Abstract
Changes in osmolality or extracellular NaCl concentrations are detected by specialized neurons in the hypothalamus to increase vasopressin (VP) and stimulate thirst. Recent in vitro evidence suggests this process is mediated by an NH2-terminal variant of the transient receptor potential vanilloid type 1 (TRPV1) channel expressed by osmosensitive neurons of the lamina terminalis and vasopressinergic neurons of the supraoptic nucleus. The present study tested this hypothesis in vivo by analysis of plasma VP levels during acute hypernatremia in awake control and TRPV1−/− rats. TRPV1−/− rats were produced by a Zinc-finger-nuclease 2-bp deletion in exon 13. Intravenous injection of the TRPV1 agonist capsaicin produced hypotension and bradycardia in control rats, but this response was absent in TRPV1−/− rats. Infusion of 2 M NaCl (1 ml/h iv) increased plasma osmolality, electrolytes, and VP levels in both control and TRPV1−/− rats. However, plasma VP levels did not differ between strains at any time. Furthermore, a linear regression between plasma VP versus osmolality revealed a significant correlation in both control and TRPV1−/− rats, but the slope of the regression lines was not attenuated in TRPV1−/− versus control rats. Hypotension produced by intravenous injection of minoxidil decreased blood pressure and increased plasma VP levels similarly in both groups. Finally, both treatments stimulated thirst; however, cumulative water intakes in response to hypernatremia or hypotension were not different between control and TRPV1−/− rats. These findings suggest that TRPV1 channels are not necessary for VP secretion and thirst stimulated by hypernatremia.
Keywords: thirst, antidiuretic hormone, hypernatremia, osmoreceptor
systemic hypernatremia stimulates thirst, changes in sympathetic nerve activity, and secretion of antidiuretic hormone or vasopressin (VP).Together, these responses normalize osmotic pressure and volume (3, 25, 31). This process is initiated by specialized osmosensitive neurons located in the organum vasculosum of the lamina terminalis (OVLT) and subfornical organ (SFO) (3, 14). Indeed, the presence of osmoresponsive or osmosensitive neurons in the OVLT and SFO is supported by both in vivo and in vitro electrophysiological studies (1, 2, 5, 7, 8, 16), immunocytochemical detection of the early intermediate gene c-Fos in response to hypernatremia (11, 17), and lesion studies to suggest that thirst and VP secretion in response to acute NaCl loads depend on the integrity of these structures (4, 13, 15, 24, 29, 30). In turn, OVLT and SFO neurons project mono- or polysynaptically onto numerous hypothalamic nuclei including both parvocellular and magnocellular neurons of the hypothalamic paraventricular nucleus (PVH) and supraoptic (SON). In the past decade, evidence suggests VP magnocellular neurons may also be intrinsically osmosensitive (3, 12). A series of in vitro electrophysiological studies have reported that hyperosmotic stimuli depolarize VP magnocellular neurons through a nonselective cation conductance (3, 18, 19, 22). Together, these osmosensory processes within the OVLT, SFO, and magnocellular neurons of the SON or PVH coordinate thirst and VP secretion during hyperosmotic conditions.
Recent studies have suggested that osmosensory transduction within OVLT and SON neurons is mediated by an NH2-terminal variant of the transient receptor potential vanilloid type 1 (TRPV1) channel (5, 6, 22, 34). In vitro patch-clamp recordings of isolated OVLT or SON neurons have shown that hyperosmolality increases action potential discharge through an inward cation current that is blocked by the broad-spectrum TRPV antagonist ruthenium red or a selective TRPV1 blocker SB366791, or absent in TRPV1−/− mice (5, 6, 22, 34). Despite these observations, in vivo evidence to support a prominent role for TRPV1 in osmosensory responses has been less convincing. First, thirst responses to acute NaCl loads are either normal (10, 28) or slightly attenuated (5) in TRPV1−/− versus wild-type mice. Second, acute or chronic hypernatremia in TRPV1−/− versus wild-type mice produces similar numbers of Fos-positive cells in the OVLT and SFO (10, 28) as well as VPergic neurons in the SON (28). On the other hand, Sharif-Naeini et al. (22) reported that the relationship between serum VP levels and osmolality during access to 2% NaCl drinking solution was significantly blunted in TRPV1−/− versus wild-type mice. Unfortunately, these blood samples were collected under halothane anesthesia (22). Therefore, this prompted the current set of experiments in which plasma VP levels were assessed in conscious TRPV1−/− and control rats after acute intravenous infusion of hypertonic NaCl. This experimental paradigm has a distinct advantage as rats can be chronically instrumented to collect blood samples before and after stimuli without the confound of anesthesia, stress, or pain associated with subcutanteous or intrperitoneal injections of hypertonic solutions.
MATERIALS AND METHODS
Animals.
All of the experimental procedures conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the Pennsylvania State College of Medicine. Male Sprague-Dawley rats (350–450 g, Charles River Laboratories) or TRPV1−/− rats (TGRS5530, Sage Research Laboratories, Boylestown, PA) were singly housed in dedicated metabolic cages placed in a temperature-controlled room (22 ± 1°C) with a 12-h light-dark cycle. TRPV1−/− rats were developed on a Sprague-Dawley background and have a 2-bp deletion in exon 13 using a Zinc Finger Nuclease. Animals exhibit reduced expression of TRPV1 protein in the brain homogenates and increased foot licking latency to thermal heat (see www.sageresearchlabs.com or www.horizondiscovery.com). Rats were fed standard chow (Harlan Teklad Global Diet 2018), given access to deionized water, and singly housed in metabolic cages for 1 wk before any procedures. There were no differences in 24-h water intakes between groups (control: 30.3 ± 2.3 ml vs. TRPV1−/−: 28.6 ± 1.5 ml; n = 8 per group).
General procedures.
Animals were anesthetized with isoflurane (2–3% in 100% O2) and instrumented with femoral arterial (microrenathane 0.012″ × 0.025″) and venous (Silastic 0.023″ × 0.037″) catheters fused to Tygon microbore tubing. Catheters were tunneled subcutaneously to exit between the scapulae and led through a tether-swivel harness system (Instech Laboratories). Animals were treated with buprenex (0.03 mg/kg sc), ampicillin (100 mg/kg sc), and carprofen (5 mg/kg sc) and allowed to recover for at least 3 days before experiments began. Arterial catheters were flushed daily with heparinized saline (500 U/ml). Venous catheters were flushed once every 3 days with heparinized saline (40 U/ml).
Confirmation of TRPV1 knockout.
TRPV1−/− knockouts were confirmed in two ways. First, ear biopsies were collected, digested, and analyzed through a standard PCR treated with Exo-SAP. The PCR product was isolated and subsequently sequenced by Sage Research Laboratories to confirm the 2-bp deletion from exon 13. Samples were collected at Penn State College of Medicine and sent to Sage Research Laboratories in a randomized, blinded design. Second, arterial blood pressure (ABP) and heart rate responses to the TRPV1 agonist capsaicin (0, 0.25, and 0.5 μg, intravenous bolus in 0.2 ml separated by 10 min) were measured in control and TRPV1−/− rats. Peak responses (1 s) were compared with a 2-min baseline period.
Plasma VP and thirst experiments.
Control and TRPV1−/− rats received two different treatments in a randomized order separated by a minimum of 3 days. Food and water were removed ∼1 h before experiments began. First, acute hypernatremia was produced by intravenous infusion of 2 M NaCl (1.0 ml/h). Second, hypotension was produced by intravenous injection of the arteriolar vasodilator minoxidil (5 mg/kg iv) and used as a nonosmotic stimulus for VP secretion. Blood samples (1.0 ml) were collected at baseline, 30 and 60 min from the arterial line into microcentrifuge tubes containing heparin (10 units), and then centrifuged (10,000 g). The plasma was stored at −80°C until VP levels were determined. Plasma osmolality was measured in triplicate by freezing-point depression (model 3320, Advanced Instruments). Plasma electrolytes were analyzed using whole blood using ISTAT and 6+ cartridges (Abbott). Each blood sample was replaced by an equal volume (1.0 ml) of isotonic saline (first sample) or red blood cells from the previous sample resuspended in heparinized saline (40 U/ml) at 37°C. After the 60-min blood sample, water bottles were returned to the cages. Cumulative water intakes and urine outputs were measured every 15 min for the next 60 min. ABP and heart rate were monitored throughout the experimental protocol using a BPM-832 ABP device (CWE) and Spike2 software (CED).
Analysis of plasma VP levels.
Plasma VP levels were determined by ELISA (Enzo Life Sciences). Briefly, samples were extracted using C18 Sep-Pak Cartridges (1 ml, 50 mg; Waters, Milford, MA) as described previously (21) through a 4% acetic acid wash and eluted with a 3:1 acetonitrile:4% acetic acid solution. The extract was frozen, dried using a Speed Vac (Savant Instruments), and then reconstituted in assay buffer (Enzo Life Sciences). Although 100-μl samples are used in the ELISA, 200-μl samples were extracted and reconstituted with 100 μl buffer to double the sample concentration and increase sensitivity of the assay. Each sample was assayed in duplicate. Values are expressed as picograms per milliliter of plasma. Recovery of VP was 87 ± 2% (n = 8) in preliminary trials using I125-labeled VP. Intra- and interassay coefficients of variance were 8% and 4%, respectively. Sensitivity was 1.42 pg/ml. A subset of baseline samples for control and TRPV1−/− rats (n = 4–5 per experiment) fell below the sensitivity value but displayed absorbance values above the 0 pg/ml standard. Values presented in the results represent extrapolated values based on the standard curve.
Statistical analysis.
Data are expressed as means ± SE. All variables were analyzed by a one- or two-way ANOVA with repeated measures (Systat 10.2, Systat Software). When significant F values were obtained, independent or pair t-tests with a layered Bonferroni correction were performed. Plasma VP concentrations were measured in duplicate, averaged, and log-transformed. Linear regression analysis was performed between plasma VP and osmolality (Systat 10.2, Systat Software). A P value <0.05 was considered significant in all tests.
RESULTS
Confirmation of TRPV1−/− rats.
Figure 1 illustrates the 2-bp deletion in Exon 13 of TRPV1−/− rats that was confirmed by DNA sequencing of ear biopsies. To confirm that this 2-bp deletion resulted in a disruption of TRPV1 channel function, ABP and heart rate responses to intravenous injection of capsaicin were tested in control and TRPV1−/− rats. Intravenous injection of capsaicin produced a dose-dependent decrease in mean ABP and heart rate of control rats (Fig. 1B). These responses were abolished in TRPV1−/− rats. There were no differences in baseline mean ABP (control: 113 ± 4 vs. TRPV1−/−: 106 ± 4 mmHg) and heart rate (control: 392 ± 9 vs. TRPV1−/−: 398 ± 19 beats/min).
Fig. 1.
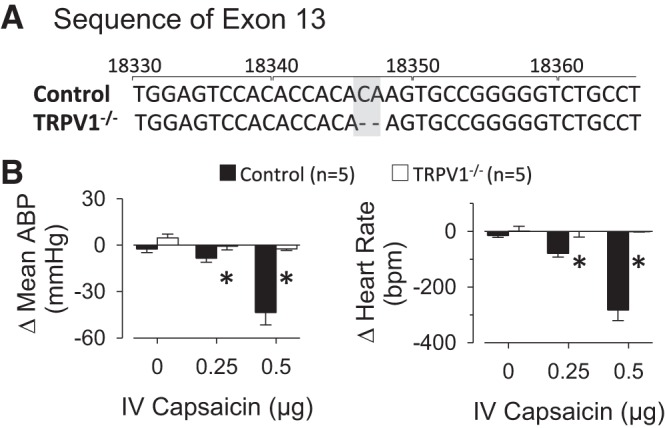
A: sequence analysis of control and transient receptor potential vanilloid type 1 (TRPV1)−/− rats. The numbers correspond to the sequence of the wild-type gene (NCBI NC_005109). Note the 2-bp deletion (shaded region) present in TRPV1−/− rats of exon 13. B: intravenous (IV) bolus administration of capsaicin produced a dose-dependent decrease in mean arterial blood pressure (ABP) and heart rate of control rats. The hypotension and bradycardia were absent in TRPV1−/− rats. *P < 0.01 vs. control rats.
Effect of acute hypernatremia on plasma VP levels in control and TRPV1−/− rats.
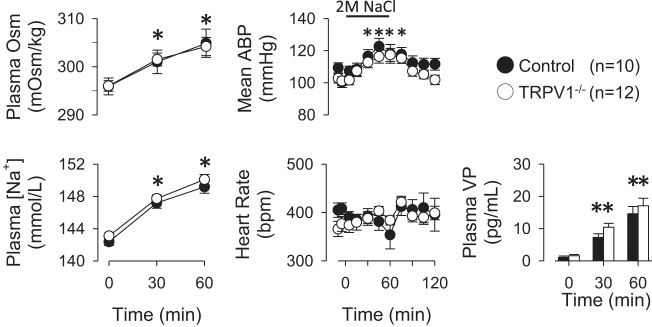
Intravenous infusion of hypertonic NaCl (2 M, 1 ml/h) significantly increased plasma osmolality and sodium concentration of both control and TRPV1−/− rats at 30 and 60 min after start of the infusion (Fig. 2). There were no significant differences between groups at any time. The infusion of 2 M NaCl also significantly increased mean ABP but did not affect heart rate of control and TRPV1−/− rats. Again, there were no differences between groups at any time. Plasma VP levels of control and TRPV1−/− rats increased significantly above baseline values at 30 and 60 min. However, there were no significant differences in plasma VP levels between control and TRPV1−/− rats at baseline or 30 and 60 min after start of the intravenous infusion of 2 M NaCl.
Fig. 2.
Left: acute infusion of 2 M NaCl (1 ml/h iv) produced a time-dependent increase in plasma osmolality and sodium concentration in both control and TRPV1−/− rats. *P < 0.05 vs. 0 min. Middle: infusion of 2 M NaCl produced a significant increase in mean ABP of both control and TRPV1−/− rats but did not statistically alter heart rate. Right: infusion of 2 M NaCl significantly increased plasma VP levels in control and TRPV1−/− rats. However, there were no differences between groups. **P < 0.05 vs. 0 min.
As expected, a linear regression analysis revealed a significant correlation between plasma VP or log plasma VP levels versus plasma osmolality of control rats (Fig. 3A). Interestingly, the same analysis revealed a significant correlation between these same variables of TRPV1−/− rats (Fig. 3B). In fact, this relationship was not blunted in TRPV1−/− rats as revealed by the slope of the linear regression line.
Fig. 3.
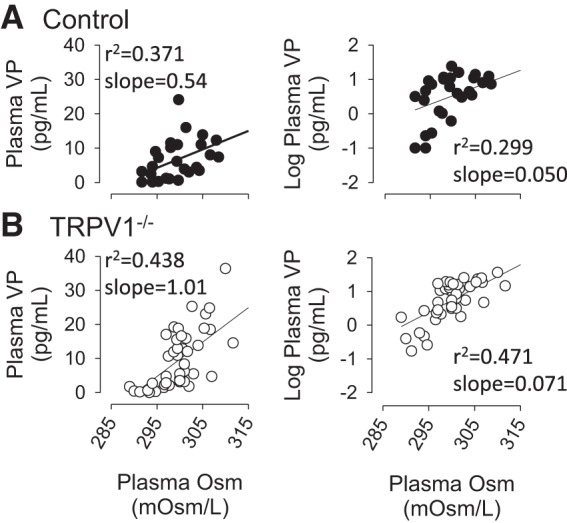
Linear regression analysis between plasma vasopressin (VP) or log plasma VP versus plasma osmolality of control (A) and TRPV1−/− (B) rats during infusion of 2 M NaCl. Correlational coefficients (r2) and slopes are reported for each plot. As expected, there was a significant correlation between plasma or log plasma VP concentrations versus plasma osmolality in control rats (P < 0.01). A significant correlation was found between plasma or log plasma VP levels versus plasma osmolality in TRPV1−/− rats (P < 0.01).
When water was returned to the cages after the 60-min blood sample, both control and TRPV1−/− rats ingested significant amounts of water (Fig. 4). However, the 60-min cumulative water intakes were not different between strains (P > 0.1). Urine volume was also not different between groups before or after water access (Fig. 4).
Fig. 4.
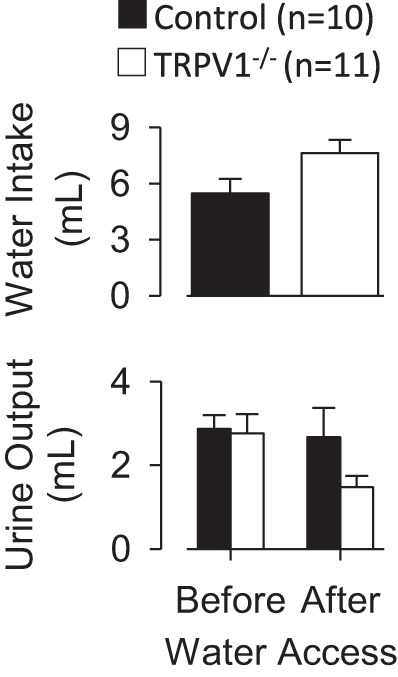
Sixty minutes of cumulative water intake (top) and (bottom) urine output of control and TRPV1−/− rats during intravenus infusion of 2 M NaCl (1 ml/h). There were no differences between groups in water intake (P > 0.1) or urine volume before (P > 0.8) or during access (P > 0.1) to water.
Effect of arterial hypotension on plasma VP levels in control and TRPV1−/− rats.
Both control and TRPV1−/− rats were also treated with the arteriolar vasodilator minoxidil to stimulate VP secretion and thirst independent of plasma osmolality or sodium concentrations. Intravenous injection of minoxidil significantly decreased mean ABP and increased heart rate in both control and TRPV1−/− rats (Fig. 5). There were no differences in mean ABP or heart rate between groups at any time. Plasma VP levels significantly increased from baseline values in both control and TRPV1−/− rats. However, there were no differences between groups at 0, 30, or 60 min (Fig. 5). When water bottles were returned to the cages after the 60-min blood sample, control and TRPV1−/− rats ingested significant amounts of water. The amount of ingested water did not differ between groups. Urine volume did not differ between groups before (control: 0.27 ± 0.15 vs. TRPV1−/−: 0.1 ± 0.1 ml) or during access to water (control: 0.1 ± 0.1 vs. TRPV1−/−: 0.1 ± 0.1 ml). Minoxidil produced a small but significant increase in plasma osmolality of control and TRPV1−/− rats (Table 1). However, there were no differences between groups. Plasma sodium concentrations did not change at any time.
Fig. 5.
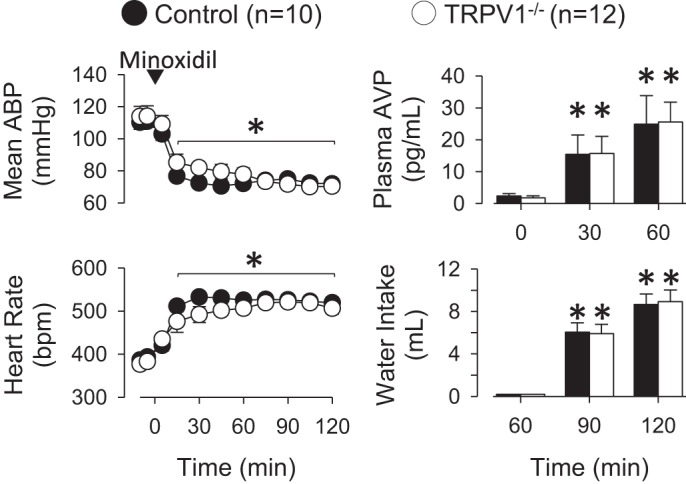
Left: mean ABP and heart rate of control and TRPV1−/− rats at baseline and after intravenous injection of minoxidil (5 mg/kg). Minoxidil significantly decreased mean ABP and increased heart rate in both groups. The magnitudes of these changes were not different between control and TRPV1−/− rats. Right: minoxidil increased plasma VP and water intake in control and TRPV1−/− rats. There were no significant differences between groups. *P < 0.05 vs. 0 min.
Table 1.
Plasma osmolality and sodium concentrations of control and TRPV1−/− rats treated with minoxidil
| Time Relative to Minoxidil Injection |
|||
|---|---|---|---|
| 0 min | 30 min | 60 min | |
| Control (n = 10) | |||
| Plasma osmolality, momol/kg | 297 ± 2 | 303 ± 1 | 300 ± 1 |
| Plasma sodium, mM | 143 ± 1 | 144 ± 1 | 144 ± 1 |
| TRPV1−/− (n = 11) | |||
| Plasma osmolality, momol/kg | 297 ± 2 | 303 ± 1* | 300 ± 1* |
| Plasma sodium, mM | 143 ± 1 | 142 ± 1 | 142 ± 1 |
Values are means ± SE (n equals number of rats) plasma osmolality and sodium concentrations of control and TRPV1−/− rats treated with minoxidil (5 mg/kg iv.
P < 0.05 vs. 0 min
DISCUSSION
In vitro electrophysiological studies suggest a product of the TRPV1 gene contributes to osmosensory transduction in hypothalamic neurons to regulate VP secretion (5, 6, 22). The current study tested this notion in vivo by analysis of plasma VP levels during an acute NaCl load using unique TRPV1−/− rats. The present findings provide several novel observations: 1) a 2-bp deletion in exon 13 of the TRPV1 gene abolished the hypotensive and bradycardic responses to intravenous injection of the TRPV1 agonist capsaicin; 2) an acute NaCl load produced similar increases in plasma osmolality, electrolytes, and VP levels between control versus TRPV1−/− rats; 3) acute hypernatremia stimulated similar increases in water intake between strains; and 4) hypotension stimulated similar increases in plasma VP and thirst between control versus TRPV1−/− rats. Collectively, these observations suggest that TRPV1 channels are not necessary for VP secretion and thirst stimulated by hypernatremia in rats.
Previous in vitro electrophysiological studies suggest that TRPV1 channels contribute to the intrinsic osmosensitivity of both OVLT and VPergic SON neurons (5, 6, 22). Therefore, a plausible hypothesis is that TRPV1 channel dysfunction or deletion should disrupt osmotically induced VP secretion and thirst. This hypothesis was tested in unique TRPV1−/− rats created by a 2-bp deletion in exon 13, which abolished capsaicin-evoked cardiovascular responses. Although an acute NaCl load produced similar increases in plasma osmolality and Na+ concentrations, plasma VP levels were surprisingly unaffected in TRPV1−/− versus control rats. This conclusion was further validated by a linear regression analysis of plasma VP (or log plasma VP) versus plasma osmolality. It is noteworthy that a previous study reported plasma VP levels of TRPV1−/− versus wild-type mice were attenuated in response to 0–48 h ingestion of 2% NaCl solution (22). Although 2% NaCl solution will provide a mixed stimulus for VP secretion including both plasma hypernatremia and volume depletion, the reasons for the apparent discrepancies are unclear aside from methodological differences. First, these studies used two different species (rats versus mice) with different targeting for the TRPV1−/− channel (2-bp deletion in exon 13 versus pore-loop domain). Second, plasma VP samples of mice were collected using halothane anesthesia (22). The present study has several advantages including: 1) plasma hypernatremia was produced by an acute NaCl infusion, 2) blood sampling was performed within the same animal, and 3) VP samples were collected in conscious, unstressed animals. Altogether, the findings indicate that disruption of the TRPV1 channel does not attenuate VP secretion stimulated by hypernatremia.
There are several potential explanations for the discordant observations between the in vitro electrophysiological studies of OVLT and SON neurons (5, 6, 22) versus the current findings. First, the in vitro electrophysiological studies have exclusively employed hypertonic mannitol solutions to investigate mechanisms underlying the intrinsic osmosensitivity of OVLT and SON neurons (5, 6, 22). Although VP secretion can be stimulated by both hypernatremia and hyperosmolality, the cellular mechanisms underlying Na+- versus osmosensing in magnocellular neurons may differ (32). This raises the possibility that TRPV1 channel may contribute to VP and thirst stimulated by hyperosmolality but not hypernatremia. However, we have previously reported that injection of hypertonic mannitol stimulates the ingestion of water in both control and TRPV1−/− mice (10). Second, other cellular mechanisms may compensate in the TRPV1−/− rat including locally release of taurine from glial cells (9), actin filaments (35), or the NaX channel (23). Clearly, future research is needed to identify cellular mechanisms regulating osmoreceptor function during both hyperosmolality and hypernatremia.
Given the potential role of TRPV1 channels within osmosensitive sites, TRPV1−/− animals may display deficits in osmotically induced thirst. Previous studies have reported thirst responses to acute NaCl loads are either normal (10, 28) or slightly attenuated (5) in TRPV1−/− versus wild-type mice. In the present study, TRPV1−/− versus control rats ingested similar amounts of water after an acute NaCl load. Moreover, if TRPV1 channels were necessary for osmoreceptor function, TRPV1−/− animals may display deficits in body fluid homeostasis during normal conditions. Previous studies have reported either a small elevation (22) or no difference (10, 28) in plasma osmolality and electrolytes of TRPV1−/− versus wild-type mice. In the present study, plasma osmolality and Na+ concentrations at baseline conditions did not differ between TRPV1−/− and control rats. Furthermore, there were no differences in baseline plasma VP levels or 24-h water intakes. Altogether, these findings suggest TRPV1−/− animals do not display profound deficits in body fluid homeostasis.
Hypotension was used as a nonosmotic experimental treatment to stimulate VP secretion and thirst. Injection of minoxidil significantly increased VP levels and stimulated the ingestion of water in both control and TRPV1−/− rats. Under this paradigm, VP secretion is largely mediated by baroreceptor unloading (27), whereas the stimulated water intake is mediated by activation of the renin-angiotensin system and the central dipsogenic action of angiotensin II (20, 26, 27). There was no a priori reason to hypothesize these responses would be affected in TRPV1−/− rats. Instead, the paradigm was chosen as a control experiment to stimulate VP secretion and thirst independent of osmoreceptor function. Neither response was affected by deletion of TRPV1 in rats.
Experimental limitations.
The current study employed a novel KO rat in which a 2-bp deletion in exon 13 was introduced to disrupt TRPV1 channel function. These animals display expected deficits such as thermal pain insensitivity (see www.horizondiscovery.com), altered brain activation to paw injection capsaicin (33), and an absence of cardiovascular responses to intraenous injection of capsaicin (Fig. 1). Despite these deficits, the current experiments yielded negative findings in regard to hypernatremia-induced VP secretion. Since the 2-bp deletion was present at development, it is possible that compensatory mechanisms such as expression of other channels preserve responses to body fluid homeostatic challenges. Furthermore, a recent report (34) provides strong in vitro evidence that a NH2-terminal variant of the TRPV1 channel underlies the electrophysiological responses of hypothalamic neurons to hypertonic mannitol. This TRPV1 variant lacks exons 1–4, is capsaicin insensitive, but does contain exon 13. In the present study, it is not clear whether the 2-bp deletion of exon 13 introduces a mutant mRNA or protein. Unfortunately, we are unaware of a pharmacological tool or agonist independent of mannitol to test whether the 2-bp deletion of exon 13 results in a nonfunctional protein. Thus the experiments employed capsaicin to examine TRPV1 function in general. Although the results confirm functional disruption of the TRPV1 channels, it remains possible that this 2-bp deletion did not disrupt the function of the NH2-terminal variant implicated in osmosensory transduction. Clearly, additional research is needed to identify the molecular identity of the putative TRPV1 NH2-terminal variant as well as other potential targets that participate in hypernatremia-induced responses.
Perspectives and Significance
Osmotically induced vasopressin secretion depends on the integrity of structures in the lamina terminalis (3, 14). In turn, these regions densely innervate magnocellular neurons of the SON and PVH to subsequently regulate VP secretion through glutamatergic inputs (3, 14). Although previous studies indicate that TRPV1 channels underlie osmosensory transduction in multiple neuronal populations within this circuit, the present in vivo findings indicate that hypernatremia-induced VP secretion is unaffected by disruption of TRPV1. These findings agree with recent observations suggesting that TRPV1 deletion does not affect hypernatremia-induced thirst. These studies may highlight potential differences between osmosensory transduction versus Na+-sensing.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-113270 (to S. D. Stocker) and an American Heart Association Established Investigator Grant (to S. D. Stocker). A. B. Tucker was supported by an American Heart Association Summer Undergraduate Research Fellowship (14UFEL3900000).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.B.T. and S.D.S. conception and design of research; A.B.T. and S.D.S. performed experiments; A.B.T. and S.D.S. analyzed data; A.B.T. and S.D.S. interpreted results of experiments; A.B.T. and S.D.S. edited and revised manuscript; A.B.T. and S.D.S. approved final version of manuscript; S.D.S. prepared figures; S.D.S. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Christopher Yengo for discussions regarding the sequence analysis.
REFERENCES
- 1.Anderson JW, Smith PM, Ferguson AV. Subfornical organ neurons projecting to paraventricular nucleus: whole-cell properties. Brain Res 921: 78–85, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JW, Washburn DL, Ferguson AV. Intrinsic osmosensitivity of subfornical organ neurons. Neuroscience 100: 539–547, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci 9: 519–531, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Buggy J, Jonhson AK. Preoptic-hypothalamic periventricular lesions: thirst deficits and hypernatremia. Am J Physiol Regul Integr Comp Physiol 233: R44–R52, 1977. [DOI] [PubMed] [Google Scholar]
- 5.Ciura S, Bourque CW. Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J Neurosci 26: 9069–9075, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciura S, Liedtke W, Bourque CW. Hypertonicity sensing in organum vasculosum lamina terminalis neurons: a mechanical process involving TRPV1 but not TRPV4. J Neurosci 31: 14669–14676, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutman MB, Ciriello J, Mogenson GJ. Effects of plasma angiotensin II and hypernatremia on subfornical organ neurons. Am J Physiol Regul Integr Comp Physiol 254: R746–R754, 1988. [DOI] [PubMed] [Google Scholar]
- 8.Honda K, Negoro H, Dyball RE, Higuchi T, Takano S. The osmoreceptor complex in the rat: evidence for interactions between the supraoptic and other diencephalic nuclei. J Physiol 431: 225–241, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussy N, Deleuze C, Desarménien MG, Moos FC. Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Prog Neurobiol 62: 113–134, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Kinsman B, Cowles J, Lay J, Simmonds SS, Browning KN, Stocker SD. Osmoregulatory thirst in mice lacking the transient receptor potential vanilloid type 1 (TRPV1) and/or type 4 (TRPV4) receptor. Am J Physiol Regul Integr Comp Physiol 307: R1092–R1100, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsen PJ, Mikkelsen JD. Functional identification of central afferent projections conveying information of acute “stress” to the hypothalamic paraventricular nucleus. J Neurosci 15: 2609–2627, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason WT. Supraoptic neurones of rat hypothalamus are osmosensitive. Nature 287: 154–157, 1980. [DOI] [PubMed] [Google Scholar]
- 13.McKinley MJ, Congiu M, Denton DA, Park RG, Penschow J, Simpson JB, Tarjan E, Weisinger RS, Wright RD. The anterior wall of the third cerebral ventricle and homeostatic responses to dehydration. J Physiol (Paris) 79: 421–427, 1984. [PubMed] [Google Scholar]
- 14.McKinley MJ, Mathai ML, McAllen RM, McClear RC, Miselis RR, Pennington GL, Vivas L, Wade JD, Oldfield BJ. Vasopressin secretion: osmotic and hormonal regulation by the lamina terminalis. J Neuroendocrinol 16: 340–347, 2004. [DOI] [PubMed] [Google Scholar]
- 15.McKinley MJ, Mathai ML, Pennington G, Rundgren M, Vivas L. Effect of individual or combined ablation of the nuclear groups of the lamina terminalis on water drinking in sheep. Am J Physiol Regul Integr Comp Physiol 276: R673–R683, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Nissen R, Bourque CW, Renaud LP. Membrane properties of organum vasculosum lamina terminalis neurons recorded in vitro. Am J Physiol Regul Integr Comp Physiol 264: R811–R815, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Oldfield BJ, Badoer E, Hards DK, McKinley MJ. Fos production in retrogradely labelled neurons of the lamina terminalis following intravenous infusion of either hypertonic saline or angiotensin II. Neuroscience 60: 255–262, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Oliet SH, Bourque CW. Gadolinium uncouples mechanical detection and osmoreceptor potential in supraoptic neurons. Neuron 16: 175–181, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Oliet SH, Bourque CW. Mechanosensitive channels transduce osmosensitivity in supraoptic neurons. Nature 364: 341–343, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Robinson MM, Evered MD. Pressor action of intravenous angiotensin II reduces drinking response in rats. Am J Physiol Regul Integr Comp Physiol 252: R754–R759, 1987. [DOI] [PubMed] [Google Scholar]
- 21.Schiltz JC, Hoffman GE, Stricker EM, Sved AF. Decreases in arterial pressure activate oxytocin neurons in conscious rats. Am J Physiol Regul Integr Comp Physiol 273: R1474–R1483, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Sharif Naeini R, Witty MF, Séguéla P, Bourque CW. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci 9: 93–98, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu H, Watanabe E, Hiyama TY, Nagakura A, Fujikawa A, Okado H, Yanagawa Y, Obata K, Noda M. Glial Nax channels control lactate signaling to neurons for brain [Na+] sensing. Neuron 54: 59–72, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Sladek CD, Johnson AK. Effect of anteroventral third ventricle lesions on vasopressin release by organ-cultured hypothalamo-neurohypophyseal explants. Neuroendocrinology 37: 78–84, 1983. [DOI] [PubMed] [Google Scholar]
- 25.Stocker SD, Monahan KD, Browning KN. Neurogenic and sympathoexcitatory actions of NaCl in hypertension. Curr Hypertens Rep 15: 538–546, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stocker SD, Smith CA, Kimbrough CM, Stricker EM, Sved AF. Elevated dietary salt suppresses renin secretion but not thirst evoked by arterial hypotension in rats. Am J Physiol Regul Integr Comp Physiol 284: R1521–R1528, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Stricker EM, Sved AF. Controls of vasopressin secretion and thirst: similarities and dissimilarities in signals. Physiol Behav 77: 731–736, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Taylor AC, McCarthy JJ, Stocker SD. Mice lacking the transient receptor vanilloid potential 1 channel display normal thirst responses and central Fos activation to hypernatremia. Am J Physiol Regul Integr Comp Physiol 294: R1285–R1293, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Thrasher TN, Keil LC. Regulation of drinking and vasopressin secretion: role of organum vasculosum laminae terminalis. Am J Physiol Regul Integr Comp Physiol 253: R108–R120, 1987. [DOI] [PubMed] [Google Scholar]
- 30.Thrasher TN, Keil LC, Ramsay DJ. Lesions of the organum vasculosum of the lamina terminalis (OVLT) attenuate osmotically-induced drinking and vasopressin secretion in the dog. Endocrinology 110: 1837–1839, 1982. [DOI] [PubMed] [Google Scholar]
- 31.Toney GM, Stocker SD. Hyperosmotic activation of CNS sympathetic drive: implications for cardiovascular disease. J Physiol 588: 3375–3384, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voisin DL, Chakfe Y, Bourque CW. Coincident detection of CSF Na+ and osmotic pressure in osmoregulatory neurons of the supraoptic nucleus. Neuron 24: 453–460, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Yee JR, Kenkel W, Caccaviello JC, Gamber K, Simmons P, Nedelman M, Kulkarni P, Ferris CF. Identifying the integrated neural networks involved in capsaicin-induced pain using fMRI in awake TRPV1 knockout and wild-type rats. Front Syst Neurosci 9: 1–13, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaelzer C, Hua P, Prager-Khoutorsky M, Ciura S, Voisin DL, Liedtke W, Bourque CW. ΔN-TRPV1: a molecular co-detector of body temperature and osmotic stress. Cell Reports 13: 23–30, 2015. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, Kindrat AN, Sharif-Naeini R, Bourque CW. Actin filaments mediate mechanical gating during osmosensory transduction in rat supraoptic nucleus neurons. J Neurosci 27: 4008–4013, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]