Abstract
Hypothalamic orexin neurons project to numerous brain areas, including the ventral tegmental area (VTA), which is involved in motivation and food-seeking behavior. Here we address how exogenously administered orexin-A and endogenous orexin 1 receptor (OX1R) activation in the VTA affects feeding behavior. We hypothesized that orexin-A and OX1R antagonist SB334867 delivered to the VTA, at doses that were subthreshold for effect when injected into the ventricle, would affect intake of palatable foods in multiple test situations. We first used a hedonic feeding model in which satiated rats selectively consume a high-fat diet (HFD). Intra-VTA orexin-A stimulated additional consumption of chow and increased HFD intake in this model. In ad libitum-fed rats given daily 30-min test sessions, intra-VTA orexin-A also increased intake of HFD and 0.1 M sucrose. Further analysis of licking patterns revealed that that VTA orexin-A increased meal size and licking burst size only toward the end of the meal. Consistent with this finding, a subthreshold dose of VTA orexin-A prevented intake suppression induced by gastrointestinal nutrient infusion. Surprisingly, intra-VTA orexin-A had no effect on operant responding for sucrose pellets on a progressive ratio schedule of reinforcement. A role for endogenous VTA OX1R stimulation is supported by our finding that bilateral VTA injection of the selective OX1R antagonist SB334867 suppressed 0.1 M sucrose intake. Together, our data suggest that OX1R activity in the VTA facilitates food intake, potentially by counteracting postingestive negative feedback that would normally suppress feeding later in a meal.
Keywords: food intake, orexin 1 receptors, orexin-A, palatable foods, ventral tegmental area
mounting evidence supports a critical role for hypothalamic orexin neurons in the control of food intake. Lateral, third, and fourth intracerebroventricular injection of orexin-A increases food intake, as does injection of orexin-A into specific hypothalamic nuclei (14, 19) and the nucleus of the solitary tract (18). Orexin is expressed as a prepro-gene that is posttranslationally modified to yield two isoforms: orexin-A and orexin-B (29). Orexin-A seems to be the primary isoform involved in feeding control through its actions at the orexin 1 receptor (OX1R). This is based on observations that selective antagonists for the OX1R decrease food intake and activation of orexin-2 receptor by orexin-B typically does not affect feeding (10, 13). The decrease in food intake after peripheral OX1R antagonist treatment seems to occur independent of changes in malaise (17). OX1R activity in some brain regions strongly influences spontaneous physical activity and arousal, which could in turn affect feeding. However, hindbrain application of orexin-A and the OX1R antagonist SB334867 affect feeding without altering physical activity (24), suggesting that at least in some locations, OX1R have a physiological role in the control of feeding.
Orexin neurons appear to play a role in both food and drug reward. Studies using conditioned place preference (CPP) as a model of drug reinforcement report that mice lacking the prepro-orexin gene fail to express a CPP for a morphine-paired environment (23). In other studies, exposure to the CPP compartment previously paired with morphine, cocaine, or food leads to increased activation of orexin neurons in the lateral hypothalamus (LH), and expression of morphine-CPP can be attenuated by blockade with orexin-A antagonist before the posttraining preference test session (11). These observations indicate that LH orexin neurons are activated by environmental cues that signal availability of reinforcing stimuli. Consistent with this, rats trained to expect a palatable food “treat” show increased activation of LH and perifornical area orexin neurons in response to the food-paired environment (5). Orexin neurons can also be activated by pharmacological manipulations that elicit intake of palatable food, such as injection of the μ-opioid receptor agonist [d-Ala2,N-MePhe4,Gly-ol]-enkephalin (DAMGO) into the nucleus accumbens (35). In addition, orexin neurons are sensitive to glucose levels within the physiological range, and their responsiveness is modulated by the feeding-relevant hormones leptin and ghrelin (30). These cells are therefore in a position to integrate information about energy balance and environmental cues relating to food.
Orexin neurons project to many food intake-relevant nuclei where cells express OX1R (15, 20, 26). The majority of research on the role of orexins in food intake has focused on hypothalamic, thalamic, and other forebrain sites of action, and some attention has been given to the role of caudal brain stem OX1R (2, 5, 18). In addition to those regions, previous research has shown that the orexin pathway to the ventral tegmental area (VTA) can influence behavior and that it may play a role in reward and motivation. Direct application of orexin-A to the VTA induces a CPP (33), suggesting that stimulation of VTA orexin receptors can serve as an appetitive unconditioned stimulus. Additional data comes from studies examining orexin involvement in the effects of drugs of abuse. Blockade of VTA OX1R blunts the development of locomotor sensitization to cocaine, and in the same study, OX1R activity was reported to play a critical role in cocaine-induced plasticity in VTA dopamine neurons, a process hypothesized to contribute to relapse in addiction (4). Moreover, a combination of unilateral VTA lesion and intra-VTA OX1R antagonist on the intact side was shown to prevent the acquisition of morphine-CPP (12). Intra-VTA orexin-A injection can also reinstate a previously extinguished morphine-CPP (11).
Data on feeding effects of VTA orexin have been limited thus far. One previous study reported no effect of intra-VTA orexin-A injection on chow intake (32). In this context, additional studies have implicated VTA OX1R in the response to other orexigenic treatments. Specifically, blockade of OX1R in the VTA blunts the orexigenic effects of lateral intracerebroventricular ghrelin (7) and intranucleus accumbens DAMGO injection (35), suggesting that orexin input to the VTA may play a role in feeding under high-motivation conditions. Here, we investigate whether direct application of orexin-A to the VTA can increase palatable food intake, and whether blockade of VTA OX1R suppresses palatable food intake even in the absence of some other exogenous stimulus for feeding.
We first used a paradigm sometimes referred to as a “hedonic eating” or “dessert” model to determine the effects of intra-VTA orexin-A on feeding in rats that consume a large chow meal and then choose to eat more when given access to high-fat diet (HFD) (5). We also examined the effect of VTA orexin-A on HFD intake and sucrose intake in nondeprived rats trained to have short-term access to these foods on most days. Using licking microstructural analysis, we examined the behavioral mechanisms through which VTA orexin-A increases sucrose solution intake. To address the question of whether endogenous OX1R stimulation in the VTA affects feeding, we asked whether blockade of VTA OX1R with the selective antagonist SB334867 decreases sucrose intake. Because of the previous evidence of a role for brain orexin-A in motivation, we examined the effect of VTA orexin-A on motivation for sucrose reinforcement by assessing operant responding on a progressive ratio schedule. Our data suggest that the primary effect of VTA orexin-A on feeding occurs late in the meal. Therefore, the final study we performed is a more direct test of the hypothesis that OX1R activity in the VTA counteracts postingestive negative feedback induced by gastrointestinal nutrient infusion.
MATERIALS AND METHODS
Subjects.
Naïve male Wistar rats (Harlan, Indianapolis, IN) were maintained individually in temperature-controlled vivariums on a 12-h light:12-h dark cycle in plastic cages. Rats had ad libitum access to distilled water and rat chow (Purina 5001) except where otherwise noted. All experimental procedures were approved by the Florida State University Institutional Animal Care and Use Committee and conformed to the standard of the NIH “Guide for the Care and Use of Laboratory Animals” (National Research Council, 1996).
Surgery.
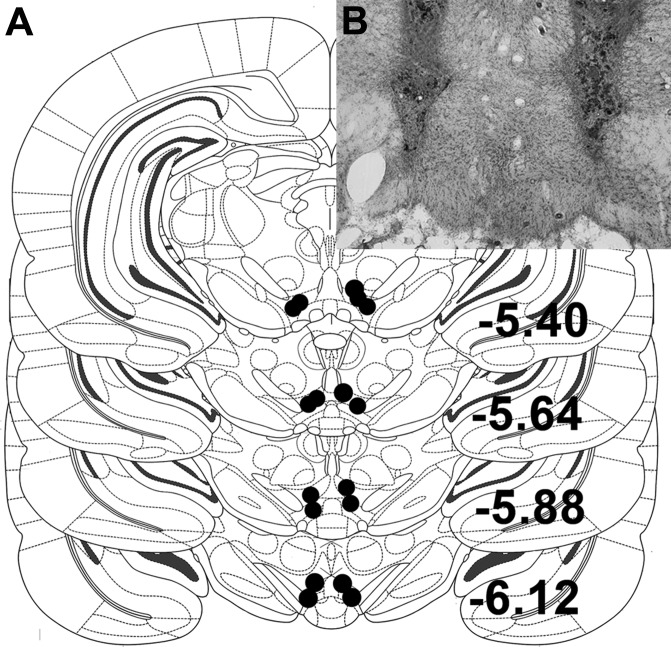
Under 2–4% isoflurane delivered at a rate of 1 l/min, rats were implanted with unilateral 26-gauge guide cannulas (Plastics One, Roanoke, VA) targeting VTA. Coordinates for unilateral VTA cannulas were 0.7 mm lateral to midline, 5.4 mm posterior to bregma, and 7.5 mm ventral to skull surface. In experiment 4, rats were implanted with bilateral 26-gauge guide cannulas (Plastics One) targeting VTA. Coordinates for bilateral VTA cannulas were 0.6 mm lateral to midline, 5.4 mm posterior to bregma, and 7.0 mm ventral to skull surface. Injectors (33 gauge) extending 2.0 mm below the end of the guide cannulas were used in all experiments. Rats were given 1 wk to recover from surgery before the start of experimentation. VTA placements were verified histologically at the end of behavioral testing. Injection sites within the boundaries of the VTA, as drawn in the atlas of Paxinos and Watson (25), were considered correct, and only data from rats with correct placements were included in the analysis (87% hit rate) (Fig. 1).
Fig. 1.
A: diagram of representative ventral tegmental area (VTA) injection placements based on the atlas of Paxinos and Watson [adapted with permission from Elsevier (25)]. Additional subjects' injection sites were identified in similar locations at points between the anterior-posterior levels displayed here. B: representative image of a bilateral VTA injection site in a thionin-stained coronal section (black box identifies the location at which the photograph was taken).
Drugs and injections.
Orexin-A (Bachem, Torrance, CA) was dissolved in sterile 0.9% saline. The OX1R antagonist SB334867, obtained from Tocris Bioscience (Ellisville, MO), was dissolved in 100% dimethyl sulfoxide (DMSO); this was used to dissolve the compound at sufficiently high concentration to deliver the desired dose intraparenchymally. We have used this approach previously and determined that this DMSO vehicle does not adversely affect behavior or brain tissue (18). In all experiments, the corresponding vehicle control was used for comparison with drug. Injections were made with a 10-μl syringe (Hamilton, Reno, NV) connected to a 33-gauge injector (Plastics One) via the Tygon tubing (VWR, Radnor, PA). Injections were delivered at a rate of 0.25 μl/min to the VTA, and injections were 0.5 μl in volume. For VTA administration of orexin-A, we utilized doses that were below threshold for effect when delivered to the third ventricle (5). When we used the OX1R antagonist, rats received intra-VTA injections of 10 nmol of SB334867; this dose was chosen based on a pilot study in which we observed that this dose was subthreshold for effect when delivered to the fourth ventricle. All experiments utilized a within-subjects counterbalanced design, and experimental days were separated by 48 h. All experiments were conducted during the midlight phase.
Experiment 1: effect of unilateral VTA orexin-A on feeding in a dessert model.
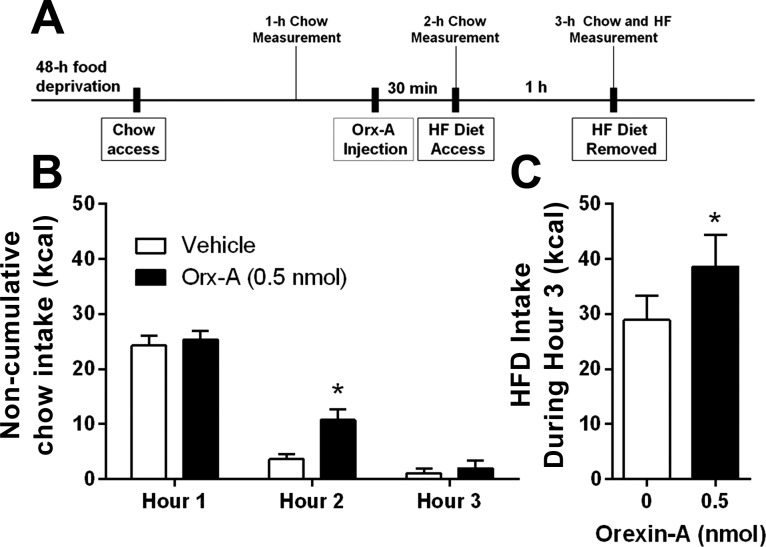
Unilateral VTA-cannulated rats (n = 8) were food deprived for 48 h and were subsequently given ad libitum access to chow. Ninety minutes after the initiation of chow access, rats received intra-VTA injection of saline vehicle or 0.5 nmol orexin-A. Chow remained available and HFD (60% fat, Research Diets) was introduced 30 min postinjection. Intake of chow was measured at 1, 2, and 3 h. HFD was measured from hour 2 to hour 3. Rats recovered for 6 days before the second food deprivation period began. Drug treatments were presented in counterbalanced order.
Experiment 2: effect of unilateral VTA orexin-A on HFD intake.
Naïve rats (n = 10) with VTA cannulas were habituated to receive 30 min access to a preweighed hopper filled with HFD in their home cages 5 or 6 days per week during the midlight phase, always at the same time of day. Chow was removed from the cage during these test sessions. After the 30-min test period, HFD was removed from the cage and weighed, and chow was returned. The experiment began after rats were habituated to this test situation for 8 days, at which point HFD intakes were stable (less than 10% variation over 3 consecutive days). On experimental days, rats received intra-VTA injections of either 0.1 or 0.5 nmol of orexin-A or saline vehicle 30 min before the HFD intake test.
Experiment 3: effects of unilateral VTA orexin-A or OX1R antagonist on sucrose intake.
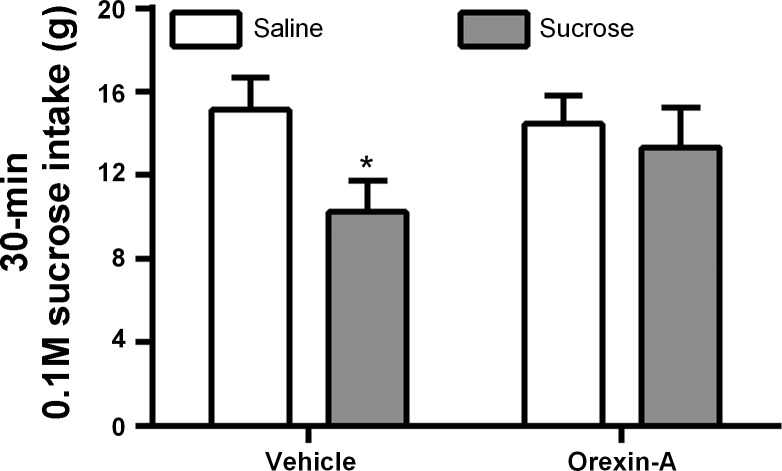
VTA-cannulated rats (n = 15) were given 30 min access to 0.1 M sucrose in a preweighed bottle placed on their home cage with no access to chow or water during the session 5 or 6 days per week. After 16 days, sucrose intake was stable across days and the experiment began. On experiment days, food and water were removed before injections. Rats received intra-VTA injections of either 0.1 or 0.5 nmol of orexin-A or saline vehicle 30 min before the sucrose test session. In the same cohort of rats, we utilized this design to test the effects of the OX1R antagonist on sucrose intake. Five days after the final orexin treatment day, rats received intra-VTA injections of either 10 nmol of SB334867 or DMSO vehicle 30 min before the sucrose test session.
Experiment 4: effect of bilateral VTA OX1R blockade on sucrose intake.
The same design as described above was used in a separate group of bilaterally VTA-cannulated rats (n = 9) that received 30-min access to 0.1 M sucrose in a preweighed bottle placed on their home cage. Rats received bilateral intra-VTA injections of either 10 nmol of SB334867 (5 nmol per hemisphere) or DMSO vehicle 30 min before the sucrose test session.
Experiment 5: effect of unilateral VTA orexin-A on operant responding.
VTA-cannulated rats (n = 11) were trained to make operant responses for 45-mg sucrose pellets (TestDiet, Richmond, IN). Training was conducted in operant conditioning chambers enclosed in sound-attenuating cabinets (Coulbourn Instruments, Allentown, PA) as described by Kay et al. (18). Two levers were present in each chamber; presses on the active lever were reinforced, whereas inactive lever presses were not reinforced (rats rarely pressed the inactive lever). For all training and testing sessions, there was a 5-s timeout after each reinforcement. During both training and test sessions, a cue light was illuminated above the active lever. The locations of the active and inactive levers were counterbalanced across subjects. Training progressed as follows: first, rats were trained on a fixed ratio one schedule (FR1) where each response resulted in delivery of one sucrose pellet. FR1 training was conducted for 9 days. Next, rats were moved to a FR3 schedule where three responses were required to achieve one sucrose pellet for 7 days. After this training, all rats showed less than 10% day-to-day variation in responding and were switched to a progressive ratio (PR) schedule that followed the algorithm of Richardson and Roberts (27): 1, 2, 4, 6, 9, 12, 16, 20, 28, 36, 48, etc., . . . lever presses for reinforcement. PR sessions ended when the rat failed to press the active lever for 30 min, with a maximum duration of 2 h. Rats were then returned to home cages with ad libitum food and water access. Experimentation began after 8 days of PR training, at which point rats showed stable responding on the PR schedule. On testing days, rats received intra-VTA injection of saline vehicle, 0.1 nmol, or 0.5 nmol orexin-A 30 min before the start of the PR session.
Experiment 6: effect of unilateral VTA orexin-A on licking microstructure for sucrose.
After 5 days of recovery from the PR experiment, a subset (n = 9) of the VTA-cannulated rats used in the PR experiment were trained to lick a spout for 0.1 M sucrose in the operant chambers described above during 45-min sessions. Each chamber was equipped with a recessed drinking spout located 2 cm above the grid floor. Licks were detected via photobeam positioned at the base of the spout. Licks were recorded by Graphic State 3.03 software (Coulbourn Instruments, Allentown, PA) and stored on the computer for later analysis. Licking data were then analyzed by a custom macro. A meal was defined as at least three licks, and the criterion for the end of a meal was a pause of 600 or more seconds (3, 31). Intermeal interval was defined as the time between the last lick of one meal and the first lick of the next. Within each meal, a licking burst was defined as series of licks separated by an interlick interval (ILI) of <1 s (31). Variables obtained from the custom macro included meal duration, burst duration, within-meal burst number, mean number of licks per burst, mean licks per minute over the course of the meal, and number of licks in the first minute of the meal, size, and average interburst interval. Within-burst interlick interval was calculated as an average of interlick intervals below 250 ms, because this captures more than 95% of interlick intervals (8). Furthermore, the first meal of the session was divided into thirds based on burst number. This allowed for assessment of drug effects that may occur early in the meal, when postingestive negative feedback is minimal and licking would be largely driven by oral sensory cues, and effects that may occur late in the meal when licking behavior would be more strongly influenced by postingestive satiation signals.
On experimental days, rats received intra-VTA injections of either saline vehicle or 0.5 nmol of orexin-A 30 min before the test session. When the session ended, rats were removed from the chamber and returned to their home cage.
Experiment 7: effect of unilateral VTA orexin-A on the response to intragastric nutrient infusion.
Rats (n = 8) were trained to consume 0.1 M sucrose in daily home cage test sessions as described in experiment 3, but these rats were also first connected to infusion lines in the operant chambers described in experiment 5. No manipulanda, spouts, or food were available during this time; these chambers were simply used for infusions. The infusion lines consisted of polyethylene tubing (PE; VWR, Radnor, PA) protected by a stainless steel spring. The PE tubing was connected to an infusion swivel (Instech Solomon, Plymouth Meeting, PA) mounted on a counterbalanced lever at the top of the chamber. This conserved the rats' ability to move freely around the chamber when the infusion line was connected to the back-mounted intragastric catheter port. When daily 0.1 M sucrose intakes were stable, rats received an additional habituation test session in which they entered the chamber to receive an intragastric infusion of 0.9% saline at a rate of 1 ml/min for 5 min and were then returned to their home cage and given access to the preweighed sucrose bottle for 30 min.
This experiment had four conditions that were administered to all rats in a counterbalanced order separated by minimum of 48 h. Rats received an intra-VTA injection of either saline vehicle or 0.025 nmol orexin-A 40 min before the start of the intake test. This dose was chosen based on a pilot study in which we observed no effect of intra-NTS 0.025 nmol of orexin-A. Thirty minutes after the intra-VTA injections, rats were connected to the infusion lines. They then received 5 ml of either 0.9% saline or 25% sucrose (5 kcal) delivered at a rate of 1 ml/min for a total of 5 min. We chose this sucrose load and timing to induce postingestive negative feedback that would significantly suppress subsequent voluntary intake for a short period of time. After the intragastric infusion, rats were disconnected from the infusion line, returned to their home cages, and given access to 0.1 M sucrose for 30 min. The four experimental conditions were the following: VTA vehicle/IG saline; VTA vehicle/IG sucrose; VTA orexin-A/IG saline; and VTA orexin-A/IG sucrose.
Statistical analysis.
Data are reported as means ± SE. Effects were evaluated by within-subjects Student's t-test or within-subjects one-way ANOVA, as appropriate. Drug effects on licking variables across segments of the meal in experiment 6 were assessed by two-factor ANOVA with drug and segment as factors. Similarly, in experiment 7, a two-factor ANOVA was used to assess the effect of intragastric sucrose and VTA orexin-A on sucrose intake. Pairwise planned comparisons were made with Holm-Sidak tests. P values of < 0.05 were taken as significant.
RESULTS
Experiment 1: effect of unilateral VTA orexin-A on feeding in a dessert model.
After 48 h of food deprivation, there was no difference in 1-h chow intake between groups before injections. Intra-VTA orexin-A significantly increased chow intake at the 2-h measurement, which occurred 30 min postinjection [t(7) = 3.17, P < 0.01] (Fig. 2A). When rats were exposed to HFD after refeeding on chow, intra-VTA orexin-A significantly elevated HFD intake from hour 2 to 3 [t(7) = 2.13, P < 0.05] (Fig. 2B). There was no effect on chow intake from hour 2 to 3 (Fig. 2A).
Fig. 2.
Effect of unilateral VTA Orexin-A on feeding in a dessert model. A: experimental timeline. B: after a 48-h food deprivation, rats showed no difference in a 1-h chow intake. Intra-VTA orexin-A significantly increased chow intake at 2 h (30 min postinjection). *P < 0.05. There was no effect on chow intake from hour 2 to 3. C: rats were given access to high-fat diet (HFD) from hour 2 to 3. During this time, intra-VTA orexin-A significantly elevated HFD intake. *P < 0.05.
Experiment 2: effect of unilateral VTA OX1R stimulation on HFD intake.
VTA injection of orexin-A significantly elevated 30-min HFD intake [F(2, 18) = 4.07, P < 0.05]. Here, only the 0.5-nmol dose significantly increased HFD intake (P < 0.05) relative to vehicle (Fig. 3).
Fig. 3.
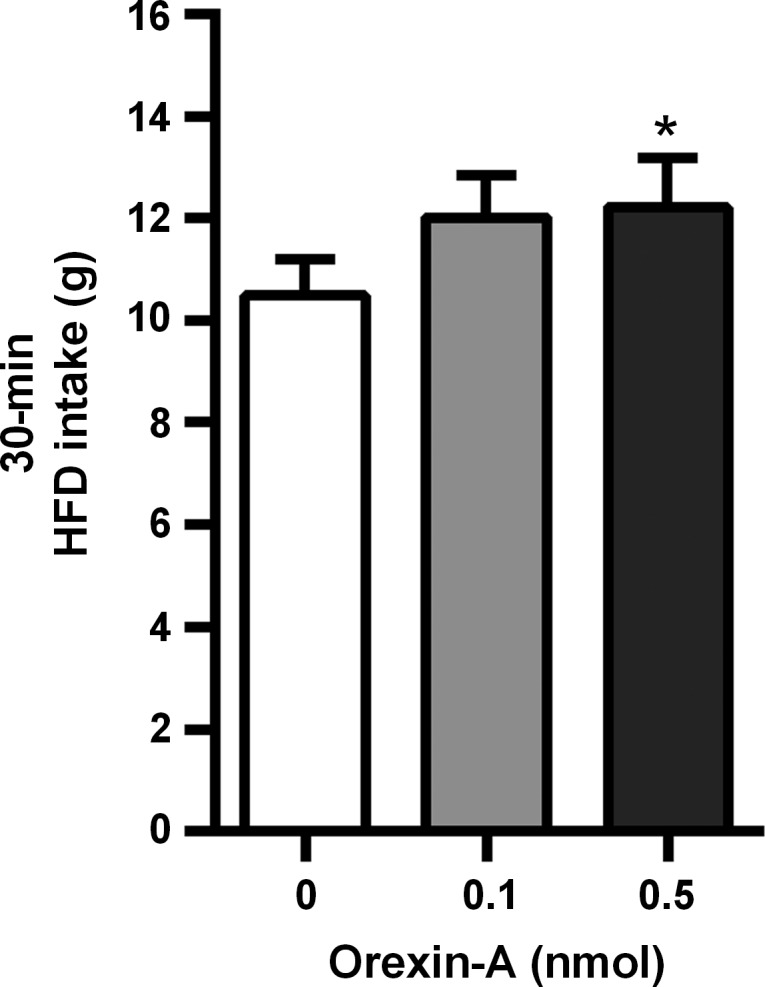
Effect of unilateral VTA OX1R stimulation on HFD intake. Intra-VTA injection of 0.5 nmol orexin-A significantly increased HFD intake during a 30-min test session. *P < 0.05.
Experiment 3: effects of unilateral VTA OX1R stimulation or blockade on sucrose intake.
Intra-VTA orexin-A strongly and significantly increased 30-min home cage sucrose intake [F(2, 28) = 15.29, P < 0.0001]. Both the 0.1- and 0.5-nmol dose significantly elevated sucrose intake relative to intra-VTA vehicle injection (Fig. 4). The same rats failed to suppress 30-min sucrose intake after unilateral VTA injection of SB334867, tested 5 days after the orexin-A experiment ended. There was a nonsignificant trend toward an increase in 30-min sucrose intake after SB334867 from a mean of 14.42 ± 1.48 g after vehicle and 16.85 ± 1.86 g after SB334867.
Fig. 4.
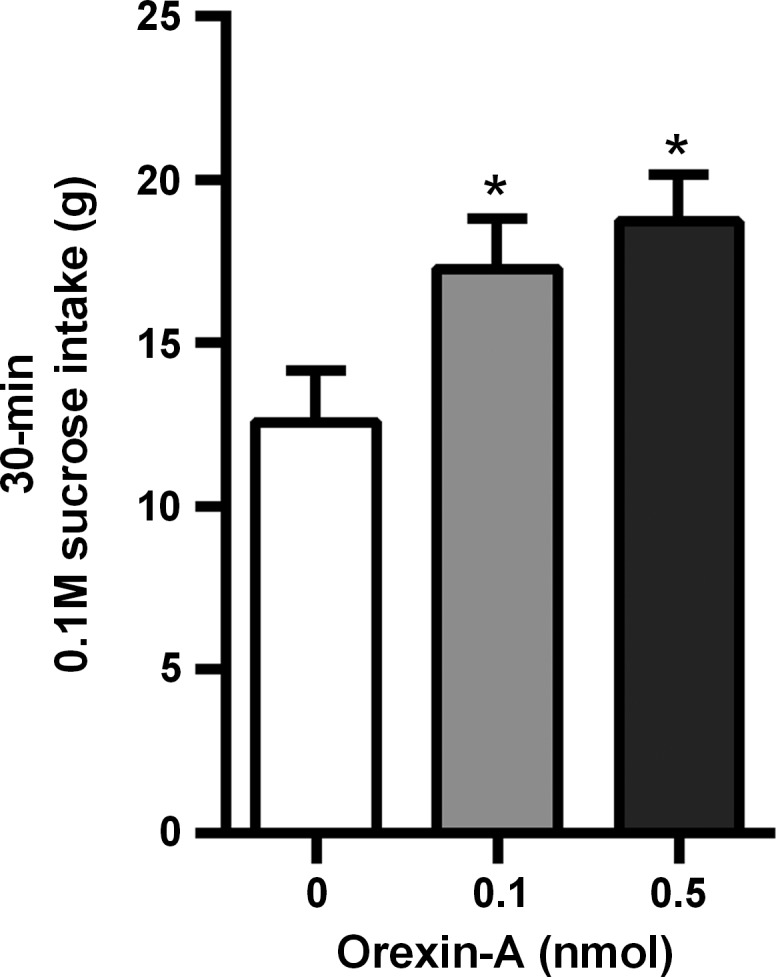
Effect of unilateral VTA OX1R stimulation on sucrose intake. Intra-VTA injection of 0.1 and 0.5 nmol orexin-A significantly elevated a 30-min home cage sucrose intake. *P < 0.05.
Experiment 4: effect of bilateral VTA OX1R blockade on sucrose intake.
Bilateral intra-VTA injection of SB334867 significantly suppressed 30-min sucrose intake [t(8) = 2.01, P < 0.05] (Fig. 5).
Fig. 5.
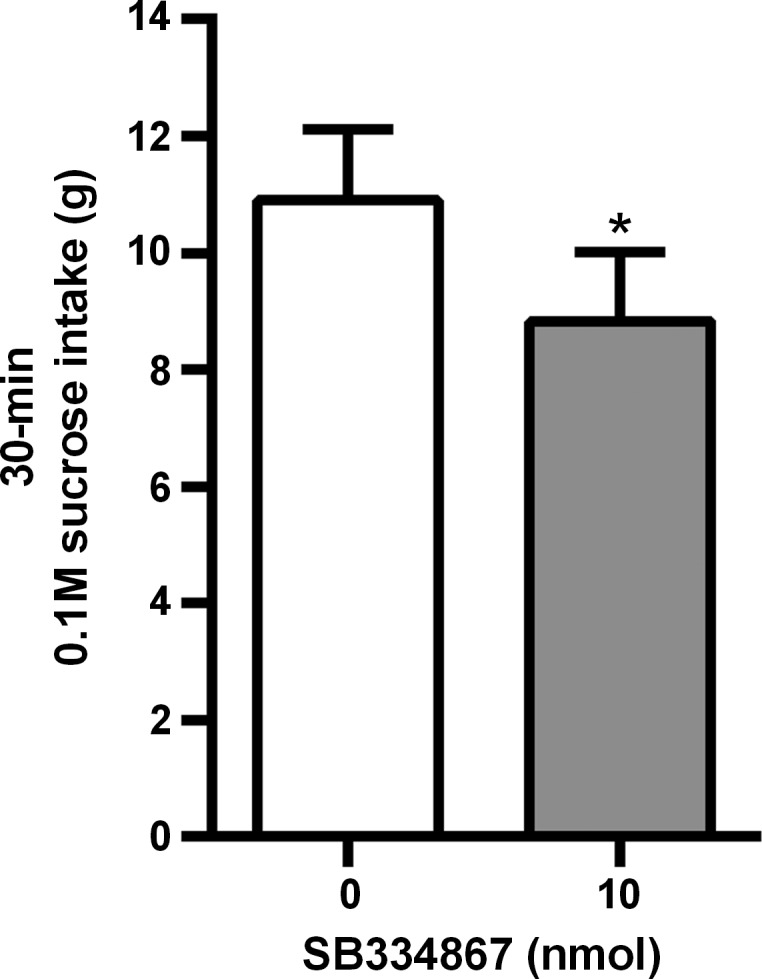
Effect of bilateral VTA OX1R blockade on sucrose intake. Bilateral intra-VTA injection of SB334867 significantly suppressed a 30-min sucrose intake. *P < 0.05.
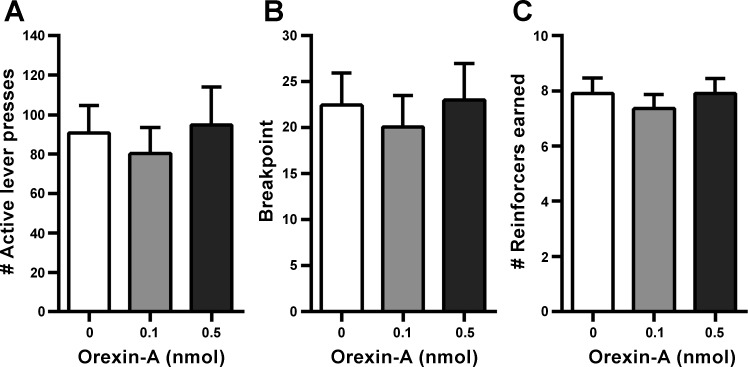
Experiment 5: effect of unilateral VTA OX1R stimulation on operant responding.
Unilateral VTA orexin-A injection had no effect on breakpoint (the last completed ratio of the session), active lever presses, or number of reinforcers earned in rats responding under a PR schedule for sucrose (Fig. 6). VTA orexin-A had a tendency to increase the duration of the operant responding test session, although this did not reach significance [means: vehicle = 61.27 ± 7.46 min; 0.1 nmol = 52.36 ± 5.65 min; 0.5 nmol = 78.36 ± 8.77 min; F(2, 20) = 3.01, P = 0.07].
Fig. 6.
Effect of unilateral VTA OX1R stimulation on operant responding (A–C). In rats responding on a progressive ratio, intra-VTA orexin-A did not affect total active lever presses, breakpoint, or the number of sucrose reinforcers earned.
Experiment 6: effect of unilateral VTA OX1R stimulation licking microstructure for sucrose.
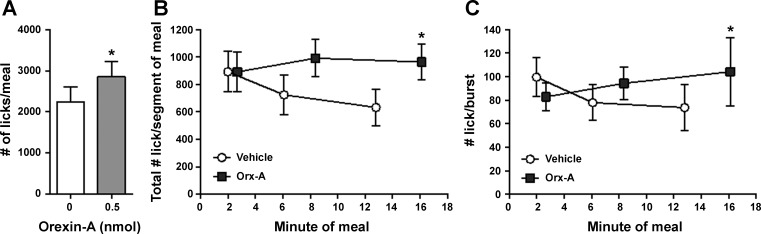
Unilateral VTA orexin-A significantly increased first meal size [t(8) = 2.88, P < 0.05] (Fig. 7A) but had no effect on number of bursts taken within that meal or meal duration (Table 1). The number of licks in the first minute of the first meal tended to be suppressed after intra-VTA orexin-A, but this did not reach significance (Table 1). When the first meal was segmented into thirds by total number of licking bursts within the meal, effects of orexin-A emerged near the end of the meal, with no effects on licking behavior toward the beginning of the meal. There was a significant main effect of orexin-A on number of licks/meal segment [F(1,8) = 8.266, P < 0.05], and pairwise comparisons show that licks/segment were only significantly elevated by VTA orexin-A in the third segment of the meal (P < 0.05). There was a significant interaction between orexin-A and meal segment for burst size [F(2,16) = 4.667, P < 0.05], where orexin-A increased burst size only during the final third of the meal (P < 0.05) (Fig. 7).
Fig. 7.
Effect of unilateral VTA OX1R stimulation licking microstructure for sucrose. A: unilateral intra-VTA orexin-A significantly increased first meal size. *P < 0.05. B and C: when the first meal was segmented into thirds by total number of licking bursts within the meal, effects of orexin-A emerged near the end of the meal. VTA orexin-A significantly increased the total number of licks in third segment of the meal (B) and also significantly increased burst size (number of licks/burst) during the final third of the meal (C). *P < 0.05.
Table 1.
Licking microstructural variables measured in experiment 6
| Variable | Vehicle | Orexin-A |
|---|---|---|
| Meal duration, min | 17.5 ± 3.92 | 21.2 ± 2.95 |
| Number of licks in the 1st minute of meal | 237.2 ± 19.7 | 198.1 ± 35.1† |
| Average ingestion rate, licks/min | 154.4 ± 26.0 | 148.9 ± 22.6 |
| Average burst duration, s | 13.1 ± 2.3 | 14.7 ± 2.4 |
| Number of bursts | 29.6 ± 4.59 | 34.2 ± 5.06 |
| Average within-burst ILI | 151.6 ± 2.4 | 146.8 ± 2.8* |
Data are means ± SE.
VTA, ventral tegmental area; ILI, interlick interval. In experiment 6, rats licking for 0.1 M sucrose were treated intra-VTA with vehicle or 0.5 nmol orexin-A.
P < 0.05;
P = 0.088.
Experiment 7: effect of unilateral VTA orexin-A on the response to intragastric nutrient infusion.
Intragastric sucrose infusion significantly suppressed 30-min sucrose intake [F(1,7) = 30.96, P < 0.01] (Fig. 8). As expected, 0.025 nmol orexin-A injected into the VTA had no effect when followed by saline intragastric infusion. However, this dose of orexin-A significantly attenuated the suppressive effect of the intragastric sucrose infusion on 30-min sucrose intake [interaction between IG infusion and orexin-A; F(1,7) = 8.56, P < 0.05] (Fig. 8).
Fig. 8.
Effect of unilateral VTA orexin-A on the response to intragastric nutrient infusion. Intragastric sucrose infusion suppressed 30 min sucrose intake; VTA orexin-A attenuated the suppressive effect of the intragastric sucrose infusion on 30 min sucrose intake.
DISCUSSION
The goal of the present experiments was to evaluate the hypothesis that orexin-A acts in the VTA to promote palatable food intake. To do this we manipulated VTA orexin signaling under multiple different feeding conditions. Overall, the results support our hypothesis. Importantly, we found that VTA administration of orexin-A, at doses that were ineffective when delivered to the cerebral ventricles (2, 5), increased intake of palatable, energy-dense HFD both in rats that had just consumed a large chow meal and in rats given acute daily access to HFD without a chow preload. In addition, VTA orexin-A increased intake of 0.1 M sucrose solution in short test sessions. Whereas unilateral blockade of VTA OX1R was not sufficient to affect sucrose intake, bilateral blockade of VTA OX1R with OX1R antagonist SB334867 significantly suppressed sucrose intake during a 30-min test. This finding suggests that endogenous OX1R stimulation in the VTA plays a role in promoting intake of sucrose under these test conditions. Together these data suggest that both exogenous and endogenous VTA OX1R stimulation promotes palatable food intake.
To further examine the behavioral mechanisms through which VTA orexin-A influences intake of palatable foods, we determined the effect of VTA orexin-A on licking behavior for 0.1 M sucrose solution. Rats were trained to lick a spout for 0.1 M sucrose during daily 45-min sessions. Under these conditions, rats took only one meal during the session, and that meal was significantly larger after VTA orexin-A treatment. When the meal was segmented into thirds, to allow examination of potential effects early versus late in the meal, we found that VTA orexin-A increased licking and burst size near the end of the meal, with no effects near the start of the meal. The effect of VTA orexin-A on the pattern of licking for sucrose suggests that the drug's effect may depend on the accumulation of nutrients in the gut as the meal progresses. Furthermore, because the effects of intra-VTA orexin-A were evident only in the later part of the meal, it is possible that VTA OX1R stimulation increases sucrose intake by attenuating or opposing postingestive negative feedback signals that normally suppress licking at that time. Similar findings were reported by Baird et al. (2) who found that both third and fourth intracerebroventricular-injected orexin-A increased meal size for 0.1 M sucrose by prolonging the meal without affecting microstructural measures early in the meal,suggesting that OX1R activation suppressed inhibitory postingestive feedback. Given that cerebrospinal fluid flows in a rostrocaudal direction, our data suggest that the VTA could be one of the sites mediating the effects of third ventricular treatment.
Previous reports indicate that peripheral delivery of the ORX1R receptor antagonist SB-334867 or reduction of ORX1R expression in the paraventricular thalamic nucleus (PVT) reduces HFD intake following a chow preload (5, 6). In the present study, VTA orexin-A-treated rats significantly increased chow intake during refeeding and HFD intake once chow intake had ceased. Furthermore, previous studies report increased neuronal activity within VTA neurons in rats that are expecting palatable chocolate meal (5). This observation suggests that VTA activity might contribute to intake of palatable food. The data we present here are in agreement with these previous findings and extend them by providing novel evidence that orexin action in the VTA stimulates HFD intake in the presence or absence of a caloric preload. In addition, the finding that orexin-A in the VTA influences both refeeding from chow and HFD following a caloric preload, along with the observed effects on licking behavior during a meal, suggests that VTA orexin may interact with postingestive processes that would typically inhibit feeding after a large meal. We directly tested this hypothesis in experiment 7 and found that an otherwise subthreshold dose of orexin-A could prevent gastrointestinal nutrient infusion-induced intake suppression. These data strongly support the idea that VTA orexin-A attenuates postingestive negative feedback following a nutrient load.
Surprisingly, we found that the same dose of VTA orexin-A that robustly increased 0.1 M sucrose intake did not affect operant responding for sucrose pellets on a PR schedule. This suggests that activation of OX1R in the VTA does not increase motivation to obtain sucrose pellets, at least under the conditions of this PR experiment. This was contrary to our original hypothesis, because the VTA has a well-established role in motivation, and orexin neurons have been implicated in food reward (1). Previously, we have shown that activation of hindbrain OX1R increases motivation for sucrose reinforcement, demonstrated by an increased breakpoint on a PR schedule, while blockade of hindbrain OX1R significantly decreased PR responding and breakpoint (18). These findings suggested that hindbrain orexin increases motivation for sucrose pellets, but our current results do not support a role for VTA orexin to affect operant responding for food reinforcement. This does not rule out the possibility that VTA OX1R play a role in other aspects of food reward that were not assessed here (e.g., CPP, cue-induced feeding).
Under the conditions of the PR experiment, rats consumed few kilocalories and therefore would have experienced relatively little postingestive negative feedback. Given our findings that VTA orexin-A affected food intake primarily late in the meal and perhaps by interacting with postingestive feedback signals, it is possible that the lack of effect on PR responding is related to the lack of significant postingestive negative feedback in this test situation. Previous work demonstrated that third ventricular orexin-A infusion significantly increased operant responding on a PR schedule of reinforcement under very similar experimental conditions, so substantial postingestive negative feedback is clearly not required for orexin-A to influence motivation presumably through actions on its receptors in other brain regions (5). It is also notable that in our sucrose solution intake experiments (experiments 3, 6, and 7) where we do find a robust effect of VTA orexin, rats also consumed very few kilocalories during those test sessions. A key difference between the intake and PR experiments is that the small number of 45-mg pellets consumed in the PR study would not be expected to produce significant gastric distention, whereas the 0.1 M sucrose intake was of sufficient volume that a distention signal should have been generated. This difference highlights gastric distention as a specific type of postingestive negative feedback signal with which orexin-A in the VTA may interact, and this will be an important subject for future investigation.
Previous work has established that orexin-A plays a role in spontaneous physical activity (19). However, orexin-A effects on feeding have been demonstrated without concurrent effects on activity in some cases, so these effects can occur independent of each other (24). We did not examine the effect of VTA orexin-A on physical activity or arousal here, and it is known that the VTA can influence physical activity (34), so it is possible that some of the effects we observed on feeding are related to general arousal or activity. Some observations from the present studies suggest that this may not have been a substantial factor. In the operant responding experiment, VTA orexin-A had no effect on active lever presses or inactive lever presses. If VTA orexin-A increased general arousal or physical activity, we might expect to see increased lever pressing, particularly increased pressing on the inactive lever, but instead there were no effects of orexin-A on active or inactive lever pressing. Although beyond the scope of the present studies, it will be worthwhile to directly investigate whether VTA OX1R influence physical activity in the future.
Perspectives and Significance
The present results indicate that VTA orexin-A influences feeding, particularly intake of palatable foods. While the VTA is best known for its role in reward and motivated behaviors, our findings suggest that VTA orexin receptor stimulation promotes intake not by increasing motivation for food, but potentially through attenuating or opposing postingestive negative feedback processes that begin to occur after a meal has been initiated. The idea that the VTA responds to postingestive negative feedback signals is relatively new but supported by other recent work. For example, Mietlicki-Baase and colleagues (22) demonstrated that receptors for the satiation signal amylin in the VTA play a physiological role in the control of feeding. In addition, the adiposity signal leptin acts in the VTA to control palatable food intake (16) and operant responding for sucrose (11). The VTA has also been identified as a site where amylin and leptin interact to reduce food intake (21). The orexin neuron-to-VTA pathway has already been implicated in the effects of other manipulations that affect food intake. For example, OX1R activity in the VTA plays a mediatory role in the feeding responses to intracerebroventricular ghrelin (7) and intra-NAc DAMGO (35). OX1R activity also appears to mediate the VTA DA neuronal response to reduced glucose levels (30). Thus the VTA seems to be a site of integration for diverse signals that influence food intake, perhaps more so that previously appreciated. Future studies will be necessary to determine the mechanisms through which OX1R activity in the VTA influences palatable food intake, and how these receptors interact with other signals at this location.
GRANTS
This work was funded by the National Institutes of Health Grant R01DK095757 to D. L. Williams.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.J.T., J.F.D., and D.L.W. conception and design of research; S.J.T., K.H., K.K., H.E.G., C.B.M., and A.E.K. performed experiments; S.J.T. and D.L.W. analyzed data; S.J.T., J.F.D., and D.L.W. interpreted results of experiments; S.J.T. prepared figures; S.J.T. drafted manuscript; S.J.T., J.F.D., and D.L.W. edited and revised manuscript; S.J.T., K.H., K.K., H.E.G., C.B.M., A.E.K., J.F.D., and D.L.W. approved final version of manuscript.
REFERENCES
- 1.Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res 1314: 74–90, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baird JP, Choe A, Loveland JL, Beck J, Mahoney CE, Lord JS, Grigg LA. Orexin-A hyperphagia: hindbrain participation in consummatory feeding responses. Endocrinology 150: 1202–1216, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baird JP, Grill HJ, Kaplan JM. Effect of hepatic glucose infusion on glucose intake and licking microstructure in deprived and nondeprived rats. Am J Physiol Regul Integr Comp Physiol 277: R1136–R1143, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49: 589–601, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience 167: 11–20, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Choi DL, Davis JF, Magrisso IJ, Fitzgerald ME, Lipton JW, Benoit SC. Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience 210: 243–248, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cone JJ, McCutcheon JE, Roitman MF. Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neurosci 34: 4905–4913, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci 106: 217–228, 1992. [PubMed] [Google Scholar]
- 9.Davis JF, Choi DL, Schurdak JD, Fitzgerald MF, Clegg DJ, Lipton JW, Figlewicz DP, Benoit SC. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol Psychiatry 69: 668–674, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dube MG, Kalra SP, Kalra PS. Food intake elicited by central administration of orexins/hypocretins: identification of hypothalamic sites of action. Brain Res 842: 473–477, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437: 556–559, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res 183: 43–51, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haynes AC, Chapman H, Taylor C, Moore GBT, Cawthorne MA, Tadayyon M, Clapham JC, Arch JR. Anorectic, thermogenic and anti-obesity activity of a selective orexin-1 receptor antagonist in ob/ob mice. Regul Pept 104: 153–159, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JRS. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept 96: 45–51, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience 103: 777–797, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51: 801–810, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Ishii Y, Blundell JE, Halford JCG, Upton N, Porter R, Johns A, Rodgers RJ. Differential effects of the selective orexin-1 receptor antagonist SB-334867 and lithium chloride on the behavioural satiety sequence in rats. Physiol Behav 81: 129–140, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Kay K, Parise EM, Lilly N, Williams DL. Hindbrain orexin 1 receptors influence palatable food intake, operant responding for food, and food-conditioned place preference in rats. Psychopharmacology (Berl) 231: 419–427, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotz CM, Teske JA, Levine JA, Wang C. Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regul Pept 104: 27–32, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435: 6–25, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Mietlicki-Baase EG, Olivos DR, Jeffrey BA, Hayes MR. Cooperative interaction between leptin and amylin signaling in the ventral tegmental area for the control of food intake. Am J Physiol Endocrinol Metab 308: E1116–E1122, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mietlicki-Baase EG, Rupprecht LE, Olivos DR, Zimmer DJ, Alter MD, Pierce RC, Schmidt HD, Hayes MR. Amylin receptor signaling in the ventral tegmental area is physiologically relevant for the control of food intake. Neuropsychopharmacology 38: 1685–1697, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci 26: 398–405, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parise EM, Lilly N, Kay K, Dossat AM, Seth R, Overton JM, Williams DL, Em P, Lilly N, Kay K, Am D, Seth R, Jm O. Evidence for the role of hindbrain orexin-1 receptors in the control of meal size. Am J Physiol Regul Integr Comp Physiol 301: R1692–R1699, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic-Elsevier, 2007. [Google Scholar]
- 26.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18: 9996–10015, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Sahu A. Interactions of neuropeptide Y, hypocretin-I (orexin A) and melanin-concentrating hormone on feeding in rats. Brain Res 944: 232–238, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JRS, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92: 573–585, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Sheng Z, Santiago AM, Thomas MP, Routh VH. Metabolic regulation of lateral hypothalamic glucose-inhibited orexin neurons may influence midbrain reward neurocircuitry. Mol Cell Neurosci 62: 30–41, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci 112: 678–694, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Sweet DC, Levine AS, Billington CJ, Kotz CM. Feeding response to central orexins. Brain Res 821: 535–538, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Taslimi Z, Arezoomandan R, Omranifard A, Ghalandari-Shamami M, Riahi E, Vafaei AA, Rashidy-Pour A, Haghparast A. Orexin A in the ventral tegmental area induces conditioned place preference in a dose-dependent manner: involvement of D1/D2 receptors in the nucleus accumbens. Peptides 37: 225–232, 2012. [DOI] [PubMed] [Google Scholar]
- 34.Tsujino N, Sakurai T. Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci 7: 28, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR. Appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol 284: R1436–R1444, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci 27: 11075–11082, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng H, Patterson LM, Berthoud HRR. Orexin-A projections to the caudal medulla and orexin-induced c-Fos expression, food intake, and autonomic function. J Comp Neurol 485: 127–142, 2005. [DOI] [PubMed] [Google Scholar]