Abstract
Recent evidence indicates the adaptor protein SH2B3 has a major role in the progression of renal diseases. SH2B3 is highly expressed by hematopoietic cells and regulates cytokine signaling, inducing cell-specific effects. Additionally, its expression in other cell types suggests that SH2B3 may have a more extensive role within the kidney. Ex vivo studies have determined targets of SH2B3 cell-specific signaling, while in vivo studies have observed the SH2B3 overall affects in the progression of renal diseases. This mini-review covers the function of SH2B3-expressing cell types that contribute to renal pathologies and their regulation by SH2B3.
Keywords: SH2B adaptor protein 3, LNK, hypertension, hematopoietic, lymphocyte
sh2b adaptor protein 3 (SH2B3), originally cloned and characterized as LNK, is a signal transduction regulator shown to modulate many cytokine signaling cascades and cellular motility. Evidence suggests that it is involved in the progression of several human diseases, including type 1 diabetes, autoimmune, cardiovascular, and hypertension (4). Of note, genome-wide association studies have demonstrated that polymorphisms in SH2B3 are associated with systolic and diastolic blood pressure variation in African Americans and subjects of European ancestry (6, 15). Further discoveries of the mechanisms of action of SH2B3 may therefore lead to a greater understanding of disease mechanisms.
As a member of the family of SH2B adaptor proteins, SH2B3 is composed of several functional domains: the Src-homolog 2 (SH2) domain that is essential for its binding with targets for their inhibition, the pleckstrin homology (PH) domain that recognizes phosphoinositides and controls its translocation to the cell membrane, and the dimerization domains that mediate the formation of homo- and heterodimers between members of the SH2-B family. SH2B3 has been shown to bind and negatively regulate several targets, such as Janus kinases and other tyrosine kinases regulating cytokine receptors. Recent studies have provided new insight into the potential role of SH2B3 in the kidney and renal pathologies. In part, its role in the development of hypertension-dependent renal damage has been determined to be mediated via regulation of the hematopoietic cells. Even so, endothelial function and other nonhematopoietic cellular expression suggest a more extensive function of SH2B3 throughout the kidney. Recent reviews have substantially addressed the cell type-specific molecular mechanism of SH2B3 (3, 4, 7). While no anatomic differences in the kidney due to mutations of SH2B3 or expression alterations have been reported, SH2B3 has been shown to have profound influences on the pathogenesis of renal diseases (26, 28). This mini-review highlights the known and possible functions of SH2B3 in kidney diseases.
SH2B3 is a powerful regulator of immune function.
For most if not all immune cells studied thus far, SH2B3 has been shown to be a major regulator of proliferation and differentiation. SH2B3 was first identified in T cells (11) and has since been found to function in all cells derived from hematopoietic stem cells. A normal kidney contains mononuclear phagocytes that comprise the majority of immune cells [dendritic cells (DCs) and macrophages] (22, 30) and differentiated pro- and anti-inflammatory T cells. Upon renal damage and inflammation, additional immune cells are recruited and transmigrate across the renal endothelium to participate in the inflammatory response. Ultimately, the balance of proinflammatory and reparative states of hematopoietic and nonhematopoietic renal cells directs the progression and outcome of renal pathologies. The following details the known roles of SH2B3 that alter the inflammatory balance in the kidney.
Macrophages.
Macrophages are major participants in determining the outcome of immune responses. The macrophage origin, activation state, and microenvironment determine their behavior in response to injury. Macrophages exist in healthy tissues as a mixture of classically activated, proinflammatory and alternatively activated, anti-inflammatory states. In most renal diseases, macrophages accumulate in the renal interstitium. Their abundant infiltration into kidneys may promote excessive fibrosis, tissue destruction, and the renal disease progression such as glomerulonephritis (18).
Macrophage colony-stimulating factor (M-CSF) is a cytokine that is involved in the activation of macrophages. While M-CSF may function in renal repair (35), it has also been observed as a driver of a proinflammatory macrophage phenotype (2). SH2B3 was shown to inhibit M-CSF signaling, thereby limiting macrophage activation (8). Thus macrophages lacking SH2B3 expression are easily activated, produce more reactive oxygen species (ROS), and have increased migration that is an indicator of cell activation (8). A recent study confirmed this by observing increased migration of macrophages and ROS production in the kidneys of untreated, control mice lacking SH2B3 expression (28), but the exact source of ROS was undetermined.
DCs.
Kidneys contain a network of heterogeneous populations of DCs (14, 25, 30). These are categorically composed of plasmacytoid DCs and two conventional DCs (cDC1 and cDC2) that originate via the circulation (21). These three DC types appear to have distinct functions based on their presence in renal injury and migratory behaviors. Plasmacytoid DCs are rare in healthy kidneys but become abundant after injury. cDC1 migrate from tissues to lymph nodes to present antigens to CD4+ T-cells (12). cDC2 display lower migration and present glycolipid and other antigens (19). In a healthy kidney, the cDC2 type dominates the renal cortex with the presence of a smaller population of cDC1. SH2B3 expression in DCs has been shown to control the production of IFNγ and the induction of naïve CD4+ T-cells to an IFNγ-producing fate and enhanced renal injury (20). This suggests that SH2B3 may have more of a regulatory action in the cDC1 subtype, but further studies are needed to identify specific functions of SH2B3 in these DC subtypes.
Mast cells.
In a normal kidney, mast cells (MCs) are infrequently found but significantly increase in number in diseased kidneys. They regulate many adaptive and innate immune functions and are directly and indirectly involved in the recruitment and function of other immune cell types (10). Upon renal injury, MCs migrate from the bone marrow and infiltrate the kidney where they differentiate. SH2B3 has been shown to inhibit MC proliferation and migration via inhibition of stem cell factor (SCF) signaling through the c-kit receptor (29). SCF signaling in MCs is known to regulate their migration, survival, proliferation, maturation, and secretion of MC mediators. Unlike most other terminal hematopoietic cells, MCs do not lose tyrosine-protein kinase Kit (or CD117) expression upon full differentiation (17). Therefore, SH2B3 is presumed to have a continued effect on MC function in the kidney via inhibition of MC SCF signaling. To date, no studies have examined the influence of MC SH2B3 function in kidney diseases.
T cells.
T cells have been indicated as a major contributor to the pathophysiology of kidney diseases (9). SH2B3 expression is at low levels in T cells but modulated depending on the activation and proliferative T cell states (16). Little is known of the specific function of SH2B3 in these cells. Bone marrow adoptive transfer studies have attempted to identify physiological effects of T cell SH2B3 expression (24, 26), but these studies also transferred other monocytes, DCs, MCs, and macrophages along with the T cells that may have contributed to the observed phenotypes. Katayama et al. (13) addressed this limitation by adoptively transferring splenocytes from both SH2B3−/− and wild-type mice. The cells were labeled with the proliferation stain CFSE, and subsequently coinjected into wild-type mice. They found that, of the CD4+ and CD8+ T cells measured, only the SH2B3−/− CD8+ T cells proliferated, suggesting a T cell-specific effect. Furthermore, these cells were specifically hypersensitive to IL-15 due to disinhibition of JAK3 activation and STAT5 phosphorylation and had a longer in vivo survival. A similar expansion of CD8+ T cells was reported for ANG II-induced hypertension in SH2B3−/− mice (28). This latter study found an increased production of IFNγ by these cells, normally the most prominent IFNγ-producing T cell in the kidney, and CD4+ and double negative T cells. Deficiency of IFNγ was observed to attenuate the ANG II-induced hypertension, suggesting that SH2B3 is important in regulating directly or indirectly IFNγ-producing T cells that impact renal injury.
Endothelial cells.
Endothelial cells (ECs) actively participate in modulating inflammation. They are the major regulatory interface for the trafficking of hematopoietic cells into the kidney and the secretion of cytokines. Furthermore, they regulate other renal cells in the kidney, including glomerular podocytes (27). ECs highly express SH2B3 that is upregulated by TNF-α stimulation (1, 33). In ECs, SH2B3 was shown to negatively regulate integrin signaling via inhibition of the integrin-linked kinase, thereby restricting EC adhesion and migration. Similar regulation of integrins and cell motility by SH2B3 has been observed in platelets, MCs, and megakaryocytes (29, 31, 32), suggesting that SH2B3 is a common regulator of integrins. In ECs, altered SH2B3 inhibition of integrins may affect both endothelial permeability and repair in renal diseases, but further studies are needed. The absence of SH2B3 was previously shown to impair endothelial acetylcholine-stimulated relaxation and reduced aortic nitric oxide (NO) levels (28). This may also be integrin dependent as stimulation by acetylcholine has been observed to increase EC NO production integrin dependently (23).
Perspective and Concluding Remarks
Within the kidney in normal and pathological states, there is a consensus that SH2B3 negatively inhibits proinflammatory cell signaling. The majority of the inflammatory effects of SH2B3 in the kidney have been attributed to T cells due to their potentiation of renal diseases. New evidence, however, suggests that SH2B3 has a much broader physiological function. The cell-specific expression profile of SH2B3 has yet to be fully determined, but SH2B3 mRNA has been found in both the cortical and outer medullary regions of the kidney (26). The cell types discussed above may account for this expression, but the function of SH2B3 in integrins and actin cytoskeleton regulation suggests that SH2B3 may be expressed by additional renal cell types. For example, integrins in focal adhesions are important for glomerular podocyte function (34) and the paracellular permeability of kidney proximal tubule cells (5). To accurately determine SH2B3 expression in these cells, consideration of the cell functional states may be necessary as has been shown for hematopoietic cells.
Recent genetic studies in rodents have linked SH2B3 to disease. Saleh et al. (28) demonstrated that mice lacking SH2B3 have an exaggerated hypertensive response to ANG II. In contrast, Rudemiller et al. (26) mutated the SH2 domain of SH2B3 in rats and demonstrated an attenuation of salt-sensitive hypertension, renal damage, and renal inflammation. These changes were attributed, via bone marrow adoptive transfer, to a shift of infiltrating T cells or other cells of hematopoietic origin to an anti-inflammatory fate through other cell types that may contribute to the disease phenotype.
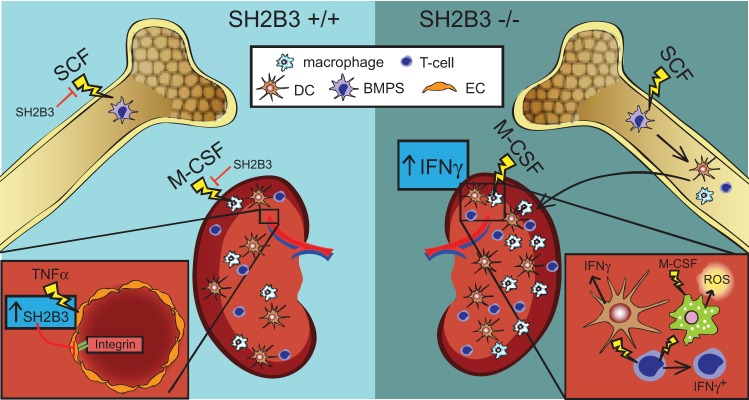
The present review addresses the known extent of SH2B3 functions in cell types that contribute to the pathogenesis of renal diseases. These studies have largely studied the function of SH2B3 by mutating the SH2B3 gene in rodents. Figure 1 shows the changes that occur due to the absence of SH2B3. Normally, SH2B3 inhibits bone marrow progenitor cell SFC signaling, EC integrin signaling, and M-CSF macrophage activation. In ECs, TNF-α increases the expression of SH2B3. In the absence of SH2B3, bone marrow progenitor cells are more proliferative and differentiated, macrophages are sensitized to M-CSF signaling and produce more ROS, DCs produce more IFNγ, and T cells differentiate to more of an IFNγ-producing fate.
Fig. 1.
SH2B3 function in the kidney. Left: known SH2B3 function in the kidney. Right: effects seen in the absence of SH3B3 in the kidney. BMPC, bone marrow progenitor cell; DC, dendritic cell; EC, endothelial cell; M-CSF, macrophage colony-stimulating factor; ROS, reactive oxygen species; SCF, stem cell factor.
GRANTS
This research was partially supported by National Heart, Lung, and Blood Institute Grants HL108880 (to A. Staruschenko) and HL116264 (to D. L. Mattson), American Diabetes Association Grant 1-15-BS-172, Juvenile Diabetes Research Foundation Grant 1-INO-2016-223-A-N, and American Heart Association Grant 16EIA26720006 (to A. Staruschenko).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.B. prepared figures; G.B. drafted manuscript; G.B., D.L.M., and A.S. edited and revised manuscript; G.B., D.L.M., and A.S. approved final version of manuscript.
REFERENCES
- 1.Boulday G, Coulon F, Fraser CC, Soulillou JP, Charreau B. Transcriptional up-regulation of the signaling regulatory protein LNK in activated endothelial cells. Transplantation 74: 1352–1354, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Cao Q, Wang Y, Zheng D, Sun Y, Wang C, Wang XM, Lee VW, Wang Y, Zheng G, Tan TK, Wang YM, Alexander SI, Harris DC. Failed renoprotection by alternatively activated bone marrow macrophages is due to a proliferation-dependent phenotype switch in vivo. Kidney Int 85: 794–806, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Dale BL, Madhur MS. Linking inflammation and hypertension via LNK/SH2B3. Curr Opin Nephrol Hypertens 25: 87–93, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devalliere J, Charreau B. The adaptor Lnk (SH2B3): an emerging regulator in vascular cells and a link between immune and inflammatory signaling. Biochem Pharmacol 82: 1391–1402, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Elias BC, Mathew S, Srichai MB, Palamuttam R, Bulus N, Mernaugh G, Singh AB, Sanders CR, Harris RC, Pozzi A, Zent R. The integrin beta1 subunit regulates paracellular permeability of kidney proximal tubule cells. J Biol Chem 289: 8532–8544, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox ER, Young JH, Li Y, Dreisbach AW, Keating BJ, Musani SK, Liu K, Morrison AC, Ganesh S, Kutlar A, Ramachandran VS, Polak JF, Fabsitz RR, Dries DL, Farlow DN, Redline S, Adeyemo A, Hirschorn JN, Sun YV, Wyatt SB, Penman AD, Palmas W, Rotter JI, Townsend RR, Doumatey AP, Tayo BO, Mosley TH Jr, Lyon HN, Kang SJ, Rotimi CN, Cooper RS, Franceschini N, Curb JD, Martin LW, Eaton CB, Kardia SL, Taylor HA, Caulfield MJ, Ehret GB, Johnson T; International Consortium for Blood Pressure Genome-wide Association Studies (ICBP-GWAS), Chakravarti A, Zhu X, Levy D. Association of genetic variation with systolic and diastolic blood pressure among African Americans: the Candidate Gene Association Resource study. Hum Mol Genet 20: 2273–2284, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gery S, Koeffler HP. Role of the adaptor protein LNK in normal and malignant hematopoiesis. Oncogene 32: 3111–3118, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Gueller S, Goodridge HS, Niebuhr B, Xing H, Koren-Michowitz M, Serve H, Underhill DM, Brandts CH, Koeffler HP. Adaptor protein Lnk inhibits c-Fms-mediated macrophage function. J Leukoc Biol 88: 699–706, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holdsworth SR, Summers SA. Role of mast cells in progressive renal diseases. J Am Soc Nephrol 19: 2254–2261, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Li Y, Tanaka K, Moore KG, Hayashi JI. Cloning and characterization of Lnk, a signal transduction protein that links T-cell receptor activation signal to phospholipase C gamma 1, Grb2, and phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA 92: 11618–11622, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen CJ, Dunbar PR, Wadley RB, Jeet V, Vulink AJ, Hart DN, Radford KJ. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med 207: 1247–1260, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katayama H, Mori T, Seki Y, Anraku M, Iseki M, Ikutani M, Iwasaki Y, Yoshida N, Takatsu K, Takaki S. Lnk prevents inflammatory CD8(+) T-cell proliferation and contributes to intestinal homeostasis. Eur J Immunol 44: 1622–1632, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Leone DA, Kozakowski N, Kornauth C, Waidacher T, Neudert B, Loeffler AG, Haitel A, Rees AJ, Kain R. The phenotypic characterization of the human renal mononuclear phagocytes reveal a co-ordinated response to injury. PLoS One 11: e0151674, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Köttgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O'Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet 41: 677–687, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, He X, Schembri-King J, Jakes S, Hayashi J. Cloning and characterization of human Lnk, an adaptor protein with pleckstrin homology and Src homology 2 domains that can inhibit T cell activation. J Immunol 164: 5199–5206, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Lyman SD, Jacobsen SE. c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood 91: 1101–1134, 1998. [PubMed] [Google Scholar]
- 18.Matsuda M, Shikata K, Makino H, Sugimoto H, Ota Z. Glomerular expression of macrophage colony-stimulating factor and granulocyte-macrophage colony-stimulating factor in patients with various forms of glomerulonephritis. Lab Invest 75: 403–412, 1996. [PubMed] [Google Scholar]
- 19.McCarthy NE, Jones HA, Marks NA, Shiner RJ, Ind PW, Al-Hassi HO, English NR, Murray CM, Lambert JR, Knight SC, Stagg AJ. Inhaled allergen-driven CD1c up-regulation and enhanced antigen uptake by activated human respiratory-tract dendritic cells in atopic asthma. Clin Exp Allergy 37: 72–82, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Mori T, Iwasaki Y, Seki Y, Iseki M, Katayama H, Yamamoto K, Takatsu K, Takaki S. Lnk/Sh2b3 controls the production and function of dendritic cells and regulates the induction of IFN-gamma-producing T cells. J Immunol 193: 1728–1736, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, Carotta S, O'Keeffe M, Bahlo M, Papenfuss A, Kwak JY, Wu L, Shortman K. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol 8: 1217–1226, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Nelson PJ, Rees AJ, Griffin MD, Hughes J, Kurts C, Duffield J. The renal mononuclear phagocytic system. J Am Soc Nephrol 23: 194–203, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paredes-Gamero EJ, Medeiros VP, Farias EH, Justo GZ, Trindade ES, Andrade-Lopes AL, Godinho RO, de Miranda A, Ferreira AT, Tersariol IL, Nader HB. Heparin induces rat aorta relaxation via integrin-dependent activation of muscarinic M3 receptors. Hypertension 56: 713–721, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Garcia A, Ambesi-Impiombato A, Hadler M, Rigo I, LeDuc CA, Kelly K, Jalas C, Paietta E, Racevskis J, Rowe JM, Tallman MS, Paganin M, Basso G, Tong W, Chung WK, Ferrando AA. Genetic loss of SH2B3 in acute lymphoblastic leukemia. Blood 122: 2425–2432, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pindjakova J, Griffin MD. The renal lymph node and immune tolerance to filtered antigens. J Am Soc Nephrol 24: 519–521, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Rudemiller NP, Lund H, Priestley JR, Endres BT, Prokop JW, Jacob HJ, Geurts AM, Cohen EP, Mattson DL. Mutation of SH2B3 (LNK), a genome-wide association study candidate for hypertension, attenuates Dahl salt-sensitive hypertension via inflammatory modulation. Hypertension 65: 1111–1117, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saleem MA. One hundred ways to kill a podocyte. Nephrol Dial Transplant 30: 1266–1271, 2015. [DOI] [PubMed] [Google Scholar]
- 28.Saleh MA, McMaster WG, Wu J, Norlander AE, Funt SA, Thabet SR, Kirabo A, Xiao L, Chen W, Itani HA, Michell D, Huan T, Zhang Y, Takaki S, Titze J, Levy D, Harrison DG, Madhur MS. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest 125: 1189–1202, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon C, Dondi E, Chaix A, de Sepulveda P, Kubiseski TJ, Varin-Blank N, Velazquez L. Lnk adaptor protein down-regulates specific Kit-induced signaling pathways in primary mast cells. Blood 112: 4039–4047, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Soos TJ, Sims TN, Barisoni L, Lin K, Littman DR, Dustin ML, Nelson PJ. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int 70: 591–596, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Takizawa H, Eto K, Yoshikawa A, Nakauchi H, Takatsu K, Takaki S. Growth and maturation of megakaryocytes is regulated by Lnk/Sh2b3 adaptor protein through crosstalk between cytokine- and integrin-mediated signals. Exp Hematol 36: 897–906, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Takizawa H, Nishimura S, Takayama N, Oda A, Nishikii H, Morita Y, Kakinuma S, Yamazaki S, Okamura S, Tamura N, Goto S, Sawaguchi A, Manabe I, Takatsu K, Nakauchi H, Takaki S, Eto K. Lnk regulates integrin alphaIIbbeta3 outside-in signaling in mouse platelets, leading to stabilization of thrombus development in vivo. J Clin Invest 120: 179–190, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan M, Li Y, Xue H, Li Q, Li J. TNF-alpha induces Lnk expression through PI3K-dependent signaling pathway in human umbilical vein endothelial cells. J Surg Res 136: 53–57, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, Guo L, Blattner SM, Mundel P, Kretzler M, Wu C. Formation and phosphorylation of the PINCH-1-integrin linked kinase-alpha-parvin complex are important for regulation of renal glomerular podocyte adhesion, architecture, and survival. J Am Soc Nephrol 16: 1966–1976, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, Chang I, Singh A, Harris RC. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest 122: 4519–4532, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]