Abstract
The mechanisms by which prostanoids contribute to the maintenance of whole body water homeostasis are complex and not fully understood. The present study demonstrates that an EP3-dependent feedback mechanism contributes to the regulation of water homeostasis under high-salt conditions. Rats on a normal diet and tap water were placed in metabolic cages and given either sulprostone (20 μg·kg−1·day−1) or vehicle for 3 days to activate EP3 receptors in the thick ascending limb (TAL). Treatment was continued for another 3 days in rats given either 1% NaCl in the drinking water or tap water. Sulprostone decreased expression of cyclooxygenase 2 (COX-2) expression by ∼75% in TAL tubules from rats given 1% NaCl concomitant with a ∼60% inhibition of COX-2-dependent PGE2 levels in the kidney. Urine volume increased after ingestion of 1% NaCl but was reduced ∼40% by sulprostone. In contrast, the highly selective EP3 receptor antagonist L-798106 (100 μg·kg−1·day−1), which increased COX-2 expression and renal PGE2 production, increased urine volume in rats given 1% NaCl. Sulprostone increased expression of aquaporin-2 (AQP2) in the inner medullary collecting duct plasma membrane in association with an increase in phosphorylation at Ser269 and decrease in Ser261 phosphorylation; antagonism of EP3 with L-798106 reduced AQP2 expression. Thus, although acute activation of EP3 by PGE2 in the TAL and collecting duct inhibits the Na-K-2Cl cotransporter and AQP2 activity, respectively, chronic activation of EP3 in vivo limits the extent of COX-2-derived PGE2 synthesis, thereby mitigating the inhibitory effects of PGE2 on these transporters and decreasing urine volume.
Keywords: COX-2, PGE2, EP3 receptors, AQP2, water homeostasis
prostaglandin e2 (PGE2), a highly abundant lipid metabolite synthesized by cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2), modulates an array of physiological functions in the kidney including tubular salt and water transport (9, 36). For instance, PGE2 contributes to the adjustment of renal function following volume expansion by acting as a mediator of diuresis, and studies show that PGE2 levels are increased to mediate natriuresis in response to high salt intake (10, 23, 44). Thus the use of selective inhibitors of COX-2 (Coxibs) or nonsteroidal anti-inflammatory drugs (NSAIDs) may result in salt and water retention and ultimately to an increase in blood pressure (3, 34, 42). Alternatively, Coxibs attenuate water excretion in pathological polyuric states, thus enhancing antidiuresis (19, 38).
The mechanism by which PGE2 regulates water excretion is complex and likely due to the differential expression of distinct PGE2 receptors (EP1-4) along the nephron that signal through multiple pathways (2, 32). The EP3 receptor modulates urinary concentrating mechanisms in the kidney, is mainly expressed in medullary thick ascending limb (TAL), cortical and inner medullary collecting ducts, and also is present in renal vasculature (5, 37). We previously showed that COX-2-derived PGE2 synthesis in the TAL is regulated by feedback inhibition via EP3 receptors (8, 41). This mechanism was first described in vivo following treatment with Coxibs (41), which resulted in an increase in COX-2 expression along the TAL and is consistent with data showing that treatment with rofecoxib caused an increase in renal COX-2, but not COX-1 expression (13). Moreover, hypertonicity and high salt intake increased COX-2 expression and activity in the TAL concomitant with increased apical expression of EP3 receptors (8). Antagonism of EP3 receptors increased COX-2-derived PGE2 synthesis in response to high-salt conditions, suggesting that the EP3/COX-2 negative feedback axis may limit the extent of COX-2-dependent PGE2 production by the TAL in response to elevated salt levels (8).
Although the vasopressin-mediated increase in water permeability along the collecting duct is a major determinant of whole-body water balance, a role for PGE2 via various EP receptors may provide additional mechanisms that regulate water homeostasis (32). Studies have demonstrated COX-2 feedback inhibition in various cell types in vitro (4, 7) and in vivo (8, 13, 30, 41); however, the functional implications of this phenomenon have not been defined. Since PGE2 inhibits Na-K-2Cl cotransporter (NKCC2) activity in the TAL (18), the nephron segment responsible for establishing the medullary interstitial gradient for water reabsorption along the collecting duct, we determined whether the effects of EP3-mediated regulation of COX-2 in the TAL were associated with changes in the rate of water excretion. In the present study, we show that EP3 receptors can be manipulated in a bidirectional manner to alter water excretion in response to high salt intake.
METHODS
Chemicals and reagents.
All chemicals were of the highest grade commercially available. The anti-COX-2 antibody was purchased from Abcam (Cambridge, MA) and used at a 1:1,000 dilution for immunoblot analysis. An antibody against total AQP2 recognized the 20-amino acid residue (CLKGLEPDTDWEEREVRRRQ), and affinity-purified rabbit polyclonal antibodies to Ser(P)261-AQP2 or Ser(P)269-AQP2 were a gift from Dr. Mark Knepper and used as previously described (14). Tissue culture media was obtained from Life Technologies (Grand Island, NY). Collagenase (type 1A) was from Sigma (St. Louis, MO), and polyvinylidene difluoride (PVDF) membranes were obtained from Amersham (Arlington Heights, IL). The EP3 receptor agonist (sulprostone) and EP3 receptor antagonist (L-798106) were purchased from Sigma and Santa Cruz Biotechnology (Santa Cruz, CA), respectively.
Animals.
Male Sprague-Dawley rats (190–200 g, Charles River Laboratory) were maintained on a standard diet and given tap water ad libitum. Experimental procedures were conducted in accordance with institutional and international guidelines for the welfare of animals (animal welfare assurance number A3362-01 or A5848-01, Office of Laboratory Animal Welfare, PHS, NIH). Animals were given sulprostone (20 μg·kg−1·day−1) or L-798106 (100 μg·kg−1·day−1) subcutaneously via an osmotic minipump, then placed in metabolic cages for 6 days, beginning 3 days before ingestion of 1% NaCl in the drinking water for 3 days. Food consumption, water intake, body weight, and 24-h urine collection in response to treatment with sulprostone or L-798106, and varying Na+ intakes were determined. Urine samples were centrifuged at 15,000 rpm for 15 min, and the supernatant was frozen in aliquots at −80°C until assay. Control groups consisted of animals given vehicle or treated with sulprostone or L-798106 alone for 6 days. At the end of the protocol, kidneys were removed and processed for immunohistochemistry, morphometric analysis, real-time RT-PCR, Western blot analysis, and PGE2 analysis.
Isolation of TAL tubules.
TAL tubules were isolated from rats as previously described (14). Briefly, kidneys were perfused with sterile 0.9% saline via retrograde perfusion of the aorta. The outer medulla (OM) was excised, minced with a sterile blade, and incubated for 10 min at 37°C in a 0.1% collagenase solution gassed with 95% oxygen. The suspension was sedimented on ice, mixed with HBSS containing 2% BSA (Life Technologies), and the supernatant containing the crude suspension of tubules was collected. The collagenase digestion step was repeated three times; the remaining undigested tissue and the combined supernatants were centrifuged for 10 min, resuspended in HBSS, and filtered through a 53-μm nylon mesh membrane (Fisher Scientific, Springfield, NJ). The filtered solution was discarded, and tubules retained on the mesh were resuspended in HBSS and centrifuged at 500 rpm for 10 min; pelleted tubules were used in experiments.
Isolation of total RNA and amplification of cDNA fragments.
Total RNA was isolated using the TRIzol Reagent (Ambion, Carlsbad, CA). Chloroform (0.2 ml) was added at room temperature for 2–3 min followed by centrifugation at 4°C at 12,000 rpm for 15 min. Isopropanol (3 vol) was added to the recovered supernatant, and the mixture was incubated at room temperature for 10 min, then centrifuged at 4°C at 12,000 rpm for 15 min. The supernatant was discarded, and the pellet was washed in 1 ml of 75% EtOH, mixed gently, and centrifuged for 5 min at 7,500 rpm at 4°C; the supernatant was removed, and the pellet was dried for 5–10 min. Total RNA was treated with deoxyribonuclease I for 30 min. A 3-μg aliquot was used for cDNA synthesis using the Superscript Preamplification system (Life Technologies) in a 20-μl reaction mixture containing Superscript II reverse transcriptase (200 U/μl) and random hexamers (50 ng/μl). The reaction was incubated at room temperature for 10 min to allow extension of the primers by reverse transcriptase, then at 42°C for 50 min, 70°C for 15 min, and 4°C for 5 min.
Quantitative real-time RT-PCR analysis.
A 0.5-μg aliquot of total RNA was converted to cDNA using random primers and PowerScript RT (Clontech, Mountain View, CA). The cDNA from each RNA sample was placed in a 20-μl RT-PCR mixture using a FastStart DNA Master SYBR Green I kit (Roche, Mannheim, Germany) supplemented with 3 mM MgCl2 and Platinum Taq polymerase (Invitrogen, Carlsbad, CA). Quantitative real-time PCR (qRT-PCR) was used to determine the accumulation of mRNA, using specific primer pairs for rat COX-2: (forward: 5′- GTCTCTCAATGAGTACCGCA-3′; reverse: 5′-GAGCTCCAAGTTCTACCATG-3′), rat EP3 (forward: 5′-GAGACGGCTATCCAGCTTAT-3′; reverse: 5′- GGCGATTAGGAAGGAATTGC-3′), and rat AQP2 (forward: 5′-GTAGAGCTCTTCCTGACCAT-3′; reverse: 5′-GATCCAGAAGACCCAGTGAT-3′). Input cDNAs were normalized using β-actin, and the efficiency of primer pair amplification was determined using a standard curve generated using protocols described previously. The 2(−ΔΔCT) method was used to evaluate changes in COX-2 and EP3 mRNA accumulation.
Measurement of PGE2.
PGE2 content in rat TAL tubules and OM tissues were determined by liquid chromatography-tandem mass spectrometry (LC-MS-MS) using a modified method according to the previously published assay (43). Briefly, frozen OM tissues were homogenized and extracted for prostaglandins and quantified by LC-MS/MS. Analysis of PGE2 levels in TAL tubules also was determined by using an enzyme immunoassay kit (catalog no. RPN22210; GE Healthcare) according to the manufacturer's instructions. The data were normalized by protein amount, which was determined by a Bradford protein assay (Bio-Rad, Hercules, CA).
Western blot analysis.
Tissue was solubilized with Triton X-100 lysis buffer after protease inhibitors (Roche) were added for COX-2. The tissue was homogenized in ice-cold sucrose solution (250 mM sucrose, 10 mM triethanolamine, pH 7.6, containing 1 mg/ml leupeptin, 0.1 mg/ml PMSF, and 1× HALT phosphatase inhibitor cocktail, Pierce, Rockland, IL) for total AQP2, pS261-AQP2, and pS269-AQP2. Protein samples were heated in boiling water with loading buffer, and concentration was determined with a Bio-Rad protein assay kit (Hercules, CA). Equal amounts of protein were separated by SDS-PAGE and electrophoretically transferred to nitrocellulose membranes. Following blocking, membranes were probed at 4°C overnight with appropriate primary antibodies, followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies (Amersham Pharmacia Biotech, Pittsburgh, PA). Membranes were washed, and proteins were detected by enhanced chemiluminescence (ECL; Amersham, Pittsburgh, PA).
Immunohistochemistry analysis.
The kidneys from rats were fixed by retrograde perfusion via the abdominal aorta with 3% paraformaldehyde in 0.1 M cacodylate buffer, pH 7.4, and then tissues were immersion fixed in 4% paraformaldehyde for 24 h at room temperature and embedded in paraffin wax. The paraffin-embedded tissues were cut into 3-μm sections on a rotary microtome (Leica Microsystems, Herlev, Denmark). For the AQP2 assay, the sections were deparaffinated and rehydrated. To expose antigens, kidney sections were boiled in a target retrieval solution (1 mmol/l Tris, pH 9.0, with 0.5 mM EGTA) for 10 min. After cooling, the nonspecific binding was prevented by incubating the sections in 50 mM NH4Cl in PBS for 30 min, followed by blocking in PBS containing 1% BSA, 0.05% saponin, and 0.2% gelatin. Samples were permeabilized in PBS plus 1% BSA and 0.1% Triton X-100 for 10 min; blocking was performed for 2–3 h in PBS/1% BSA. Sections were incubated with primary antibodies diluted in PBS with 0.1% BSA. The sections were treated sequentially with either primary anti-AQP2 polyclonal rabbit antibodies (1:1,000 dilution) and secondary antibody diluted 1:500 in PBS containing 1% BSA (1:200 dilution of donkey anti-rabbit-conjugated with Alexa Fluor 488; Invitrogen). The slides were examined using a Zeiss AX10 microscope equipped for epifluorescence illumination.
Statistics.
All data are presented as means ± SE. Statistical analyses were performed using one-way ANOVA followed by Tukey's multiple comparisons test and an unpaired Student's t-test using GraphPad Prism software (GraphPad Software, San Diego, CA). Differences with P < 0.05 were considered statistically significant.
RESULTS
Selective activation of EP3 in the TAL inhibits COX-2 expression and PGE2 synthesis induced by high salt intake.
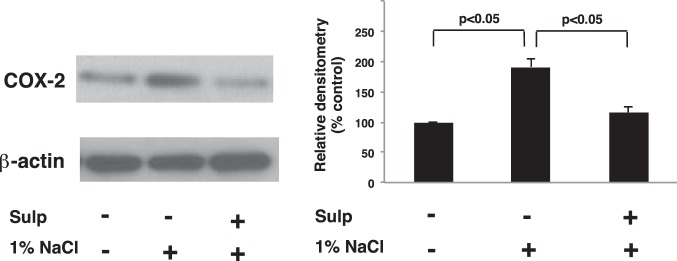
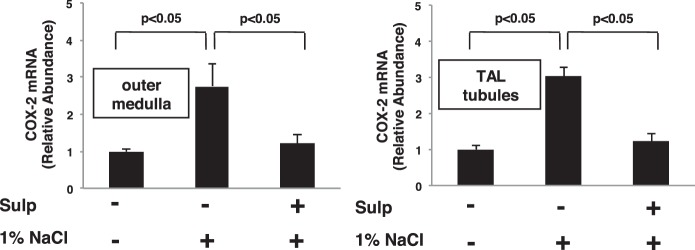
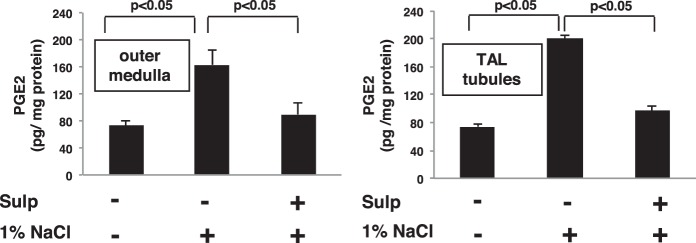
We recently showed that COX-2 expression induced by Coxibs in the TAL is under the control of an EP3-dependent feedback mechanism (41). Since the effects of EP3 activation on COX-2 expression induced by other stimuli is not known, we determined whether EP3 feedback inhibition regulates COX-2 expression in the TAL induced by high salt intake. Rats were pretreated with either vehicle or sulprostone for 3 days while ingesting a normal diet and tap water, then given 1% NaCl in the drinking for 3 days while continuing to receive either vehicle (DMSO) or sulprostone. Western blot analysis showed that sulprostone inhibits the increase in COX-2 protein expression in mTAL tubules induced by 1% NaCl in the drinking water (Fig. 1). We previously showed that sulprostone alone had a negligible effect on COX-2 expression by Western blot analysis, EP3 receptors are expressed on the apical membrane of the TAL, and that EP1 receptors are absent from this segment of the nephron (41). The suppression of COX-2 protein expression via EP3 activation may involve a mechanism that inhibits either COX-2 gene transcription or mRNA stability as the increase in COX-2 mRNA induced by 1% NaCl in OM and mTAL tubules was inhibited by sulprostone (Fig. 2). The decreases in COX-2 protein and mRNA levels were associated with a simultaneous reduction in PGE2 content in the OM and mTAL tubules from sulprostone-treated rats that consumed 1% NaCl compared with those consuming tap water (Fig. 3). These findings suggest that EP3 receptor activation attenuates COX-2 expression and activity induced by high salt intake and are consistent with a COX-2-dependent increase in PGE2 synthesis by the TAL in response to high salt (8).
Fig. 1.
Activation of EP3 in the thick ascending limb (TAL) attenuates cyclooxygenase 2 (COX-2) protein expression induced by hypertonic saline. COX-2 protein expression was determined by Western blot analysis in medullary (m) TAL tubules isolated from vehicle and sulprostone (sulp)-treated rats. Rats were given 1% NaCl in the drinking water for 3 days after pretreatment for 3 days with either vehicle or sulp. COX-2 increased in response to hypertonic NaCl compared with control and was decreased in TAL tubules from rats treated with sulp. The data in the histogram are presented as means ± SE; n = 3.
Fig. 2.
EP3 activation inhibits COX-2 mRNA accumulation induced by high salt. COX-2 mRNA increased in response to 1% NaCl compared with control in outer medulla and TAL tubules. Treatment with sulprostone decreased COX-2 mRNA levels induced by 1% NaCl, suggesting that activation of EP3 exerts a negative feedback effect on COX-2. The data are presented as means ± SE; n = 3.
Fig. 3.
Stimulation of EP3 in the TAL decreases PGE2 synthesis induced by high NaCl intake. PGE2 content in the outer medulla (OM) was determined by liquid chromatography-tandem mass spectrometry and in supernatant from isolated mTAL tubules by ELISA. In both instances, PGE2 levels increased after rats consumed 1% NaCl compared with tap water; this effect was prevented in rats treated with sulprostone. The data are presented as means ± SE; n = 3.
EP3 receptor modulation alters water excretion upon high salt intake.
As PGE2 antagonizes the hydrosmotic effects of AVP, we determined whether the decrease in PGE2 levels resulting from suppression of COX-2 by EP3 activation with sulprostone would alter urinary water excretion. Rats were given vehicle, sulprostone, or the highly selective EP3 antagonist L-798106 for 3 days while ingesting a normal-salt diet and water ad libitum; each group was then given 1% NaCl in the drinking water for an additional 3 days. In rats consuming 1% NaCl in the water drinking, sulprostone decreased urine volume compared with vehicle-treated rats (Fig. 4). The effect was evident 24 h after rats were switched from tap water to hypertonic saline and was sustained for the 3 days, as shown in Fig. 4A and, in fact, persisted for at least 10 days (not shown). The decrease in urine volume in sulprostone-treated rats also was associated with an increase in urine osmolality (Fig. 4B). In contrast, treatment with L-798106 increased urine volume in rats given 1% NaCl compared with vehicle-treated rats; the kinetics and duration of the effect were similar to that observed with sulprostone (Fig. 5). No differences in urine volume were observed for rats given vehicle, sulprostone, or L-798106 under normal-salt conditions (Figs. 4 and 5). There also were no differences in food intake between vehicle- and drug-treated groups receiving either tap water or 1% NaCl; however, changes in water intake paralleled changes in urine output (Fig. 6, A–D). Neither sulprostone nor L-798106 altered water intake and urine output in rats ingesting tap water. In contrast, sulprostone decreased urine volume and water intake (Fig. 6, A and B), while L-798106 increased urine volume and water intake in rats given 1% NaCl in the drinking water (Fig. 6, C and D). Collectively, the data indicate that while modulation of EP3 does not affect water excretion under normal-salt conditions, the diuretic effect of PGE2 to maintain water balance is influenced in a bidirectional manner by the degree of EP3 activation in response to hypertonic saline intake.
Fig. 4.
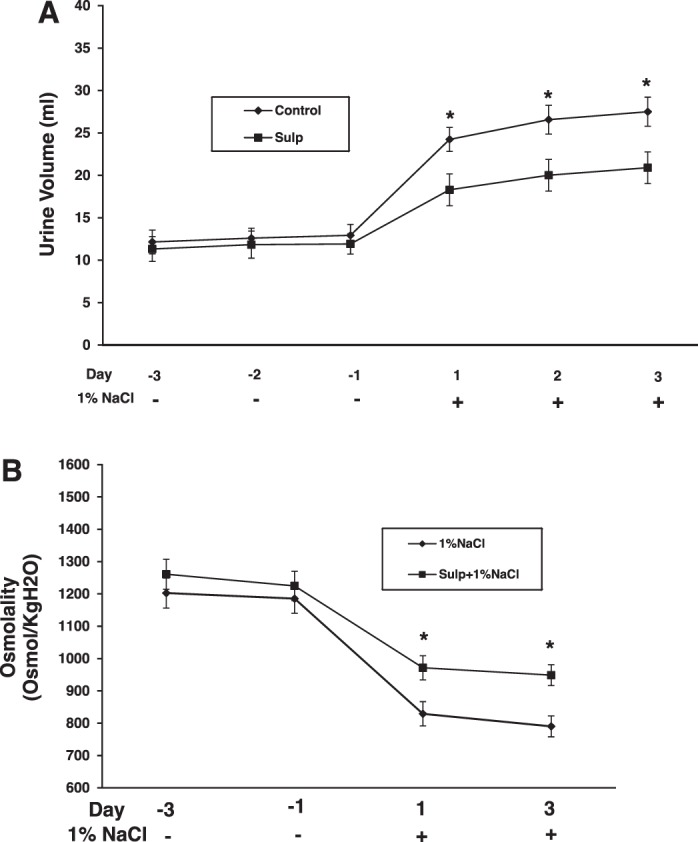
Sulprostone attenuates urine output in rats ingesting high salt. The increase in urine production induced by 1% NaCl intake was attenuated by sulprostone (A) and accompanied by an increase in urine osmolality (B). Data are shown as means ± SE; n = 6/group in A and n = 4 in B. *P < 0.05.
Fig. 5.
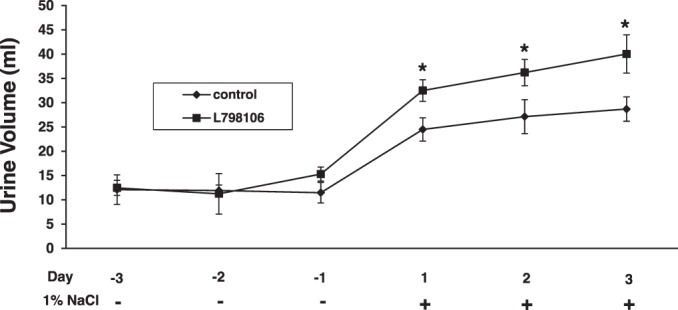
Inhibition of EP3 increases urine excretion in rats ingesting high salt. The increase in urine production induced by 1% NaCl intake was augmented in rats treated with L-798106, a selective EP3 receptor antagonist, suggesting that inhibition of EP3 facilitates the diuretic effects of PGE2. Data are shown as means ± SE; n = 6/group. *P < 0.05.
Fig. 6.
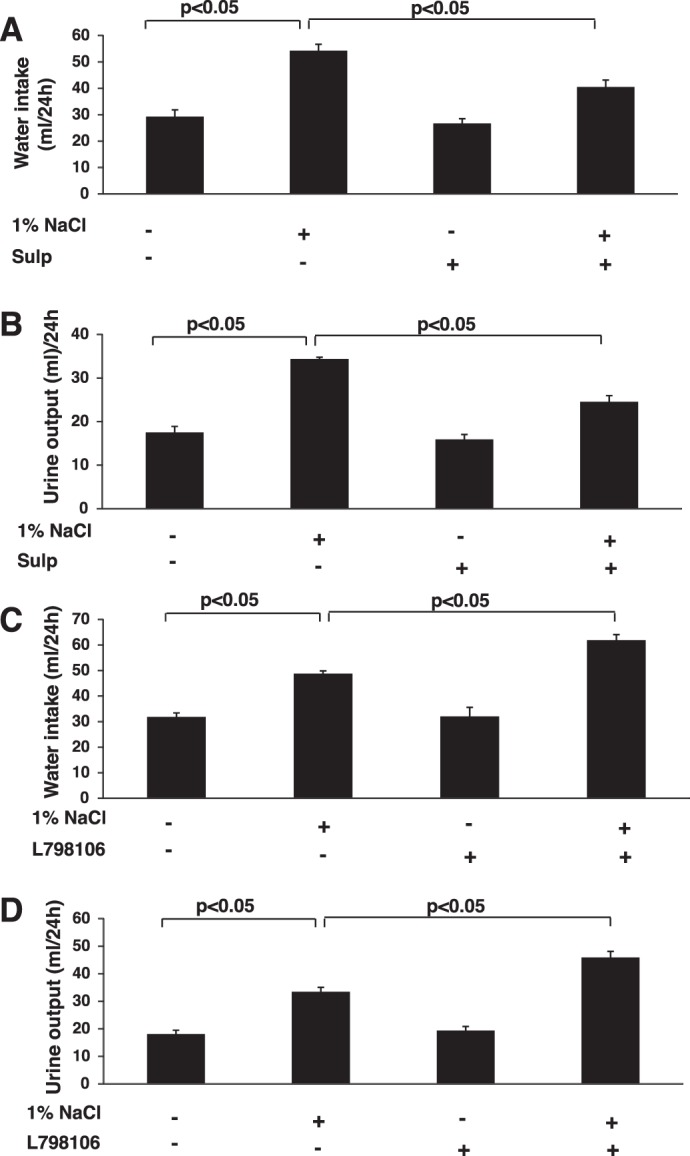
Effects of EP3 activation and inhibition on water intake and urine output under normal- and high-salt conditions. Water intake and urine output were determined after pretreatment with sulprostone (A and B) or L-798106 (C and D) for 3 days in rats ingesting tap water or 1% NaCl for 3 days concomitant with the continuation of drug treatment. The data are presented as means ± SE; n = 6.
EP3 receptors modulate AQP2 expression in the inner medulla.
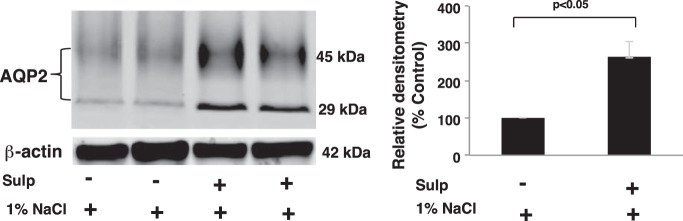
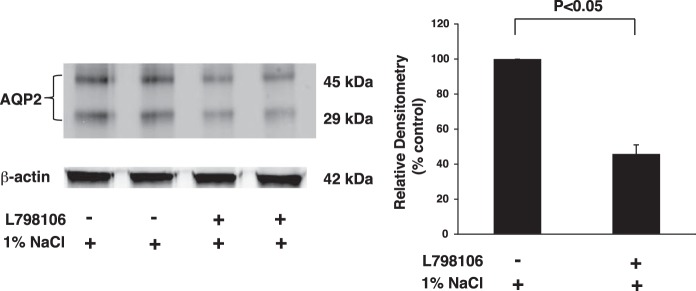
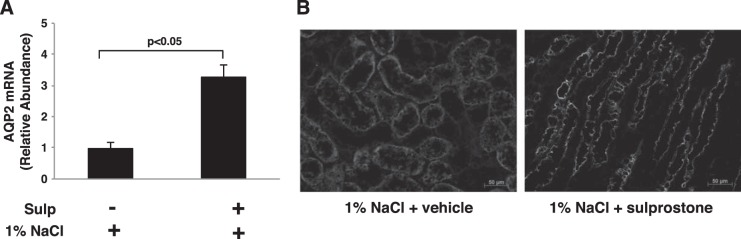
Water permeability and trafficking of the water channel AQP2, which plays a major role in water reabsorption and urine excretion, are regulated by PGE2 (31). Accordingly, effects of the EP3/COX-2 negative feedback axis on AQP2 expression and cellular localization were determined in rats given vehicle or sulprostone for 3 days while ingesting 1% NaCl in the drinking water. Sulprostone increased inner medullary expression of both nonglycosylated (29 kDa) and glycosylated (45 kDa) AQP2 compared with vehicle-treated rats (Fig. 7). Conversely, AQP2 expression was reduced by >50% in rats treated with the EP3 receptor antagonist L-798106 (Fig. 8). The nearly three-fold increase in AQP2 protein expression was accompanied by a similar increase in AQP2 mRNA accumulation (Fig. 9A), suggesting that a mechanism involving increased gene transcription and/or mRNA stability was initiated following EP3 activation in the context of hypertonic saline intake. As glycosylation may be important for the exit of AQP2 from the Golgi complex and subsequent targeting to the membrane, we evaluated the effects of sulprostone on AQP2 localization in the inner medullary collecting duct (IMCD). Diffuse staining of AQP2 was observed in the IMCD obtained from rats given 1% NaCl for 3 days and pretreated with vehicle (Fig. 9B, left). In contrast, the intracellular staining pattern in rats that received sulprostone is consistent with enhanced expression at the plasma membrane (Fig. 9B, right). Overall, the data are consistent with the notion that increased expression and function of AQP2 in the collecting duct contribute to the antidiuretic effect of sulprostone and diuretic effect of L-798106 under high-salt conditions.
Fig. 7.
Sulprostone increases aquaporin-2 (AQP2) protein expression in the inner medulla. Expression of AQP2 protein was analyzed by Western blotting of inner medullary homogenates. The results show that AQP2 increased after activation of EP3 and are consistent with data showing a decrease in urine excretion in response to elevated NaCl intake in rats treated with sulprostone. The lanes represent data from individual rats; 3 similar experiments were performed. The data in the histogram are presented as means ± SE; n = 6.
Fig. 8.
L-798106 decreases AQP2 protein expression in the inner medulla. Expression of AQP2 protein was analyzed by Western blotting of inner medullary homogenates after inhibition of EP3 receptors. The data are consistent with those showing an increase in urine output in response to elevated NaCl intake in rats treated with L-798106. The lanes represent data from individual rats; 3 similar experiments were performed. The data in the histogram are presented as means ± SE; n = 6.
Fig. 9.
Sulprostone increases AQP2 mRNA accumulation and trafficking to the plasma membrane in response to hypertonic saline. Rats were pretreated with vehicle or sulprostone for 3 days while ingesting a normal diet and tap water. AQP2 mRNA assessed by qRT-PCR (A) and AQP2 expression determined by immunohistochemical analysis (B) were increased in sulprostone-treated rats that were then given 1% NaCl in the drinking water for 3 days. The data in A are presented as means ± SE; n = 4; representative figures are shown in B.
Effects of EP3/COX-2 negative feedback on site-specific phosphorylation of AQP2.
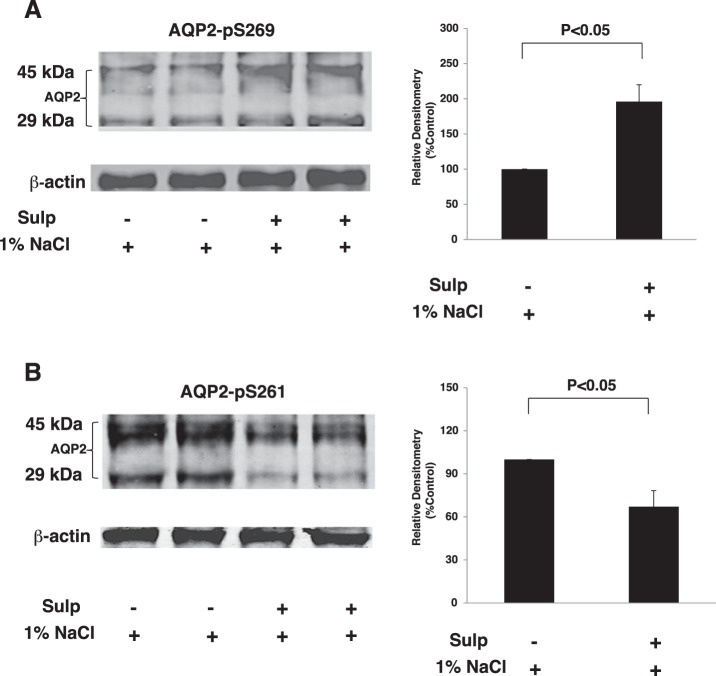
AQP2 accumulation at the plasma membrane is associated with increases in phosphorylation of Ser269, which alter subsequent protein-protein interactions that reduce internalization of AQP2 (24). Since PGE2 can induce AQP2 internalization (26) and sulprostone increased AQP2 abundance and decreased PGE2 production, we evaluated phosphorylation of AQP2 at Ser261 and Ser269 under high-salt conditions. Phosphorylation of AQP2 at Ser269 increased in the inner medulla isolated from rats pretreated with sulprostone for 3 days and ingesting 1% NaCl for an additional 3 days compared with vehicle-treated rats (Fig. 10A). In contrast, there was a concomitant decrease in phosphorylation of AQP2 at Ser261 in sulprostone-treated rats ingesting hypertonic saline (Fig. 10B). These data suggest that under high-salt conditions, chronic activation of EP3 receptors can modulate phosphorylation of AQP2, subsequent to AVP-induced Ser256 phosphorylation, at Ser261 and Ser269 residues in a manner that promotes AQP2 trafficking to the apical membrane, thereby mediating a decrease in water excretion.
Fig. 10.
Effects of sulprostone on AQP2 phosphorylation. The inner medulla was isolated from vehicle- or sulprostone-treated rats given 1% NaCl in the drinking water for 3 days. Western blot analysis was performed using a phospho-specific antibody against Ser269 (A) or Ser261 (B) residue of AQP2. The samples in each lane are from individual rats, and the blot is a representative figure; n = 6.
DISCUSSION
The present study was conducted to determine whether the feedback mechanism by which EP3 receptors regulate COX-2-dependent PGE2 production in the TAL was associated with altered urine output under normal- and high-salt conditions. We demonstrated that sulprostone inhibited COX-2 expression induced by high salt in the TAL concomitant with a decrease in PGE2 levels, indicating that activation of EP3 receptors downregulates COX-2-derived PGE2 synthesis in this segment of the nephron. Conversely, COX-2-derived PGE2 production in response to high-salt conditions increases when EP3 receptors in the TAL are blocked with L-798106 (8). Accordingly, urine output decreased when EP3 receptors were activated, and increased subsequent to EP3 inhibition. Activation of EP3 under high-salt conditions increased the total expression and plasma membrane abundance of AQP2 in association with an increase in its phosphorylation at Ser269 and decrease in phosphorylation at Ser261, an effect that was reversed when EP3 receptors were blocked. The data suggest that EP3 feedback inhibition contributed to an increase in AQP2, which altered the excretion of an appropriate water load in response to high-salt intake. Collectively, these findings are consistent with our previous study showing that the increase in PGE2 synthesis induced by high salt is regulated by EP3 receptor feedback inhibition and support the hypothesis that renal production of this prostanoid is an important determinant of water excretion (8, 31).
The observations in this study may, at first glance, appear counterintuitive as PGE2 exhibits several actions that antagonize the effects of AVP (6, 11, 35). Among several EP3 signaling pathways, acute Gi-mediated inhibition of cAMP decreases NKCC2 activity in the TAL and antagonizes AVP-dependent AQP2-dependent water reabsorption in the collecting duct (27, 33). However, chronic regulation of these transporters via EP3 receptors, the expression of which is increased in response to hypertonic conditions, is subject to feedback inhibition that limits the extent of COX-2-derived PGE2 production (8, 17, 20). Thus the short-term signaling events may be superseded by the feedback inhibition axis, which limits the production of PGE2. Accordingly, when presented with high-salt conditions in the context of a coordinated induction of COX-2-derived PGE2 synthesis and upregulation of EP3 in the TAL, sulprostone mitigates the increase in PGE2 required to offset AVP-dependent increases in AQP2 expression and function (19). In the absence of PGE2, AQP2 expression and phosphorylation at Ser269 increased along with trafficking to the plasma membrane and the ability to reabsorb water. Similarly, treatment with a COX-2 inhibitor ameliorated lithium-induced nephrogenic diabetes insipidus by upregulating NKCC2 and AQP2 (19).
Previous studies have noted the phenomenon of increased COX-2 protein expression subsequent to COX inhibition in the kidney; however, neither the mechanism responsible for the increase nor functional implications have been addressed (4, 7, 13, 30). For instance, increased COX-2 expression was observed in a study showing that COX-2 inhibition prevented the downregulation of renal transport proteins after bilateral ureteral obstruction (30). The present study considered the functional implications of COX-2 feedback inhibition via the EP3-dependent mechanism that operates in response to COX-2 inhibition and high salt intake (8, 41). Our recent studies showed that EP3, the predominant prostanoid receptor expressed in the TAL, is expressed on the apical membrane under normal- and high-salt conditions (8, 41). Thus, even though sulprostone acts as a dual EP3/EP1 agonist and has a similar affinity as PGE2 for these receptors (21, 37), the TAL does not express EP1, a finding that is confirmed by observations showing functional effects via activation of EP3, but not EP1, in the TAL (1, 41). The effects of PGE2 on AQP2 also do not appear to be EP1 dependent since an EP1 antagonist had no effect on AVP-dependent AQP2 targeting, and EP1 receptor stimulation does not decrease AQP2 membrane targeting or water permeability in the collecting duct (32). In addition, PGE2 inhibits the AVP-induced increase in water permeability in IMCDs via EP1 receptors, an effect that would tend to increase urine volume rather than decrease it, as shown in response to sulprostone in the present study (25). As a complementary approach, we studied the contribution of the COX-2-PGE2-EP3 axis to the regulation of urine output by using a highly selective EP3 receptor antagonist, L-798106. In contrast to EP3 activation with sulprostone, blocking EP3 increased COX-2 expression and activity by relieving the effects of EP3 feedback inhibition (8, 41). The net result in the kidney is an increase in COX-2-derived PGE2 production in the context of minimal EP3 signaling and the residual functional capabilities of the remaining EP subtypes. Thus the increase in urine output observed under high-salt conditions in rats treated with L-798106 may, for instance, have occurred via activation of other EP such as EP2, which are vasodilatory and important for mediating the natriuretic actions of PGE2 (3). We currently are determining whether the effects of PGE2 generated via the EP3/COX-2 feedback mechanism in the TAL exerts paracrine effects on other nephron segments and/or if a similar feedback mechanism is operative in the collecting duct.
Previous studies showed that basal water permeability is not affected by treatment with sulprostone alone (12). In contrast, chronic treatment with sulprostone significantly decreased urine output, COX-2 expression, and PGE2 production in rats ingesting high salt, suggesting that the natriuretic response that limits salt and water retention may have become impaired and that EP3 receptor activation is participating in urine volume regulation under these conditions. Expression and trafficking of AQP2 to the apical membrane in the collecting duct is regulated by the increase in cAMP-mediated signaling after stimulation of vasopressin 2 receptors by AVP, leading to PKA activation. Subsequently, AQP2 is phosphorylated and redistributed to the plasma membrane from intracellular vesicles after phosphorylation at Ser256 by PKA, which is necessary for AVP-mediated apical translocation (28). Phosphorylation of AQP2 at Ser261 and Ser269 also is critical for AQP2 trafficking to the membrane and its overall function (15, 16, 39). In the present study, we showed that chronic EP3 activation under high-salt conditions was associated with increased AQP2 phosphorylation at Ser269 and decreased phosphorylation at Ser261. Phosphorylation of AQP2 at Ser269 depends on prior phosphorylation at Ser256 and was observed exclusively in the apical membrane of the collecting duct where it enhances retention of AQP2, suggesting that activation of the EP3-COX-2-PGE2 axis facilitates AVP-mediated AQP2 trafficking by limiting renal PGE2 production (14). While Ser269 is the only phosphorylated form detected in the apical plasma membrane, AQP2 trafficking in response to AVP also is regulated by endocytosis and exocytosis, which help determine its steady-state abundance there (22, 29). A decrease in AQP2 phosphorylation at Ser261 in the present study suggests that phosphorylation at this site, which is associated with a different distribution mechanism from total AQP2 as well as AQP2 phosphorylated at Ser256, follows ubiquitination and endocytosis and may stabilize AQP2 ubiquitination and intracellular localization (15, 39). Thus the sulprostone-mediated reduction in phosphorylation at Ser261 is consistent with the steady-state redistribution of AQP2 from vesicles to the apical membrane and a decrease in urine output (40).
Urinary volume and concentration are regulated primarily by AVP, yet PGE2 plays an important role by providing an alternative regulatory mechanism. EP3 contributes to the maintenance of low urine osmolality under normal conditions, and genetic deletion of EP3 revealed that compensatory mechanisms regulate urine osmolality in its absence (5). Indeed, the expression and cell-type restricted signaling of EP subtypes contribute to the multifaceted effects of PGE2 on water excretion by the kidney. The contribution of feedback regulation of COX-2 by EP3 provides an additional layer of functional regulation and may supplant the short-term regulatory effects attributed to PGE2 in isolated cell and tubule preparations. For instance, it is interesting to speculate that the paradoxical increase in PGE2 production in P2Y2-deficient mice subjected to a model of lithium-induced polyuria and associated with a decrease in EP3 expression is related to increases in COX-2-derived PGE2 synthesis observed by pharmacological inhibition of EP3 (8, 41, 45). The present study indicates that alterations in EP3 activation status affect water balance in response to high salt intake and suggest that additional roles for the COX-2-PGE2-EP3 axis may be revealed in clinical disorders of water balance.
GRANTS
This work was supported by grants from the American Heart Association (12GRNT12060350), Fondecyt (1130741), and the National Heart, Lung, and Blood Institute (HL34300).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.H., M.Q.-M., and N.R.F. provided conception and design of research; S.H., A.D., M.Q.-M., and H.J. performed experiments; S.H., A.D., M.Q.-M., and N.R.F. analyzed data; S.H., A.D., M.Q.-M., H.J., and N.R.F. interpreted results of experiments; S.H., A.D., M.Q.-M., and N.R.F. prepared figures; S.H., M.Q.-M., and N.R.F. drafted manuscript; S.H., A.D., M.Q.-M., H.J., and N.R.F. edited and revised manuscript; N.R.F. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Lisa Satlin, Mount Sinai School of Medicine, for determination of urine osmolality (P30 DK079307; The Pittsburgh Center for Kidney Research: Physiology Core).
REFERENCES
- 1.Aarab L, Siaume-Perez S, Chabardes D. Cell-specific coupling of PGE2 to different transduction pathways in arginine vasopressin- and glucagon-sensitive segments of the rat renal tubule. Br J Pharmacol 126: 1041–1049, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol 41: 661–690, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Zhao M, He W, Milne GL, Howard JR, Morrow J, Hebert RL, Breyer RM, Hao CM. Increased dietary NaCl induces renal medullary PGE2 production and natriuresis via the EP2 receptor. Am J Physiol Renal Physiol 295: F818–F825, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferguson S, Hebert RL, Laneuville O. NS-398 upregulates constitutive cyclooxygenase-2 expression in the M-1 cortical collecting duct cell line. J Am Soc Nephrol 10: 2261–2271, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Fleming EF, Athirakul K, Oliverio MI, Key M, Goulet J, Koller BH, Coffman TM. Urinary concentrating function in mice lacking EP3 receptors for prostaglandin E2. Am J Physiol Renal Physiol 275: F955–F961, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Ge Y, Strait KA, Stricklett PK, Yang T, Kohan DE. Role of prostaglandins in collecting duct-derived endothelin-1 regulation of blood pressure and water excretion. Am J Physiol Renal Physiol 293: F1805–F1810, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Hao CM, Komhoff M, Guan Y, Redha R, Breyer MD. Selective targeting of cyclooxygenase-2 reveals its role in renal medullary interstitial cell survival. Am J Physiol Renal Physiol 277: F352–F359, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Hao S, Hernandez A, Quiroz-Munoz M, Cespedes C, Vio CP, Ferreri NR. PGE2 EP3 receptor downregulates COX-2 expression in the medullary thick ascending limb induced by hypertonic NaCl. Am J Physiol Renal Physiol 307: F736–F746, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Harris RC, Breyer MD. Physiological regulation of cyclooxygenase-2 in the kidney. Am J Physiol Renal Physiol 281: F1–F11, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest 94: 2504–2510, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hebert RL, Jacobson HR, Breyer MD. Prostaglandin E2 inhibits sodium transport in rabbit cortical collecting duct by increasing intracellular calcium. J Clin Invest 87: 1992–1998, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hebert RL, Jacobson HR, Fredin D, Breyer MD. Evidence that separate PGE2 receptors modulate water and sodium transport in rabbit cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 265: F643–F650, 1993. [DOI] [PubMed] [Google Scholar]
- 13.Hocherl K, Kammerl MC, Schumacher K, Endemann D, Grobecker HF, Kurtz A. Role of prostanoids in regulation of the renin-angiotensin-aldosterone system by salt intake. Am J Physiol Renal Physiol 283: F294–F301, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Hoffert JD, Fenton RA, Moeller HB, Simons B, Tchapyjnikov D, McDill BW, Yu MJ, Pisitkun T, Chen F, Knepper MA. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem 283: 24617–24627, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffert JD, Nielsen J, Yu MJ, Pisitkun T, Schleicher SM, Nielsen S, Knepper MA. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am J Physiol Renal Physiol 292: F691–F700, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA 103: 7159–7164, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen BL, Mann B, Skott O, Kurtz A. Differential regulation of renal prostaglandin receptor mRNAs by dietary salt intake in the rat. Kidney Int 56: 528–537, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Kaji M, Chase J, HS, Eng JP, Diaz J. Prostaglandin E2 inhibits Na-K-2Cl cotransport in medullary thick ascending limb cells. Am J Physiol Cell Physiol 271: C354–C361, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Kim GH, Choi NW, Jung JY, Song JH, Lee CH, Kang CM, Knepper MA. Treating lithium-induced nephrogenic diabetes insipidus with a COX-2 inhibitor improves polyuria via upregulation of AQP2 and NKCC2. Am J Physiol Renal Physiol 294: F702–F709, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Kim JA, Sheen MR, Lee SD, Jung JY, Kwon HM. Hypertonicity stimulates PGE2 signaling in the renal medulla by promoting EP3 and EP4 receptor expression. Kidney Int 75: 278–284, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Kiriyama M, Ushikubi F, Kobayashi T, Hirata M, Sugimoto Y, Narumiya S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol 122: 217–224, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knepper MA, Nielsen S. Kinetic model of water and urea permeability regulation by vasopressin in collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 265: F214–F224, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Mann B, Hartner A, Jensen BL, Kammerl M, Kramer BK, Kurtz A. Furosemide stimulates macula densa cyclooxygenase-2 expression in rats. Kidney Int 59: 62–68, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Moeller HB, Praetorius J, Rutzler MR, Fenton RA. Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc Natl Acad Sci USA 107: 424–429, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nadler SP, Zimpelmann JA, Hebert RL. PGE2 inhibits water permeability at a post-cAMP site in rat terminal inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 262: F229–F235, 1992. [DOI] [PubMed] [Google Scholar]
- 26.Nejsum LN, Zelenina M, Aperia A, Frøkiær J, Nielsen S. Bidirectional regulation of AQP2 trafficking and recycling: involvement of AQP2-S256 phosphorylation. Am J Physiol Renal Physiol 288: F930–F938, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA 92: 1013–1017, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen S, Knepper MA. Vasopressin activates collecting duct urea transporters and water channels by distinct physical processes. Am J Physiol Renal Fluid Electrolyte Physiol 265: F204–F213, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Norregaard R, Jensen BL, Li C, Wang W, Knepper MA, Nielsen S, Frøkiær J. COX-2 inhibition prevents downregulation of key renal water and sodium transport proteins in response to bilateral ureteral obstruction. Am J Physiol Renal Physiol 289: F322–F333, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Olesen ET, Fenton RA. Is there a role for PGE2 in urinary concentration? J Am Soc Nephrol 24: 169–178, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Olesen ET, Rutzler MR, Moeller HB, Praetorius HA, Fenton RA. Vasopressin-independent targeting of aquaporin-2 by selective E-prostanoid receptor agonists alleviates nephrogenic diabetes insipidus. Proc Natl Acad Sci USA 108: 12949–12954, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ortiz PA. cAMP increases surface expression of NKCC2 in rat thick ascending limbs: role of VAMP. Am J Physiol Renal Physiol 290: F608–F616, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Schlondorff D. Renal complications of nonsteroidal anti-inflammatory drugs. Kidney Int 44: 643–653, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Sonnenburg WK, Smith WL. Regulation of cyclic AMP metabolism in rabbit cortical collecting tubule cells by prostaglandins. J Biol Chem 263: 6155–6160, 1988. [PubMed] [Google Scholar]
- 36.Stokes JB. Integrated actions of renal medullary prostaglandins in the control of water excretion. Am J Physiol Renal Fluid Electrolyte Physiol 240: F471–F480, 1981. [DOI] [PubMed] [Google Scholar]
- 37.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem 282: 11613–11617, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi N, Chernavvsky DR, Gomez RA, Igarashi P, Gitelman HJ, Smithies O. Uncompensated polyuria in a mouse model of Bartter's syndrome. Proc Natl Acad Sci USA 97: 5434–5439, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamma G, Robben JH, Trimpert C, Boone M, Deen PM. Regulation of AQP2 localization by S256 and S261 phosphorylation and ubiquitination. Am J Physiol Cell Physiol 300: C636–C646, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Trepiccione F, Pisitkun T, Hoffert JD, Poulsen SB, Capasso G, Nielsen S, Knepper MA, Fenton RA, Christensen BM. Early targets of lithium in rat kidney inner medullary collecting duct include p38 and ERK1/2. Kidney Int 86: 757–767, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Vio CP, Quiroz-Munoz M, Cespedes C, Ferreri NR. Prostaglandin E2 EP3 receptor regulates cyclooxygenase-2 expression in the kidney. Am J Physiol Renal Physiol 303: F449–F457, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White WB. Cardiovascular effects of the cyclooxygenase inhibitors. Hypertension 49: 408–418, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Yang P, Chan D, Felix E, Madden T, Klein RD, Shureiqi I, Chen X, Dannenberg AJ, Newman RA. Determination of endogenous tissue inflammation profiles by LC/MS/MS: COX- and LOX-derived bioactive lipids. Prostaglandins Leukot Essent Fatty Acids 75: 385–395, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann JB, Briggs JP. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Physiol Renal Physiol 274: F481–F489, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Pop IL, Carlson NG, Kishore BK. Genetic deletion of the P2Y2 receptor offers significant resistance to development of lithium-induced polyuria accompanied by alterations in PGE2 signaling. Am J Physiol Renal Physiol 302: F70–F77, 2012. [DOI] [PubMed] [Google Scholar]