Abstract
Here, we tested the hypothesis that animals with severe pulmonary arterial hypertension (PAH) display increased sensitivity to vascular permeability induced by activation of store-operated calcium entry. To test this hypothesis, wild-type and transient receptor potential channel 4 (TRPC4) knockout Fischer 344 rats were given a single injection of Semaxanib (SU5416; 20 mg/kg) followed by 3 wk of exposure to hypoxia (10% oxygen) and a return to normoxia (21% oxygen) for an additional 2–3 wk. This Semaxanib/hypoxia/normoxia (i.e., SU5416/hypoxia/normoxia) treatment caused PAH, as evidenced by development of right ventricular hypertrophy, pulmonary artery medial hypertrophy, and occlusive lesions within precapillary arterioles. Pulmonary artery pressure was increased fivefold in Semaxanib/hypoxia/normoxia-treated animals compared with untreated, Semaxanib-treated, and hypoxia-treated controls, determined by isolated perfused lung studies. Thapsigargin induced a dose-dependent increase in permeability that was dependent on TRPC4 in the normotensive perfused lung. This increase in permeability was accentuated in PAH lungs but not in Semaxanib- or hypoxia-treated lungs. Fluid accumulated in large perivascular cuffs, and although alveolar fluid accumulation was not seen in histological sections, Evans blue dye conjugated to albumin was present in bronchoalveolar lavage fluid of hypertensive but not normotensive lungs. Thus PAH is accompanied by a TRPC4-dependent increase in the sensitivity to edemagenic agents that activate store-operated calcium entry.
Keywords: edema, canonical transient receptor potential 4, TRPC4, calcium channels, Semaxanib, Sugen 5416, filtration coefficient
pulmonary arterial hypertension (PAH) is a progressive vasculopathy characterized by medial hypertophy and hyperplasia, distal extension of smooth muscle into typically nonmuscularized arterioles, and complex occlusive lesion formation in small precapillary arterioles and supernumerary vessels (1, 19, 20, 22, 40, 42, 49, 53, 57, 58, 63–66, 71). Endothelial dysfunction contributes to this vasculopathy in at least two ways. First, in small precapillary arterioles endothelial cell apoptosis is thought to lead to exuberant overgrowth of apoptosis resistant cells that display disordered angiogenesis (19, 20, 22, 49, 63–65). In this case, hyperproliferation causes endothelial accumulation within the blood vessel, where in the most severe form of remodeling the cells form multiple luminal slits rather than open vascular channels, e.g., plexogenic arteriopathy. Second, pulmonary artery endothelial cells become disordered with apparent structural abnormalities (28, 52). The functional consequence(s) of these changes is less clear, although an imbalance in the normal production of endothelial vasodilators and vasoconstrictors accompanies PAH (37, 39, 41, 55).
In addition to the abnormal production of vasoactive mediators, conduit and resistance artery endothelial cells may possess an abnormally hyperpermeable barrier. While the contribution of endothelial hyperpermeability to remodeling in PAH has not received considerable attention, its potential importance has been recognized for decades (59). This issue has been revisited in recent studies of group I PAH (i.e., heritable PAH). Mutations in bone morphogenetic protein receptor II (BMPR-II) constitute a principal genetic cause of the heritable disease (56). Animals harboring endothelial BMPR-II mutations spontaneously develop PAH (67), and their endothelium is hyperpermeable and proinflammatory (32, 34, 47). The mechanisms responsible for such increased susceptibility to vascular leak remain incompletely explored, although Prewitt et al. (47) have recently suggested that BMPR-II deficiency impairs normal caveolae function. In their studies, BMPR-II deficiency increased paracellular and transcellular protein flux, dependent on Src kinase activation; inhibition of Src kinase reduced the hyperpermeability response. The pathophysiological significance of pulmonary arterial hyperpermeability in PAH is unknown. Nonetheless, investigators have speculated that disrupted endothelium may promote growth factor access to underlying smooth muscle, allow delivery of inflammatory mediators and immune cells to the vascular wall, and decrease vascular and potentially airway compliance.
A hyperpermeable endothelial barrier may display increased sensitivity to circulating inflammatory mediators. Many inflammatory mediators activate store-operated calcium entry, and the resulting calcium influx increases paracellular transport leading to pulmonary edema, especially in extra-alveolar blood vessels (for review, see Refs. 13–15, 18). Transient receptor potential channel 4 (TRPC4) contributes to the molecular anatomy of store-operated calcium entry channels (6, 16, 17), and it has recently been incriminated in the pathogenesis of PAH (5). Fischer rats (F344) subjected to a single Semaxanib (i.e., Sugen 5416) injection followed by 3 wk of hypoxia (10%) and a return to normoxia for 2–5 wk (Semaxanib/hypoxia/normoxia) develop severe PAH that culminates in right heart failure and death (5, 31). TRPC4 deficiency confers a survival benefit in these animals (5). Whereas TRPC4 deficiency does not reduce either the pulmonary arterial pressure or the Fulton index, it decreases the extent of occlusive lesion formation and appears to preserve cardiac output (5). Fischer rats with severe PAH display an exaggerated permeability response to thapsigargin that appears to require TRPC4 (23). Based on these studies, we tested the hypothesis that animals with severe PAH display increased sensitivity to permeability induced by activation of store-operated calcium entry.
MATERIALS AND METHODS
Animals.
All experimental procedures were performed in accordance with current provisions of the U.S. Animal Welfare Act and were approved by the Institutional Animal Care and Use Committee of the University of South Alabama. Male TRPC4 wild-type (TRPC4+/+), heterozygous (TRPC4+/−), and null littermate (TRPC4−/−) F344 rats were anesthetized with Nembutal (65 mg/kg body wt). TRPC4−/− F344 rats were generated by Transposagen Biopharmaceuticals (Lexington, KY), as part of the Knockout Rat Consortium (Trpc4tm1Bni, targeted mutation 1, Bernd Nilius), and were bred and genotyped both at Transposagen and at the University of South Alabama as previously described (5).
Genotyping of the TRPC4-KO rats.
Rat tail snips were collected according to the guidelines of the University of South Alabama Animal Care and Use Committee. DNA was extracted from tail snips, as described previously (12), and 2 μl of the resulting DNA solution were subjected to PCR analysis using three primers (primer A, 5′-GTGTTGGTCTCCATTACTTCAGCT-3′; primer B, 5′-ATTCTTCCCTTTGAGCCCACT-3′; and transposon primer, 5′-CTGACCTAAGACAGGGAATT-3′) in a total volume of 20 μl containing 1× GoTaq Green PCR master mix (Promega, Madison, WI) and 1 mmol/l of each primer. The cycling parameters were denaturation at 94°C for 5 min; then 30 cycles of 94°C for 30 s, 54°C for 30 s, and 72°C for 1 min; and extension at 72°C for 7 min.
Isolated lung and assessment of endothelial permeability.
Animals were anesthetized using Nembutal (65 mg/kg body wt). Once a surgical plane was achieved, as defined by the absence of a withdrawal reflex following toe and tail pinch, animals were intubated and ventilated, a sternotomy was performed, and pulmonary artery and left ventricular catheters were placed. Heart and lungs were removed en bloc and suspended in a humidified chamber, where mechanical ventilation and flow were established. Rat lungs were perfused at constant flow (40 ml·min−1·kg body wt−1) with either buffer (in mmol/l: 119.0 NaCl, 4.7 KCl, 1.17 MgSO4, 1.0 Na2HPO4, 1.18 KH2PO4, 23 NaHCO3, and 5.5 glucose) containing 4% BSA and physiological (2.2 mmol/l) CaCl2, adjusted to pH 7.4 at 38°C, or with buffer and BSA plus 6% autologous whole blood.
Hemodynamic measurements and the filtration coefficient (Kf) were measured as previously described (44, 45), using zone 3 conditions. Baseline Kf was calculated as the rate of weight gain obtained 13 to 15 min after a 10 cmH2O increase in pulmonary venous pressure, normalized per 100 g predicted wet lung weight. Kf, the product of specific endothelial hydraulic permeability and surface area for exchange, is a sensitive measure of lung endothelial permeability when surface area is fully recruited. To determine the optimal dose of thapsigargin in subsequent experimental protocols, dose-response studies were conducted in the isolated lung preparation. Thapsigargin was added to the perfusate reservoir over a range of concentrations (0–300 nM), and a second Kf value was determined 15 min after later.
Evans blue dye albumin measurements in the bronchoalveolar lavage fluid.
To evaluate albumin extravasation, Evans blue dye was added to a 5% (wt/vol) solution of bovine serum albumin (BSA) to give a final concentration of 1.5 mg/ml, and the mixture was dialyzed against excessive 5% BSA overnight at 4°C in a Slide-A-Lyzer Dialysis Cassettes (Pierce). During the isolated lung perfusion, after lungs were challenged with 150 nM thapsigargin for 15 min, 2.5 ml albumin-bound Evans blue were added to the circulating perfusate 5 min after a 10 cmH2O-raise of venous pressure and kept circulating at this higher venous pressure for 10 more min. After double occlusion, whole lung bronchoalveolar lavage was performed by instilling 2 ml of PBS. Evans blue dye albumin in bronchoalveolar lavage fluid was determined by measuring absorption at 620 nM and extrapolated against the standard curve of Evans blue-conjugated albumin.
Rat PAH model.
PAH was induced by a single subcutaneous injection of Sugen 5416 (20 mg/kg; Semaxanib; Cayman Chemical, Ann Arbor, MI) on day 1, followed by exposure to 3 wk of normobaric hypoxia (Hx; 10% O2) and then reexposure to normoxia (Nx; 21% O2) for 2–3 additional wk (1, 60). The hemodynamic and histopathological parameters of PAH were compared among three experimental groups of age- and weight-matched rats (n = 5–10 each): male Fischer 344 (F344) TRPC4+/+, male F344 TRPC4−/−, and male F344 TRPC4+/−. Each experimental group included a set of normoxia time control rats.
Lung histology.
The trachea was ligated and lungs were submersion fixed with 10% formalin, paraffin embedded and prepared for light microscopy. The left lungs were cut in a horizontal plane in the middle of lung, and 5-μm slices were stained with hematoxylin and eosin for examination.
Statistical analysis.
Quantitative data are presented as mean ± SE. Group means were compared using one- or two-way ANOVA with Bonferroni (Sidak) post hoc test as appropriate. P < 0.05 was considered statistically significant.
RESULTS
Thapsigargin induces a TRPC4-dependent increase in lung permeability.
Previous studies in Sprague-Dawley (11) and Fischer 344 (23) rats have established that thapsigargin increases endothelial cell permeability, in part dependent on TRPC4. In addition, Tiruppathi et al. (61) demonstrated that TRPC4 plays an important role in lung endothelial cell permeability, using TRPC4 knockout mice. Here, we sought to determine sensitivity to the thapsigargin-induced increase in permeability in the normotensive F344 rat and evaluate the importance of TRPC4 to this response.
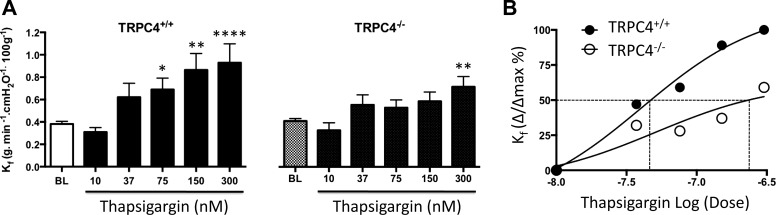
To test this idea, thapsigargin was tested over a range of concentrations, from 10 to 300 nM (Fig. 1A). In the normotensive TRPC4+/+ F344 rat, thapsigargin induced a dose-dependent increase in Kf; 75 nM represented the threshold concentration where Kf first became significantly increased (P < 0.05). Near maximal responses were observed at 150 nM thapsigargin. Compared with previous studies using Sprague-Dawley rats (11), baseline Kf values were higher and the sensitivity to thapsigargin was lower in Fischer rats. Baseline Kf values were similar between normotensive TRPC4+/+ and TRPC4−/− rats (P = 0.99). However, the normotensive TRPC4−/− rats were relatively insensitive to thapsigargin, as Kf did not significantly increase (P < 0.05) until the 300-nM concentration (Fig. 1, A and B). These data support the idea that TRPC4 channels contribute to the store-operated calcium influx that increases endothelial cell permeability.
Fig. 1.
Thapsigargin induces a dose-dependent increase in filtration coefficient (Kf) that requires transient receptor potential channel 4 (TRPC4) expression. A: after an isogravimetric state was established, a baseline Kf was measured and thapsigargin at the indicated concentration was added to the reservoir and allowed to recirculate for 15 min before a second Kf was measured. Thapsigargin induced a dose-dependent increase in permeability in lungs from wild-type (TRPC+/+) and TRPC4 knockout (TRPC4−/−) rats, estimated by Kf. Data are expressed as mean ± SE; n = 6–10 per concentration. *P < 0.05; **P < 0.01; ****P < 0.0001, significant difference using one-way ANOVA with Bonferroni's post hoc test. B: concentration-response curves generated from data obtained in Fig. 1A reveal the half maximal concentration for increased permeability in TRPC4+/+ lungs is ∼49 nM, similar to that previously reported in Sprague-Dawley rat lungs (11). Within the range of concentrations tested, the apparent half-maximal concentration is right shifted to at least 220 nM in TRPC4−/− lungs.
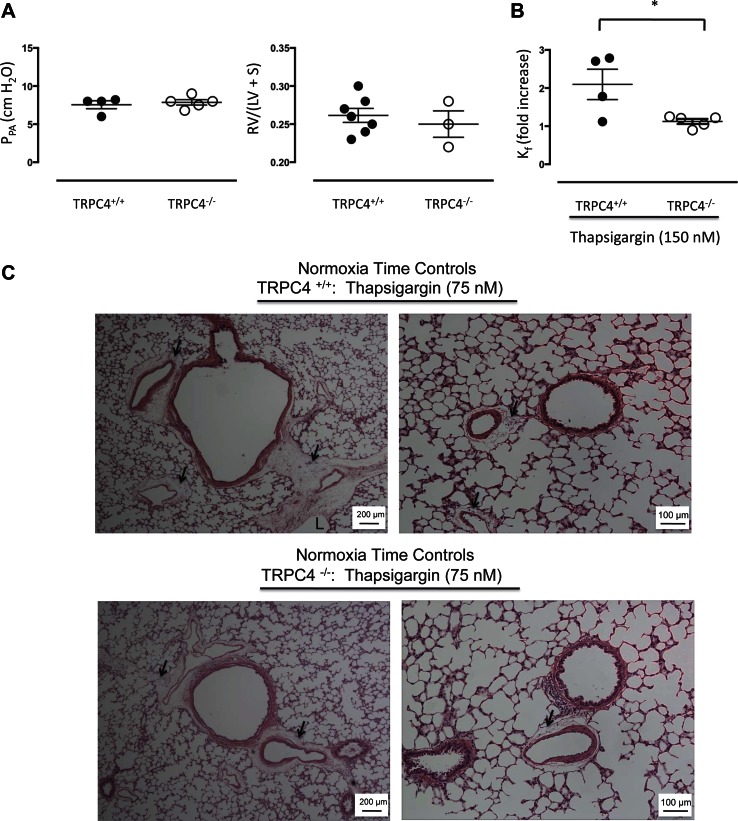
In wild-type F344 rats, the threshold concentration for thapsigargin to increase Kf was 75 nM as previously reported (23), and 150 nM yielded a near-maximal effect, given the range of concentrations examined. Therefore, we studied the permeability response to thapsigargin at 150 nM in additional experiments. Baseline perfusion pressures in normotensive isolated lungs were ∼10 cmH2O in both wild-type and TRPC4−/− rats, and Fulton indexes for these animals were <0.30, indicating baseline hemodynamic values were similar and largely unaffected by loss of TRPC4 expression (Fig. 2A). Baseline Kf values were slightly lower in TRPC4+/+ (0.12 ± 0.03) than in TRPC4−/− (0.21 ± 0.03) rats (P < 0.05). Thapsigargin (150 nM) increased Kf by approximately twofold in normotensive wild-type controls (Fig. 2B; P < 0.05). This permeability evoking effect of thapsigargin was abolished altogether in TRPC4−/− rat lungs (P < 0.05 vs. wild type).
Fig. 2.
The TRPC4-dependent increase in permeability corresponds with formation of perivascular fluid cuffs and not with alveolar flooding. A: lungs were isolated and perfused as described in materials and methods. Baseline perfusion pressures were <10 cmH2O and the Fulton index (RV/LV + S) was <0.30 in TRPC4+/+ and TRPC4−/− lungs, characteristic features of a normotensive circulation. B: thapsigargin increased Kf ∼2-fold in TRPC4+/+ lungs, and the increase in permeability was significantly reduced in TRPC4−/− lungs. *P < 0.05 using unpaired t-test. C: thapsigargin promoted fluid accumulation in perivascular cuffs, especially in TRPC4+/+ lungs but did not cause alveolar flooding. Arrows identify perivascular cuffs and L denotes a dilated lymphatic channel.
Thapsigargin promotes fluid accumulation in perivascular cuffs but does not cause alveolar flooding (4, 11, 35, 36, 70). We examined lung histology following thapsigargin treatment (75 nM) in normotensive wild-type and TRPC4−/− rat lungs. As seen in Fig. 2C, consistent with previous reports (4, 11, 35, 36, 70), fluid accumulation was extensive and limited to perivascular cuffs around arteries and veins of all sizes. Dilated lymphatic channels were visible. In all cases, fluid was retained in the interstitial spaces and could not be seen in the alveoli. Fluid accumulation did not appear to be as extensive in lungs from TRPC4−/− rats, although histology was not quantified because images were assessed in parallel with physiological parameters, e.g., Kf. Thus thapsigargin increases endothelial cell permeability, especially in extra-alveolar arteries and veins, dependent on the expression of TRPC4.
PAH reveals a greater sensitivity to the thapsigargin-induced increase in permeability.
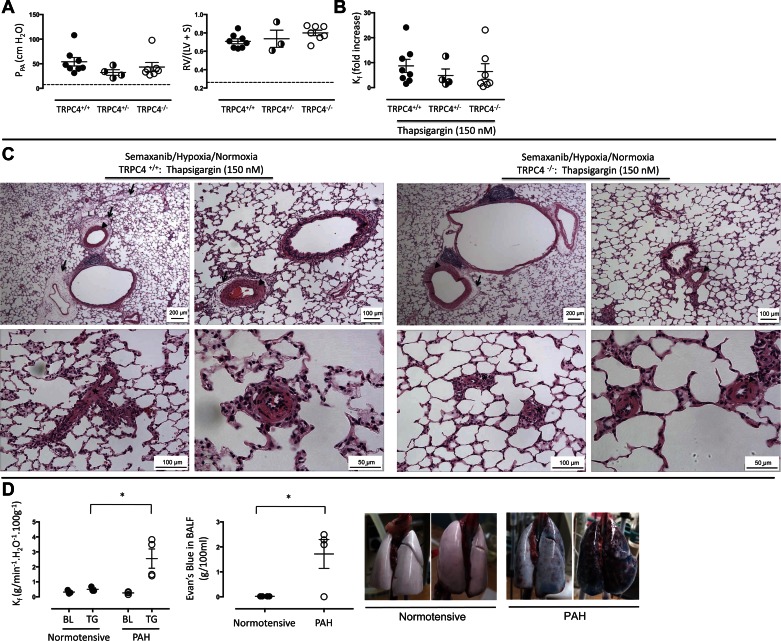
We next examined endothelial cell barrier integrity, and its sensitivity to thapsigargin, in animals with severe PAH; experimental PAH was induced using the Semaxanib/hypoxia/normoxia model (see materials and methods). The baseline pulmonary artery perfusion pressure in isolated TRPC4+/+ lungs was ∼50 cmH2O (≈ 38 mmHg; Fig. 3A), which is approximately fivefold higher than normotensive values. This is a large elevation in pulmonary artery pressure, especially considering the perfusion rate in these isolated lung studies was roughly 20% of the baseline cardiac output in vivo. There was no difference in pulmonary artery perfusion pressures among TRPC4+/+, TRPC4+/−, and TRPC4−/− rat groups (P = 0.99). The Fulton index was threefold higher in all three hypertensive groups when compared with normotensive controls (P < 0.05), although there was no difference in the Fulton index among TRPC4+/+, TRPC4+/−, and TRPC4−/− groups (P = 0.99 between TRPC4+/+ and TRPC+/− and P = 0.24 between TRPC4+/+ and TRPC4−/−). Thapsigargin (150 nM) increased Kf nearly 10-fold in hypertensive TRPC4+/+ lungs (Fig. 3B), which represents a tremendous potentiation above normotensive controls (see Fig. 2B; P < 0.05), and in this case, TRPC4 deletion was unable to rescue the hyperpermeability response. We previously reported that at lower thapsigargin concentrations (75 nM), TRPC4 deletion is protective against the increase in permeability (23). Thus this higher thapsigargin concentration appears to recruit additional store-operated calcium entry mechanisms that are not dependent on TRPC4 in the hypertensive circulation.
Fig. 3.
Hyperpermeability response to thapsigargin is revealed in animals with severe pulmonary arterial hypertension. A: lungs from Semaxanib/hypoxia/normoxia-treated animals were isolated and perfused as described in materials and methods. Baseline pulmonary perfusion pressure was ∼50 cmH2O and the Fulton index was ∼0.75 in TRPC4+/+, TRPC4+/−, and TRPC4−/− lungs; there were no differences among groups in either of measurements. The dashed line reflects average values reported in normotensive TRPC4+/+ lungs from Fig. 1 for comparison. B: thapsigargin (150 nM) increased Kf ∼10-fold in TRPC4+/+ lungs. This hyperpermeability response to thapsigargin was not abolished in TRPC4−/− lungs at the 150-nM concentration. C: thapsigargin induced extensive perivascular fluid cuffs, especially in TRPC4+/+ lungs (arrows). Arteries and arterioles were remodeled, with evidence for medial hypertrophy, shown by arrowheads. Complex obliterative lesions were resolved in small precapillary arterioles (*), consistent with previous reports in the F344 rat (5). Dilated lymphatics were seen (L). As previously reported (5), complex lesions were less severe in TRPC4−/− than in TRPC4+/+ lungs. D: thapsigargin (150 nM) promotes accumulation of Evans blue dye conjugated to albumin in the bronchoalveolar lavage of pulmonary arterial hypertensive (PAH) but not normotensive lungs. PAH increases sensitivity to the thapsigargin (150 nM)-induced increase in permeability (left), consistent with the data shown in Figs. 2 and 3. This hyperpermeability response corresponds with the appearance of Evans blue dye in the bronchoalveolar lavage fluid, an effect that is not seen in normotensive lungs (middle). Two representative lung images are shown from the normotensive and PAH animals (right). The left PAH lung represents the low responder reported in the accompanying graph, whereas the right PAH lung represents one of the high responders. BL, baseline; TG, thapsigargin. *P < 0.05 using unpaired t-test.
We have previously quantified the histological changes seen in the lungs of F344 rats treated with Semaxanib/hypoxia/normoxia (5). Consistent with these previous results, severe precapillary pulmonary vascular remodeling was verified in the hypertensive animals (Fig. 3C). Medial hypertrophy with apparent distal extension of smooth muscle was seen in pulmonary arteries and arterioles. Occlusive lesions were prominent in small precapillary segments. Following the thapsgargin exposure, extensive fluid accumulation was observed in perivascular cuffs, especially surrounding arteries and veins. However, we noted that in no circumstance were cuffs seen in or around occlusive lesions; fluid did not accumulate in the attenuated interstitial compartment of these remodeled vessels. A similar degree of vascular remodeling was observed in TRPC4+/− and TRPC4−/− lungs. As we have previously quantified and reported (5), TRPC4 deletion reduced the extent of remodeling within occlusive lesions.
We noticed that the permeability defect determined by Kf was considerably more severe than what could be predicted by the accompanying histology sections. Therefore, we generated a movie documenting the thapsigargin-induced hyperpermeability response in PAH lungs from TRPC4+/+ rats (Supplemental Movie S1; Supplemental Material for this article is available online at the Journal website). Following thapsigargin application (150 nM), fluid is seen dripping from the lung, due to its clearance through open lymphatics. As filtration due to the permeability defect exceeds apparent lymphatic clearance, the lung collects water to the point that it can no longer inflate under the established ventilatory parameters. Fluid can be seen moving through the trachea coincident with ventilation. The lung surface appearance is suggestive of alveolar flooding, although as seen in representative histological sections (Fig. 3C), prominent alveolar flooding was not seen.
To further address this issue, we repeated a series of experiments in both normotensive and PAH animals. In these studies, after baseline Kf was assessed and thapsigargin (150 nM) was applied, Evans blue dye conjugated to albumin was recirculated and a second Kf was measured. Upon completion of the experiment, Evans blue dye was measured in the bronchoalveolar lavage fluid. As is seen in Fig. 3D, PAH lungs displayed an increased sensitivity to thapsigargin. Whereas Evans blue dye could not be detected in the airways of normotensive lungs, it did accumulate in the airspaces of PAH lungs. These data therefore indicate that in this model of PAH, endothelium displays a hypersensitivity to activation of store-operated calcium entry. Perhaps most importantly, these data indicate that the endothelial barrier is sufficiently fragile in PAH to enable activation of store-operated calcium entry to cause water and protein access to the airways.
Three-week hypoxia exposure is not sufficient to sustain either PAH or the increased sensitivity to thapsigargin.
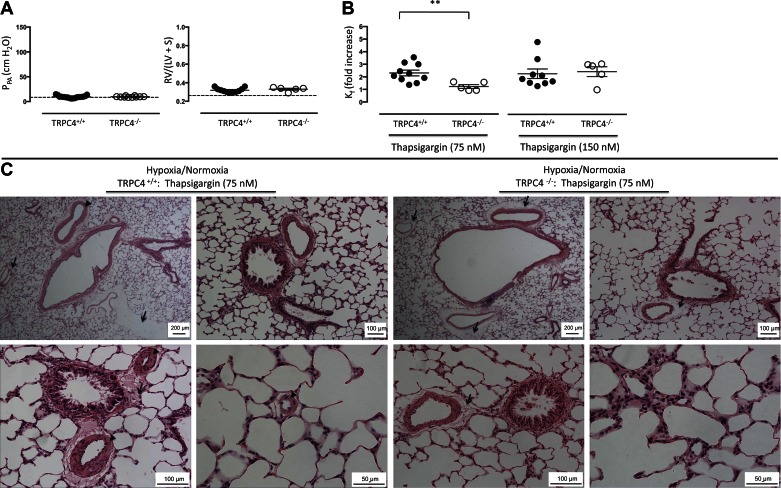
As a control for the Semaxanib/hypoxia/normoxia treatment, animals were exposed to chronic hypoxia (10% O2) for three wk followed by 2–3 additional wk of normoxia (i.e., room air, barometric pressure ∼760 mmHg), at which time the experiment was terminated and lungs were isolated and perfused. Baseline pulmonary artery perfusion pressure of these hypoxia-exposed TRPC+/+ lungs was ∼10 cmH2O (Fig. 4A), similar to normoxia controls (see Fig. 2A), and the Fulton index in these animals was 0.32 ± 0.01 vs. 0.26 ± 0.01 in the controls (P < 0.05) and 0.74 ± 0.02 in Semaxanib/Hypoxia/Normoxia-exposed animals (P < 0.05). Pulmonary artery perfusion pressures and the Fulton index were not different among the hypoxia-exposed TRPC4+/+, TRPC4+/−, and TRPC4−/− lungs (P = 0.73 and 0.41, respectively). Therefore, whereas pulmonary perfusion pressures were similar to untreated control animals, hypoxia-exposed animals retained some evidence of right ventricular remodeling 2 wk after their hypoxia exposure.
Fig. 4.
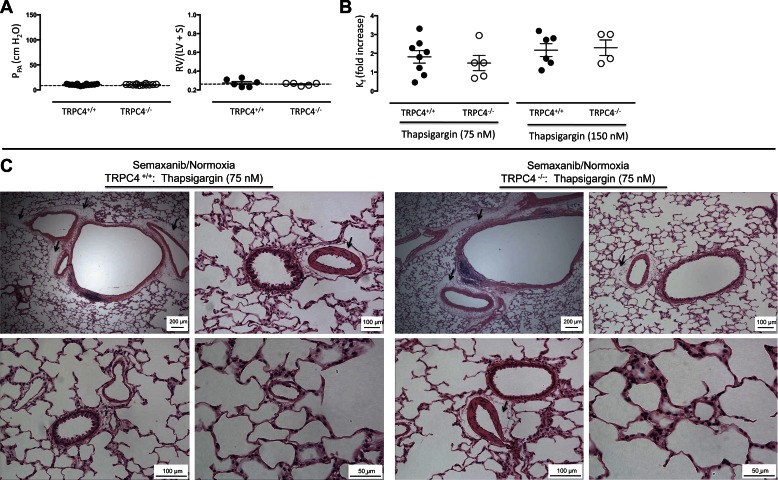
Three-week hypoxia exposure is not sufficient to sustain either the pulmonary arterial hypertension or the hyperpermeability response to thapsigargin. A: animals were exposed to hypoxia (10% oxygen) for 3 wk and then returned to normoxia for an additional 2 wk. Pulmonary perfusion pressures were ∼10 cmH2O and Fulton indexes were ∼0.30 in both TRPC4+/+ and TRPC4−/− lungs. The dashed line reflects average responses reported in normotensive TRPC4+/+ lungs from Fig. 1 for comparison. P = ns using unpaired t-test. B: thapsigargin (75 and 150 nM) increased Kf ∼2-fold in TRPC4+/+ lungs. This effect was abolished in TRPC4−/− lungs at 75 nM (P < 0.05 using unpaired t-test), but not at 150 nM, thapsigargin. C: thapsigargin induced perivascular cuffing without evidence of alveolar flooding, especially apparent in lungs from TRPC4+/+ rats (arrows). Despite the normal pulmonary artery pressures seen in A, pulmonary artery and arteriole media were remodeled, characteristic of the hypertrophy and hyperplasia that accompanies chronic hypoxia exposure (arrowhead).
Seventy-five and 150 nM thapsigargin concentrations increased Kf approximately twofold in TRPC4+/+ lungs (Fig. 4B). The magnitude of this increase was similar to what we observed in normotensive controls (see Figs. 1 and 2) and less than that in lungs from Semaxanib/hypoxia/normoxia-treated animals (Fig. 3; P < 0.05). This permeability response was abolished in TRPC4−/− lungs in response to 75 nM, but not 150 nM, thapsigargin. Histology revealed prominent perivascular fluid cuffs around arteries and veins, but again, alveolar flooding was not seen (Fig. 4C). Despite a normal pulmonary artery perfusion pressure, small arterioles and large arteries appeared muscularized. These findings suggest that the 3-wk hypoxic exposure had caused elevated pulmonary artery pressure with an associated pulmonary vascular remodeling, and further, that upon return to normoxia the pressure had normalized whereas the remodeling in blood vessels and the right ventricle had not fully regressed.
Semaxanib is not sufficient to increase either pulmonary arterial pressure or the sensitivity to thapsigargin.
As an additional control for the Semaxanib/hypoxia/normoxia treatment, animals were administered Semaxanib and then maintained under normoxia control conditions for 5–6 wk, at which time lungs were isolated and perfused. The baseline perfusion pressure was slightly elevated compared with untreated controls (control = 7.6 ± 0.4 vs. Semaxanib = 10.4 ± 0.4 cmH2O), although this difference achieved statistical significance (P < 0.05). The Fulton index in this treatment group was not different from the untreated time controls (Fig. 5A; P = 0.53), and the results in wild-type and TRPC4−/− animals were similar (P = 0.99). Thapsigargin (75 and 150 nM) increased Kf in lungs from both wild-type and TRPC4−/− rats approximately twofold (Fig. 5B), similar to the responses seen in untreated control lungs (Figs. 1 and 2). Large perivascular fluid cuffs were once again prominent in lungs from wild-type and TRPC4−/− rats, but alveolar flooding was absent (Fig. 5C).
Fig. 5.
Semaxanib inoculation is not sufficient to induce pulmonary arterial hypertension or cause a hyperpermeability response to thapsigargin. A: F344 rats received a single subcutaneous Semaxanib injection, and were then maintained under normoxia for an additional five wk. Lungs were isolated and perfused as described in materials and methods. Pulmonary perfusion pressures were ∼10 cmH2O, and the Fulton index was ∼0.30 in both TRPC4+/+ and TRPC4−/− rats. The dashed line reflects average responses reported in normotensive TRPC4+/+ lungs from Fig. 1 for comparison. P = ns using unpaired t-test. B: thapsigargin increased Kf ∼2-fold at 75 and 150 nM concentrations in both TRPC4+/+ and TRPC4−/− lungs. P = ns using unpaired t-test. C: extensive perivascular cuffs were noted in both TRPC4+/+ and TRPC4−/− lungs (arrows), while fluid was not seen in alveoli.
DISCUSSION
PAH is a vasculopathy characterized by endothelial dysfunction, in both small precapillary arterioles and resistance and conduit arteries. Thapsigargin activates store-operated calcium entry channels, which induce extra-alveolar endothelial permeability, targeting a vascular location, e.g., pulmonary artery, most prominently impacted in PAH. We recently reported that thapsigargin induces TRPC4-dependent high frequency endothelial cell cytosolic calcium transients that are coupled to a permeability defect in severe PAH (23). Our present studies extend these observations, as we now resolve that neither the hypoxia nor Semaxanib treatments alone are sufficient to recapitulate the hyperpermeability sensitivity of PAH. Moreover, the endothelial cell barrier in PAH is fragile; thapsigargin increases protein and fluid accumulation in the air spaces, an uncharacteristic physiological response following activation of store-operated calcium entry channels.
In the normoxia time control lungs, thapsigargin induced a dose-dependent increase in permeability that was dependent on TRPC4. This was not a surprising result. The importance of TRPC4 in the molecular anatomy of store-operated calcium entry channels that increase endothelial cell permeability has been established (for review, see Refs. 13–15, 18), and TRPC4 knockout mice are resistant to calcium agonists that evoke store-operated calcium entry and increase permeability (61). However, the sensitivity to thapsigargin had not been established in F344 rats. Compared with results obtained previously using Sprague-Dawley rat lungs (11), the thapsigargin-induced increase in permeability was modest, yet the half maximal concentration was nearly identical among strains. Lungs from TRPC4 knockout rats were largely insensitive to thapsigargin; a significant increase in permeability was only seen with a 300-nM concentration of thapsigargin. These findings continue to support an important role for TRPC4 in disorders of lung permeability. They also bring into question whether high concentrations of thapsigargin can activate TRPC4-independent store-operated calcium entry channels, or perhaps other nonstore-operated calcium entry channels, to disrupt the endothelial cell barrier in PAH. This issue will have to be addressed in future studies.
PAH lungs displayed enhanced sensitivity to thapsigargin; the hyperpermeability of PAH was due to TRPC4, especially at lower thapsigargin concentrations (23). Although pulmonary artery, vein and double occlusion pressures were increased in PAH lungs compared with normotensive lungs, they were not different in the wild-type and TRPC4 knockout studies. Therefore, increased hydrostatic pressure is not responsible for the observed increase in thapsigargin sensitivity. Moreover, the increased sensitivity cannot be attributed to either Semaxanib treatment or hypoxia exposure alone, since typical thapsigargin permeability responses were observed following these treatments. Endothelial dysfunction that accompanies PAH, especially in extra-alveolar segments, renders that barrier more susceptible to permeability edema, at least following activation of store-operated calcium entry channels. This finding is similar to the hyperpermeability phenotype in mice harboring a BMPR-II mutation (32, 34, 47).
Vascular permeability in chronic forms of pulmonary hypertension has not been extensively studied, although indices of endothelial dysfunction have been periodically reported for more than 50 yr (collated in Table 1, except for citations specifically discussed below). This issue has recently been tested in heritable PAH. Mice engineered to possess a heterozygous knock-in of the BMPRIIR899X mutation develop mild PAH by ∼6-wk of age (34). Coincident with PAH development, lungs in these animals exhibit a basal and lipopolysaccharide-induced hyperpermeability compared with controls. Pulmonary artery endothelial cells obtained from mice and blood outgrowth endothelial cells obtained from humans expressing dysfunctional BMPRII are similarly hyperpermeable, suggesting increased permeability may be an important factor in the pathophysiology of heritable disease. Consistent with these findings, endothelial cells isolated from heterozygous mice carrying the BMPR2Δex4−5/+ mutation displayed a basal macromolecular hyperpermeability (47). Monocrotaline-induced PAH is a nongenetic cause of PAH associated with decreased BMPRII expression (33, 51). In this case, exposure to the injurious pyrrole causes cellular apoptosis and increased permeability and is used as a model for drug-induced endothelial injury and PAH (Table 1). These studies suggest that endothelial cell barrier dysfunction is a critical determinant of vascular remodeling in PAH.
Table 1.
Examples of studies documenting increased lung permeability in commonly used models of pulmonary hypertension
| Model of Pulmonary Hypertension/Species | Permeability Measurement | Reference |
|---|---|---|
| Monocrotaline | ||
| Albino rat | Endothelial and epithelial cell injury, and interstitial edema | (9) |
| Albino rat | Increased lung weight-to-body weight ratio, extravascular albumin, and interstitial edema | (46) |
| Wistar rat | Increased albumin in bronchoalveolar lavage fluid | (10) |
| SD rat | Higher wet lung weight and increased albumin in bronchoalveolar lavage fluid | (27) |
| SD rat | Increased albumin in lung tissue | (24) |
| SD rat | Increased lung wet weight-to-dry weight ratio and lung extravascular albumin content | (59) |
| SD rat | Endothelial injury and associated subendothelial edema | (29) |
| SD rat | Lung perivascular edema and increased extravascular albumin | (50) |
| SD rat | Increased lung wet-to-dry weight ratio | (21) |
| SD rat and F344 rat | Lung alveolar septal edema | (43) |
| SD rat | Increased bronchoalveolar lavage fluid protein and lung wet weight | (48) |
| SD rat | Increased bronchoalveolar lavage fluid protein and lung wet weight | (54) |
| F344 rat | Increased bronchoalveolar lavage fluid protein and lung wet weight | (68) |
| SD rat | Increased bronchoalveolar lavage fluid protein and lung wet weight | (69) |
| SD rat | Increased bronchoalveolar lavage fluid protein and lung wet weight | (25) |
| Pneumonectomy and monocrotaline | ||
| Wistar rat | Increased lung water content | (38) |
| BMPR II deletion | ||
| R26R mouse | Increased lung tissue albumin | (7) |
| R26R mouse | Increased lung tissue albumin | (8) |
These additional papers are not specifically highlighted in the discussion, but they provide supportive evidence for an endothelial cell permeability defect in pulmonary arterial hypertension. Endpoints assessing endothelial barrier integrity and/or permeability are highlighted.
SD, Sprague-Dawley rat; F344, Fischer rat; R26R, ROSA26 reporter mouse; BMPR II, bone morphogenetic protein receptor II.
Yet hyperpermeability is not a consistent finding among all forms of chronic pulmonary hypertension. In the few examples studied, store-operated calcium entry-dependent permeability responses were ablated in pulmonary hypertension due to both chronic hypoxia and congestive heart failure. In the former example, 1-wk hypoxia (10%) exposure led to increased pulmonary artery pressure and right ventricular hypertrophy, but the thapsigargin-induced increase in permeability was abolished (62). Note that this experimental design is different than the studies reported here. In our work, animals were exposed to hypoxia (10%) for 3 wk and allowed to return to normoxia for 2 wk, as a control for the Semaxanib/hypoxia/normoxia exposure. The discrepancy among these results suggests that hypoxic hypertension is accompanied by loss of thapsigargin sensitivity, which is rescued upon the return to normoxia. Alternatively, our current studies may reflect the relative insensitivity of Fischer rats to hypoxia (26).
Development of heart failure was also accompanied by loss of the thapsigargin-induced increase in permeability, both in the dog pacing (30) and in the rat aortocaval fistula (3) models, due at least in part to a downregulation of TRPC1, TRPC3, and TRPC4 (3). Whereas store-operated calcium entry channel expression and function in endothelium was lost in heart failure, TRPV4 channel expression and activation remained intact. This is an interesting contrast since the TRPC4-induced, and not the TRPV4-induced, increase in permeability occurs in extra-alveolar vessels, which is the vascular location most relevant to PAH (2, 4, 35). Endothelial cell barrier dysfunction may be one of the discriminating features among the various forms (e.g., classifications) of pulmonary hypertension.
The extensive permeability response seen in PAH reveals important and unanswered questions regarding how fluid accumulates within interstitial spaces, airways and alveoli. We observed large increases in Kf and extensive perivascular fluid cuff formation, but alveolar flooding was not seen by histology. Supplemental Movie S1 illustrates fluid dripping from the lung due to its clearance through open lymphatics and the appearance of fluid within the trachea. Evans blue dye was detected in bronchoalveolar lavage fluid. What remains undetermined is how, using histological approaches that have previously detected alveolar edema fluid, can the alveoli appear devoid of fluid and protein by histology while Evans blue dye is detected by bronchoalveolar lavage. Future studies will be required to identify possible vascular leaks sites to track the origin of fluid recovered by bronchoalveolar lavage.
In summary, we demonstrate that TRPC4 controls endothelial cell barrier integrity in both normotensive and PAH circulations. The PAH circulation, in particular, is susceptible to permeability induced by agents that activate store-operated calcium entry. While the importance of endothelial hyperpermeability in evolution of PAH remains uncertain, we must now consider its role in promoting the vascular remodeling that increases pulmonary vascular resistance, and we must evaluate whether the fragile nature of the endothelium in this disease increases the risk of certain PAH patients to development of pulmonary edema.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-66299 (to T. Stevens and M. Alexeyev) and HL-60024 (to T. Stevens).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.Z. and M.A. performed experiments; C.Z. analyzed data; C.Z., M.I.T., and T.S. interpreted results of experiments; C.Z. prepared figures; C.Z., M.I.T., N.F.V., and T.S. edited and revised manuscript; T.S. conception and design of research; T.S. drafted manuscript; T.S. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Viktoriya Pastukh for assistance genotyping Fischer rats, Brian Hulon for generating Supplemental Movie S1, and Joanne Brookfield for assistance in preparing Supplemental Movie S1 for publication. We also thank Benjamin Gumbs for work with animals in the Department of Comparative Medicine. We thank Dr. Ivan F. McMurtry for careful review and discussion of the manuscript, and Ed Crockett, Boniface Obiako, and Dr. James Parker for experimental assistance in working with animals.
REFERENCES
- 1.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 121: 2747–2754, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez DF, Gjerde EA, Townsley MI. Role of EETs in regulation of endothelial permeability in rat lung. Am J Physiol Lung Cell Mol Physiol 286: L445–L451, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez DF, King JA, Townsley MI. Resistance to store depletion-induced endothelial injury in rat lung after chronic heart failure. Am J Respir Crit Care Med 172: 1153–1160, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res 99: 988–995, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alzoubi A, Almalouf P, Toba M, O'Neill K, Qian X, Francis M, Taylor MS, Alexeyev M, McMurtry IF, Oka M, Stevens T. TRPC4 inactivation confers a survival benefit in severe pulmonary arterial hypertension. Am J Pathol 183: 1779–1788, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brough GH, Wu S, Cioffi D, Moore TM, Li M, Dean N, Stevens T. Contribution of endogenously expressed Trp1 to a Ca2+-selective, store-operated Ca2+ entry pathway. FASEB J 15: 1727–1738, 2001. [PubMed] [Google Scholar]
- 7.Burton VJ, Ciuclan LI, Holmes AM, Rodman DM, Walker C, Budd DC. Bone morphogenetic protein receptor II regulates pulmonary artery endothelial cell barrier function. Blood 117: 333–341, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Burton VJ, Holmes AM, Ciuclan LI, Robinson A, Roger JS, Jarai G, Pearce AC, Budd DC. Attenuation of leukocyte recruitment via CXCR1/2 inhibition stops the progression of PAH in mice with genetic ablation of endothelial BMPR-II. Blood 118: 4750–4758, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler WH. An ultrastructural study of the pulmonary lesion induced by pyrrole derivatives of the pyrrolizidine alkaloids. J Pathol 102: 15–19, 1970. [DOI] [PubMed] [Google Scholar]
- 10.Cassee FR, Boere AJ, Bos J, Fokkens PH, Dormans JA, van Loveren H. Effects of diesel exhaust enriched concentrated PM2.5 in ozone preexposed or monocrotaline-treated rats. Inhal Toxicol 14: 721–743, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Chetham PM, Babal P, Bridges JP, Moore TM, Stevens T. Segmental regulation of pulmonary vascular permeability by store-operated Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 276: L41–L50, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Cinelli P, Rettich A, Seifert B, Burki K, Arras M. Comparative analysis and physiological impact of different tissue biopsy methodologies used for the genotyping of laboratory mice. Lab Anim 41: 174–184, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Cioffi DL, Barry C, Stevens T. Store-operated calcium entry channels in pulmonary endothelium: the emerging story of TRPCS and Orai1. Adv Exp Med Biol 661: 137–154, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Cioffi DL, Lowe K, Alvarez DF, Barry C, Stevens T. TRPing on the lung endothelium: calcium channels that regulate barrier function. Antioxid Redox Signal 11: 765–776, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cioffi DL, Stevens T. Regulation of endothelial cell barrier function by store-operated calcium entry. Microcirculation 13: 709–723, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Cioffi DL, Wu S, Alexeyev M, Goodman SR, Zhu MX, Stevens T. Activation of the endothelial store-operated ISOC Ca2+ channel requires interaction of protein 4.1 with TRPC4. Circ Res 97: 1164–1172, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Cioffi DL, Wu S, Chen H, Alexeyev M, St Croix CM, Pitt BR, Uhlig S, Stevens T. Orai1 determines calcium selectivity of an endogenous TRPC heterotetramer channel. Circ Res 110: 1435–1444, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cioffi DL, Wu S, Stevens T. On the endothelial cell ISOC. Cell Calcium 33: 323–336, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Cool CD, Kennedy D, Voelkel NF, Tuder RM. Pathogenesis and evolution of plexiform lesions in pulmonary hypertension associated with scleroderma and human immunodeficiency virus infection. Hum Pathol 28: 434–442, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, Tuder RM. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol 155: 411–419, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farhat MY, Chen MF, Bhatti T, Iqbal A, Cathapermal S, Ramwell PW. Protection by oestradiol against the development of cardiovascular changes associated with monocrotaline pulmonary hypertension in rats. Br J Pharmacol 110: 719–723, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fishman AP. Changing concepts of the pulmonary plexiform lesion. Physiol Res 49: 485–492, 2000. [PubMed] [Google Scholar]
- 23.Francis M, Xu N, Zhou C, Stevens T. TRPC4 encodes high frequency calcium transients in severe pulmonary arterial hypertension. Am J Pathol 186: 1701–1709, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganey PE, Sprugel KH, Hadley KB, Roth RA. Monocrotaline pyrrole-induced cardiopulmonary toxicity is not altered by metergoline or ketanserin. J Pharmacol Exp Ther 237: 226–231, 1986. [PubMed] [Google Scholar]
- 25.Ganey PE, Sprugel KH, White SM, Wagner JG, Roth RA. Pulmonary hypertension due to monocrotaline pyrrole is reduced by moderate thrombocytopenia. Am J Physiol Heart Circ Physiol 255: H1165–H1172, 1988. [DOI] [PubMed] [Google Scholar]
- 26.He LS, Chang SW, Voelkel NF. Pulmonary vascular reactivity in Fischer rats. J Appl Physiol (1985) 70: 1861–1866, 1991. [DOI] [PubMed] [Google Scholar]
- 27.Hilliker KS, Bell TG, Lorimer D, Roth RA. Effects of thrombocytopenia on monocrotaline pyrrole-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 246: H747–H753, 1984. [DOI] [PubMed] [Google Scholar]
- 28.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43: 13S–24S, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Ilkiw R, Todorovich-Hunter L, Maruyama K, Shin J, Rabinovitch M. SC-39026, a serine elastase inhibitor, prevents muscularization of peripheral arteries, suggesting a mechanism of monocrotaline-induced pulmonary hypertension in rats. Circ Res 64: 814–825, 1989. [DOI] [PubMed] [Google Scholar]
- 30.Ivey CL, Roy BJ, Townsley MI. Ablation of lung endothelial injury after pacing-induced heart failure is related to alterations in Ca2+ signaling. Am J Physiol Heart Circ Physiol 275: H844–H851, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Jiang B, Deng Y, Suen C, Taha M, Chaudhary KR, Courtman DW, Stewart DJ. Marked strain-specific differences in the SU5416 rat model of severe pulmonary arterial hypertension. Am Rev Respir Crit Care Med 54: 461–468, 2015. [DOI] [PubMed] [Google Scholar]
- 32.Johnson JA, Hemnes AR, Perrien DS, Schuster M, Robinson LJ, Gladson S, Loibner H, Bai S, Blackwell TR, Tada Y, Harral JW, Talati M, Lane KB, Fagan KA, West J. Cytoskeletal defects in Bmpr2-associated pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 302: L474–L484, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long L, Crosby A, Yang X, Southwood M, Upton PD, Kim DK, Morrell NW. Altered bone morphogenetic protein and transforming growth factor-beta signaling in rat models of pulmonary hypertension: potential for activin receptor-like kinase-5 inhibition in prevention and progression of disease. Circulation 119: 566–576, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Long L, Ormiston ML, Yang X, Southwood M, Graf S, Machado RD, Mueller M, Kinzel B, Yung LM, Wilkinson JM, Moore SD, Drake KM, Aldred MA, Yu PB, Upton PD, Morrell NW. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med 21: 777–785, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowe K, Alvarez D, King J, Stevens T. Phenotypic heterogeneity in lung capillary and extra-alveolar endothelial cells. Increased extra-alveolar endothelial permeability is sufficient to decrease compliance. J Surg Res 143: 70–77, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowe K, Alvarez DF, King JA, Stevens T. Perivascular fluid cuffs decrease lung compliance by increasing tissue resistance. Crit Care Med 38: 1458–1466, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMurtry IF, Abe K, Ota H, Fagan KA, Oka M. Rho kinase-mediated vasoconstriction in pulmonary hypertension. Adv Exp Med Biol 661: 299–308, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Murugesan P, Hildebrandt T, Bernlohr C, Lee D, Khang G, Doods H, Wu D. Inhibition of kinin B1 receptors attenuates pulmonary hypertension and vascular remodeling. Hypertension 66: 906–912, 2015. [DOI] [PubMed] [Google Scholar]
- 39.Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, Oka M. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 287: L665–L672, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Newman JH, Fanburg BL, Archer SL, Badesch DB, Barst RJ, Garcia JG, Kao PN, Knowles JA, Loyd JE, McGoon MD, Morse JH, Nichols WC, Rabinovitch M, Rodman DM, Stevens T, Tuder RM, Voelkel NF, Gail DB. Pulmonary arterial hypertension: future directions: report of a National Heart, Lung and Blood Institute/Office of Rare Diseases workshop. Circulation 109: 2947–2952, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Oka M, Hasunuma K, Webb SA, Stelzner TJ, Rodman DM, McMurtry IF. EDRF suppresses an unidentified vasoconstrictor mechanism in hypertensive rat lungs. Am J Physiol Lung Cell Mol Physiol 264: L587–L597, 1993. [DOI] [PubMed] [Google Scholar]
- 42.Palevsky HI, Schloo BL, Pietra GG, Weber KT, Janicki JS, Rubin E, Fishman AP. Primary pulmonary hypertension. Vascular structure, morphometry, and responsiveness to vasodilator agents. Circulation 80: 1207–1221, 1989. [DOI] [PubMed] [Google Scholar]
- 43.Pan LC, Wilson DW, Segall HJ. Strain differences in the response of Fischer 344 and Sprague-Dawley rats to monocrotaline induced pulmonary vascular disease. Toxicology 79: 21–35, 1993. [DOI] [PubMed] [Google Scholar]
- 44.Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. Am J Physiol Lung Cell Mol Physiol 286: L231–L246, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Parker JC, Townsley MI. Physiological determinants of the pulmonary filtration coefficient. Am J Physiol Lung Cell Mol Physiol 295: L235–L237, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plestina R, Stoner HB. Pulmonary oedema in rats given monocrotaline pyrrole. J Pathol 106: 235–249, 1972. [DOI] [PubMed] [Google Scholar]
- 47.Prewitt AR, Ghose S, Frump AL, Datta A, Austin ED, Kenworthy AK, de Caestecker MP. Heterozygous null bone morphogenetic protein receptor type 2 mutations promote SRC kinase-dependent caveolar trafficking defects and endothelial dysfunction in pulmonary arterial hypertension. J Biol Chem 290: 960–971, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qin L, D'Alessandro-Gabazza CN, Aoki S, Gil-Bernabe P, Yano Y, Takagi T, Boveda-Ruiz D, Ramirez Marmol AY, San Martin Montenegro VT, Toda M, Miyake Y, Taguchi O, Takei Y, Morser J, Gabazza EC. Pulmonary hypertension is ameliorated in mice deficient in thrombin-activatable fibrinolysis inhibitor. J Thromb Haemost 8: 808–816, 2010. [DOI] [PubMed] [Google Scholar]
- 49.Rai PR, Cool CD, King JA, Stevens T, Burns N, Winn RA, Kasper M, Voelkel NF. The cancer paradigm of severe pulmonary arterial hypertension. Am J Respir Crit Care Med 178: 558–564, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reindel JF, Ganey PE, Wagner JG, Slocombe RF, Roth RA. Development of morphologic, hemodynamic, and biochemical changes in lungs of rats given monocrotaline pyrrole. Toxicol Appl Pharmacol 106: 179–200, 1990. [DOI] [PubMed] [Google Scholar]
- 51.Reynolds AM, Holmes MD, Danilov SM, Reynolds PN. Targeted gene delivery of BMPR2 attenuates pulmonary hypertension. Eur Respir J 39: 329–343, 2012. [DOI] [PubMed] [Google Scholar]
- 52.Rosenberg HC, Rabinovitch M. Endothelial injury and vascular reactivity in monocrotaline pulmonary hypertension. Am J Physiol Heart Circ Physiol 255: H1484–H1491, 1988. [DOI] [PubMed] [Google Scholar]
- 53.Schnader J, Schloo BL, Anderson W, Stephenson LW, Fishman AP. Chronic pulmonary hypertension in sheep: temporal progression of lesions. J Surg Res 62: 243–250, 1996. [DOI] [PubMed] [Google Scholar]
- 54.Schultze AE, Wagner JG, White SM, Roth RA. Early indications of monocrotaline pyrrole-induced lung injury in rats. Toxicol Appl Pharmacol 109: 41–50, 1991. [DOI] [PubMed] [Google Scholar]
- 55.Shimoda LA, Sham JS, Sylvester JT. Altered pulmonary vasoreactivity in the chronically hypoxic lung. Physiol Res 49: 549–560, 2000. [PubMed] [Google Scholar]
- 56.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 54: S43–54, 2009. [DOI] [PubMed] [Google Scholar]
- 57.Smith P, Heath D. Electron microscopy of the plexiform lesion. Thorax 34: 177–186, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith P, Heath D, Yacoub M, Madden B, Caslin A, Gosney J. The ultrastructure of plexogenic pulmonary arteriopathy. J Pathol 160: 111–121, 1990. [DOI] [PubMed] [Google Scholar]
- 59.Sugita T, Hyers TM, Dauber IM, Wagner WW, McMurtry IF, Reeves JT. Lung vessel leak precedes right ventricular hypertrophy in monocrotaline-treated rats. J Appl Physiol Respir Environ Exercise Physiol 54: 371–374, 1983. [DOI] [PubMed] [Google Scholar]
- 60.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 15: 427–438, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, Malik AB. Impairment of store-operated Ca2+ entry in TRPC4(−/−) mice interferes with increase in lung microvascular permeability. Circ Res 91: 70–76, 2002. [DOI] [PubMed] [Google Scholar]
- 62.Townsley MI, King JA, Alvarez DF. Ca2+ channels and pulmonary endothelial permeability: insights from study of intact lung and chronic pulmonary hypertension. Microcirculation 13: 725–739, 2006. [DOI] [PubMed] [Google Scholar]
- 63.Tuder RM, Cool CD, Yeager M, Taraseviciene-Stewart L, Bull TM, Voelkel NF. The pathobiology of pulmonary hypertension. Endothelium Clin Chest Med 22: 405–418, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 144: 275–285, 1994. [PMC free article] [PubMed] [Google Scholar]
- 65.Tuder RM, Voelkel NF. Angiogenesis and pulmonary hypertension: a unique process in a unique disease. Antioxid Redox Signal 4: 833–843, 2002. [DOI] [PubMed] [Google Scholar]
- 66.Tuder RM, Voelkel NF. Plexiform lesion in severe pulmonary hypertension: association with glomeruloid lesion. Am J Pathol 159: 382–383, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.West J, Fagan K, Steudel W, Fouty B, Lane K, Harral J, Hoedt-Miller M, Tada Y, Ozimek J, Tuder R, Rodman DM. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res 94: 1109–1114, 2004. [DOI] [PubMed] [Google Scholar]
- 68.White SM, Roth RA. Pulmonary platelet sequestration is increased following monocrotaline pyrrole treatment of rats. Toxicol Appl Pharmacol 96: 465–475, 1988. [DOI] [PubMed] [Google Scholar]
- 69.White SM, Wagner JG, Roth RA. Effects of altered platelet number on pulmonary hypertension and platelet sequestration in monocrotaline pyrrole-treated rats. Toxicol Appl Pharmacol 99: 302–313, 1989. [DOI] [PubMed] [Google Scholar]
- 70.Wu S, Cioffi EA, Alvarez D, Sayner SL, Chen H, Cioffi DL, King J, Creighton JR, Townsley M, Goodman SR, Stevens T. Essential role of a Ca2+-selective, store-operated current (ISOC) in endothelial cell permeability: determinants of the vascular leak site. Circ Res 96: 856–863, 2005. [DOI] [PubMed] [Google Scholar]
- 71.Yamaki S, Wagenvoort CA. Plexogenic pulmonary arteriopathy: significance of medial thickness with respect to advanced pulmonary vascular lesions. Am J Pathol 105: 70–75, 1981. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.