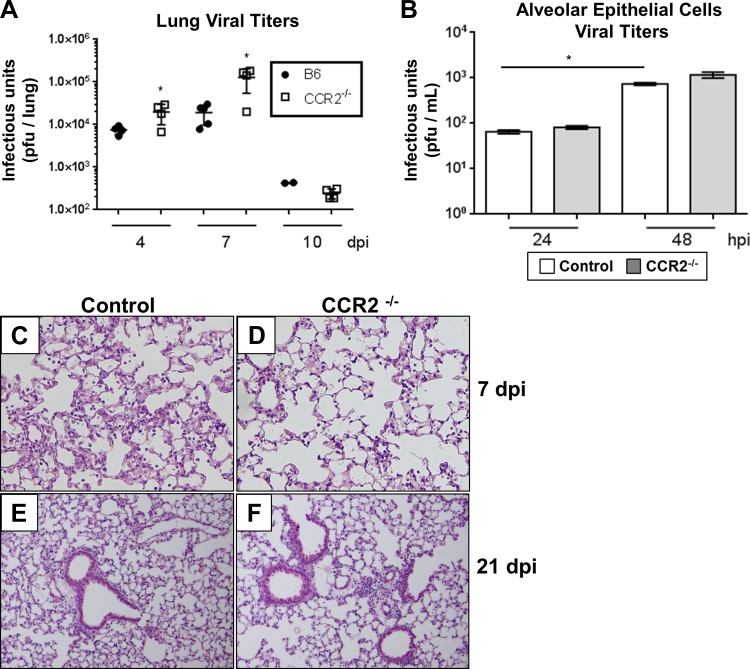
To assess whether recruited CCR2+ cells in the lung were important in controlling γHV-68 infection, C57BL/6J control or CCR2−/− mice were infected i.n. with 5×103 pfu γHV-68 (Strain WUMS). Lungs were harvested at 4, 7, or 10 days postinfection (dpi) and the amount of infectious virus present in the lung was quantified by plaque assay. In contrast to a previous study in which γHV-68 infection in CCR2−/− mice showed no increase in viral lytic gene expression in the lungs at 6 dpi (11), we observed an increase in viral titer in the lungs of CCR2−/− mice at both 4 and 7 dpi (Fig. 1A). However, no difference in eventual viral clearance was observed between control and CCR2−/− mice and almost no infectious virus was recovered from the lungs of either group by 10 dpi. To assess the role that CCR2 signaling plays in structural lung cells, we isolated alveolar epithelial cells from Control or CCR2−/− mice and infected them with γHV-68 over a 48-h time course (Fig. 1B). In contrast to results in vivo, no increase in viral replication was observed in the isolated CCR2−/− epithelial cells, indicating that recruited hematopoietic CCR2+ cells are important in limiting lytic replication of γHV-68 in vivo. We next assessed lung histopathology of infected control or CCR2−/− lungs either at 7 dpi, a time correlating with the peak of acute lytic infection, or at 21 dpi after the virus had been cleared from the lungs. No overt pathological changes were noted at either time point; however, a reduction in the total number of infiltrating leukocytes was observed in CCR2−/− lungs especially at 7 dpi by microscopic observation of H&E-stained FFPE sections (Fig. 1, C–F). Importantly, no pulmonary fibrosis was noted after challenge with γHV-68 in the absence of BMT.
Fig. 1.
γHV-68 early replication is increased in the lungs of CCR2−/− mice. A: at the indicated time after infection, lungs were harvested and infectious virus was quantified by plaque assay on NIH 3T12 monolayers (mean ± SE), n = 4–5 mice per group, repeated once with same result. Two mice were below the detection threshold in the B6 10 dpi group and are not represented on the graph. B: isolated alveolar epithelial cells were infected at multiplicity of infection of 1.0. At the indicated time, cell culture supernatants were harvested and viral replication determined by plaque assay (n = 3 per group, repeated 3 times with similar results). Statistical significance was calculated by Student's t-test, *P < 0.05. C and D: H&E staining of FFPE sections from infected B6 or CCR2−/− mice at 7 dpi. E and F: γHV-68-infected B6 or CCR2−/− lung sections at 21 dpi. Images representative of 3–4 mice per group.
CCR2−/− mice recruit neutrophils instead of monocytes to the lungs in response to γHV-68 infection.
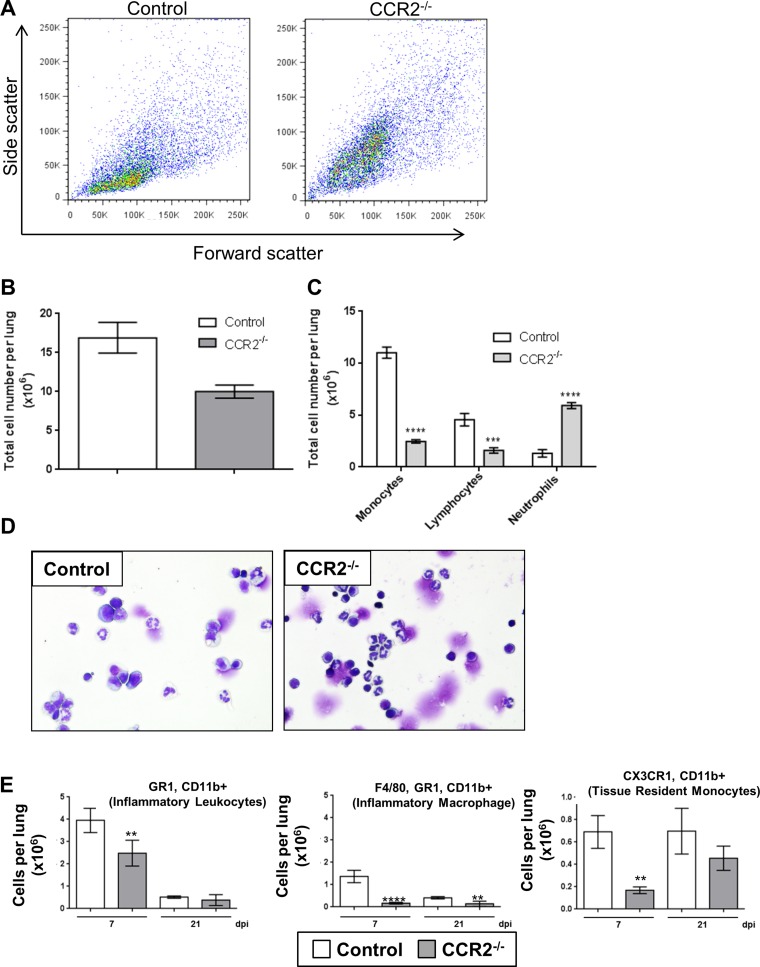
Previous studies have noted an increase in neutrophil recruitment in the absence of CCR2 in response to various viral infections (8, 11, 27, 44, 62). To characterize changes in immune cell recruitment following γHV-68 infection in the absence of CCR2, control C57BL/6J or CCR2−/− mice were infected i.n. with γHV-68 and lungs were harvested at either 7 or 21 dpi. Initial characterization of the lung cells by flow cytometry at 7 dpi revealed a shift in infected CCR2−/− mice from a forward scatter low, side scatter low population (likely monocytes and macrophages) to a forward scatter high, side scatter high population (likely neutrophils) (Fig. 2A). Total cells recovered by collagenase digestion of the lungs of infected CCR2−/− mice were lower than infected control mice at 7 dpi (Fig. 2B), corroborating the reduced inflammatory infiltrate noted in the histological analysis in Fig. 1. These cells were further characterized by cytospin and differential staining which revealed a decrease in the fraction of mononuclear cells and a large increase in the fraction of segmented granulocyte cells in the lungs at 7 dpi (Fig. 2, C and D). Flow cytometric analysis staining for CD11b, CX3CR1 (fractalkine receptor), GR-1, and the macrophage-specific marker F4/80 revealed that, while there was a small decrease in the number of total CD11b+,GR-1+ cells (the fraction containing both LY6C+ monocytes, and LY6G+ neutrophils) in the lungs of CCR2−/− mice at 7 dpi, these mice failed to recruit F4/80+ macrophages or inflammatory monocytes characterized by CD11b+,CX3CR1+ staining following γHV-68 infection (Fig. 2E). Taken together, these data show that CCR2−/− mice largely retain CD11b+,GR-1+ neutrophils, but do not recruit CD11b+,GR-1+,F4/80+ monocytes.
Fig. 2.
CCR2−/− mice recruit neutrophils to the lungs instead of monocytes in response to γHV-68 infection. A: representative dot plot showing side scatter (granularity) and forward scatter (cell size) flow cytometry profiles of collagenase-digested lung cells from B6 or CCR2−/− mice infected for 7 days with γHV-68. B: quantification of total leukocyte numbers present in the lungs at 7 dpi. C: quantification of differential stains showing numbers of leukocyte subpopulations present in the lungs at 7 dpi. D: representative images of differential stained collagenase-digested lung cells at 7 dpi. E: flow cytometric analysis of collagenase-digested lung cells at either 7 or 21 dpi stained for the indicated cell surface marker. Graphs display means ± SE, n = 4–5 mice per group (repeated twice with similar results), **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with B6.
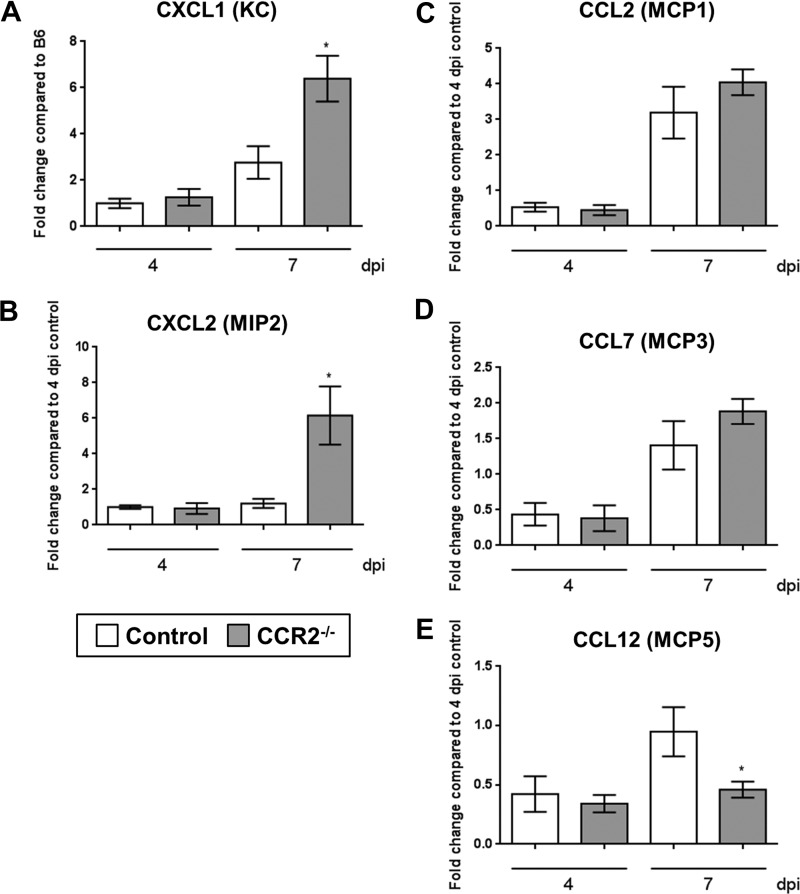
To further characterize the differences in the immune response to γHV-68 between control and CCR2−/− mice, we measured transcript expression of the neutrophil-recruiting chemokines CXCL1 and CXCL2 (also known as KC and MIP-2 in the mouse) as well as the monocyte recruitment chemokines CCL2, CCL7, and CCL12 [also known as monocyte chemoattractant protein (MCP) 1, 3, and 5, respectively] by qRT-PCR. Consistent with the increased numbers of neutrophils observed in the lungs of CCR2−/− mice by flow cytometry and histology, expression of both KC and MIP-2 were elevated in CCR2−/− mice at 7 dpi compared with control mice (Fig. 3, A and B). Although expression of CCR2 ligands has been shown to be elevated in CCR2 knockout mice due to the loss of feedback inhibition in the absence of the receptor (34), we did not observe a significant increase in CCL2 or CCL7 at either 4 or 7 dpi and noted a decrease in CCL12 expression at 7 dpi (Fig. 3, C–E).
Fig. 3.
Expression of neutrophil and monocyte recruitment genes is altered in the lungs of CCR2−/− mice in response to γHV-68. B6 or CCR2−/− mice (n = 4–5 mice per group) were infected for 4 or 7 days. Whole lungs were harvested and total RNA was extracted with TRIzol. Expression of the indicated transcripts was measured by qRT-PCR. Graphs display means ± SE relative to B6 control mice at 4 dpi. Statistical significance was calculated by ANOVA, *P < 0.05.
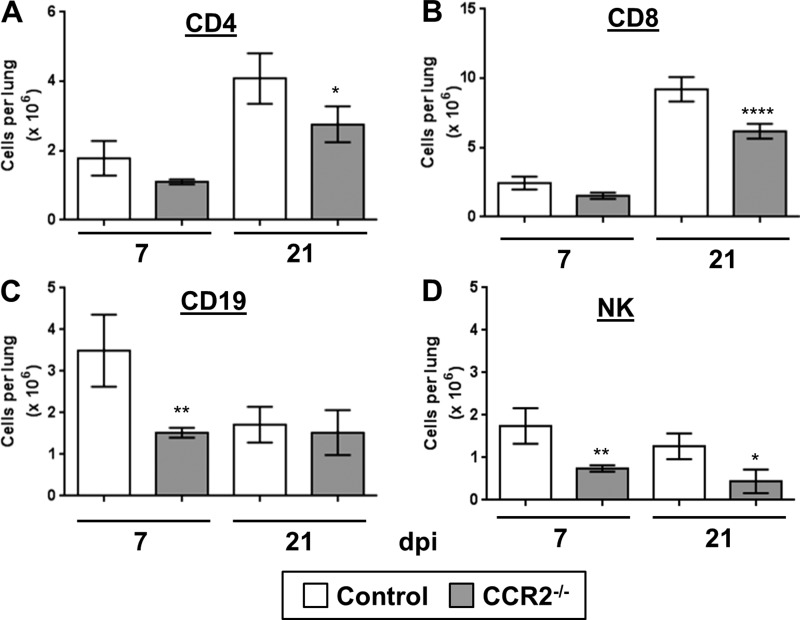
CCR2 is also expressed on a variety of lymphocytes including immature B cells and certain subpopulations of NK cells and T cells; thus deletion of CCR2 could directly impair the ability of lymphocytes to migrate in response to infection. To assess whether the migration of lymphocytes to the lungs was impaired in CCR2−/− mice in response to γHV-68, we analyzed the numbers of accumulated T cells (CD4 and CD8 positive) (Fig. 4, A and B), B cells (Fig. 4C), and NK cells (Fig. 4D) present at either 7 or 21 dpi by flow cytometry. Overall, numbers of all lymphocyte subtypes were lower in CCR2−/− mice at both time points compared with control mice; this was partly due to the fact that CCR2−/− mice had lower overall numbers of CD45+ cells in the lungs (Fig. 2B). Reduction in lymphocyte numbers was not as extreme as what was observed for GR-1-, F4/80-, or CX3CR1-positive monocytes/macrophages.
Fig. 4.
Deletion of CCR2 impairs recruitment of lymphocytes to the lungs in response to γHV-68. Purified leukocytes from the lungs of WT or CCR2−/− mice were analyzed at either 7 or 21 dpi by flow cytometry. All cells were first gated on CD45+ and then stained with CD4 (T helper cells), CD8 (cytotoxic T cells), CD19 (B cells), or NK1.1 (NK cells). Shown are the average absolute numbers of cells per lung digest; error bars represent ± SE, n = 4–5 mice per group (repeated once with similar results) *P < 0.05, **P < 0.01, ****P < 0.0001 compared with control.
Loss of CCR2 signaling exacerbates pathology of γ-herpesvirus induced pulmonary fibrosis following BMT.
We previously reported syngeneic BMT mice developed a severe pulmonary fibrosis and pneumonitis following γHV-68 infection (14, 65). This was driven in part by a switch from a Th1-mediated immune response to Th17. CCR2 knockout mice have been shown to be resistant in commonly used models of bleomycin- or FITC-induced pulmonary fibrosis (18, 33, 34, 54). Thus, to ascertain what role CCR2 may be playing during γHV-68-induced fibrosis post-BMT, we performed bone marrow transplantation by lethally irradiating wild-type (WT) C57BL/6J mice and reconstituting them with WT C57BL/6J (WT BMT) or CCR2−/− bone marrow (CCR2−/− BMT) stem cells followed by challenge with γHV-68.
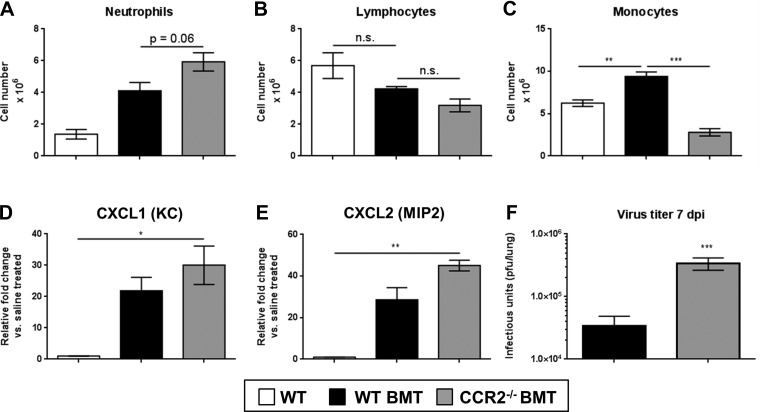
To confirm that CCR2−/− transplanted mice failed to recruit monocytes, lungs were harvested 7 dpi and leukocyte populations were identified by differential stain from collagenase-digested lung preparations (Fig. 5, A–C). Similar to nontransplanted mice, CCR2−/− BMT mice displayed a marked reduction in the numbers of monocytic cells in the lungs compared with WT BMT mice. Previously, BMT mice were shown to have a neutrophilic infiltration after γHV-68 challenge compared with non-BMT mice (14), and we saw a similar outcome in γHV-68-infected nontransplanted CCR2−/− mice (Fig. 2D). Similarly, we noted a further increase in neutrophil numbers in the lungs of CCR2−/− BMT mice as well as a corresponding increase in levels of CXCL1 (KC) and CXCL2 (MIP2) transcript expression and a ∼1-log increase in viral replication (Fig. 5, D–F).
Fig. 5.
Recruitment of neutrophils is increased in response to γHV-68 following CCR2−/− BMT. Mice at 5 wk post-BMT or control nontransplanted mice were infected for 7 days with 5 × 103 pfu γHV-68. A–C: numbers of lung infiltrating leukocyte populations as assessed by differential stain of single-cell suspensions from lung homogenates. D and E: qRT-PCR gene expression analysis of the neutrophil chemoattractant genes CXCL1 and CXCL2. F: lung-associated viral titer as assessed by plaque assay on 3T12 fibroblast monolayers. Statistical significance was determined by ANOVA (A–E) or Student's t-test (F), *P < 0.05, **P < 0.01, ***P < 0.001, n = 4–5 mice per group (repeated twice with similar results).
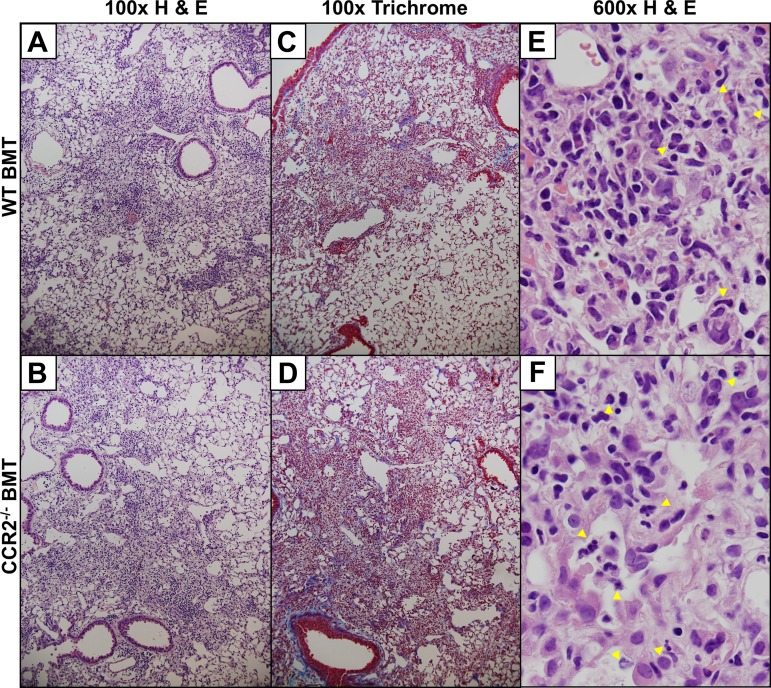
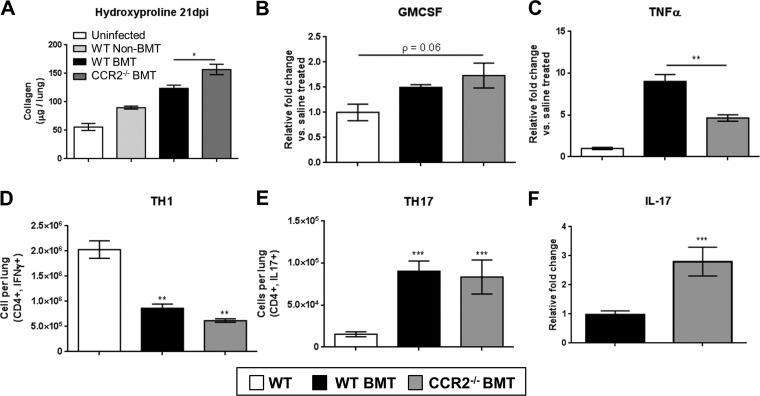
Histological comparison of FFPE lung sections revealed that WT BMT mice and CCR2−/− BMT mice displayed a marked fibrosis with large numbers of inflammatory cells seen by H&E stain and blue-stained ECM-rich fibrotic nodes evident by Masson's trichrome stain (Fig. 6, A–D). Examination of H&E-stained lung sections under high magnification revealed robust leukocyte infiltration into areas of dense fibrosis with large numbers of segmented granulocytes visible in CCR2−/− BMT mice (Fig. 6, E and F). To assess the degree of ECM deposition, levels of hydroxyproline were quantified in viral-infected mice and were found to be significantly increased in the lungs of CCR2−/− BMT mice compared with WT BMT (Fig. 7A). Increased levels of GM-CSF and low levels of TNFα have previously been correlated with the protective effect seen in nontransplanted CCR2−/− mice in response to bleomycin and FITC (32, 34). We measured levels of these two cytokines after γHV-68 infection in chimeric BMT mice and found analogous results in the CCR2−/− BMT mice (Fig. 7, B and C) despite the fact that the CCR2−/− BMT mice developed more ECM deposition. Alterations in T cell polarization, namely an increase in TH17 cells in conjunction with a concomitant decrease in numbers of TH1 cells, has previously been linked to development of γ-herpesvirus-induced fibrosis following BMT. We next analyzed numbers of TH1 and TH17 cells (IFNγ or IL17a expressing, respectively) by flow cytometry to assess whether the increased fibrotic pathology in CCR2−/− BMT mice was associated with a further increase in the numbers of lung infiltrating TH17 cells. Interestingly, there was no change in numbers of TH1 or TH17 cells in the CCR2−/− BMT mice compared with WT BMT (Fig. 7, D and E). However there was an approximately threefold increase the expression of IL-17 transcript as measured by qRT-PCR (Fig. 7F). Thus it is likely that the increased ECM deposition in CCR2−/− BMT mice is attributed to the higher levels of IL17 transcript detected in the lungs of CCR2−/− BMT mice compared with WT BMT mice. In summary, the absence of CCR2 signaling did not alter the switch from TH1-mediated immunity to TH17 in response to γHV-68 following BMT and loss of CCR2 worsened fibrotic outcomes after BMT.
Fig. 6.
Fibrotic pathology is preserved in the absence of CCR2 signaling. A and B: H&E staining of FFPE lung sections showing either B6-to-B6 syngeneic transplant (WT BMT) or CCR2−/−-to-B6 transplant at 21 dpi. C and D: Masson's trichrome staining of FFPE lung sections showing either B6-to-B6 syngeneic transplant or CCR2−/−-to-B6 transplant at 21 dpi. E and F: high-power (×600 magnification) fields of fibrotic areas from either WT or CCR2−/− BMT (respectively) lung sections harvested 21 dpi and stained with H&E. Yellow arrowheads show the location of infiltrating segmented/banded granulocytes. Images are representative of 3 separate experiments n = 3–4 mice per group per experiment.
Fig. 7.
Loss of CCR2 signaling exacerbates γ-herpesvirus-induced pulmonary fibrosis following BMT. A: 21 dpi, lungs were harvested from the indicated transplant group and total amount of lung associated collagen was assessed via hydroxyproline assay (n = 6–7 mice per group). B and C: total RNA was harvested from lungs at 7 dpi (n = 4–5 mice per group) and assayed via TaqMan qRT-PCR for the expression of the indicated transcript. D and E: cells from collagenase-digested lungs (7 dpi) were assayed by flow cytometry for CD4+,IFN-γ+ (TH1) or CD4+,IL-17+ (TH17) cells. F: total RNA was harvested from lungs at 7 dpi (n = 6–9 mice per group) and assayed via TaqMan qRT-PCR for the expression of the indicated transcript. All mean values are displayed with error bars representing ±SE, Statistical significance was determined by ANOVA or t-test in H. *P < 0.05, **P < 0.01, ***P < 0.001. All experiments were repeated twice with same result.
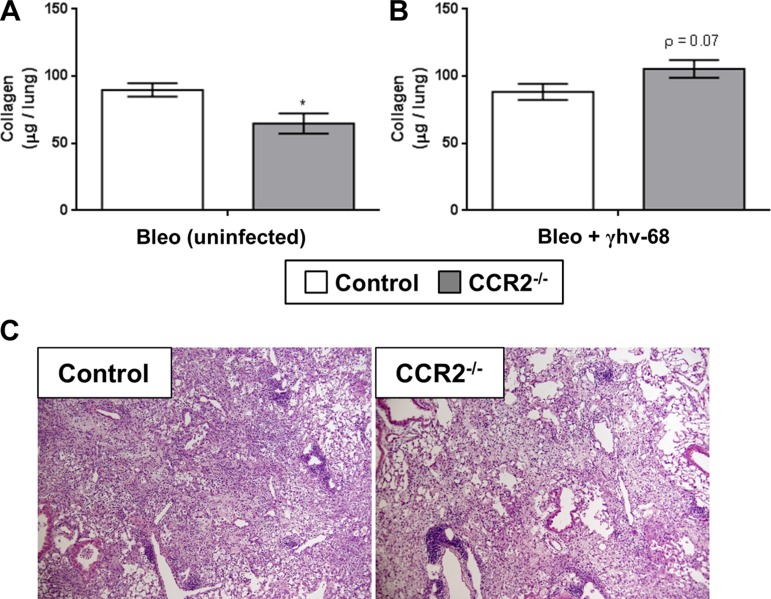
CCR2−/− mice have previously shown protection from bleomycin-induced pulmonary fibrosis (18). Conversely, γHV-68 has been shown to exacerbate established bleomycin-induced fibrosis (4). To address the question of whether infection with γHV-68 could exacerbate bleomycin-induced pulmonary fibrosis in the context of a normally protected CCR2−/− background, WT or CCR2−/− mice were first given an intratracheal injection of bleomycin; 14 days after treatment, mice were infected with γHV-68 or left untreated as control, and 14 dpi (21 days postbleomycin treatment) lungs were harvested and deposition of ECM was measured by hydroxyproline and FFPE lung sections were examined. As expected, in the absence of γHV-68 infection CCR2−/− mice showed a marked reduction in the amount of lung-associated hydroxyproline compared with WT uninfected mice (Fig. 8A). Interestingly, when infected with γHV-68, both WT and CCR2−/− mice had comparable levels of hydroxyproline (Fig. 8B) and both groups showed similar pathology in the lungs 14 days after γHV-68 infection following bleomycin treatment (Fig. 8C), indicating that γHV-68 can exacerbate bleomycin-induced pulmonary fibrosis in normally protected CCR2−/− mice even in the absence of BMT.
Fig. 8.
Deletion of CCR2 is not protective against γHV-68 exacerbation of pulmonary fibrosis following bleomycin. C57/Bl6 mice were first challenged with bleomycin for 14 days and either subsequently infected with 5 × 103 pfu MHV-68 or left uninfected for a further 7 days (21 days total). A: lungs were harvested 7 dpi (21 days postbleomycin) and total lung-associated collagen was quantitated by hydroxyproline assay. B: H&E stain of FFPE lungs sections of either WT or CCR2−/− mice after bleomycin treatment and MHV-68 infection. Statistical significance was calculated by Student's t-test (n = 5–7 mice per group), *P < 0.05.
CCR2−/− BMT mice develop γHV-68-induced fibrosis despite reduced fibrocyte accumulation.
Fibrocytes are a circulating leukocyte subpopulation distinct from monocytes that are recruited to sites of inflammation and mediate processes such as wound healing, tissue repair/remodeling, and fibrosis (10, 20, 24). CD45+, collagen I-expressing fibrocytes are recruited to the lungs in a CCR2-dependent fashion (31, 33). Furthermore, γHV-68 exacerbation of FITC-induced pulmonary fibrosis increased recruitment of fibrocytes to the lungs of mice (58).
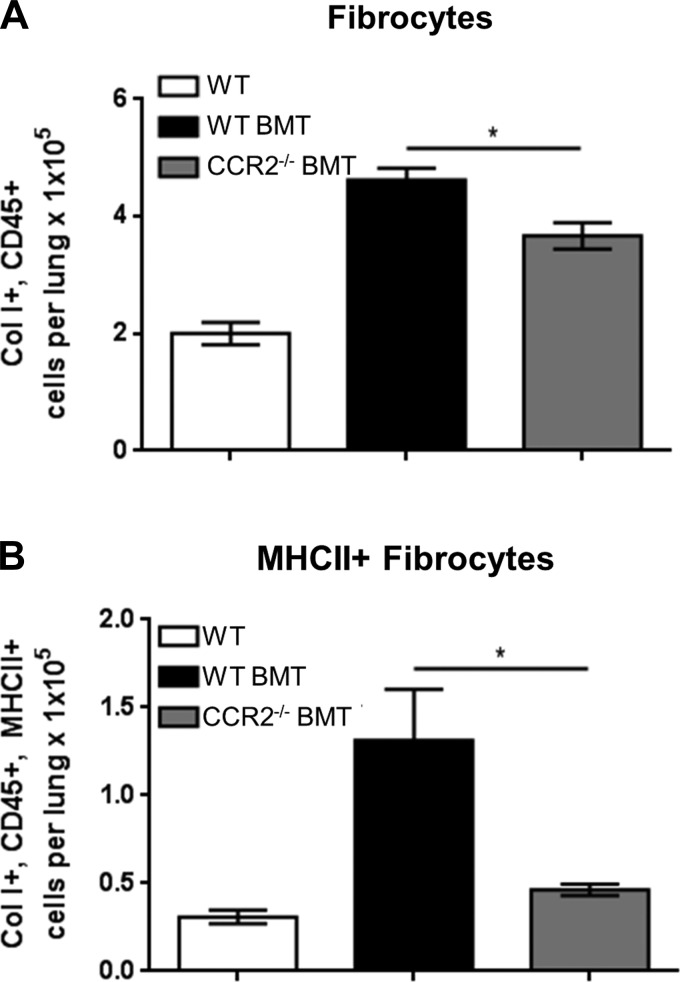
Given that mice transplanted with CCR2−/− bone marrow still develop pulmonary fibrosis in response to γHV-68 infection, we sought to measure numbers of CD45+ collagen I+ fibrocytes recruited to the lungs. Flow cytometry was performed on collagenase-digested lung leukocytes and numbers of fibrocytes were quantified by flow cytometry. Following WT BMT, an increase in total fibrocytes was observed at 7 dpi compared with nontransplanted WT mice infected with γHV-68 (Fig. 9A). Consistent with previous reports, absolute numbers of fibrocytes recruited to the lungs were decreased in CCR2−/− BMT mice compared with WT BMT (Fig. 9A). Interestingly, there was a marked reduction in the numbers of MHCII+ fibrocytes recruited to the lungs of CCR2−/− BMT mice, indicating a change in the activation state between WT and CCR2−/− BMT mice (Fig. 9B).
Fig. 9.
CCR2−/− BMT mice recruit fewer fibrocytes in response to γ-herpesvirus infection. Control C57Bl/6, WT BMT, or CCR2−/− BMT mice (n = 4–5 mice per group) were infected i.n. with 5 × 103 pfu γHV-68. At 7 dpi, cells were harvested from collagenase-digested lungs and stained with indicated antibodies and analyzed via flow cytometry. A: total numbers of CD45+,Col I+ cells. B: CD45+,Col I+,MHCII+ cells. Average number of cells per lung is shown ± SE. Statistical significance was calculated by ANOVA, *P < 0.05 (n = 3–4 mice per group).
Loss of CCR2 signaling alters LY6C+ and LY6G+ cell populations in response to γ-herpesvirus challenge following BMT.
Differential stain of collagenase-digested single-cell lung suspensions indicated a marked reduction in the numbers of mononuclear cells and an accumulation of neutrophilic granulocytes in the absence of CCR2 signaling. To further characterize the subpopulations of cells present or absent in the lungs following γHV-68 challenge after BMT, we utilized flow cytometry and various markers for both monocytes and granulocytes at 7 dpi.
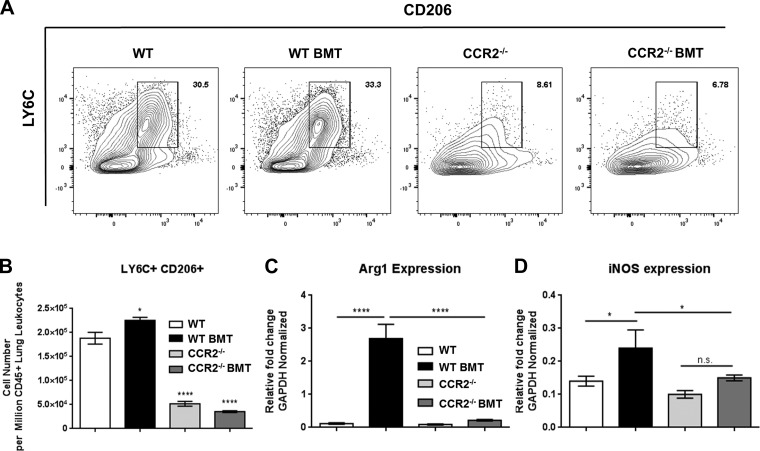
A previous study indicated an infiltration of alternatively activated (M2) skewed monocytes and macrophage during γHV-68-induced pulmonary fibrosis using a mouse strain deficient in the IFNγ receptor (IFNγR−/−) (35). Thus we first compared numbers of CD45+,CD11B+,LY6C+,CD206+ (mannose receptor) cells to assess whether M2-like monocytes/macrophage were recruited to the lungs during γHV-68 challenge following BMT. In agreement with the previous study, WT BMT mice recruited significantly more CD206+ M2-like cells to the lungs following γHV-68 challenge after BMT (Fig. 10A, quantified in Fig. 10B). Interestingly, numbers of CD206+ LY6C+ cells were markedly reduced in both BMT and non-BMT CCR2−/− mice (Fig. 10A, quantified in Fig. 10B). We next measured transcript levels of inducible nitric oxide synthetase (iNOS; a marker for M1 traditionally skewed monocytes/macrophage), and arginase 1 (Arg1; a marker for M2 alternatively activated monocytes) via qRT-PCR of RNA prepared from Percoll-purified, collagenase-digested lung leukocytes. Correlating with the increase in numbers of lung infiltrating CD206+,LY6C+ cells in WT BMT mice at 7 dpi, a significant increase in Arg1 transcript level was also detected (Fig. 10C). We also measured a significant increase in iNOS expression, indicating a mixed activation phenotype of monocytes recruited to the lungs of BMT mice in response to γHV-68 (Fig. 10D). Furthermore, levels of both Arg1 and iNOS were significantly reduced in CCR2−/− BMT mice compared with WT BMT, suggesting that that neither M1 nor M2 skewed monocyte/macrophages accumulate in infected CCR2−/− mice.
Fig. 10.
Reduced recruitment of CD206+, Arg1-expressing cells in CCR2−/− BMT mice. A: flow cytometric analysis of single-cell suspensions prepared from collagenase-digested lungs of γHV-68 infected mice at 7 dpi. Cells were first gated on CD45, CD11B double positive before being gated by LY6C and CD206. Shown are concatenated plots, combining data from all mice in a particular group. B: quantification of cell number from the LY6C+,CD206+ gate in A. C and D: RNA was prepared from Percoll-purified leukocytes taken from collagenase-digested lung tissue at 7 dpi. Transcript expression analysis was conducted via qRT-PCR for the indicated transcript. Statistical significance was determined by ANOVA *P < 0.05, ****P < 0.0001, n = 4–5 mice per group (repeated once with similar results).
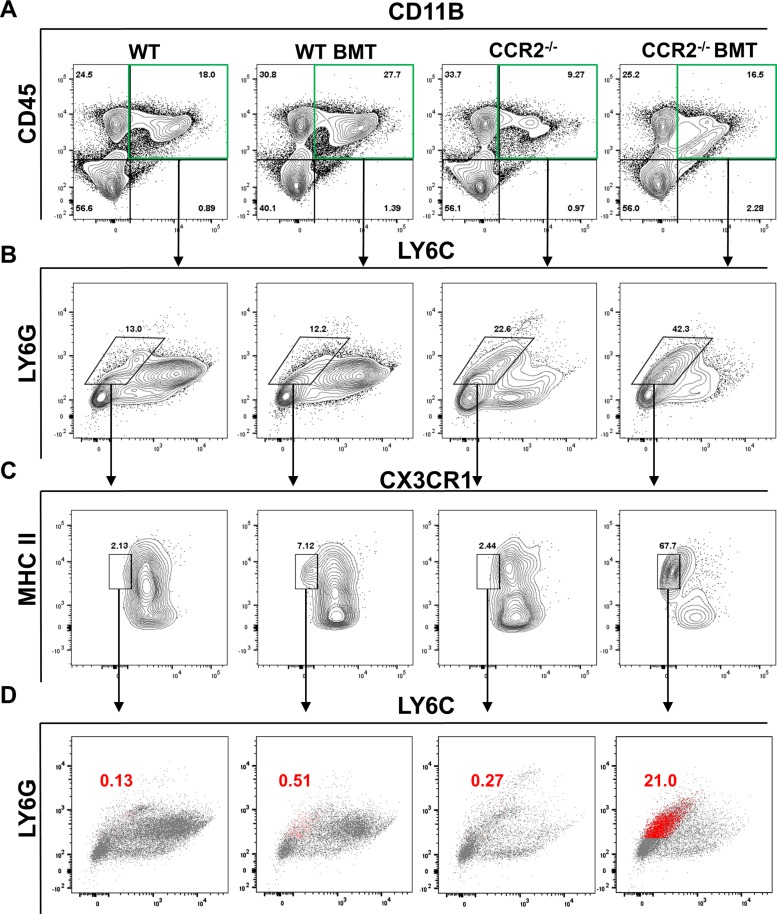
To further analyze granulocytic cells recruited to the lungs following γHV-68 challenge, we next assessed subpopulations of LY6G+ cells in the lungs at 7 dpi via flow cytometry. WT BMT mice exhibited an increased infiltration of CD11B+,CD45+ cells and, as expected, both non-BMT and BMT CCR2−/− mice had far fewer of these cells (Fig. 11A, top right quadrant). We next assessed LY6C and LY6G expression on CD11B+,CD45+ cells. Both non-BMT and BMT WT mice had significant infiltration of LY6C+,LY6G− cells while, in agreement with increased numbers of segmented and banded granulocytes observed via differential stain, CCR2−/− mice displayed an increase in LY6G+,LY6C− cells following γHV-68 challenge (Fig. 11B). To further separate granulocytes from any LY6C−/intermediate tissue resident monocytes that may be present, we gated the remaining cells based on CX3CR1 (Fractalkine receptor) and major histocompatibility complex (MHC) II staining. Interestingly, while we expected most of the granulocytes present to be both CX3CR1− and MHC II− we instead found a population of MHC II+ granulocytes that was present in WT BMT mice and to a much larger extent in CCR2−/− BMT mice (Fig. 11C). Backgating of this MHCII+ population based on LY6G and LY6C staining revealed the vast majority to be LY6C− and LY6G intermediate (Fig. 11D).
Fig. 11.
Recruitment of granulocytes is altered in CCR2−/− BMT mice following γHV-68 challenge. A–C: flow cytometric analysis of single-cell suspension prepared from collagenase-digested lung tissue at 7 dpi. Autofluorescent CD11c+ alveolar macrophage were first gated out (not shown) after which cells were gated by CD45, CD11B double positivity (A, top right quadrant). Subsequent gating was carried out using staining for LY6C and LY6G (B) and for CX3CR1 and MHC II (C). D: MHCII+ cells from C were back gated onto the LY6G/LY6C plot from B and are highlighted in red (shown as a percentage of CD45+,CD11B+ cells). Shown are concatenated plots combining data from all mice in a particular group, n = 4–5 mice per group (repeated once with similar results).
Taken together, our data indicate that CCR2−/− BMT mice failed to recruit CD206+, Arg1-expressing alternatively activated monocytes in response to γHV-68. We also identified a novel population of LY6G intermediate, MHCII+ granulocytes that was elevated in WT BMT lungs and to an even greater extent in CCR2−/− BMT mice but virtually absent from the lungs of virus-challenged nontransplanted mice.
Treatment of CCR2−/− BMT mice with an αGR1 antibody reduces but does not eliminate granulocyte infiltration and alters TH1/TH17 skewing following γ-herpesvirus challenge.
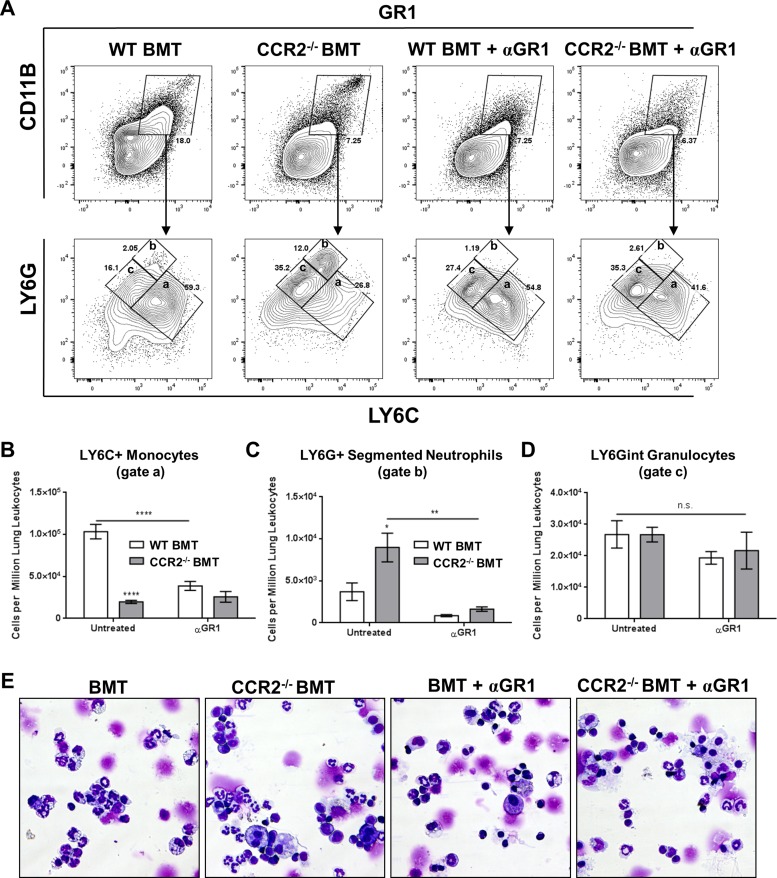
A marked increase in the number of lung infiltrating neutrophils was observed in CCR2−/− mice infected with γHV-68 both before and after BMT (Figs. 2C and 5A). To assess the role that these cells may play in the progression of pulmonary fibrosis, neutrophils and other GR1+ cells were depleted in mice at 5 wk post-BMT starting 3 days prior to infection and continuing every 3–4 days using intraperitoneal injections of an αGR1 antibody (500 μg per dose). Numbers of lung infiltrating GR1+ cells, most of which were neutrophils as judged by forward vs. side scatter and high expression of both GR1 and CD11B, were markedly reduced at 7 dpi as assessed by flow cytometry in the antibody-treated mice (Fig. 12A, top row).
Fig. 12.
Treatment of BMT mice with αGR1 antibody reduces but does not eliminate granulocyte infiltration in response to γMHV-68. A: flow cytometry plots showing staining of single-cell suspensions prepared from collagenase-digested lung tissue at 7 dpi. GR1, CD11B double positive cells were first gated (top row) after which subsequent gating was carried out using LY6C and LY6G (bottom row). B through D: quantification of inset gates a, b, and c from LY6C/LY6G staining in A (n = 4–5 mice per group, repeated once with similar results); statistical analysis was carried out by ANOVA; *P < 0.05, **P < 0.01, ****P < 0.0001. E: representative images of differential stained lung leukocytes from collagenase-digested lungs.
GR1 is a shared epitope expressed on granulocytes as LY6G and as LY6C on granulocytes and monocytes. Thus we further gated GR1+ cells by LY6G and LY6C staining to identify subpopulations of leukocytes that had been depleted by the αGR1 antibody (Fig. 12A, bottom row). As expected, untreated CCR2−/− BMT mice displayed a marked reduction in the numbers of LY6C+ monocytes due to a lack of recruitment of inflammatory cells in the absence of CCR2 signaling (Fig. 12A, gate a, quantified in Fig. 12B). As αGR1 also targets an epitope on LY6C, we noted decreased numbers of LY6C+ monocytes even in WT BMT mice that normally have robust infiltration of inflammatory monocytes in response to γHV-68 (Fig. 12A, gate a, quantified in Fig. 12B). Correlating with the increase in neutrophilic infiltration in CCR2−/− mice, we observed a robust increase in LY6G+ cells in untreated CCR2−/− BMT mice at 7 dpi (Fig. 12A, gate b, quantified in Fig. 12C). Treatment with αGR1 antibody reduced numbers of LY6G high neutrophils in WT and CCR2−/− BMT mice (Fig. 12A, gate b, quantified in Fig. 12C). However, numbers of LY6G intermediate granulocytes remained consistent across all groups, indicating that certain populations of LY6G-expressing granulocytes are resistant to depletion using αGR1 antibodies (Fig. 12A, gate c, quantified in Fig. 12D). To further identify this αGR1-resistant population, single-cell suspensions from collagenase-digested lung preparations were analyzed by differential stain at 7 dpi. As expected, the lungs of untreated CCR2−/− BMT mice had an excess of segmented neutrophils compared with WT BMT (Fig. 12E). Interestingly, after αGR1 treatment there was substantial reduction in these segmented polymorphonuclear leukocytes in both WT and CCR2−/− BMT lungs; however, numbers of banded neutrophilic granulocytes were relatively unchanged.
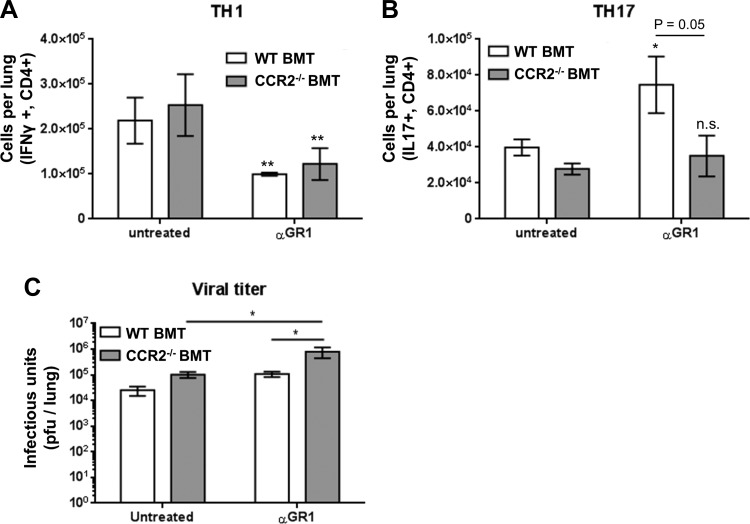
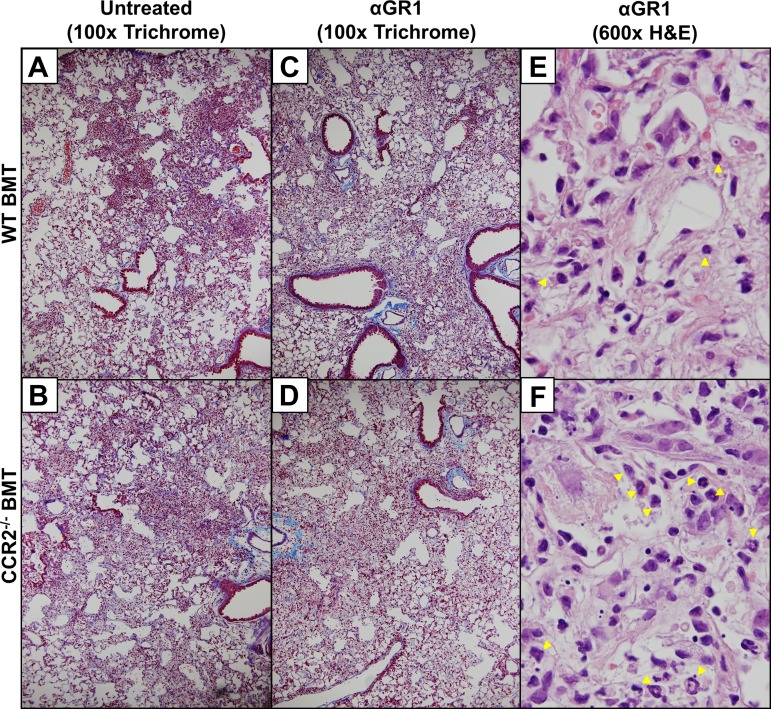
We next assessed numbers of TH1 and TH17 cells in the lungs of WT or CCR2−/− BMT mice 7 dpi by flow cytometry and intracellular cytokine staining for either IL-17 (TH17) or IFNγ (TH1). Depletion of GR1+ cells resulted in a significant decrease in the numbers of lung associated TH1 cells at 7 dpi in both WT and CCR2−/− BMT mice (Fig. 13A). Interestingly, numbers of TH17 cells were significantly increased in WT BMT mice following αGR1 treatment, indicating that certain populations of GR1-expressing monocytes normally may dampen TH17 responses. Consistent with this, αGR1 treatment had no effect on TH17 cells in the CCR2−/− BMT mice where monocyte recruitment was already blunted (Fig. 13B). Viral burden was measured in the lungs at 7 dpi via plaque assay and found to be ∼1-log higher in both WT BMT and CCR2−/− BMT following αGR1+ treatment compared with control-treated BMT mice of both genotypes (Fig. 13C). Microscopic examination of FFPE lung sections at 21 dpi revealed similar evidence of fibrotic pathology in both control and αGR1-treated mice following γHV-68 challenge in both WT and CCR2−/− BMT mice with collagen-rich fibrotic nodes evident by Masson's trichrome staining (Fig. 14, A–D), in agreement with the maintenance of LY6G intermediate cells as observed by flow cytometry in αGR1-treated mice. Analysis of H&E-stained lung sections under high magnification revealed numerous segmented and banded granulocytes present in areas of dense fibrosis in both WT and CCR2−/− BMT mice at 21 dpi (Fig. 14, E and F).
Fig. 13.
Loss of GR1+ cells contributes to TH1 and TH17 responses during γ-herpesvirus-induced fibrosis following BMT. A and B: 7 dpi, cells were harvested from collagenase-digested lungs of either control WT BMT, control CCR2−/− BMT, or αGR1-treated WT or CCR2−/− BMT mice (n = 4–5 mice per group, repeated once with similar results) and stained for CD4+,IFN-γ+ (TH1) or CD4+,IL-17+ (TH17) cells. C: lungs were harvested 7 dpi and infectious virus was quantified by plaque assay on NIH 3T12 monolayers. Mean values per lung are shown ±SE. Statistical significance was calculated by ANOVA, *P < 0.05, **P < 0.01.
Fig. 14.
Depletion of GR1+ cells does not eliminate γ-herpesvirus-induced fibrosis following BMT. WT BMT or CCR2−/− BMT mice (n = 4–5 mice per group) were infected with 5×103 pfu γMVH-68. A and B: lung sections from untreated mice stained with Masson's trichrome 21 dpi. C and D: lung sections from mice given 500 μg of αGR1 antibody intraperitoneally every 3 days until 12 dpi and then harvested at 21 dpi and stained with Masson's trichrome. E and F: high-power (×600 magnification) fields of fibrotic areas from either αGR1-treated WT or CCR2−/− BMT (respectively) lung sections harvested 21 dpi and stained with H&E. Yellow arrowheads show the location of infiltrating segmented/banded granulocytes. Images are representative of 2 separate experiments; n = 3–4 mice per group per experiment.
DISCUSSION
Human herpesviruses, while largely innocuous in the normal immunocompetent population, can cause severe morbidity and mortality following stem cell transplant. DNA of various herpesviruses, including HHV-6 and HCMV, was detected with high frequency in HSCT patients suffering from IPS, and EBV is a major cause of posttransplant pneumonia (48, 63). Immune reconstitution following myeloablation undoubtedly contributes to an altered immunological response to herpesvirus infections; however, the cellular mechanisms that contribute to these pathologies are not well elucidated.
Deletion of CCR2 in nontransplanted mice resulted in a ∼1-log increase in lung associated viral titer, compared with WT mice, following γHV-68 challenge at 4 and 7 dpi. Marked reductions in the numbers of proinflammatory monocytes (CD11B+,GR1+,F4−80+) as well as various subclasses of lymphocytes including CD4+, CD8+, B cells, and NK cells were also noted. The reduced capacity of CCR2−/− mice to recruit cells involved in both innate and adaptive immunity most likely explains the increase in viral burden observed in the lungs of CCR2−/− mice at 7 dpi. Several of these leukocyte populations are implicated in the control and maintenance of γ-herpesvirus infections. However, even in the face of this drastic reduction in the recruitment of leukocytes in CCR2−/− mice, no defect in eventual viral clearance was observed and levels of infectious virus were almost undetectable in both WT and CCR2−/− mice by 10 dpi. Many chemokine/cytokine receptors are upregulated following γHV-68 infection (45, 46), implying that, while CCR2+ cells are important for the initial control of virus replication, other chemokine receptors such as CXCR3 and CXCR4 undoubtedly serve redundant functions and facilitate migration of activated lymphocytes sufficient for long-term control of γ-herpesvirus replication.
Given that CCR2−/− mice have been shown to be protected in many other models of lung fibrosis (7, 18, 34), we were surprised to find that CCR2−/− BMT mice develop exuberant pneumonitis and fibrosis following γHV-68 infection. Because we had previously shown that protection from FITC-induced lung fibrosis was associated with reduced recruitment of fibrocytes (32), we looked for accumulation of fibrocytes in these mice. As anticipated, absolute numbers of CD45+ collagen 1+ fibrocytes were reduced in CCR2−/− BMT mice compared with WT BMT mice; however, the accumulation of fibrocytes in the lungs of both groups of BMT mice was greater than in nontransplanted controls. One possibility may be that that numbers of fibrocytes recruited in the CCR2−/− BMT mice were still sufficiently high post-γHV-68 that fibrosis was unchanged. We have previously suggested that fibrocytes promote fibrogenesis via paracrine effects (24, 25); thus the number recruited in CCR2−/− BMT mice may secrete sufficiently high levels of profibrotic mediators. Another possibility may be that, in the context of viral infection, the fibrocytes play an important host-defense role rather than a fibrogenic role. Interestingly, fibrocytes have been shown to present viral antigens to T cells, and fibrocytes can promote T cell proliferation in sepsis models (5, 38). In this regard, it is interesting that the reduction in CD45+,collagen 1+,MHC-II+ fibrocytes is far more dramatic in CCR2−/− BMT mice (Fig. 9B). This may explain, in part, the impaired early viral clearance in the CCR2−/− BMT mice.
BMT mice infected with γHV-68 exhibited an accumulation of lung infiltrating LY6C+ monocytes at 7 dpi, many of which were positive for the alternative macrophage marker CD206 (mannose receptor). Additionally, BMT mice had higher levels of Arg1 transcript, indicating an increase in the infiltration or differentiation of alternatively activated monocytes/macrophages. Mice transplanted with CCR2−/− bone marrow and challenged with γHV-68 failed to recruit LY6C+ monocytes and Arg1 expression was comparable to that of non-BMT mice at 7 dpi. Alternatively activated macrophages expressing both CD206 and arginase I have been implicated in the progression of pulmonary fibrosis following γHV-68 infection in IFNγR−/− mice and in a bleomycin model of pulmonary fibrosis (35, 60). In contrast, several studies have detailed a protective role for M2 macrophages during progression of pulmonary fibrosis (42, 57). Thus the role of M1 or M2 skewed macrophages during progression of pulmonary fibrosis is highly context dependent. However, during γ-herpesvirus infection following BMT, it appears that the loss of Arg1 expression and CD206+ monocytes correlates with a more severe fibrotic outcome.
CCR2−/− BMT mice exhibited a ∼1.5-fold increase in LY6G+ granulocyte infiltration compared with WT BMT mice following γHV-68 challenge. Previous studies have demonstrated that neutrophils can both secrete and be activated by IL-17 (17). Given that development of pneumonitis and fibrosis in γHV-68-infected BMT mice is dependent on IL-17 (65), we wondered whether neutrophils may contribute to the progression of γHV-68-induced pulmonary fibrosis post-BMT and expected neutrophil depletion to improve outcomes. Following depletion with an αGR1 antibody, both WT BMT and CCR2−/− BMT mice had a marked reduction in the numbers of certain populations of LY6G+ granulocytes (mostly highly LY6G expressing segmented neutrophils) and LY6C+ monocytes. Counter to our initial hypothesis, the reduction in highly GR1+ segmented neutrophils did not correspond to reduced IL-17 levels. In fact, WT BMT mice treated with αGR1 exhibited elevated levels of IL-17-expressing TH17 cells, and CCR2−/− BMT mice had no reduction in TH17 numbers despite reductions in the numbers of Th1 cells in both genotypes of mice treated with αGR1. Furthermore, no protection from pulmonary fibrosis was observed after administration of αGR1.
Interestingly, there was a population of LY6G+ intermediate cells that was resistant to αGR1 therapy. Based on the low levels of LY6G expression, this population correlates with the unusual population of LY6G low, MHC II+ granulocyte like cells that were in abundance in both WT and CCR2−/− BMT lungs. Certain populations of granulocytes have been shown to express MHC II and drive T cell cytokine responses, including IL-17 (1, 40). Other populations of granulocytes such as eosinophils are well characterized to play pathogenic roles in the progression of pulmonary fibrosis following bleomycin treatment and in allergic and nonallergic models of asthma (9, 22, 23). Furthermore, stimulation of eosinophils with IL-17a, IL-17f, and the TH17-associated cytokine IL-23 induced secretion of the profibrotic mediators TGFβ and IL-11 (3). Thus it is plausible that these αGR1-resistant, MHC II+ granulocytes contribute to the IL-17-mediated progression of γ-herpesvirus-induced pulmonary fibrosis following BMT.
Our previous study suggested that the process of BMT impairs the function of CD11c+ antigen-presenting cells (APCs) in the lung to promote Th17, rather than Th1 responses (65). Adoptive transfer of CD11C+ APCs from nontransplanted mice restores TH1 and TH17 balance in BMT mice; however, the specific subset of CD11C+ cells responsible for this effect has not been clarified. CCR2 is present on the surface of a variety of proinflammatory CD11C+ DC populations. Interestingly, these CCR2+ CD11C+ DCs are implicated in the progression of fibrosis in a variety of disease models including CCL4-induced liver fibrosis and bleomycin-induced pulmonary fibrosis (6, 30, 37). Contrary to these studies, γHV-68-infected CCR2−/− BMT mice developed more severe pulmonary fibrosis compared with their WT BMT counterparts. Higher levels of IL-17 transcript were detected at 7 dpi in CCR2−/− BMT compared with WT BMT which correlated with an increased accumulation of collagen I as measured by hydroxyproline assay at 21 dpi. Recently it was found that CCR2+ monocytes are protective in a silica model of pulmonary fibrosis preventing the transition from nodular to diffuse fibrosis by altering genes involved in tissue remodeling (50). Thus it is possible that the lack of recruited CCR2+,LY6C+ monocytes coupled with the increased infiltration of αGR1-resistant LY6G intermediate granulocytes contributes to the increased progression of pulmonary fibrosis in CCR2−/− BMT mice.
In conclusion, our results suggest that CCR2 deficiency is not protective in γHV-68-induced pneumonitis and fibrosis following BMT and in fact CCR2+ cells may play a protective role in limiting progression of pulmonary fibrosis in response to γ-herpesviruses. This is most likely due to the fact that CCR2−/− BMT mice still accumulate sufficient TH17 cells in response to γHV-68 infection, and our earlier work has shown that IL-17 can directly promote fibroblast activation in this context (65). These data provide new evidence that CCR2 is dispensable for at least some forms of lung fibrosis and suggest that anti-CCR2 therapies may be ineffective in IL-17-mediated fibrotic disease. Taken together, our results imply that different pathogenic mechanisms regulate infectious vs. sterile insults leading to lung fibrosis.
GRANTS
This work was supported by NIH grants AI117229 and HL115618 awarded to B. B. Moore. S. J. Gurczynski was supported by T32HL07749.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.J.G., D.N.O., and B.B.M. conception and design of research; S.J.G., M.C.P., and C.A.W. performed experiments; S.J.G., M.C.P., D.N.O., and B.B.M. analyzed data; S.J.G. and B.B.M. interpreted results of experiments; S.J.G. prepared figures; S.J.G. and B.B.M. drafted manuscript; S.J.G., M.C.P., D.N.O., and B.B.M. edited and revised manuscript; S.J.G., M.C.P., D.N.O., C.A.W., and B.B.M. approved final version of manuscript.
REFERENCES
- 1.Abi Abdallah DS, Egan CE, Butcher BA, Denkers EY. Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T cell differentiation. Int Immunol 23: 317–326, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afessa B, Peters SG. Major complications following hematopoietic stem cell transplantation. Semin Respir Crit Care Med 27: 297–309, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Al-Muhsen S, Letuve S, Vazquez-Tello A, Pureza MA, Al-Jahdali H, Bahammam AS, Hamid Q, Halwani R. Th17 cytokines induce pro-fibrotic cytokines release from human eosinophils. Respir Res 14: 34, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashley SL, Jegal Y, Moore TA, van Dyk LF, Laouar Y, Moore BB. γ-Herpes virus-68, but not Pseudomonas aeruginosa or influenza A (H1N1), exacerbates established murine lung fibrosis. Am J Physiol Lung Cell Mol Physiol 307: L219–L230, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balmelli C, Ruggli N, McCullough K, Summerfield A. Fibrocytes are potent stimulators of anti-virus cytotoxic T cells. J Leukoc Biol 77: 923–933, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Bantsimba-Malanda C, Marchal-Somme J, Goven D, Freynet O, Michel L, Crestani B, Soler P. A role for dendritic cells in bleomycin-induced pulmonary fibrosis in mice? Am J Respir Crit Care Med 182: 385–395, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Baran CP, Opalek JM, McMaken S, Newland CA, O'Brien JM Jr, Hunter MG, Bringardner BD, Monick MM, Brigstock DR, Stromberg PC, Hunninghake GW, Marsh CB. Important roles for macrophage colony-stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 176: 78–89, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boivin N, Menasria R, Gosselin D, Rivest S, Boivin G. Impact of deficiency in CCR2 and CX3CR1 receptors on monocytes trafficking in herpes simplex virus encephalitis. J Gen Virol 93: 1294–1304, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Broide DH. Immunologic and inflammatory mechanisms that drive asthma progression to remodeling. J Allergy Clin Immunol 121: 560–570; quiz 571–562, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1: 71–81, 1994. [PMC free article] [PubMed] [Google Scholar]
- 11.Cadillac JM, Sigler RE, Weinberg JB, Lutzke ML, Rochford R. Gammaherpesvirus-induced lung pathology is altered in the absence of macrophages. Lung 183: 239–251, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Chambers RC. Abnormal wound healing responses in pulmonary fibrosis: focus on coagulation signalling. Eur Respir Rev 17: 130–137, 2008. [Google Scholar]
- 13.Chambers RC, Mercer PF. Mechanisms of alveolar epithelial injury, repair, and fibrosis. Ann Am Thorac Soc 12, Suppl 1: S16–S20, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coomes SM, Farmen S, Wilke CA, Laouar Y, Moore BB. Severe gammaherpesvirus-induced pneumonitis and fibrosis in syngeneic bone marrow transplant mice is related to effects of transforming growth factor-beta. Am J Pathol 179: 2382–2396, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coomes SM, Wilke CA, Moore TA, Moore BB. Induction of TGF-beta 1, not regulatory T cells, impairs antiviral immunity in the lung following bone marrow transplant. J Immunol 184: 5130–5140, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebrahimi B, Dutia BM, Brownstein DG, Nash AA. Murine gammaherpesvirus-68 infection causes multi-organ fibrosis and alters leukocyte trafficking in interferon-gamma receptor knockout mice. Am J Pathol 158: 2117–2125, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol 170: 2106–2112, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Gharaee-Kermani M, McCullumsmith RE, Charo IF, Kunkel SL, Phan SH. CC-chemokine receptor 2 required for bleomycin-induced pulmonary fibrosis. Cytokine 24: 266–276, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest 113: 243–252, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herzog EL, Bucala R. Fibrocytes in health and disease. Exp Hematol 38: 548–556, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hildebrandt GC, Duffner UA, Olkiewicz KM, Corrion LA, Willmarth NE, Williams DL, Clouthier SG, Hogaboam CM, Reddy PR, Moore BB, Kuziel WA, Liu C, Yanik G, Cooke KR. A critical role for CCR2/MCP-1 interactions in the development of idiopathic pneumonia syndrome after allogeneic bone marrow transplantation. Blood 103: 2417–2426, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Huaux F, Gharaee-Kermani M, Liu T, Morel V, McGarry B, Ullenbruch M, Kunkel SL, Wang J, Xing Z, Phan SH. Role of eotaxin-1 (CCL11) and CC chemokine receptor 3 (CCR3) in bleomycin-induced lung injury and fibrosis. Am J Pathol 167: 1485–1496, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huaux F, Liu T, McGarry B, Ullenbruch M, Xing Z, Phan SH. Eosinophils and T lymphocytes possess distinct roles in bleomycin-induced lung injury and fibrosis. J Immunol 171: 5470–5481, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Kleaveland KR, Moore BB, Kim KK. Paracrine functions of fibrocytes to promote lung fibrosis. Expert Rev Respir Med 8: 163–172, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleaveland KR, Velikoff M, Yang J, Agarwal M, Rippe RA, Moore BB, Kim KK. Fibrocytes are not an essential source of type I collagen during lung fibrosis. J Immunol 193: 5229–5239, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci USA 94: 12053–12058, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim JK, Obara CJ, Rivollier A, Pletnev AG, Kelsall BL, Murphy PM. Chemokine receptor Ccr2 is critical for monocyte accumulation and survival in West Nile virus encephalitis. J Immunol 186: 471–478, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauermann N, Burian J, von Garnier C, Dirnhofer S, Germano D, Schuett C, Tamm M, Bingisser R, Eriksson U, Hunziker L. Interferon-gamma regulates idiopathic pneumonia syndrome, a Th17+CD4+ T cell-mediated graft-versus-host disease. Am J Respir Crit Care Med 178: 379–388, 2008. [DOI] [PubMed] [Google Scholar]
- 29.McMillan TR, Moore BB, Weinberg JB, Vannella KM, Fields WB, Christensen PJ, van Dyk LF, Toews GB. Exacerbation of established pulmonary fibrosis in a murine model by gammaherpesvirus. Am J Respir Crit Care Med 177: 771–780, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell C, Couton D, Couty JP, Anson M, Crain AM, Bizet V, Renia L, Pol S, Mallet V, Gilgenkrantz H. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am J Pathol 174: 1766–1775, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol 166: 675–684, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol 166: 675–684, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol 35: 175–181, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore BB, Paine R, Christensen PJ, Moore TA, Sitterding S, Ngan R, Wilke CA, Kuziel WA, Toews GB. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol 167: 4368–4377, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Mora AL, Torres-Gonzalez E, Rojas M, Corredor C, Ritzenthaler J, Xu J, Roman J, Brigham K, Stecenko A. Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis. Am J Respir Cell Mol Biol 35: 466–473, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naik PN, Horowitz JC, Moore TA, Wilke CA, Toews GB, Moore BB. Pulmonary fibrosis induced by gamma-herpesvirus in aged mice is associated with increased fibroblast responsiveness to transforming growth factor-beta. J Gerontol A Biol Sci Med Sci 67: 714–725, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakashima T, Liu T, Yu H, Ding L, Ullenbruch M, Hu B, Wu Z, Oguro H, Phan SH. Lung bone marrow-derived hematopoietic progenitor cells enhance pulmonary fibrosis. Am J Respir Crit Care Med 188: 976–984, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nemzek JA, Fry C, Moore BB. Adoptive transfer of fibrocytes enhances splenic T cell numbers and survival in septic peritonitis. Shock 40: 106–114, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neurohr C, Huppmann P, Leuchte H, Schwaiblmair M, Bittmann I, Jaeger G, Hatz R, Frey L, Uberfuhr P, Reichart B, Behr J; Munich Lung Transplant Group. Human herpesvirus 6 in bronchoalveolar lavage fluid after lung transplantation: a risk factor for bronchiolitis obliterans syndrome? Am J Transplant 5: 2982–2991, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Ostanin DV, Kurmaeva E, Furr K, Bao R, Hoffman J, Berney S, Grisham MB. Acquisition of antigen-presenting functions by neutrophils isolated from mice with chronic colitis. J Immunol 188: 1491–1502, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panoskaltsis-Mortari A, Griese M, Madtes DK, Belperio JA, Haddad IY, Folz RJ, Cooke KR; American Thoracic Society Committee on Idiopathic Pneumonia Syndrome. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med 183: 1262–1279, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, Thompson RW, Cheever AW, Murray PJ, Wynn TA. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog 5: e1000371, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 114: 438–446, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poo YS, Nakaya H, Gardner J, Larcher T, Schroder WA, Le TT, Major LD, Suhrbier A. CCR2 deficiency promotes exacerbated chronic erosive neutrophil-dominated chikungunya virus arthritis. J Virol 88: 6862–6872, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarawar SR, Cardin RD, Brooks JW, Mehrpooya M, Tripp RA, Doherty PC. Cytokine production in the immune response to murine gammaherpesvirus 68. J Virol 70: 3264–3268, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarawar SR, Lee BJ, Anderson M, Teng YC, Zuberi R, Von Gesjen S. Chemokine induction and leukocyte trafficking to the lungs during murine gammaherpesvirus 68 (MHV-68) infection. Virology 293: 54–62, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Sarawar SR, Lee BJ, Giannoni F. Cytokines and costimulatory molecules in the immune response to murine gammaherpesvirus-68. Viral Immunol 17: 3–11, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Seo S, Renaud C, Kuypers JM, Chiu CY, Huang ML, Samayoa E, Xie H, Yu G, Fisher CE, Gooley TA, Miller S, Hackman RC, Myerson D, Sedlak RH, Kim YJ, Fukuda T, Fredricks DN, Madtes DK, Jerome KR, Boeckh M. Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood 125: 3789–3797, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shankar G, Cohen DA. Idiopathic pneumonia syndrome after bone marrow transplantation: the role of pre-transplant radiation conditioning and local cytokine dysregulation in promoting lung inflammation and fibrosis. Int J Exp Pathol 82: 101–113, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shichino S, Abe J, Ueha S, Otsuji M, Tsukui T, Kosugi-Kanaya M, Shand FH, Hashimoto S, Suzuki HI, Morikawa T, Inagaki Y, Matsushima K. Reduced supply of monocyte-derived macrophages leads to a transition from nodular to diffuse lesions and tissue cell activation in silica-induced pulmonary fibrosis in mice. Am J Pathol 185: 2923–2938, 2015. [DOI] [PubMed] [Google Scholar]
- 51.Soubani AO, Pandya CM. The spectrum of noninfectious pulmonary complications following hematopoietic stem cell transplantation. Hematol Oncol Stem Cell Ther 3: 143–157, 2010. [DOI] [PubMed] [Google Scholar]
- 52.Sparks-Thissen RL, Braaten DC, Hildner K, Murphy TL, Murphy KM, Virgin HW 4th. CD4 T cell control of acute and latent murine gammaherpesvirus infection requires IFNgamma. Virology 338: 201–208, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Stoolman JS, Vannella KM, Coomes SM, Wilke CA, Sisson TH, Toews GB, Moore BB. Latent infection by gammaherpesvirus stimulates profibrotic mediator release from multiple cell types. Am J Physiol Lung Cell Mol Physiol 300: L274–L285, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun L, Louie MC, Vannella KM, Wilke CA, LeVine AM, Moore BB, Shanley TP. New concepts of IL-10-induced lung fibrosis: fibrocyte recruitment and M2 activation in a CCL2/CCR2 axis. Am J Physiol Lung Cell Mol Physiol 300: L341–L353, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thrall RS, McCormick JR, Jack RM, McReynolds RA, Ward PA. Bleomycin-induced pulmonary fibrosis in the rat: inhibition by indomethacin. Am J Pathol 95: 117–130, 1979. [PMC free article] [PubMed] [Google Scholar]
- 56.Torres-Gonzalez E, Bueno M, Tanaka A, Krug LT, Cheng DS, Polosukhin VV, Sorescu D, Lawson WE, Blackwell TS, Rojas M, Mora AL. Role of endoplasmic reticulum stress in age-related susceptibility to lung fibrosis. Am J Respir Cell Mol Biol 46: 748–756, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trujillo G, O'Connor EC, Kunkel SL, Hogaboam CM. A novel mechanism for CCR4 in the regulation of macrophage activation in bleomycin-induced pulmonary fibrosis. Am J Pathol 172: 1209–1221, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vannella KM, Luckhardt TR, Wilke CA, van Dyk LF, Toews GB, Moore BB. Latent herpesvirus infection augments experimental pulmonary fibrosis. Am J Respir Crit Care Med 181: 465–477, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varelias A, Gartlan KH, Kreijveld E, Olver SD, Lor M, Kuns RD, Lineburg KE, Teal BE, Raffelt NC, Cheong M, Alexander KA, Koyama M, Markey KA, Sturgeon E, Leach J, Reddy P, Kennedy GA, Yanik GA, Blazar BR, Tey SK, Clouston AD, MacDonald KP, Cooke KR, Hill GR. Lung parenchyma-derived IL-6 promotes IL-17A-dependent acute lung injury after allogeneic stem cell transplantation. Blood 125: 2435–2444, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venosa A, Malaviya R, Choi H, Gow AJ, Laskin JD, Laskin DL. Characterization of distinct macrophage subpopulations during nitrogen mustard-induced lung injury and fibrosis. Am J Respir Cell Mol Biol 54: 436–446, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Versluys AB, Rossen JW, van Ewijk B, Schuurman R, Bierings MB, Boelens JJ. Strong association between respiratory viral infection early after hematopoietic stem cell transplantation and the development of life-threatening acute and chronic alloimmune lung syndromes. Biol Blood Marrow Transplant 16: 782–791, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wareing MD, Lyon A, Inglis C, Giannoni F, Charo I, Sarawar SR. Chemokine regulation of the inflammatory response to a low-dose influenza infection in CCR2−/− mice. J Leukoc Biol 81: 793–801, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Xuan L, Jiang X, Sun J, Zhang Y, Huang F, Fan Z, Guo X, Dai M, Liu C, Yu G, Zhang X, Wu M, Huang X, Liu Q. Spectrum of Epstein-Barr virus-associated diseases in recipients of allogeneic hematopoietic stem cell transplantation. Transplantation 96: 560–566, 2013. [DOI] [PubMed] [Google Scholar]
- 64.Yoshihara S, Yanik G, Cooke KR, Mineishi S. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 13: 749–759, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Zhou X, Loomis-King H, Gurczynski SJ, Wilke CA, Konopka KE, Ptaschinski C, Coomes SM, Iwakura Y, van Dyk LF, Lukacs NW, Moore BB. Bone marrow transplantation alters lung antigen-presenting cells to promote T17 response and the development of pneumonitis and fibrosis following gammaherpesvirus infection. Mucosal Immunol 9: 610–620, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]