Abstract
The overload-induced increase in muscle mass is accompanied by protein accretion; however, the initiating events are poorly understood. Regulated in Development and DNA Damage 1 (REDD1), a repressor of the mechanistic target of rapamycin in complex 1 (mTORC1), blunts the elevation in protein synthesis induced by acute muscle contractions. Therefore, this study was designed to determine whether REDD1 alters the rate of the overload-induced increase in muscle mass. Wild-type (WT) and REDD1-null mice underwent unilateral functional overload (OV) of the plantaris, while the contralateral sham leg served as a control. After 3 and 5 days of OV, puromycin incorporation was used as a measurement of protein synthesis. The percent increase in plantaris wet weight and protein content was greater in REDD1-null mice after 3, 5, and 10 days OV. The overload-stimulated rate of protein synthesis in the plantaris was similar between genotypes after 3 days OV, but translational capacity was lower in REDD1-null mice, indicating elevated translational efficiency. This was likely due to elevated absolute mTORC1 signaling [phosphorylation of p70S6K1 (Thr-389) and 4E-BP1 (Ser-65)]. By 5 days of OV, the rate of protein synthesis in REDD1-null mice was lower than WT mice with no difference in absolute mTORC1 signaling. Additionally, markers of autophagy (LC3II/I ratio and p62 protein) were decreased to a greater absolute extent after 3 days OV in REDD1-null mice. These data suggest that loss of REDD1 augments the rate of the OV-induced increase in muscle mass by altering multiple protein balance pathways.
Keywords: resistance exercise, autophagy, ribosome biogenesis, protein synthesis
the increase in muscle mass following long-term overload is due, at least in part, to accretion of muscle protein. This can be achieved by enhancing the rate of protein synthesis relative to protein breakdown, decreasing the rate of protein breakdown relative to protein synthesis, or a combination of the two (30). It is well accepted that an increase in the rate of protein synthesis contributes considerably to muscle protein accretion and the subsequent increase in muscle mass (2, 12), but there is also evidence that reduced protein breakdown via autophagy (lysosome-mediated protein breakdown) may also contribute (11). Thus, a better understanding of the factors that alter protein balance following muscle overload is merited, as overload fails to increase muscle mass to the same magnitude in populations such as the aged.
Enhanced rates of protein synthesis and muscle mass following overload requires signaling through the mechanistic target of rapamycin in complex 1 (mTORC1) pathway. Indeed, pharmacological inhibition of this pathway using rapamycin negates both the acute enhancement in the rate of protein synthesis, as well as the long-term increase in muscle mass induced by overload (2, 8, 12). Activated mTORC1 signaling enhances the rate of protein synthesis through phosphorylation of at least two known downstream targets, the 70-kDa ribosomal protein S6 kinase 1 (p70S6K1) and the eIF4E binding protein 1 (4E-BP1) (15). Phosphorylation of these targets increases mRNA translation initiation and elongation, as well as stimulation of ribosome biogenesis, resulting in a subsequent increase in translational capacity (15, 28). Additionally, activation of mTORC1 affects protein balance through inhibition of protein degradation by phosphorylating and inhibiting proteins required for initiation of autophagy, such as uncoordinated-51 like autophagy activating kinase 1 (ULK1), as well as other substrates (10, 37). Indeed, Fry et al. (11) showed that the activation state of autophagy, as assessed by the ratio of LC3 II to LC3 I, was reduced within 3 h following a bout of resistance exercise in humans, and the reduction persisted for up to 24 h postexercise. Thus, muscle overload impacts protein balance by enhancing protein synthesis and likely through suppressing autophagy.
Regulated in Development and DNA Damage 1 (REDD1; aka DDIT4, RTP801, dig2) is a known repressor of the mTORC1 signaling pathway (5, 6, 16). We recently reported that the maximal activation state of mTORC1 and the maximal rate of protein synthesis were lower following a single bout of muscle contractions in wild-type (WT) mice relative to mice in which the REDD1 gene was disrupted (16). Additionally, it has been shown that REDD1 expression is required for full activation of autophagy (31). Overall, the available data would suggest that REDD1 expression limits the rate of the overload-induced increase in muscle mass and protein accretion by altering protein synthesis, as well as autophagy. Therefore, the objective of the present study was to determine whether REDD1 affects the rate of the overload-induced increase in muscle mass and protein accretion following synergistic ablation-induced muscle overload in association with changes in protein synthesis and markers of autophagy.
METHODS
Animals.
All experiments were performed on male mice 5–7 mo of age. Mice with a genetic disruption in the REDD1 gene (referred to hereafter as REDD1-null mice) were generated by Lexicon Genetics (The Woodlands, TX), and permission to use them was generously granted by Dr. Elena Feinstein (Quark Pharmaceuticals). Breeding pairs on a B6/129F1 background were obtained from Dr. David Williamson (SUNY Buffalo) and bred in The Pennsylvania State University College of Medicine animal facility. Male wild-type (WT), age-matched B6/129F1 control mice were obtained from Taconic (Hudson, NY). All mice were housed in a temperature- (25°C) and light (12:12-h light-dark)-controlled environment on corn cob bedding within the vivarium at the Pennsylvania State University College of Medicine. Mice were provided rodent chow (Harlan-Teklad 2018, Indianapolis, IN) and water ad libitum. The Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine approved the animal facilities and all experimental protocols, and all animal work was performed within this facility. Details pertaining to the phenotype of REDD1-null mice have been described elsewhere (4).
Experimental design and functional overload.
All mice were housed 2–5 per cage until the day of the experiment. Unilateral functional overload of the plantaris was induced by partial removal of the synergist muscles (soleus and gastrocnemius) taking care not to affect blood flow or innervation of the plantaris. Under deep isoflurane anesthesia (∼3%) and using sterile surgical technique, the rear hind limbs were shaved, cleaned, and then disinfected with iodine solution. A small incision was made through the skin on both legs to expose the distal tendons of the triceps surae muscle complex. On the left leg, the tendons of the gastrocnemius and soleus were separated from the plantaris tendon, and the distal ∼1/3 of the gastrocnemius and soleus were removed leaving the plantaris intact. The contralateral leg underwent the same procedure, except that the gastrocnemius and soleus were left intact. The incision site was closed using 7-0 silk suture. All mice were given a subcutaneous injection of buprenorphine at a dose of 0.05 mg/kg in 1 ml of sterile saline immediately following surgery and again 5 h into recovery. No adverse effects were observed with this volume of subcutaneous injection. All mice were singly housed during the recovery for the remainder of the experimental period (1, 3, 5, or 10 days).
Protein synthesis.
Protein synthesis was assessed using the SUnSET method (13) with antibodies against puromycin (Kerafast, Boston, MA). Following a 17-h overnight fast, mice were administered an intraperitoneal injection of 0.04 μmol/g body wt of puromycin (AG Scientific; San Diego, CA) dissolved in PBS 30 min prior to the removal of the plantaris muscles. Western blot analysis procedures were performed to visualize puromycin incorporation into the protein of the plantaris, and measurements were expressed relative to total protein loaded from the Ponceau-S-stained membrane, as previously described (32). The anti-mouse secondary antibody used in this study reacts nonspecifically with proteins in mouse muscle with molecular weights of ∼25 kDa and ∼50 kDa. Consequently, quantification of the puromycin Western blots excluded these bands.
Western blot analysis.
Whole muscle protein was extracted by glass on glass homogenization in buffer consisting of 50 mM HEPES (pH 7.4), 0.1% Triton-X 100, 4 mM EGTA, 10 mM EDTA, 15 mM Na4P2O7, 100 mM β-glycerophosphate, 25 mM NaF, 5 mM Na3VO4, and 10 μl/ml protease inhibitor cocktail (Sigma Aldrich no. P8340; St. Louis, MO). The muscle extract was centrifuged at 10,000 g for 10 min, and the protein content of the supernatant fraction was quantified using the Bradford method (3). For analysis, proteins in the supernatant were fractionated on Bio-Rad (Hercules, CA) Criterion precast gels and were transferred to PVDF membranes (Pall Lifesciences, Port Washington, NY), as previously described (14). Membranes were stained with Ponceau-S to confirm effective transfer and equal protein loading. Membranes were incubated with appropriate antibodies overnight at 4°C. Antibodies against phospho-p70S6K1 (Thr-389) (cat. no. 9205), phospho-4E-BP1 (Ser-65) (cat. no.9451), LC3B (cat. no. 2775), p62 (cat. no. 5114), phospho-ULK1 (Ser-757) (cat. no. 6888), and total ULK1 (cat. no. 8054) were obtained from Cell Signaling Technology (Danvers, MA). Antibodies against total p70S6K1 and total 4E-BP1 were custom made by Bethyl Laboratories (Montgomery, TX). Antibodies against REDD1 (cat. no. 10638-1-AP) were obtained from ProteinTech (Chicago, IL). Monoclonal antibodies against puromycin were produced in-house and are available through Kerafast (Boston, MA). Antibodies against ubiquitin were a kind gift from Dr. Vincent Chau (Pennsylvania State College of Medicine). Following incubation with appropriate secondary antibodies (Bethyl Laboratories, Montgomery, TX) (cat. no. A120-101P, A90-116P), the antigen-antibody complex was visualized by enhanced chemiluminescence using Clarity Reagent (Bio-Rad) and a ProteinSimple Fluorchem M imaging system (Santa Clara, CA). All blots were quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
RNA extraction, cDNA synthesis, and qRT-PCR.
All procedures were performed as previously described (34). Briefly, RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA). RNA quantity was determined spectrophotometrically by 260-to-280 nm ratio. cDNA was synthesized using a Superscript VILO cDNA synthesis kit (Invitrogen) from 2 μg of RNA. Quantitative RT-PCR was performed using GoTaq qPCR Master Mix (Promega, Fitchburg, WI) or PowerUp SYBR Green Master Mix (Thermo Fisher Scientific, Waltham, MA). Each sample was analyzed in triplicate for each transcript. A melting curve analysis was performed for each primer pair to ensure that a single product was efficiently amplified. Relative expression levels of the 45S pre-rRNA externally transcribed spacer (ETS) and p62 were normalized by using the ΔΔ Ct method with GAPDH as a housekeeping gene. The two sets of primer sequences used for GAPDH were the folowing: forward 5′-CCA AGT GTT CAT GCC ACG TG-3′ and reverse 5′-CGA GCG ACT GCC ACA AAA A-3′. Primer sequences for GAPDH were as follows: forward 5′-ATG TTC CAG TAT GAC TCC ACT CAC G 3′ and reverse 5′-GAA GAC ACC AGT AGA CTC CAC GAC A-3′ and forward 5′-GTT GTC TCC TGC GAC TTC A-3′ and reverse 5′-TGC TGT AGC CGT ATT CAT TG-3′. Primer sequences for p62 were as follows: forward 5′-TGT GGT GGG AAC TCG CTA TA-3′ and reverse 5′-GAG AAG CTA TCA GAG AGG TGG CC-3′.
Statistical analysis.
All data are presented as means ± SE. Percent change comparisons between wild-type mice and REDD1-null mice within a time point were performed using Student's t-test. Where appropriate, two-way ANOVA was used to determine the effect of genotype and overload on the dependent variables. An unpaired Student's t-test was used post hoc when significance was observed, as previously described (16, 17). Analysis of select relationships was performed using the Pearson product moment correlation. All analyses were performed using GraphPad Prism software (La Jolla, CA). Statistical significance was set at P ≤ 0.05 for all analyses.
RESULTS
Loss of REDD1 augments the rate of the overload-induced increase in muscle mass.
To determine whether REDD1 alters the rate of the overload-induced increase in muscle mass, unilateral functional overload of the plantaris was performed, and muscle samples were collected 1, 3, 5, and 10 days following surgery. Muscle wet weight was enhanced after 1 day of overload in WT mice (P < 0.05), but this effect was not detectable after 3 days (Table 1). A significant increase in muscle wet weight and percent change in muscle wet weight (overloaded muscle vs. contralateral leg) was observed after 3 days of overload only in REDD1-null mice (P < 0.05) (Table 1 and Fig. 1A). Importantly, this was not due to an already elevated increase in sham muscle mass in REDD1-null mice, as the mean wet weight was lower than that in the WT mice (Table 1). Although both WT and REDD1-null mice showed an increase in muscle wet weight after 5 and 10 days of overload, the percent change remained significantly greater (P < 0.05) in REDD1-null mice compared with that observed in WT mice at these time points (Table 1 and Fig. 1A). Further examination of the early time points (i.e., 3 and 5 days) showed that the percent change in muscle protein with overload was greater in REDD1-null mice relative to WT mice (P < 0.05) (Fig. 1B), suggesting that the augmented rate of the overload-induced increase in muscle mass of REDD1-null mice was at least partly due to protein accretion. In support of this idea, strong, positive correlations were observed between the percent change in muscle wet weight and the percent change in total protein in the plantaris of REDD1-null mice after both 3 and 5 days of overload (P = 0.006 and P = 0.001, respectively) (Table 2). However, others have shown that factors other than protein accretion may contribute to the increase in muscle mass during these early time points. For example, transient edema is often observed after overload surgery (7). Indeed, in the present study the increase in muscle wet weight observed in WT mice on day 1 after surgery was likely due to edema. However, there was no difference in wet weight between the overloaded and sham legs on day 3, suggesting that any edema present on day 1 had resolved by day 3. Also, while others (e.g., Ref. 1) have reported changes in protein content after 3 and 5 days that appear to conflict with the data herein, the mean muscle weight of the overloaded plantaris in WT mice after 5 and 10 days is strikingly similar to those values previously reported at these time points (∼28 and 35 mg, respectively), showing consistency with other studies (27). Overall, these data indicate that loss of REDD1 augments the rate of the overload-induced increase in muscle mass through increased accretion of muscle protein during the initial periods of overload.
Table 1.
Characteristics of REDD1+/+ and REDD1−/− mice
| BW, g (Initial) | BW, g (Sacrifice) | Tibia Length, mm | Sham Plantaris Wet Weight, mg | Overload Plantaris Wet Weight, mg | |
|---|---|---|---|---|---|
| REDD1+/+ (1-day overload) | 32.4 ± 1.4 | 30.7 ± 1.4* | — | 21.3 ± 0.6 | 25.0 ± 0.5^ |
| REDD1−/− (1-day overload) | 37.6 ± 1.9 | 34.8 ± 2.0* | — | 21.4 ± 0.9 | 23.9 ± 1.5 |
| REDD1+/+ (3-day overload) | 31.3 ± 0.5 | 27.8 ± 0.5* | 18.7 ± 0.1 | 20.8 ± 0.4 | 21.8 ± 1.3 |
| REDD1−/− (3-day overload) | 30.1 ± 0.5 | 26.3 ± 0.7* | 17.9 ± 0.1¥ | 17.2 ± 0.6 | 22.7 ± 0.7^§ |
| REDD1+/+ (5-day overload) | 31.7 ± 0.9 | 28.2 ± 0.7* | 18.5 ± 0.2 | 19.4 ± 0.5 | 25.8 ± 0.8^ |
| REDD1−/− (5-day overload) | 31.6 ± 0.7 | 28.1 ± 0.7* | 17.7 ± 0.1¥ | 17.8 ± 0.7 | 27.8 ± 2.7^ |
| REDD1+/+ (10-day overload) | 31.9 ± 0.8 | — | 18.7 ± 0.3 | 19.9 ± 0.5 | 34.0 ± 1.3^ |
| REDD1−/− (10-day overload) | 29.1 ± 0.8 | — | 17.7 ± 0.2¥ | 18.3 ± 1.7 | 44.7 ± 1.4^§ |
Data are expressed as means ± SE. BW, body weight. REDD1+/+, mice with REDD1 gene. REDD1−/−, mice with disrupted REDD1 gene.
Significantly different than Initial BW, P < 0.05.
Significantly different than REDD1+/+ within time point, P < 0.05.
Significantly different than Sham within genotype, P < 0.05.
Genotype × Overload interaction. n = 3–8 from one or two independent experiments.
Fig. 1.
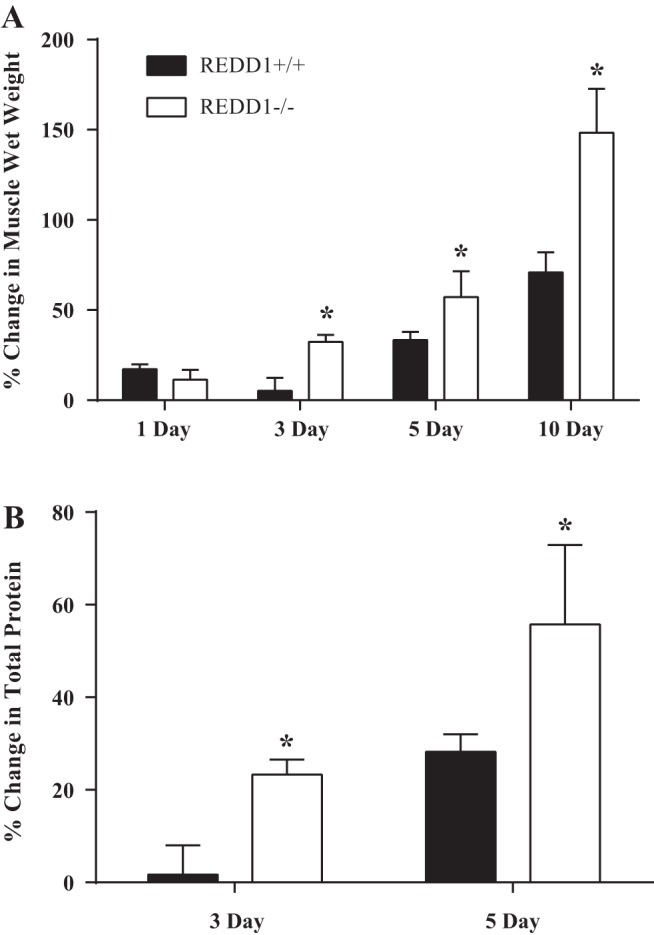
Loss of REDD1 augments the rate of the overload-induced increase in muscle mass and protein accretion following unilateral synergistic ablation-induced overload. A: percent change in muscle wet weight between the sham control muscle and overloaded muscle after 1, 3, 5, and 10 days. B: percent change in total protein between sham control muscle and the overloaded muscle after 3 and 5 days. *Significant difference between genotypes within the time point. n = 3–8 per group from 1–2 independent experiments. Significance was set at P ≤ 0.05.
Table 2.
Pearson product-moment correlation matrix for REDD1-null mice after 3 and 5 days of overload
| % Change in Total Protein | P Value | |
|---|---|---|
| % Change in Muscle Wet Weight (3-day overload) | r = 0.89 | P = 0.006 |
| % Change in Muscle Wet Weight (5-day overload) | r = 0.97 | P = 0.001 |
Augmented rate of increased muscle mass and protein accretion occurs independent of translational capacity.
Accretion of muscle protein can occur by a change in translational efficiency, i.e., the rate of protein synthesis per unit of RNA, translational capacity, i.e., the number of ribosomes available for protein synthesis, or both. The ribosome content of a cell has been proposed to impose an upper limit to the rate of protein synthesis (20). Ribosomes are composed of both ribosomal RNA and ribosomal proteins. Given that nearly 90% of total RNA consists of ribosomal RNA, changes in RNA content are used as an indication of a change in ribosome biogenesis (34). Indeed, it was recently shown that the magnitude of increased muscle mass following various intensities of muscle overload were strongly correlated with changes in RNA content (29). Given the importance of ribosome biogenesis for the increase in muscle mass, we determined whether an increase in translational capacity in the absence of REDD1 was an initiating event that led to the augmented rate of increased mass and protein accretion in REDD1-null mice observed by 3 days of overload. As shown in Fig. 2A, the magnitude of the increase in total RNA after 3 days of overload was the same in both WT and REDD1-null mice, such that there was no difference between genotypes after either 1 or 3 days of overload. Likewise, there was also no difference between genotypes for the overload-induced increase in the relative abundance of the ETS of the 45S pre-rRNA after either 1 or 3 days of overload (Fig. 2B), suggesting that ribosomal RNA transcription and ribosome biogenesis were similar for both genotypes. No difference between genotypes was observed in translational capacity after 1 day of overload (Fig. 2C). However, this measure was significantly lower in the overloaded plantaris of REDD1-null mice compared with WT mice after 3 days of overload (P < 0.05) (Fig. 2C). These data suggest that the augmented rate of the increase in muscle mass and protein accretion in REDD1-null mice occurred in a manner that was independent of translational capacity. Further, the reduced translational capacity in REDD1-null mice compared with WT mice was not due to impaired ribosome biogenesis, but rather an increase in muscle mass.
Fig. 2.
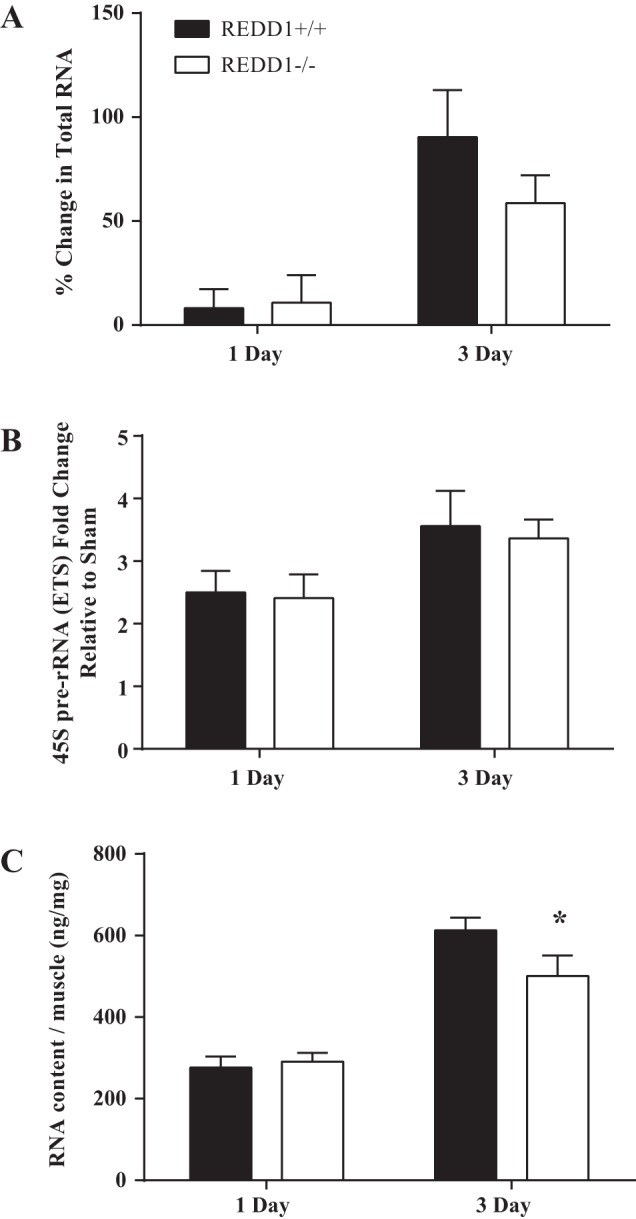
Loss of REDD1 alters translational capacity independent of a change in ribosome biogenesis. A: percent change in total RNA between the sham control muscle and the overloaded muscle after 1 and 3 days. B: relative abundance of the external transcribed spacer (ETS) of the 45S pre-rRNA was determined by quantitative RT-PCR in the overloaded muscle of both wild-type and REDD1-null mice after 1 and 3 days. C: translational capacity [RNA content per milligram of muscle (ng/mg)] of the overloaded muscle of wild-type and REDD1-null mice after 1 and 3 days of overload. *Significant difference between overloaded muscles of genotypes within a time point. n = 6 or 7 per group. Significance was set at P ≤ 0.05.
Overload-induced translation efficiency and mTORC1 signaling are altered in REDD1-null mice.
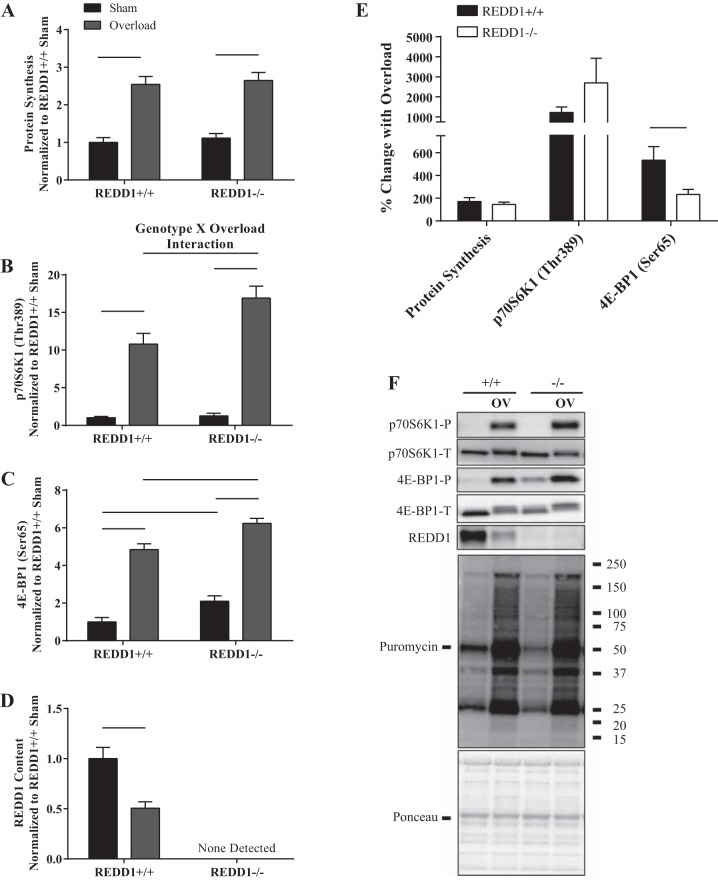
Since the augmented rate of overload-induced increase in muscle mass and protein accretion in REDD1-null mice occurred independent of translational capacity, we next examined changes in translational efficiency. As shown in Fig. 3, A and F, protein synthesis was increased in the overloaded leg compared with the sham leg after 3 days of overload (P < 0.05). However, there was no difference in the overload-induced percent change in the rate of synthesis or absolute value between genotypes (Fig. 3, A, E, and F). Given that the translational capacity in the overloaded plantaris of REDD1-null mice was lower at this time point (Fig. 2C), the observation that the rate of protein synthesis was maintained would suggest that translational efficiency was increased. Because of the small size of the plantaris (particularly in the sham leg), protein synthesis and RNA content could not be measured in the same animals. However, when the individual values obtained for protein synthesis in the overloaded muscle presented in Fig. 3A were normalized using the average total RNA value in the overloaded plantaris in Fig. 2C, translation efficiency was found to be 38% higher (P < 0.05) in REDD1-null mice compared with WT mice at this time point. This was likely due to an increase in the absolute overload-induced activation of mTORC1, as evidenced by increased phosphorylation of p70S6K1 (Thr-389) and 4E-BP1 (Ser-65) in the plantaris of REDD1-null mice relative to the values observed in WT mice after 3 days of overload (P < 0.05) (Fig. 3, B, C, and F). Moreover, an interaction between genotype and overload was observed for phosphorylation of p70S6K1 (Thr-389) (P < 0.05) (Fig. 3B). However, although the absolute values for p70S6K1 and 4E-BP1 phosphorylation were greater in REDD1-null compared with WT mice in response to overload, the percent change in the overload-induced increase in p70S6K1 (Thr-389) phosphorylation was not different between genotypes (Fig. 3E), and the overload-induced difference in 4E-BP1 (Ser-65) phosphorylation was less in REDD1-null compared with WT mice (Fig. 3E). The latter effect was most likely due to a higher baseline value (Fig. 3, B and C). Lastly, REDD1 expression in the overloaded plantaris of WT mice was significantly reduced to 50% (P < 0.05) of the value observed in the nonoverloaded muscle (Fig. 3D). Thus, although speculative, the loss of REDD1 enhanced translational efficiency, which was likely due to a greater absolute level of mTORC1 activation, resulting in an increase in mRNA translation initiation, elongation, or both.
Fig. 3.
Loss of REDD1 alters absolute mTORC1 signaling and translational efficiency following 3 days of overload. A: protein synthesis was determined by the SUnSET method. The phosphorylated to total protein ratio of p70S6K1 (Thr-389) (B) and 4E-BP1 (Ser-65) (C) were determined by Western blot analysis. D: REDD1 content was determined by Western blot analysis. E: percent change in indicated measures between the sham and overloaded muscle. F: representative Western blots. Lines connecting bars indicate significant differences. OV, overload; +/+, wild-type mice; −/−, REDD1-null mice; P, phosphorylated protein; T, total protein. n = 7/group from two independent experiments.
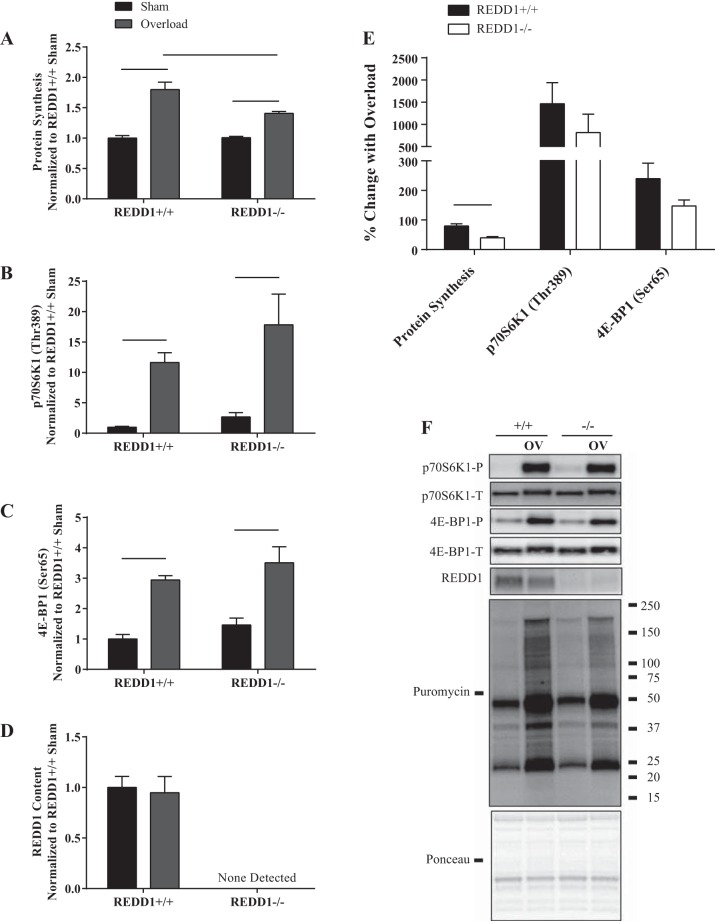
After 5 days, protein synthesis was still higher in the overloaded compared with the sham leg in both WT and REDD1-null mice (Fig. 4, A and F). However, the percent change in the overload-induced rate of protein synthesis was blunted by nearly 50% in REDD1-null mice compared with WT mice (Fig. 4E). Additionally, although phosphorylation of p70S6K1 (Thr-389) and 4E-BP1 (Ser-65) was elevated in response to overload in both WT and REDD1-null mice (Fig. 4, B, C, and F), the absolute value was no longer different between genotypes. These data suggest that a failure to sustain greater absolute overload-induced phosphorylation of mTORC1 substrates in REDD1-null mice in combination with reduced translational capacity leads to a reduction in the rate of protein synthesis. In all, increased translational efficiency may have contributed to the augmented rate of the overload-induced increase in muscle mass and protein accretion at early time points (<3 days of overload), but by itself, it would not explain the continued growth in REDD1-null mice.
Fig. 4.
Loss of REDD1 blunts the overload-induced rate of protein synthesis but does not alter mTORC1 signaling after 5 days of overload. A: protein synthesis was determined by the SUnSET method. The phosphorylated-to-total protein ratio of p70S6K1 (Thr-389) (B) and 4E-BP1 (Ser-65) (C) were determined by Western blot analysis. D: REDD1 content was determined by Western blot analysis. E: percent change in indicated measures between the sham and overloaded muscle. F: representative Western blots. Lines connecting bars indicate significant differences. OV, overload; +/+, wild-type mice; −/−, REDD1-null mice; P, phosphorylated protein; T, total protein. n = 6/8 per group from two independent experiments.
Loss of REDD1 augments the suppression of markers of autophagy following overload.
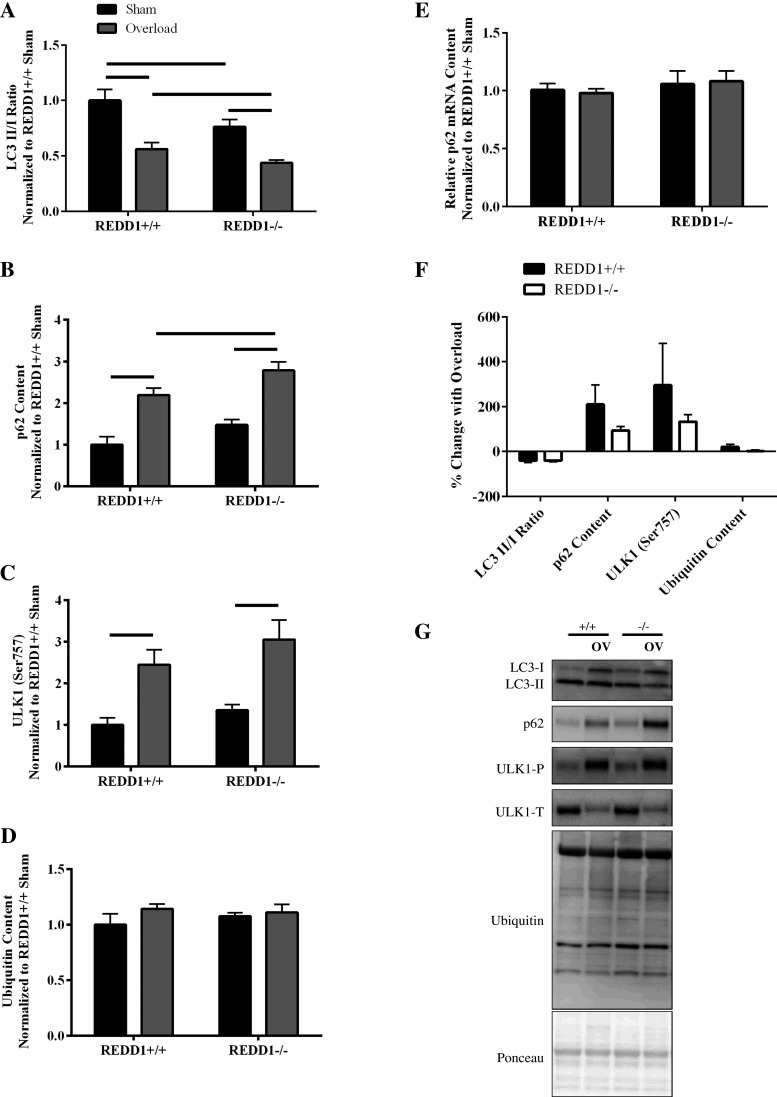
The data presented thus far indicate that the augmented rate of the overload-induced increase in muscle mass and protein accretion in REDD1-null mice compared with WT cannot be fully explained by a change in translational capacity or translational efficiency. Therefore, we investigated markers of other processes affected by muscle overload that alter protein balance. Others have shown that markers of autophagy activation are reduced following a single bout of resistance exercise in humans (11). For instance, a significant reduction in the ratio of LC3 II to LC3 I in human skeletal muscle was reported following a single bout of resistance exercise that persisted for up to 24 h (11). In addition, increased expression of p62 is another, and complementary marker used to assess repressed autophagy (25, 31). Given that REDD1 regulates autophagy (31) and that markers of autophagy are reduced following a bout of resistance exercise in humans, it is possible that loss of REDD1 would lead to a further suppression in markers of this degradative process. Consistent with this idea, although the percent reduction in the LC3 II to LC3 I ratio was not different between genotypes with overload (Fig. 5F), the absolute ratio was lower in the overloaded plantaris of REDD1-null mice (P < 0.05) relative to that of WT mice (Fig. 5, A and G). Similarly, the percent increase in p62 protein content with overload was not different between genotypes (Fig. 5F), whereas the absolute p62 protein content was greater in the overloaded plantaris of REDD1-null mice (P < 0.05) relative to that of WT mice (Fig. 5, B and G). Importantly, the changes in p62 protein content observed with both overload and genotype were not due to a corresponding alteration in the relative abundance of p62 mRNA (Fig. 5E). The observation that the LC3 II/I ratio was lower and p62 content higher in REDD1-null compared with WT mice is suggestive that autophagy is repressed to a greater absolute extent in mice lacking REDD1, although the magnitude of the repression with overload is unaffected by the protein. Additional evidence supporting a role of REDD1 in the regulation of autophagy is garnered from significant correlations observed between REDD1 expression and either the ratio of LC3 II to LC3 I (r = 0.87: P = 0.002) or p62 protein content (r = −0.52: P = 0.028) in both the sham and overloaded plantaris of WT mice. To determine the potential contribution of mTORC1 to the repression of autophagy, we analyzed the phosphorylation of ULK1 (Ser-757). Phosphorylation of ULK1 (Ser-757) was increased in response to overload in mice of both genotypes (both magnitude and the absolute value) (Fig. 5, C, F, and G), suggesting the possibility that REDD1 regulates autophagy independent of mTORC1 following overload or that it enables a more effective repression by mTORC1. Lastly, there was no genotype or overload effect observed for total protein ubiquitylation (Fig. 5, D, F, and G). These data suggest that a greater absolute suppression in protein degradation as assessed via markers of autophagy following overload in REDD1-null mice contributed to the augmented rate of the overload-induced increase in muscle mass and protein accretion.
Fig. 5.
Loss of REDD1 leads to a greater absolute suppression of autophagy markers following 3 days of overload. A: The LC3 II-to-LC3 I ratio. B: p62 protein content. C: ratio of phosphorylated ULK1 (Ser-757) to total ULK1. D: total ubiquitin content were determined by Western blot analysis. E: relative p62 mRNA content was determined by quantitative RT-PCR. F: percent change in indicated measures between the sham and overloaded muscle. G: representative Western blots. Lines connecting bars indicate significant difference. OV, overload; +/+, wild-type mice; −/−, REDD1-null mice; P, phosphorylated protein; T, total protein. n = 6/7 per group from two independent experiments.
DISCUSSION
Though numerous groups have shown a change in REDD1 mRNA and/or protein expression following a single bout of resistance exercise or induction of muscle contractions (9, 16, 18, 33), the role of the mTORC1 repressor in the anabolic response is just beginning to be realized through the use of mice with a disruption in the REDD1 gene. We previously reported that REDD1-null mice had increased absolute activation of mTORC1 signaling and absolute rates of protein synthesis relative to WT mice following a single bout of muscle contractions (16). Similarly, in the present study, translational efficiency was enhanced in REDD1-null mice compared with WT mice after 3 days of overload in conjunction with elevated mTORC1 activity. However, although translation efficiency was elevated in REDD1-null mice compared with WT mice, absolute rates of protein synthesis in the overloaded muscle were not different between genotypes at this time point, most likely because of a reduction in translational capacity in REDD1-null mice compared with WT mice. Thus, the increase in translational efficiency in muscle in the overloaded leg of REDD1-null mice was sufficient to maintain protein synthesis at values equivalent to those observed in WT mice, even though the capacity for mRNA translation was reduced in null animals. Although not directly tested, it is tempting to speculate that the increase in translational efficiency was a consequence of the greater absolute mTORC1 activation that occurs in REDD1-null mice compared with WT mice in response to overload. Although mTORC1 signaling and protein synthesis are concomitantly increased in response to both an acute bout of muscle contraction (16) and overload, the mechanisms involved may differ between the models as synergistic ablation results in a continuous, prolonged increase in the rate of synthesis, while the response to muscle contractions is transient. However, the contribution of translation efficiency to the sustained growth with longer periods of overload in REDD1-null mice remains in question, as the global rate of protein synthesis after 5 days of overload was lower in the overloaded muscle of REDD1-null mice compared with that observed in WT mice with no significant difference in the absolute value of mTORC1 signaling between genotypes.
Because changes in protein synthesis do not fully account for the augmented and sustained overload-induced rate of increased muscle mass and protein accretion in the absence of REDD1, other pathways affecting protein balance were likely altered. Indeed, others have reported that a single bout of resistance exercise in humans leads to repression of autophagy (11). Similar to a resistance-training bout in humans, the results of the present study suggest that autophagy is repressed in response to overload and that the magnitude of the repression is greater in mice lacking REDD1. Although speculative, this effect may contribute to the sustained increase in muscle mass observed after 10 days of overload in REDD1-null mice. On the basis of the newly described role of REDD1 in the regulation of autophagy (31), the apparent difference in autophagy in the overloaded muscle between genotypes would likely continue to diverge at later time points. For instance, we show that REDD1 expression is no longer reduced after 5 days of overload in WT mice, and others show that REDD1 expression is increased in the muscle at 14 days of overload (32). Thus, the ability to sustain the overload-induced suppression of autophagy would likely persist in REDD1-null mice, whereas the failure to decrease REDD1 with overload in WT mice (i.e., 5 days of overload) and an eventual increase in REDD1 expression (i.e., 14 days of overload) would blunt the ability to suppress this degradative pathway. Importantly, the overload-induced change in markers of autophagy may have been independent of mTORC1-mediated control of autophagy initiation, as there was no difference between genotypes in either the percent change in ULK1 (Ser-757) phosphorylation or its absolute level in response to overload. This finding supports the newly proposed idea that REDD1 regulates autophagy in an mTORC1-independent manner (31). It is important to note that this report did not directly measure autophagy flux; rather, it measured markers of this process. As the expression of the markers used in this report are dynamic during autophagy (e.g., degradation of LC3 II and p62), it might be useful in future studies to utilize autophagy inhibitors to gain a better understanding of the role of REDD1 in the overload-induced suppression of this process. However, these inhibitory methods are not without fault, as many have nonspecific side effects, they might disrupt the lysosome-dependent activation of mTORC1, or they may induce toxicity, which could all confound the results. As such, the recent recommendations for measurement and assessment of autophagy (25) suggest the combination of the LC3 II/I ratio together with p62 content as indirect measures of autophagy flux.
Recently, Hamilton et al. (19) proposed that certain molecular brakes act to limit the absolute magnitude of mTORC1 signaling during the overload-induced increase in muscle mass. In that report, expression of ER stress proteins (i.e., BiP and CHOP) was increased after 3 days of overload and remained so through 12 days (19). As ER stress represses mTORC1 signaling at the same time as it enhances REDD1 expression (21), it follows that REDD1 should be considered as one of the molecular brakes. However, no overload-induced change in REDD1 protein expression was reported in the Hamilton et al. paper (19). Potential explanations for the discordant results include species analyzed (rat vs. mouse) or sex of the rodents (female vs. male). Alternatively, it may be due to the fact that the overload-induced repression in REDD1 protein expression occurs in a transient manner, i.e., only prior to, or at the early stages of, the onset of the increase in muscle mass (i.e., 3 days of overload; see Fig. 3C), while its expression returns to control values after an increase in muscle mass has ensued (i.e., 5 days of overload; see Fig. 4C). Thus, in the Hamilton et al. (19) report, REDD1 expression may have transiently decreased, and subsequently returned to control levels at their earliest time point of measurement (3 days of overload), in which growth was already apparent. Interestingly, a transient change in REDD1 expression has also been reported in rats subjected to unilateral hindlimb immobilization (22–24), i.e., REDD1 expression is elevated during the initial 3 days of immobilization, but returns to control values at later times. As such, it can be proposed that changes in REDD1 expression only manifest during the initial response of muscle to overload or disuse, suggesting that REDD1 plays an important role in regulating the initiating events leading to changes in muscle mass.
The functional consequences of suppressed autophagy with overload, particularly in the absence of REDD1, remains to be determined. Recent data suggest that impaired autophagy via deletion of the Atg7 gene is detrimental to skeletal muscle, and results in muscle atrophy, accumulation of protein aggregates, appearance of abnormal mitochondria, concentric membranous structures, induction of oxidative stress, and activation of the unfolded protein response (26). This is paradoxical to the findings reported here showing that a suppression in autophagy markers occurs in parallel with an overload-induced increase in muscle mass, which is typically associated with increased muscle function and muscle health. It is possible that lysosome-mediated breakdown of certain proteins or cellular structures remain intact within an enlarging muscle. This possibility is consistent with the idea that there are two major forms of autophagy, including nonselective autophagy, which is associated with stimuli such as starvation, and selective autophagy, which is triggered by and functions to remove aggregate/misfolded proteins and damaged organelles (36). Hence, it is likely that selective autophagic removal of certain damaged proteins and/or organelles remains intact with overload despite an overall repression of autophagy.
In conclusion, the data show that REDD1 modulates the rate of the overload-induced increase in muscle mass and protein accretion. When viewed in the context of previous work (16), the results of the present study are consistent with a model in which REDD1 limits the absolute level of mTORC1 activation and translation efficiency during the early time points of muscle overload, as well as suppresses activation of autophagy. Thus, REDD1 expression affects multiple pathways that augment the rate of the overload-induced increase in muscle mass.
Perspectives and Significance
Skeletal muscle is a highly plastic organ that adapts in size and function to various external stimuli, including nutrient status and mechanical load. Although it is generally accepted that muscle mass is important for functional independence and survival in various diseased populations where muscle mass is compromised, the factors that lead to changes in muscle mass with different anabolic/catabolic stimuli are not fully defined. The work described within is a continuation of a line of research aimed at defining the role of REDD1 in the regulation of muscle metabolism. As REDD1 expression is elevated in skeletal muscle during atrophic conditions such as disuse, and its expression is decreased in skeletal muscle during hypertrophic conditions such as increased mechanical overload, REDD1 represents a potentially important regulator of muscle metabolism, particularly as it relates to changes in muscle mass.
GRANTS
This project was supported by National Institutes of Health Grants R01 DK-15658 (to L. S. Jefferson) and F32 AA023422 (to J. L. Steiner).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.S.G., L.S.J., and S.R.K. conception and design of research; B.S.G., C.L., J.L.S., and G.A.N. performed experiments; B.S.G., C.L., and G.A.N. analyzed data; B.S.G., L.S.J., and S.R.K. interpreted results of experiments; B.S.G. prepared figures; B.S.G. drafted manuscript; B.S.G., C.L., J.L.S., G.A.N., L.S.J., and S.R.K. edited and revised manuscript; B.S.G., C.L., J.L.S., G.A.N., L.S.J., and S.R.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Chen Yang for overseeing the breeding and maintenance of the REDD1-null mice.
REFERENCES
- 1.Adams GR, Haddad F, Baldwin KM. Time course of changes in markers of myogenesis in overloaded rat skeletal muscles. J Appl Physiol (1985) 87: 1705–1712, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 4.Britto FA, Begue G, Rossano B, Docquier A, Vernus B, Sar C, Ferry A, Bonnieu A, Ollendorff V, Favier FB. REDD1 deletion prevents dexamethasone-induced skeletal muscle atrophy. Am J Physiol Endocrinol Metab 307: E983–E993, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Corradetti MN, Inoki K, Guan KL. The stress-inducted proteins RTP801 and RTP801L are negative regulators of the mammalian target of rapamycin pathway. J Biol Chem 280: 9769–9772, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Dennis MD, Coleman CS, Berg A, Jefferson LS, Kimball SR. REDD1 enhances protein phosphatase 2A-mediated dephosphorylation of Akt to repress mTORC1 signaling. Sci Signal 7: ra68, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiPasquale DM, Cheng M, Billich W, Huang SA, van Rooijen N, Hornberger TA, Koh TJ. Urokinase-type plasminogen activator and macrophages are required for skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol 293: C1278–C1285, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587: 1535–1546, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drummond MJ, Fujita S, Abe T, Dreyer HC, Volpi E, Rasmussen BB. Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc 40: 691–698, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan D, Kim J, Shaw RJ, Guan KL. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 7: 643–644, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Volpi E, Rasmussen BB. Skeletal muscle autophagy and protein breakdown following resistance exercise are similar in younger and older adults. J Gerontol A Biol Sci Med Sci 68: 599–607, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman CA, Frey JW, Mabrey DM, Jacobs BL, Lincoln HC, You JS, Hornberger TA. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J Physiol 589: 5485–5501, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodman CA, Hornberger TA. Measuring protein synthesis with SUnSET: a valid alternative to traditional techniques? Exerc Sport Sci Rev 41: 107–115, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon BS, Kazi AA, Coleman CS, Dennis MD, Chau V, Jefferson LS, Kimball SR. RhoA modulates signaling through the mechanistic target of rapamycin complex 1 (mTORC1) in mammalian cells. Cell Signal 26: 461–467, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon BS, Kelleher AR, Kimball SR. Regulation of muscle protein synthesis and the effects of catabolic states. Int J Biochem Cell Biol 45: 2147–2157, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon BS, Steiner JL, Lang CH, Jefferson LS, Kimball SR. Reduced REDD1 expression contributes to activation of mTORC1 following electrically induced muscle contraction. Am J Physiol Endocrinol Metab 307: E703–E711, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon BS, Williamson DL, Lang CH, Jefferson LS, Kimball SR. Nutrient-induced stimulation of protein synthesis in mouse skeletal muscle is limited by the mTORC1 repressor REDD1. J Nutr 145: 708–713, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greig CA, Gray C, Rankin D, Young A, Mann V, Noble B, Atherton PJ. Blunting of adaptive responses to resistance exercise training in women over 75 y. Exp Gerontol 46: 884–890, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton DL, Philp A, MacKenzie MG, Patton A, Towler MC, Gallagher IJ, Bodine SC, Baar K. Molecular brakes regulating mTORC1 activation in skeletal muscle following synergist ablation. Am J Physiol Endocrinol Metab 307: E365–E373, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iadevaia V, Liu R, Proud CG. mTORC1 signaling controls multiple steps in ribosome biogenesis. Semin Cell Dev Biol 36: 113–120, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Jin HO, Seo SK, Woo SH, Kim ES, Lee HC, Yoo DH, An S, Choe TB, Lee SJ, Hong SI, Rhee CH, Kim JI, Park IC. Activating transcription factor 4 and CCAAT/enhancer-binding protein-beta negatively regulate the mammalian target of rapamycin via Redd1 expression in response to oxidative and endoplasmic reticulum stress. Free Radic Biol Med 46: 1158–1167, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Kelleher AR, Gordon BS, Kimball SR, Jefferson LS. Changes in REDD1, REDD2, and atrogene mRNA expression are prevented in skeletal muscle fixed in a stretched position during hindlimb immobilization. Physiol Rep 2: e00246, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelleher AR, Kimball SR, Dennis MD, Schilder RJ, Jefferson LS. The mTORC1 signaling repressors REDD1/2 are rapidly induced and activation of p70S6K1 by leucine is defective in skeletal muscle of an immobilized rat hindlimb. Am J Physiol Endocrinol Metab 304: E229–E236, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelleher AR, Pereira SL, Jefferson LS, Kimball SR. REDD2 expression in rat skeletal muscle correlates with nutrient-induced activation of mTORC1: responses to aging, immobilization, and remobilization. Am J Physiol Endocrinol Metab 308: E122–E129, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Arozena AA, Adachi H, Adams CM, Adams PD, Adeli K, Adhihetty PJ, Adler SG, Agam G, Agarwal R, Aghi MK, Agnello M, Agostinis P, Aguilar PV, Aguirre-Ghiso J, Airoldi EM, Ait-Si-Ali S., Akematsu T., Akporiaye ET, Al-Rubeai M, Albaiceta GM, Albanese C, Albani D, Albert ML, Aldudo J, Algul H, Alirezaei M, Alloza I, Almasan A, Almonte-Beceril M, Alnemri ES, Alonso C, Altan-Bonnet N, Altieri DC, Alvarez S, Alvarez-Erviti L, Alves S, Amadoro G, Amano A, Amantini C, Ambrosio S, Amelio I, Amer AO, Amessou M, Amon A, An ZY, Anania FA, Andersen SU, Andley UP, Andreadi CK, Andrieu-Abadie N, Anel A, Ann DK, Anoopkumar-Dukie S, Antonioli M, Aoki H, Apostolova N, Aquila S, Aquilano K, Araki K, Arama E, Aranda A, Araya J, Arcaro A, Arias E, Arimoto H, Ariosa AR, Armstrong JL, Arnould T, Arsov I, Asanuma K, Askanas V, Asselin E, Atarashi R, Atherton SS, Atkin JD, Attardi LD, Auberger P, Auburger G, Aurelian L, Autelli R, Avagliano L, Avantaggiati ML, Avrahami L, Awale S, Azad N, Bachetti T, Backer JM, Bae DH, Bae JS, Bae ON, Bae SH, Baehrecke EH, Baek SH, Baghdiguian S, Bagniewska-Zadworna A, Bai H, Bai J, Bai XY, Bailly Y, Balaji KN, Balduini W, Ballabio A, Balzan R, Banerjee R, Banhegyi G, Bao HJ, Barbeau B, Barrachina MD, Barreiro E, Bartel B, Bartolome A, Bassham DC, Bassi MT, Bast RC, Basu A, Batista MT, Batoko H, Battino M, Bauckman K, Baumgarner BL, Bayer KU, Beale R, Beaulieu JF, Beck GR, Becker C, Beckham JD, Bedard PA, Bednarski PJ, Begley TJ, Behl C, Behrends C, Behrens GMN, Behrns KE, Bejarano E, Belaid A, Belleudi F, Benard G, Berchem G, Bergamaschi D, Bergami M, Berkhout B, Berliocchi L, Bernard A, Bernard M, Bernassola F, Bertolotti A, Bess AS, Besteiro S, Bettuzzi S, Bhalla S, Bhattacharyya S, Bhutia SK, Biagosch C, Bianchi MW, Biard-Piechaczyk M, Billes V, Bincoletto C, Bingol B, Bird SW, Bitoun M, Bjedov I, Blackstone C, Blanc L, Blanco GA, Blomhoff HK, Boada-Romero E, Bockler S, Boes M, Boesze-Battaglia K, Boise LH, Bolino A, Boman A, Bonaldo P, Bordi M, Bosch J, Botana LM, Botti J, Bou G, Bouche M, Bouchecareilh M, Boucher MJ, Boulton ME, Bouret SG, Boya P, Boyer-Guittaut M, Bozhkov PV, Brady N, Braga VMM, Brancolini C, Braus GH, Bravo-San Pedro JM, Brennan LA, Bresnick EH, Brest P, Bridges D, Bringer MA, Brini M, Brito GC, Brodin B, Brookes PS, Brown EJ, Brown K, Broxmeyer HE, Bruhat A, Brum PC, Brumell JH, Brunetti-Pierri N, Bryson-Richardson RJ, Buch S, Buchan AM, Budak H, Bulavin DV, Bultman SJ, Bultman SJ, Bumbasirevic V, Burelle Y, Burke RE, Burmeister M, Butikofer P, Caberlotto L, Cadwell K, Cahova M, Cai DS, Cai JJ, Cai Q, Calatayud S, Camougrand N, Campanella M, Campbell GR, Campbell M, Campello S, Candau R, Caniggia I, Cantoni L, Cao LZ, Caplan AB, Caraglia M, Cardinali C, Cardoso SM, Carew JS, Carleton LA, Carlin CR, Carloni S, Carlsson SR, Carmona-Gutierrez D, Carneiro LAM, Carnevali O, Carra S, Carrier A, Carroll B, Casas C, Casas J, Cassinelli G, Castets P, Castro-Obregon S, Cavallini G, Ceccherini I, Cecconi F, Cederbaum AI, Cena V, Cenci S, Cerella C, Cervia D, Cetrullo S, Chaachouay H, Chae HJ, Chagin AS, Chai CY, Chakrabarti G, Chamilos G, Chan EYW, Chan MTV, Chandra D, Chandra P, Chang CP, Chang RCC, Chang TY, Chatham JC, Chatterjee S, Chauhan S, Che YS, Cheetham ME, Cheluvappa R, Chen CJ, Chen G, Chen GC, Chen GQ, Chen HZ, Chen JW, Chen JK, Chen M, Chen MZ, Chen PW, Chen Q, Chen Q, Chen SD, Chen S, Chen SSL, Chen W, Chen WJ, Chen WQ, Chen WL, Chen XM, Chen YH, Chen YG, Chen Y, Chen YY, Chen YS, Chen YJ, Chen YQ, Chen YJ, Chen Z, Chen Z, Cheng A, Cheng CHK, Cheng H, Cheong HS, Cherry S, Chesney J, Cheung CHA, Chevet E, Chi HC, Chi SG, Chiacchiera F, Chiang HL, Chiarelli R, Chiariello M, Chieppa M, Chin LS, Chiong M, Chiu GNC, Cho DH, Cho SG, Cho WC, Cho YY, Cho YS, Choi AMK, Choi EJ, Choi EK, Choi JY, Choi ME, Choi SI, Chou TF, Chouaib S, Choubey D, Choubey V, Chow KC, Chowdhury K, Chu CT, Chuang TH, Chun T, Chung HW, Chung TJ, Chung YL, Chwae YJ, Cianfanelli V, Ciarcia R, Ciechomska IA, Ciriolo MR, Cirone M, Claerhout S, Clague MJ, Claria J, Clarke PGH, Clarke R, Clementi E, Cleyrat C, Cnop M, Coccia EM, Cocco T, Codogno P, Coers J, Cohen EEW, Colecchia D, Coletto L, Coll NS, Colucci-Guyon E, Comincini S, Condello M, Cook KL, Coombs GH, Cooper CD, Cooper JM, Coppens I, Corasaniti MT, Corazzari M, Corbalan R, Corcelle-Termeau E, Cordero MD, Corral-Ramos C, Corti O, Cossarizza A, Costelli P, Costes S, Costes S, Coto-Montes A, Cottet S, Couve E, Covey LR, Cowart LA, Cox JS, Coxon FP, Coyne CB, Cragg MS, Craven RJ, Crepaldi T, Crespo JL, Criollo A, Crippa V, Cruz MT, Cuervo AM, Cuezva JM, Cui TX, Cutillas PR, Czaja MJ, Czyzyk-Krzeska MF, Dagda RK, Dahmen U, Dai CS, Dai WJ, Dai Y, Dalby KN, Valle LD, Dalmasso G, D′Amelio M, Damme M, Darfeuille-Michaud A, Dargemont C, Darley-Usmar VM, Dasarathy S, Dasgupta B, Dash S, Dass CR, Davey HM, Davids LM, Davila D, Davis RJ, Dawson TM, Dawson VL, Daza P, de Belleroche J, de Figueiredo P, de Figueiredo RCBQ, de la Fuente J, De Martino L, De Matteis A, De Meyer GRY, De Milito A, De Santi M, de Souza W, De Tata V, De Zio D, Debnath J, Dechant R, Decuypere JP, Deegan S, Dehay B, Del Bello B, Del Re DP, Delage-Mourroux R, Delbridge LMD, Deldicque L, Delorme-Axford E, Deng YZ, Dengjel J, Denizot M, Dent P, Der CJ, Deretic V, Derrien B, Deutsch E, Devarenne TP, Devenish RJ, Di Bartolomeo S, Di Daniele N, Di Domenico F, Di Nardo A, Di Paola S, Di Pietro A, Di Renzo L, DiAntonio A, Diaz-Araya G, Diaz-Laviada I, Diaz-Meco MT, Diaz-Nido J, Dickey CA, Dickson RC, Diederich M, Digard P, Dikic I, Dinesh-Kumar SP, Ding C, Ding WX, Ding ZF, Dini L, Distler JHW, Diwan A, Djavaheri-Mergny M, Dmytruk K, Dobson RCJ, Doetsch V, Dokladny K, Dokudovskaya S, Donadelli M, Dong XC, Dong XN, Dong Z, Donohue TM, Doran KS, D′Orazi G, Dorn GW, Dosenko V, Dridi S, Drucker L, Du J, Du LL, Du LH, du Toit A, Dua P, Duan L, Duann P, Dubey VK, Duchen MR, Duchosal MA, Duez H, Dugail I, Dumit VI, Duncan MC, Dunlop EA, Dunn WA, Dupont N, Dupuis L, Duran RV, Durcan TM, Duvezin-Caubet S, Duvvuri U, Eapen V, Ebrahimi-Fakhari D, Echard A, Eckhart L, Edelstein CL, Edinger AL, Eichinger L, Eisenberg T, Eisenberg-Lerner A, Eissa NT, El-Deiry WS, El-Khoury V, Elazar Z, Eldar-Finkelman H, Elliott CJH, Emanuele E, Emmenegger U, Engedal N, Engelbrecht AM, Engelender S, Enserink JM, Erdmann R, Erenpreisa J, Eri R, Eriksen JL, Erman A, Escalante R, Eskelinen EL, Espert L, Esteban-Martinez L, Evans TJ, Fabri M, Fabrias G, Fabrizi C, Facchiano A, Faergeman NJ, Faggioni A, Fairlie WD, Fan CH, Fan DP, Fan J, Fang SY, Fanto M, Fanzani A, Farkas T, Faure M, Favier FB, Fearnhead H, Federici M, Fei E, Felizardo TC, Feng H, Feng YB, Feng YC, Ferguson TA, Fernandez AF, Fernandez-Barrena MG, Fernandez-Checa JC, Fernandez-Lopez A, Fernandez-Zapico ME, Feron O, Ferraro E, Ferreira-Halder CV, Fesus L, Feuer R, Fiesel FC, Filippi-Chiela EC, Filomeni G, Fimia GM, Fingert JH, Finkbeiner S, Finkel T, Fiorito F, Fisher PB, Flajolet M, Flamigni F, Florey O, Florio S, Floto RA, Folini M, Follo C, Fon EA, Fornai F, Fortunato F, Fraldi A, Franco R, Francois A, Francois A, Frankel LB, Fraser IDC, Frey N, Freyssenet DG, Frezza C, Friedman SL, Frigo DE, Fu DX, Fuentes JM, Fueyo J, Fujitani Y, Fujiwara Y, Fujiya M, Fukuda M, Fulda S, Fusco C, Gabryel B, Gaestel M, Gailly P, Gajewska M, Galadari S, Galili G, Galindo I, Galindo MF, Galliciotti G, Galluzzi L, Galluzzi L, Galy V, Gammoh N, Gandy S, Ganesan AK, Ganesan S, Ganley IG, Gannage M, Gao FB, Gao F, Gao JX, Nannig LG, Vescovi EG, Garcia-Macia M, Garcia-Ruiz C, Garg AD, Garg PK, Gargini R, Gassen NC, Gatica D, Gatti E, Gavard J, Gavathiotis E, Ge L, Ge PF, Ge SF, Gean PW, Gelmetti V, Genazzani AA, Geng JF, Genschik P, Gerner L, Gestwicki JE, Gewirtz DA, Ghavami S, Ghigo E, Ghosh D, Giammarioli AM, Giampieri F, Giampietri C, Giatromanolaki A, Gibbings DJ, Gibellini L, Gibson SB, Ginet V, Giordano A, Giorgini F, Giovannetti E, Girardin SE, Gispert S, Giuliano S, Gladson CL, Glavic A, Gleave M, Godefroy N, Gogal RM, Gokulan K, Goldman GH, Goletti D, Goligorsky MS, Gomes AV, Gomes LC, Gomez H, Gomez-Manzano C, Gomez-Sanchez R, Goncalves DAP, Goncu E, Gong QQ, Gongora C, Gonzalez CB, Gonzalez-Alegre P, Gonzalez-Cabo P, Gonzalez-Polo RA, Goping IS, Gorbea C, Gorbunov NV, Goring DR, Gorman AM, Gorski SM, Goruppi S, Goto-Yamada S, Gotor C, Gottlieb RA, Gozes I, Gozuacik D, Graba Y, Graef M, Granato GE, Grant GD, Grant S, Gravina GL, Green DR, Greenhough A, Greenwood MT, Grimaldi B, Gros F, Grose C, Groulx JF, Gruber F, Grumati P, Grune T, Guan JL, Guan KL, Guerra B, Guillen C, Gulshan K, Gunst J, Guo CY, Guo L, Guo M, Guo WJ, Guo XG, Gust AA, Gustafsson AB, Gutierrez E, Gutierrez MG, Gwak HS, Haas A, Haber JE, Hadano S, Hagedorn M, Hahn DR, Halayko AJ, Hamacher-Brady A, Hamada K, Hamai A, Hamann A, Hamasaki M, Hamer I, Hamid Q, Hamid Q, Han F, Han WD, Handa JT, Hanover JA, Hansen M, Harada M, Harhaji-Trajkovic L, Harper JW, Harrath AH, Harris AL, Harris J, Hasler U, Hasselblatt P, Hasui K, Hawley RG, Hawley TS, He CC, He CY, He FT, He G, He RR, He XH, He YW, He YY, Heath JK, Hebert MJ, Heinzen RA, Helgason GV, Hensel M, Henske EP, Her CT, Herman PK, Hernandez A, Hernandez C, Hernandez-Tiedra S, Hetz C, Hiesinger PR, Higaki K, Hilfiker S, Hill BG, Hill JA, Hill WD, Hino K, Hofius D, Hofman P, Hoglinger GU, Hohfeld J, Holz MK, Hong YG, Hood DA, Hoozemans JJM, Hoppe T, Hsu C, Hsu CY, Hsu LC, Hu D, Hu GC, Hu HM, Hu HB, Hu MC, Hu YC, Hu ZW, Hua F, Hua Y, Huang CH, Huang HL, Huang KH, Huang KY, Huang SL, Huang SQ, Huang WP, Huang YR, Huang Y, Huang YF, Huber TB, Huebbe P, Huh WK, Hulmi JJ, Hur GM, Hurley JH, Husak Z, Hussain SNA, Hussain S, Hwang JJ, Hwang SM, Hwang TIS, Ichihara A, Imai Y, Imbriano C, Inomata M, Into T, Iovane V, Iovanna JL, Iozzo RV, Ip NY, Irazoqui JE, Iribarren P, Isaka Y, Isakovic AJ, Ischiropoulos H, Isenberg JS, Ishaq M, Ishida H, Ishii I, Ishmael JE, Isidoro C, Isobe KI, Isono E, Issazadeh-Navikas S, Itahana K, Itakura E, Ivanov AI, Iyer AKV, Izquierdo JM, Izumi Y, Izzo V, Jaattela M, Jaber N, Jackson DJ, Jackson WT, Jacob TG, Jacques TS, Jagannath C, Jain A, Jana NR, Jang BK, Jani A, Janji B, Jannig PR, Jansson PJ, Jean S, Jendrach M, Jeon JH, Jessen N, Jeung EB, Jia KL, Jia LJ, Jiang H, Jiang HC, Jiang LW, Jiang T, Jiang XY, Jiang XJ, Jiang XJ, Jiang Y, Jiang YJ, Jimenez A, Jin C, Jin HC, Jin L, Jin MY, Jin SK, Jinwal UK, Jo EK, Johansen T, Johnson DE, Johnson GVW, Johnson JD, Jonasch E, Jones C, Joosten LAB, Jordan J, Joseph AM, Joseph B, Joubert AM, Ju DW, Ju JF, Juan HF, Juenemann K, Juhasz G, Jung HS, Jung JU, Jung YK, Jungbluth H, Justice MJ, Jutten B, Kaakoush NO, Kaarniranta K, Kaasik A, Kabuta T, Kaeffer B, Kagedal K, Kahana A, Kajimura S, Kakhlon O, Kalia M, Kalvakolanu DV, Kamada Y, Kambas K, Kaminskyy VO, Kampinga HH, Kandouz M, Kang C, Kang R, Kang TC, Kanki T, Kanneganti TD, Kanno H, Kanthasamy AG, Kantorow M, Kaparakis-Liaskos M, Kapuy O, Karantza V, Karim MR, Karmakar P, Kaser A, Kaushik S, Kawula T, Kaynar AM, Ke PY, Ke ZJ, Kehrl JH, Keller KE, Kemper JK, Kenworthy AK, Kepp O, Kern A, Kesari S, Kessel D, Ketteler R, Kettelhut ID, Khambu B, Khan MM, Khandelwal VKM, Khare S, Kiang JG, Kiger AA, Kihara A, Kim AL, Kim CH, Kim DR, Kim DH, Kim EK, Kim HY, Kim HR, Kim JS, Kim JH, Kim JC, Kim JH, Kim KW, Kim MD, Kim MM, Kim PK, Kim SW, Kim SY, Kim YS, Kim Y, Kimchi A, Kimmelman AC, Kimura T, King JS, Kirkegaard K, Kirkin V, Kirshenbaum LA, Kishi S, Kitajima Y, Kitamoto K, Kitaoka Y, Kitazato K, Kley RA, Klimecki WT, Klinkenberg M, Klucken J, Knaevelsrud H, Knecht E, Knuppertz L, Ko JL, Kobayashi S, Koch JC, Koechlin-Ramonatxo C, Koenig U, Koh YH, Kohler K, Kohlwein SD, Koike M, Komatsu M, Kominami E, Kong DX, Kong HJ, Konstantakou EG, Kopp BT, Korcsmaros T, Korhonen L, Korolchuk VI, Koshkina NV, Kou YJ, Koukourakis MI, Koumenis C, Kovacs AL, Kovacs T, Kovacs WJ, Koya D, Kraft C, Krainc D, Kramer H, Kravic-Stevovic T, Krek W, Kretz-Remy C, Krick R, Krishnamurthy M, Kriston-Vizi J, Kroemer G, Kruer MC, Kruger R, Ktistakis NT, Kuchitsu K, Kuhn C, Kumar AP, Kumar A, Kumar A, Kumar D, Kumar D, Kumar R, Kumar S, Kundu M, Kung HJ, Kuno A, Kuo SH, Kuret J, Kurz T, Kwok T, Kwon TK, Kwon YT, Kyrmizi I, La Spada AR, Lafont F, Lahm T, Lakkaraju A, Lam T, Lamark T, Lancel S, Landowski TH, Lane DJR, Lane JD, Lanzi C, Lapaquette P, Lapierre LR, Laporte J, Laukkarinen J, Laurie GW, Lavandero S, Lavie L, LaVoie MJ, Law BYK, Law HKW, Law KB, Layfield R, Lazo PA, Le Cam L, Le Roch KG, Le Stunff H, Leardkamolkarn V, Lecuit M, Lee BH, Lee CH, Lee EF, Lee GM, Lee HJ, Lee H, Lee JK, Lee J, Lee JH, Lee JH, Lee M, Lee MS, Lee PJ, Lee SW, Lee SJ, Lee SJ, Lee SY, Lee SH, Lee SS, Lee SJ, Lee S, Lee YR, Lee YJ, Lee YH, Leeuwenburgh C, Lefort S, Legouis R, Lei JZ, Lei QY, Leib DA, Leibowitz G, Lekli I, Lemaire SD, Lemasters JJ, Lemberg MK, Lemoine A, Leng SL, Lenz G, Lenzi P, Lerman LO, Barbato DL, Leu JIJ, Leung HY, Levine B, Lewis PA, Lezoualc'h F, Li C, Li FQ, Li FJ, Li J, Li K, Li L, Li M, Li M, Li Q, Li R, Li S, Li W, Li W, Li XT, Li YM, Lian JQ, Liang CY, Liang QR, Liao YL, Liberal J, Liberski PP, Lie P, Lieberman AP, Lim HJ, Lim KL, Lim K, Lima RT, Lin CS, Lin CF, Lin F, Lin FM, Lin FC, Lin K, Lin KH, Lin PH, Lin TW, Lin WW, Lin YS, Lin Y, Linden R, Lindholm D, Lindqvist LM, Lingor P, Linkermann A, Liotta LA, Lipinski MM, Lira VA, Lisanti MP, Liton PB, Liu B, Liu C, Liu CF, Liu F, Liu HJ, Liu JX, Liu JJ, Liu JL, Liu K, Liu LY, Liu L, Liu QT, Liu RY, Liu SM, Liu SW, Liu W, Liu XD, Liu XG, Liu XH, Liu XF, Liu X, Liu XQ, Liu Y, Liu YL, Liu ZX, Liu Z, Liuzzi JP, Lizard G, Ljujic M, Lodhi IJ, Logue SE, Lokeshwar BL, Long YC, Lonial S, Loos B, Lopez-Otin C, Lopez-Vicario C, Lorente M, Lorenzi PL, Lorincz P, Los M, Lotze MT, Lovat PE, Lu BF, Lu B, Lu J, Lu Q, Lu SM, Lu SY, Lu YY, Luciano F, Luckhart S, Lucocq JM, Ludovico P, Lugea A, Lukacs NW, Lum JJ, Lund AH, Luo HL, Luo J, Luo SQ, Luparello C, Lyons T, Ma JJ, Ma Y, Ma Y, Ma ZY, Machado J, Machado-Santelli GM, Macian F, MacIntosh GC, MacKeigan JP, Macleod KF, MacMicking JD, MacMillan-Crow LA, Madeo F, Madesh M, Madrigal-Matute J, Maeda A, Maeda T, Maegawa G, Maellaro E, Maes H, Magarinos M, Maiese K, Maiti TK, Maiuri L, Maiuri MC, Maki CG, Malli R, Malorni W, Maloyan A, Mami-Chouaib F, Man N, Mancias JD, Mandelkow EM, Mandell MA, Manfredi AA, Manie SN, Manzoni C, Mao K, Mao ZX, Mao ZW, Marambaud P, Marconi AM, Marelja Z, Marfe G, Margeta M, Margittai E, Mari M, Mariani FV, Marin C, Marinelli S, Marino G, Markovic I, Marquez R, Martelli AM, Martens S, Martin KR, Martin SJ, Martin S, Martin-Acebes MA, Martin-Sanz P, Martinand-Mari C, Martinet W, Martinez J, Martinez-Lopez N, Martinez-Outschoorn U, Martinez-Velazquez M, Martinez-Vicente M, Martins WK, Mashima H, Mastrianni JA, Matarese G, Matarrese P, Mateo R, Matoba S, Matsumoto N, Matsushita T, Matsuura A, Matsuzawa T, Mattson MP, Matus S, Maugeri N, Mauvezin C, Mayer A, Maysinger D, Mazzolini GD, McBrayer MK, McCall K, McCormick C, McInerney GM, McIver SC, McKenna S, McMahon JJ, McNeish IA, Mechta-Grigoriou F, Medema JP, Medina DL, Megyeri K, Mehrpour M, Mehta JL, Mei YD, Meier UC, Meijer AJ, Melendez A, Melino G, Melino S, de Melo EJT, Mena MA, Meneghini MD, Menendez JA, Menezes R, Meng LS, Meng LH, Meng SS, Menghini R, Menko AS, Menna-Barreto RFS, Menon MB, Meraz-Rios MA, Merla G, Merlini L, Merlot AM, Meryk A, Meschini S, Meyer JN, Mi MT, Miao CY, Micale L, Michaeli S, Michiels C, Migliaccio AR, Mihailidou AS, Mijaljica D, Mikoshiba K, Milan E, Miller-Fleming L, Mills GB, Mills IG, Minakaki G, Minassian BA, Ming XF, Minibayeva F, Minina EA, Mintern JD, Minucci S, Miranda-Vizuete A, Mitchell CH, Miyamoto S, Miyazawa K, Mizushima N, Mnich K, Mograbi B, Mohseni S, Moita LF, Molinari M, Molinari M, Moller AB, Mollereau B, Mollinedo F, Monick MM, Monick MM, Montagnaro S, Montell C, Moore DJ, Moore MN, Mora-Rodriguez R, Moreira PI, Morel E, Morelli MB, Moreno S, Morgan MJ, Moris A, Moriyasu Y, Morrison JL, Morrison LA, Morselli E, Moscat J, Moseley PL, Mostowy S, Motori E, Mottet D, Mottram JC, Moussa CEH, Mpakou VE, Mukhtar H, Levy JMM, Muller S, Munoz-Moreno R, Munoz-Pinedo C, Munz C, Murphy ME, Murray JT, Murthy A, Mysorekar IU, Nabi IR, Nabissi M, Nader GA, Nagahara Y, Nagai Y, Nagata K, Nagelkerke A, Nagy P, Naidu SR, Nair S, Nakano H, Nakatogawa H, Nanjundan M, Napolitano G, Naqvi NI, Nardacci R, Narendra DP, Narita M, Nascimbeni AC, Natarajan R, Navegantes LC, Nawrocki ST, Nazarko TY, Nazarko VY, Neill T, Neri LM, Netea MG, Netea-Maier RT, Neves BM, Ney PA, Nezis IP, Nguyen HTT, Nguyen HP, Nicot AS, Nilsen H, Nilsson P, Nishimura M, Nishino I, Niso-Santano M, Niu H, Nixon RA, Njar VCO, Noda T, Noegel AA, Nolte EM, Norberg E, Norga KK, Noureini SK, Notomi S, Notterpek L, Nowikovsky K, Nukina N, Nurnberger T, O'Donnell VB, O'Donovan T, O'Dwyer PJ, Oehme I, Oeste CL, Ogawa M, Ogretmen B, Ogura Y, Oh YJ, Ohmuraya M, Ohshima T, Ojha R, Okamoto K, Okazaki T, Oliver FJ, Ollinger K, Olsson S, Orban DP, Ordonez P, Orhon I, Orosz L, O'Rourke EJ, Orozco H, Ortega AL, Ortona E, Osellame LD, Oshima J, Oshima S, Osiewacz HD, Otomo T, Otsu K, Ou JHJ, Outeiro TF, Ouyang DY, Ouyang HJ, Overholtzer M, Ozbun MA, Ozdinler PH, Ozpolat B, Pacelli C, Paganetti P, Page G, Pages G, Pagnini U, Pajak B, Pak SC, Pakos-Zebrucka K, Pakpour N, Palkova Z, Palladino F, Pallauf K, Pallet N, Palmieri M, Paludan SR, Palumbo C, Palumbo S, Pampliega O, Pan HM, Pan W, Panaretakis T, Pandey A, Pantazopoulou A, Papackova Z, Papademetrio DL, Papassideri I, Papini A, Parajuli N, Pardo J, Parekh VV, Parenti G, Park JI, Park J, Park OK, Parker R, Parlato R, Parys JB, Parzych KR, Pasquet JM, Pasquier B, Pasumarthi KBS, Patschan D, Patterson C, Pattingre S, Pattison S, Pause A, Pavenstadt H, Pavone F, Pedrozo Z, Pena FJ, Penalva MA, Pende M, Peng JX, Penna F, Penninger JM, Pensalfini A, Pepe S, Pereira GJS, Pereira PC, la Cruz VP, Perez-Perez ME, Perez-Rodriguez D, Perez-Sala D, Perier C, Perl A, Perlmutter DH, Perrotta I, Pervaiz S, Pesonen M, Pessin JE, Peters GJ, Petersen M, Petrache I, Petrof BJ, Petrovski G, Phang JM, Piacentini M, Pierdominici M, Pierre P, Pierrefite-Carle V, Pietrocola F, Pimentel-Muinos FX, Pinar M, Pineda B, Pinkas-Kramarski R, Pinti M, Pinton P, Piperdi B, Piret JM, Platanias LC, Platta HW, Plowey ED, Poggeler S, Poirot M, Polic P, Poletti A, Poon AH, Popelka H, Popova B, Poprawa I, Poulose SM, Poulton J, Powers SK, Powers T, Pozuelo-Rubio M, Prak K, Prange R, Prescott M, Priault M, Prince S, Proia RL, Proikas-Cezanne T, Prokisch H, Promponas VJ, Przyklenk K, Puertollano R, Pugazhenthi S, Puglielli L, Pujol A, Puyal J, Pyeon D, Qi X, Qian WB, Qin ZH, Qiu Y, Qu ZW, Quadrilatero J, Quinn F, Raben N, Rabinowich H, Radogna F, Ragusa MJ, Rahmani M, Raina K, Ramanadham S, Ramesh R, Rami A, Randall-Demllo S, Randow F, Rao H, Rao VA, Rasmussen BB, Rasse TM, Ratovitski EA, Rautou PE, Ray SK, Razani B, Reed BH, Reggiori F, Rehm M, Reichert AS, Rein T, Reiner DJ, Reits E, Ren J, Ren XC, Renna M, Reusch JEB, Revuelta JL, Reyes L, Rezaie AR, Richards RI, Richardson DR, Richetta C, Riehle MA, Rihn BH, Rikihisa Y, Riley BE, Rimbach G, Rippo MR, Ritis K, Rizzi F, Rizzo E, Roach PJ, Robbins J, Roberge M, Roca G, Roccheri MC, Rocha S, Rodrigues CMP, Rodriguez CI, de Cordoba SR, Rodriguez-Muela N, Roelofs J, Rogov VV, Rohn TT, Rohrer B, Romanelli D, Romani L, Romano PS, Roncero MIG, Rosa JL, Rosello A, Rosen KV, Rosenstiel P, Rost-Roszkowska M, Roth KA, Roue G, Rouis M, Rouschop KM, Ruan DT, Ruano D, Rubinsztein DC, Rucker EB, Rudich A, Rudolf E, Rudolf R, Ruegg MA, Ruiz-Roldan C, Ruparelia AA, Rusmini P, Russ DW, Russo GL, Russo G, Russo R, Rusten TE, Ryabovol V, Ryan KM, Ryter SW, Sabatini DM, Sacher M, Sachse C, Sack MN, Sadoshima J, Saftig P, Sagi-Eisenberg R, Sahni S, Saikumar P, Saito T, Saitoh T, Sakakura K, Sakoh-Nakatogawa M, Sakuraba Y, Salazar-Roa M, Salomoni P, Saluja AK, Salvaterra PM, Salvioli R, Samali A, Sanchez AMJ, Sanchez-Alcazar JA, Sanchez-Prieto R, Sandri M, Sanjuan MA, Santaguida S, Santambrogio L, Santoni G, dos Santos CN, Saran S, Sardiello M, Sargent G, Sarkar P, Sarkar S, Sarrias MR, Sarwal MM, Sasakawa C, Sasaki M, Sass M, Sato K, Sato M, Satriano J, Savaraj N, Saveljeva S, Schaefer L, Schaible UE, Scharl M, Schatzl HM, Schekman R, Scheper W, Schiavi A, Schipper HM, Schmeisser H, Schmidt J, Schmitz I, Schneider BE, Schneider EM, Schneider JL, Schon EA, Schonenberger MJ, Schonthal AH, Schorderet DF, Schroder B, Schuck S, Schulze RJ, Schwarten M, Schwarz TL, Sciarretta S, Scotto K, Scovassi AI, Screaton RA, Screen M, Seca H, Sedej S, Segatori L, Segev N, Seglen PO, Segui-Simarro JM, Segura-Aguilar J, Seiliez I, Seki E, Sell C, Semenkovich CF, Semenza GL, Sen U, Serra AL, Serrano-Puebla A, Sesaki H, Setoguchi T, Settembre C, Shacka JJ, Shajahan-Haq AN, Shapiro IM, Sharma S, She H, Shen CKJ, Shen CC, Shen HM, Shen SB, Shen WL, Sheng R, Sheng XY, Sheng ZH, Shepherd TG, Shi JY, Shi Q, Shi QH, Shi YG, Shibutani S, Shibuya K, Shidoji Y, Shieh JJ, Shih CM, Shimada Y, Shimizu S, Shin DW, Shinohara ML, Shintani M, Shintani T, Shioi T, Shirabe K, Shiri-Sverdlov R, Shirihai O, Shore GC, Shu CW, Shukla D, Sibirny AA, Sica V, Sigurdson CJ, Sigurdsson EM, Sijwali PS, Sikorska B, Silveira WA, Silvente-Poirot S, Silverman GA, Simak J, Simmet T, Simon AK, Simon HU, Simone C, Simons M, Simonsen A, Singh R, Singh SV, Singh SK, Sinha D, Sinha S, Sinicrope FA, Sirko A, Sirohi K, Sishi BJN, Sittler A, Siu PM, Sivridis E, Skwarska A, Slack R, Slaninova I, Slavov N, Smaili SS, Smalley KSM, Smith DR, Soenen SJ, Soleimanpour SA, Solhaug A, Somasundaram K, Son JH, Sonawane A, Song CJ, Song FY, Song HK, Song JX, Song W, Soo KY, Sood AK, Soong TW, Soontornniyomkij V, Sorice M, Sotgia F, Soto-Pantoja DR, Sotthibundhu A, Sousa MJ, Spaink HP, Span PN, Spang A, Sparks JD, Speck PG, Spector SA, Spies CD, Springer W, St Clair D, Stacchiotti A, Staels B, Stang MT, Starczynowski DT, Starokadomskyy P, Steegborn C, Steele JW, Stefanis L, Steffan J, Stellrecht CM, Stenmark H, Stepkowski TM, Stern ST, Stevens C, Stockwell BR, Stoka V, Storchova Z, Stork B, Stratoulias V, Stravopodis DJ, Strnad P, Strohecker AM, Strom AL, Stromhaug P, Stulik J, Su YX, Su ZL, Subauste CS, Subramaniam S, Sue CM, Suh SW, Sui XB, Sukseree S, Sulzer D, Sun FL, Sun JR, Sun J, Sun SY, Sun Y, Sun Y, Sun YJ, Sundaramoorthy V, Sung J, Suzuki H, Suzuki K, Suzuki N, Suzuki T, Suzuki YJ, Swanson MS, Swanton C, Sward K, Swarup G, Sweeney ST, Sylvester PW, Szatmari Z, Szegezdi E, Szlosarek PW, Taegtmeyer H, Tafani M, Taillebourg E, Tait SWG, Takacs-Vellai K, Takahashi Y, Takats S, Takemura G, Takigawa N, Talbot NJ, Tamagno E, Tamburini J, Tan CP, Tan L, Tan ML, Tan M, Tan YJ, Tanaka K, Tanaka M, Tang DL, Tang DZ, Tang GM, Tanida I, Tanji K, Tannous BA, Tapia JA, Tasset-Cuevas I, Tatar M, Tavassoly I, Tavernarakis N, Taylor A, Taylor GS, Taylor GA, Taylor JP, Taylor MJ, Tchetina EV, Tee AR, Teixeira-Clerc F, Telang S, Tencomnao T, Teng BB, Teng RJ, Terro F, Tettamanti G, Theiss AL, Theron AE, Thomas KJ, Thome MP, Thomes PG, Thorburn A, Thorner J, Thum T, Thumm M, Thurston TLM, Tian L, Till A, Ting JPY, Titorenko VI, Toker L, Toldo S, Tooze SA, Topisirovic I, Torgersen ML, Torosantucci L, Torriglia A, Torrisi MR, Tournier C, Towns R, Trajkovic V, Travassos LH, Triola G, Tripathi DN, Trisciuoglio D, Troncoso R, Trougakos IP, Truttmann AC, Tsai KJ, Tschan MP, Tseng YH, Tsukuba T, Tsung A, Tsvetkov AS, Tu SP, Tuan HY, Tucci M, Tumbarello DA, Turk B, Turk V, Turner RFB, Tveita AA, Tyagi SC, Ubukata M, Uchiyama Y, Udelnow A, Ueno T, Umekawa M, Umemiya-Shirafuji R, Underwood BR, Ungermann C, Ureshino RP, Ushioda R, Uversky VN, Uzcategui NL, Vaccari T, Vaccaro MI, Vachova L, Vakifahmetoglu-Norberg H, Valdor R, Valente EM, Vallette F, Valverde AM, Van den Berghe G, Van Den Bosch L, van den Brink GR, van der Goot FG, van der Klei IJ, van der Laan LJW, van Doorn WG, van Egmond M, van Golen KL, Van Kaer L, Campagne MV, Vandenabeele P, Vandenberghe W, Vanhorebeek I, Varela-Nieto I, Vasconcelos MH, Vasko R, Vavvas DG, Vega-Naredo I, Velasco G, Velentzas AD, Velentzas PD, Vellai T, Vellenga E, Vendelbo MH, Venkatachalam K, Ventura N, Ventura S, Veras PST, Verdier M, Vertessy BG, Viale A, Vidal M, Vieira HLA, Vierstra RD, Vigneswaran N, Vij N, Vila M, Villar M, Villar VH, Villarroya J, Vindis C, Viola G, Viscomi MT, Vitale G, Vogl DT, Voitsekhovskaja OV, von Haefen C, von Schwarzenberg K, Voth DE, Vouret-Craviari V, Vuori K, Vyas JM, Waeber C, Walker CL, Walker MJ, Walter J, Wan L, Wan XB, Wang B, Wang CH, Wang CY, Wang CS, Wang CR, Wang CH, Wang D, Wang F, Wang FX, Wang GH, Wang HJ, Wang HC, Wang HG, Wang HM, Wang HD, Wang J, Wang JJ, Wang M, Wang MQ, Wang PY, Wang P, Wang RC, Wang S, Wang TF, Wang X, Wang XJ, Wang XW, Wang X, Wang XJ, Wang Y, Wang Y, Wang Y, Wang YJ, Wang YP, Wang Y, Wang YT, Wang YQ, Wang ZN, Wappner P, Ward C, Ward DM, Warnes G, Watada H, Watanabe Y, Watase K, Weaver TE, Weekes CD, Wei JW, Weide T, Weihl CC, Weindl G, Weis SN, Wen LP, Wen X, Wen YF, Westermann B, Weyand CM, White AR, White E, Whitton JL, Whitworth AJ, Wiels J, Wild F, Wildenberg ME, Wileman T, Wilkinson DS, Wilkinson S, Willbold D, Williams C, Williams K, Williamson PR, Winklhofer KF, Witkin SS, Wohlgemuth SE, Wollert T, Wolvetang EJ, Wong E, Wong GW, Wong RW, Wong VKW, Woodcock EA, Wright KL, Wu CL, Wu DF, Wu GS, Wu J, Wu JF, Wu M, Wu M, Wu SZ, Wu WKK, Wu YH, Wu ZL, Xavier CPR, Xavier RJ, Xia GX, Xia T, Xia WL, Xia Y, Xiao HY, Xiao J, Xiao S, Xiao WH, Xie CM, Xie ZP, Xie ZL, Xilouri M, Xiong YY, Xu CS, Xu CF, Xu F, Xu HX, Xu HW, Xu J, Xu JZ, Xu JX, Xu L, Xu XL, Xu YQ, Xu Y, Xu ZX, Xu ZH, Xue Y, Yamada T, Yamamoto A, Yamanaka K, Yamashina S, Yamashiro S, Yan B, Yan B, Yan X, Yan Z, Yanagi Y, Yang DS, Yang JM, Yang L, Yang MH, Yang PM, Yang P, Yang Q, Yang WN, Yang WY, Yang XS, Yang Y, Yang Y, Yang ZF, Yang ZH, Yao MC, Yao PJ, Yao XF, Yao ZY, Yao ZY, Yasui LS, Ye MX, Yedvobnick B, Yeganeh B, Yeh ES, Yeyati PL, Yi F, Yi L, Yin XM, Yip CK, Yoo YM, Yoo YH, Yoon SY, Yoshida KI, Yoshimori T, Young KH, Yu HM, Yu JJ, Yu JT, Yu J, Yu L, Yu WH, Yu XF, Yu ZP, Yuan JY, Yuan ZM, Yue BYJT, Yue JB, Yue ZY, Zacks DN, Zacksenhaus E, Zaffaroni N, Zaglia T, Zakeri Z, Zecchini V, Zeng JS, Zeng M, Zeng Q, Zervos AS, Zhang DD, Zhang F, Zhang G, Zhang GC, Zhang H, Zhang H, Zhang H, Zhang HB, Zhang J, Zhang J, Zhang JW, Zhang JH, Zhang JP, Zhang L, Zhang L, Zhang L, Zhang L, Zhang MY, Zhang XN, Zhang XD, Zhang Y, Zhang Y, Zhang YJ, Zhang YM, Zhang YJ, Zhao M, Zhao WL, Zhao XN, Zhao YG, Zhao Y, Zhao YC, Zhao YX, Zhao ZD, Zhao ZZJ, Zheng DX, Zheng XL, Zheng XX, Zhivotovsky B, Zhong Q, Zhou GZ, Zhou GF, Zhou HP, Zhou SF, Zhou XJ, Zhu HX, Zhu H, Zhu WG, Zhu WH, Zhu XF, Zhu YH, Zhuang SM, Zhuang XH, Ziparo E, Zois CE, Zoladek T, Zong WX, Zorzano A, Zughaier SM. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12: 1–222, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M. Autophagy is required to maintain muscle mass. Cell Metab 10: 507–515, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Miyazaki M, McCarthy JJ, Fedele MJ, Esser KA. Early activation of mTORC1 signalling in response to mechanical overload is independent of phosphoinositide 3-kinase/Akt signalling. J Physiol 589: 1831–1846, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nader GA, McLoughlin TJ, Esser KA. mTOR function in skeletal muscle hypertrophy: increased ribosomal RNA via cell cycle regulators. Am J Physiol Cell Physiol 289: C1457–C1465, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Nakada S, Ogasawara R, Kawada S, Maekawa T, Ishii N. Correlation between ribosome biogenesis and the magnitude of hypertrophy in overloaded skeletal muscle. Plos One 11: 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips SM, Glover EI, Rennie MJ. Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J Appl Physiol (1985) 107: 645–654, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Qiao S, Dennis M, Song X, Vadysirisack DD, Salunke D, Nash Z, Yang Z, Liesa M, Yoshioka J, Matsuzawa S, Shirihai OS, Lee RT, Reed JC, Ellisen LW. A REDD1/TXNIP pro-oxidant complex regulates ATG4B activity to control stress-induced autophagy and sustain exercise capacity. Nat Commun 6: 7014, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steiner JL, Gordon BS, Lang CH. Moderate alcohol consumption does not impair overload-induced muscle hypertrophy and protein synthesis. Physiol Rep 3: 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steiner JL, Lang CH. Alcohol intoxication following muscle contraction in mice decreases muscle protein synthesis but not mTOR signal transduction. Alcohol Clin Exp Res 39: 1–10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Walden F, Casagrande V, Ostlund Farrants AK, Nader GA. Mechanical loading induces the expression of a Pol I regulon at the onset of skeletal muscle hypertrophy. Am J Physiol Cell Physiol 302: C1523–C1530, 2012. [DOI] [PubMed] [Google Scholar]
- 35.West DW, Baehr LM, Marcotte GR, Chason CM, Tolento L, Gomes AV, Bodine SC, Baar K. Acute resistance exercise activates rapamycin-sensitive and -insensitive mechanisms that control translational activity and capacity in skeletal muscle. J Physiol 594: 453–468, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan Z, Lira VA, Greene NP. Exercise training-induced regulation of mitochondrial quality. Exerc Sport Sci Rev 40: 159–164, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan HX, Russell RC, Guan KL. Regulation of PIK3C3/VPS34 complexes by MTOR in nutrient stress-induced autophagy. Autophagy 9: 1983–1995, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]