Abstract
Inflammasomes are supramolecular structures that sense molecular patterns from pathogenic organisms or damaged cells and trigger an innate immune response, most commonly through production of the proinflammatory cytokines IL-1β and IL-18, but also through less understood mechanisms independent of these cytokines. Great strides have been made in understanding these structures and their dysfunction in various inflammatory diseases, lending new insights into urological and renal problems. From a clinical perspective, benign urinary pathology almost universally involves the inflammatory process, and understanding how inflammasomes translate etiological conditions (diabetes, obstruction, stones, urinary tract infections, etc.) into acute and chronic inflammatory responses is critical to understanding these diseases at a molecular level. To date, inflammasome components have been found in the bladder, prostate, and kidney and have been shown to be activated in response to several infectious and noninfectious insults. In this review, we summarize what is known regarding inflammasomes in both the upper and lower urinary tract and describe several common disease states where they potentially play critical roles.
Keywords: inflammasomes, urinary bladder, bladder outlet obstruction, inflammation, cystitis, innate immunity, kidney
inflammation plays a major role in disorders of the urinary tract due to its constant exposure to noxious stimuli in urine, continuity with an external surface rich in microbes, and significant mechanical stressors such as high pressure and stones. Consequently, there is an interest in understanding inflammatory pathways in urinary tract pathology. Inflammation begins with activation of the innate immune system by infectious or noninfectious (sterile) stimuli, and inflammasomes serve as sensors and effectors of such stimuli. In this review, we discuss the importance of inflammasomes in some of the major diseases of the urinary tract.
Inflammasomes
The innate immune system responds to patterns found in components of pathogens (pathogen-associated molecular patterns; PAMPs) or in molecules derived from damaged or dying cells (danger-associated molecular patterns; DAMPs). These patterns are recognized by pattern recognition receptors (PRRs) found in specialized immune cells, epithelia, and other tissues.
PRRs are classified into five families (Table 1). Some members of the NLR and HIN-200 families respond to DAMPs or PAMPs by forming supramolecular structures known as inflammasomes, often with adaptor molecules such as apoptosis-associated speck-like protein (ASC). These structures activate the cysteine protease caspase-1, which cleaves pro-IL-1β (pro-IL-1β) to IL-1β and pro-IL-18 to IL-18. The mature cytokines are proinflammatory and trigger classic components of the inflammatory response. The inflammasome is named after the PRR that organizes it, and the best studied of these is the NACHT, LRR, and PYD domains-containing protein 3 (NLRP3), which plays important roles in a wide variety of inflammatory diseases including several urologic pathologies.
Table 1.
Pattern recognition receptor families
| Receptor Family | Acronym | Inflammasome-Forming Members | Location |
|---|---|---|---|
| Toll-like receptors | TLRs | None | Cell membrane |
| C-type lectins | CTLs | Cell membrane | |
| Retinoic acid-inducible gene-I (RIG-I)-like receptors | RLRs | Intracellular | |
| HIN-200 | AIM2, IFI 16 | Intracellular | |
| Nucleotide binding and oligomerization domain (NOD)-like receptors | NLRs | NLRP1, NLRP2, NLRP3, NLRP6, NLRP7, NLRP12, NLRC4 | Intracellular |
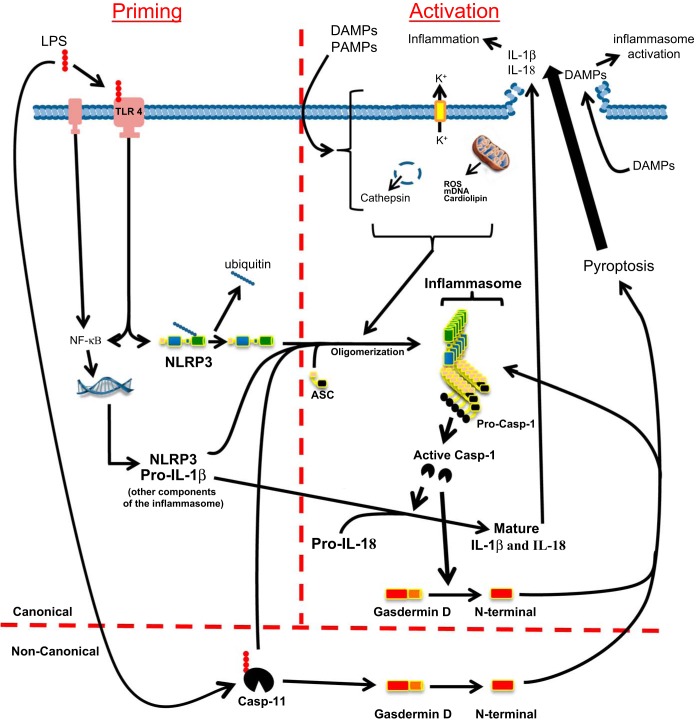
Canonical and noncanonical activation of NLRP3.
As depicted in Fig. 1, the NLRP3 inflammasome is activated by canonical and noncanonical pathways (27, 57, 100, 117). In most cell types, the canonical pathway requires two steps, priming and activation. For priming, a ligand such as LPS binds to a non-NLR receptor such as Toll-like receptor 4 (TLR4), increasing expression of inflammasome components (NLRP3, ASC, caspase-1, and pro-IL-1β; IL-18 is typically constitutively expressed) (10). Priming also promotes deubiquitination of preexisting NLRP3, thus “licensing” it for activation (47, 81). The second step, activation, is then triggered by events such as extracellular ATP binding to a purinergic receptor, reactive oxygen species (ROS), or insoluble crystals disrupting the cell membrane. Following this stimulus, numerous changes can occur such as potassium efflux from the cell, production of ROS, translocation of NLRP3 to the mitochondria, mitochondrial degradation with release of mDNA and cardiolipin, and lysosomal disruption with release of cathepsins. Not all of these changes occur with all agonists, and so the exact steps, and the necessity of those steps in response to a given signal, remain hotly debated. The net result is NLRP3 oligomerization, which triggers nucleation of ASC proteins to form long filaments. These filaments interact with pro-caspase-1, which itself forms long filaments off of the ASC. Autoproteolytic maturation of caspase-1 is then triggered by a process known as induced proximity. Active caspase is released from the complex and cleaves pro-IL-1β and pro-IL-18 to their mature forms. Active caspase-1 has recently been shown to cleave gasdermin D (95) and the N-terminal fragment of this protein is directly responsible for bringing about the lytic cell death process called pyroptosis (32, 50, 96). Pyroptosis results in the release of the mature Il-1β and IL-18 cytokines along with DAMPS such as ATP, uric acid, and high-mobility group box 1 (HMGB1) that trigger inflammasome activation in neighboring cells and further exacerbate the developing inflammation. Pyroptotic release of the ASC-containing aggregates (known as ASC-specks) can also mature pro-cytokines in the extracellular space as well as activate pro-caspase-1 within macrophages or other cells once they are reinternalized. Adding to this feed-forward effect, the N terminus of gasdermin D itself promotes further NLRP3-dependent activation of caspase-1 (50). Intriguingly, there may be an additional, perhaps redundant, mechanism leading to pyroptosis through the canonical pathway, for Shi et al. (95) found that resistance to pyroptosis in bone marrow-derived macrophages from gasdermin D null mice faded with prolonged inflammasome stimulation by canonical activators.
Fig. 1.
Schematic of the canonical and noncanonical pathways by which the NACHT, LRR, and PYD domains-containing protein 3 (NLRP3) inflammasome is activated and elicits an inflammatory response. In the canonical pathway (above the horizontal red line), 2 steps are required: priming (left of the vertical red line) and activation (right of the vertical red line). The priming step is initiated when, for example, lipopolysaccharide (LPS) from a uropathogen binds to the membrane-bound Toll-like receptor 4 (TLR4). Ligand binding then upregulates the expression of inflammasome components such as pro-IL-1β and the Nod-like receptor NLRP3 via the NF-kB pathway. Preexisting NLRP3 is also deubiquitinated, a necessary step before oligomerization can occur. In the second, activating step, a signal is received in the form of 1) potassium efflux from the cell, 2) generation of reactive oxygen species (ROS), 3) mitochondrial damage with release of mitochondrial DNA and cardiolipin, and/or 4) lysosomal disruption release of cathepsin. During this step, NLRP3 oligomerizes and recruits the adapter protein, apoptosis-associated speck-like protein containing the caspase recruitment domain (ASC). Pro-caspase-1 is recruited to complete the active inflammasome, where it is cleaved and released as active caspase-1. The activated caspase-1 then cleaves pro-IL-1β and pro-IL-18 to their active forms. Caspase-1 also cleaves gasdermin D, and the N terminus of this protein is responsible for inducing pyroptosis. Pyroptosis releases the mature cytokines [and many danger-associated molecular patterns (DAMPSs)] through a lytic cell death process. The released proinflammatory cytokines then initiate the inflammatory process while the DAMPs propagate the inflammation by triggering inflammasome activation in neighboring cells. In the noncanonical pathway, LPS (or other triggering agent) binds directly to caspase-11, which then facilitates the oligomerization of the NLRP3 inflammasome and subsequent maturation of cytokines. Caspase-11 can also stimulate pyroptosis by direct cleavage of gasdermin D.
The NLRP3 inflammasome complex can also be formed and activated by a noncanonical pathway involving caspase-11 in rodents (caspase-4 and 5 in humans) (27, 44, 57, 86, 107). These caspases are activated by directly binding LPS or intracellular bacteria. Once activated, caspase-11 then stimulates the formation and activation of the macromolecular inflammasome and the events downstream. Interestingly, caspase-11 can directly cleave gasdermin and thus can induce pyroptosis even in the absence of inflammasome formation (50).
Canonical and noncanonical activation pathways vs. canonical and noncanonical effects exerted by NLRP3.
In addition to canonical and noncanonical mechanisms that activate NLRP3, there are canonical and noncanonical effects exerted by the NLRP3 inflammasome once it is activated. While the overuse of the terms canonical and noncanonical may be somewhat confusing, their use is ingrained in the literature and will be used here for consistency. The canonical and noncanonical pathways of NLRP3 activation are described above whereas canonical effects of NLRP3 imply physiological changes induced by the end products, IL-1β or IL-18. Noncanonical effects are defined as those that do not involve these cytokines regardless of mechanism.
Inflammation in the Urinary Tract
Classification of inflammation within the urinary tract depends on location and underlying etiology. Inflammation of the upper tract includes ureteritis or nephritis, where the predominant concern is progression to renal failure. In the lower tract, urethritis, prostatitis, or cystitis presents as pain and/or disruptions in normal voiding and storage function (typically increased urinary frequency, urgency, and incontinence). Further subdivision of inflammatory pathologies depend on whether the cause is due to pathogens, such as bacterial cystitis or pyelonephritis, or sterile insults such as uric acid in diabetic uropathy or cyclophosphamide in hemorrhagic cystitis.
Inflammasomes in Sterile Pathology of the Upper Urinary Tract
In the upper tract, inflammasome components are found in tubular epithelium, podocytes, and immune cells, and expression may vary over time (22). For example, Song et al. (22) have shown that NLRP3 and NLRC4 are upregulated with aging while NLRP1 and AIM 2 appear to be stable. The increased expression of NLRP3 was found primarily in the glomeruli, which may be relevant to progressive glomerulosclerosis or other age-related kidney disorders.
Diabetic nephropathy.
The genesis of diabetes depends on genetic and environmental factors, but in the case of type 2 it appears to arise as the result of metabolic-induced inflammation as do subsequent complications including nephropathy. Hyperglycemia causes an influx of glucose into insulin-independent cells (such as kidney tubular epithelial cells), which increases metabolism, ROS production, and mitochondrial damage. In turn, these established activators of NLRP3 initiate canonical cytokine production and pyroptosis. Pyroptosis releases a cascade of DAMPs, thus amplifying and perpetuating the response. While the list of potential DAMPs is immense, three are particularly relevant to diabetic nephropathy (109).
Uric acid as a DAMP.
Hyperuricemia is an independent risk factor for nephropathy and uric acid is a well-known DAMP (125). Initially implicated in gout (25), uric acid activates NLRP3 in the kidney. Reducing serum uric acid prevents NLRP3 activation in streptozotocin-induced diabetic rats and helps preserve renal function (111). Kim et al. (53) found that, rather than having a direct effect on the tubular epithelial cells that express NLRP3, there is complex cross talk with resident macrophages. When directly exposed to uric acid, renal tubular cells increase expression of NLRP3 but do not secrete IL-1β. However, uric acid does stimulate these cells to release CXC motif ligand 12 (CXCL12; a macrophage recruiter) and HMGB1 (a DAMP), which recruits macrophages, activates their NLRP3, and cause them to release IL-1β. Uric acid also directly activates the NLRP3 in the recruited macrophage. IL-1β secreted by the macrophages diffuses back to the tubular epithelial cells to activate NF-κB, priming them for further response. This example illustrates the complex intracellular communication between immune and epithelial cells within the kidney.
ATP as a DAMP.
Hyperglycemia promotes high metabolism, cell turnover, and pyroptosis, all of which generate extracellular ATP. High glucose also stimulates ATP secretion by renal mesangial cells (99), further increasing extracellular ATP levels. ATP is a quintessential DAMP that triggers inflammasome activation by binding to the purinergic receptor P2X7 (63, 84), although the kidney may be an exception. Chen et al. (15) documented increased P2X4 expression in renal tubule cells, along with increased urinary IL-1β and IL-18, in 45 diabetic nephropathy patients. In vitro, HK-2 cells activate NLRP3 in response to high glucose and this was blocked by P2X4 antagonist small interference (si)RNA to P2X4 and the ATP scavenger apyrase. These data implicate ATP as a renal DAMP, with P2X4 as the novel purinergic receptor involved. However, P2X4 can form a complex with P2X7 to mediate inflammasome activation (39), suggesting a P2X4:P2X7 complex that might align Chen's paper with more conventional ideas of inflammasome activation by ATP.
Albumin as a DAMP.
Besides serving as a urinary marker for diagnosing diabetic nephropathy, albumin itself appears to act as a DAMP when it comes into contact with renal tubular epithelial cells. Liu et al. (61) found that rats subjected to an albumin overload, in the absence of hyperglycemia, increased priming and activation of NLRP3 in these cells. Virtually identical results were detected in vitro when cultured HK-2 cells were exposed to BSA. Thus, while we tend to think of albumin as a prevalent but benign protein, when encountered in the wrong location such as the renal tubules, it can function directly as a DAMP to propagate inflammation and nephropathy.
NLRP3 as a target for diabetic nephropathy.
Inflammation becomes a self-perpetuating cycle that persists in the chronic state of diabetes; consequently, NLRP3 is a logical target for protecting the kidney. Yang and colleagues (123) treated diabetic mice with the lectin-like domain of thrombomodulin (THBDD1), an inhibitor of NLRP3 priming and activation, and showed that albuminuria and glomerular sclerosis were decreased while renal function was preserved, even though there was no effect on hyperglycemia or hyperglycosuria. Although the nonspecific actions of THBDD1 prohibit drawing specific mechanistic conclusions, the results suggest that targeting the NLRP3 pathway may be useful independently of, or in conjunction with, blood sugar control. Further argument for this can be found in a clinical observation made by Balasubramaniam et al. (7), who found that patients with diabetic kidney disease, when treated with the IL-1 receptor antagonist anakinra for acute gout attacks, showed transient improvement in their renal function.
Obstructive nephropathy and ischemia.
Obstruction in the upper tract occurs from congenital anatomic defects, iatrogenic injury, or mechanical compression by tumors, bladder hypertrophy, or stones. Regardless of etiology, obstruction increases tubular pressures that can damage the kidney over time. Obstruction also decreases renal blood flow and creates a relative hypoxia, which brings on an inflammatory state (42). This can present in the clinics as acute renal failure or it can persist chronically and bring about fibrosis and end-stage renal failure. Since inflammasomes act as triggers to initiate an inflammatory response, it is not surprising that most studies of its role in obstructive nephropathy have focused on early periods following injury.
Unilateral ureteral obstruction model.
The most frequently utilized animal model for obstructive nephropathy involves ligating a single ureter and then comparing the obstructed kidney to the contralateral, unobstructed one (19, 106). Within days, histological changes indicative of inflammation are apparent that include disruption of tubular integrity, tubular atrophy, infiltration of inflammatory cells, accumulation of myofibroblasts, and the beginning of extracellular matrix deposition. If the obstruction is not relieved, there is progression of fibrosis and loss of function.
Role of NLRP3 in unilateral ureteral obstruction.
The expression and involvement of IL-1β in the unilateral ureteral obstruction (UUO)-induced inflammatory process suggests a role for NLRP3 (120). Recently, several investigators (80, 108, 115) have compared the inflammatory response between obstructed wild-type and NLRP3−/− mice. Wild-type kidneys display typical signs of NLRP3 priming, activation, and inflammation in the acute phase following UUO, which were diminished in NLRP3−/− mice (108, 115), implicating this inflammasome in the response to obstruction. The activity of caspase-1 in the null animals remained low over a 2-wk period, but after this initial lag IL-1β and IL-18 returned to wild-type levels (108). This suggests there are redundant pathways for cytokine maturation that may emerge beyond the acute phase of obstruction. Indeed, by 4 wk tubular damage was similar in the wild-type and NLRP3−/− mice.
Linking the canonical effects of NLRP3 with the functional deficits seen in patients during and immediately following obstructive nephropathy is critically important. Clinically, patients with renal obstruction undergo deobstructing procedures, and this can result in postobstructive diuresis with dangerous electrolyte imbalances. This is due, in part, to an attenuated expression of aquaporins in the tubules, hampering the reabsorption of water, and this condition remains until aquaporins can be restored to their normal levels (60). It has been shown that UUO causes an increase in intrarenal angiotensin II (108) and that angiotensin II increases NLRP3 priming and activation (113, 114), resulting in the canonical release of IL-1β. IL-1β, then acts on neighboring cells to decrease aquaporin-2 expression, thus reducing water resorption and causing diuresis (114). Wang, et al. (115) also found that inhibitors of the renin angiotensin system, such as angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, and a direct renin inhibitor (DRI), diminished the expression of NLRP3 inflammasome components, reduced IL-1β levels, and enhanced aquaporin-2 expression. This connection between the renin-angiotensin system and the innate immune system may provide an opportunity for clinical intervention.
Adding to the complexity of NLRP3's role in UUO is the possible involvement of noncanonical effects (62, 80). For example, Pulskens et al. (80) found that there was actually increased interstitial edema in the obstructed NLRP3−/− mouse kidney, an unexpected result since NLRP3 promotes inflammation and edema is a classic inflammatory end point. The enhanced edema in the null mouse came from increases in tubular and vascular permeability caused by reductions in components of intracellular junctions. The authors attributed this to noncanonical effects of NLRP3 maintaining basally high levels of claudin and cadherin expression. However, it has also been found that albumin-activated NLRP3 stimulates decreases in claudin-1 and thus should lead to increases in permeability (124). This decrease in claudin-1 was blocked in caspase-1−/− mice, suggesting that the effect on claudin-1 in this model is mediated through canonical mechanisms (124). Consequently, further studies on obstructed models with concurrent blockade of the downstream canonical pathway will be required to definitively conclude whether the NLRP3 preservation of vascular and tubular epithelium in the basal state is a canonical or noncanonical effect.
Finally, ischemia-reperfusion injury (97) is relevant to UUO, considering the state of ischemia created by the increased tubular pressure. Using a mouse model of renal artery occlusion, Shigeoka et al. (97) found that NLRP3−/− mice had less inflammation and less tubular injury while preserving renal function. However, ASC, caspase-1, IL-1β, and IL-18 were not required for this effect, suggesting noncanonical effects of NLRP3 in the ischemic kidney.
DAMPs released during obstruction.
Increased pressure and ischemia during obstruction produce DAMPs such as HMGB1, biglycan, hyaluron, and many others (3). HMGB1 is a nuclear protein commonly released as a DAMP from many cells, including tubular epithelial cells (82, 119, 122). Blocking its release reduced kidney injury and renal fibrosis during UUO (105). Biglycan is a component of the extracellular matrix (3, 73, 92) present in soluble form at high levels after obstruction but before the infiltration of macrophage (35, 93). It promotes priming and activation of NLRP3 without the need for a costimulating factor. Hyaluronan, another component of the ECM, similarly activates the inflammasome during kidney inflammation (3, 92). It is currently unclear which DAMPs are predominant at any given time point after UUO.
Stone disease: nephrolithiasis and crystalline nephropathy.
Precipitation of insoluble salts from urine results in formation of crystals that manifest extrarenally, as in the case of kidney stones, or intrarenally, as seen in oxalosis or gouty nephropathy (6, 103). While the mechanism of injury to the kidney by crystals was traditionally attributed to mechanical obstruction of urine flow, inflammation is emerging as an important component of this injury (3, 69, 102). Critical to this understanding was the initial discovery that gout-associated uric acid crystals activated NLRP3 (65), a fact later extended to most, if not all, crystallopathies such as asbestosis, silicosis, and nephrolithiasis (69).
Canonical vs. noncanonical effects and the crystal DAMPs.
The canonical effects of NLRP3 activated by crystals are well established and were, in fact, used to delineate much of the classic canonical pathway. The crystals themselves can be composed of numerous agents including uric acid, calcium oxalate, calcium phosphate, cysteine, adenine, certain drugs, or contrast media. We focus on calcium oxalate and cysteine as representative crystals, having already discussed uric acid in the context of diabetic uropathy.
Calcium oxalate is one of the most clinically important urinary precipitates as it accounts for the majority of kidney stones and can also be seen in metabolic derangements such as primary oxaluria and polyethylene glycol toxicity. Mulay and colleagues (69, 70) demonstrated in an acute model that calcium oxalate crystals initiate injury and cell death in tubular epithelial cells through obstructive effects and direct physical damage. This causes the release of ATP, and likely many other DAMPs, which triggers activation of the NLRP3 inflammasome in the nearby dendritic cells, likely through production of ROS (46). Similarly, Knauf et al. (54) found in a chronic model of oxalate nephropathy that NLRP3−/− mice were protected from progressive renal failure and death. Mice deficient in inflammasome components IL-1R or IL-18 had diminished inflammation and tissue injury (70). Therefore, it appears that canonical effects of NLRP3 are a critical driver of both acute and chronic inflammation induced by calcium oxalate crystals.
Cystine crystals are a devastating cause of nephrolithiasis in patients with mutations of the CTNS gene (a lysosomal cystine proton cotransporter). In a recent study, Prencipe et al. (79) found that cystine both primed and activated NLRP3 in monocytes. Clinically, their findings correlated with the observation that patients with cystinosis had higher circulating levels of IL-1β and IL-18. Taken together, the NLRP3 pathway constitutes a likely therapeutic target for crystalline nephropathies regardless of the specific type of crystal involved.
Inflammasomes in Sterile Pathology of the Lower Urinary Tract
The potential repertoire.
Our laboratory has shown that the rat bladder contains seven different PPRs known to form inflammasomes (NLRP1, 3, 6, 7, and 12, NLRC4, and Aim2) (36). All seven were present in the urothelia, with most concentrated in the layer abutting the lumen. Most were also in the detrusor with little to no expression in the interstitial layer. We have also shown that DAMPs and PAMPs instilled directly into the lumen of the bladder can activate two of the NLRs (NLRP3 and NLRC4) (37). The presence of multiple inflammasome components in the bladder illustrates how critical they are in allowing the immune system to sense a wide variety of stimuli.
Cystitis.
Cystitis results from infectious or noninfectious sources. Acute cystitis typically generates distinct symptoms such as increased frequency and urgency and incontinence. Prolonged inflammation induces fibrotic changes that culminate in organ failure and are thought to contribute to the development of transitional cell carcinomas (104).
Chemical cystitis.
Initial evidence implicating inflammasomes in sterile cystitis comes from studies of cyclophosphamide-induced hemorrhagic cystitis. Acrolein, a metabolic by product of cyclophosphamide, is stored in the bladder before excretion, where it acts as the initiator of hemorrhagic cystitis, increasing cytokine release including IL-1β and IL-18 (98). Ribeiro's group used antiserum to IL-1β (26, 83) and an IL-1 receptor antagonist (59) to demonstrate the importance of this cytokine in this inflammatory response. To investigate a role for inflammasomes, we utilized the NLRP3 inhibitor glyburide (58), which blocked cyclophosphamide-induced inflammasome activation, reduced IL-1β and IL-18 secretion, and decreased inflammation (37). Most importantly, inhibition of NLRP3 blocked the functional changes associated with irritative bladder inflammation (increased voiding frequency and decreased voiding volume).
Haldar et al. (28, 29) recently showed that acrolein caused the oxidation of mitochondrial DNA along with a concomitant epigenetic silencing of DNA repair enzymes. The oxidized mitochondrial DNA then triggered NLRP3 activation and pyroptosis. The released IL-1β, acting through IGF, induced detrusor smooth muscle hyperplasia, which is ultimately thought to bring about the functional changes by reducing bladder compliance. Thus NLRP3 appears to be a critical mediator of biochemical, histological, and physiological inflammation in this common model.
Obstructive uropathy: partial bladder outlet obstruction.
Bladder outlet obstruction (BOO) results from numerous conditions (e.g., stones, organ prolapse, posterior urethral valves), but the most prevalent is benign prostatic hyperplasia (BPH) (89). Recent studies demonstrate that BOO acutely produces an inflammatory state in the bladder (67, 75), leading to irritative symptoms. Similar to renal fibrosis in obstructive nephropathy, chronic BOO leads to bladder fibrosis (18, 67), which is difficult to treat once established (45). We have recently investigated NLRP3 in a rat model of BOO and found that acutely (12 days) there was increased inflammasome activity, bladder hypertrophy, and inflammation, which was reversed with the NLRP3 inhibitor glyburide (38). Physiologically, BOO caused irritative symptoms (increased voiding frequency, decreased voiding volume) that were ameliorated by glyburide. Thus NLRP3 appears to be a mediator of inflammation in this common condition. Although our study did not assess canonical vs. noncanonical effects of NLRP3 during BOO, Kanno et al. (48) found that many of the same inflammatory parameters were reversed in IL-1β−/− mice, suggesting a predominant role of the canonical pathway.
The mechanism by which NLRP3 is activated in BOO remains unclear but is likely to be multifactorial. For example, there are several sources of ROS during BOO. Severe and/or prolonged bladder distension restricts blood flow and creates ischemia, while emptying the bladder relieves this restriction, causing reperfusion, which produces a burst of ROS (121). Thus voiding cycles in BOO animals create cycles of ischemia-reperfusion. In addition, stronger contraction of the detrusor muscle (to push urine past the obstruction) requires a higher rate of metabolism, resulting in increased production of ROS. DAMPs are also likely involved in BOO, particularly ATP. Increased strength of a contraction creates high intravesical pressure, and elevated pressure is known to release ATP from urothelia (76). Moreover, pressure has been shown to induce necrosis and/or apoptosis. Necrosis releases large numbers of DAMPs (41), while apoptosis can also be proinflammatory under certain circumstances (17). Of course, release of cytokines by pyroptosis further releases DAMPs and propagates the resulting inflammation (57). These observations provide a basis for discerning how NLRP3 coordinates the action of the innate immune system in normal voiding conditions vs. its response to pathological pressures as encountered with BOO.
Interstitial cystitis/bladder pain syndrome.
Interstitial cystitis/bladder pain syndrome is a clinical entity diagnosed by urinary frequency, urgency, and pain. Although the etiology is unknown, current theories propose that it is caused by an alteration in the composition of the glycosaminoglycan layer lining the bladder that increases permeability, thereby exposing urinary solutes to the urothelium and triggering an inflammatory reaction (2, 40, 51, 77, 94). Such a scenario strongly suggests that inflammasomes in the urothelium could serve as sensors of those solutes and triggers of the inflammatory reaction. Indeed, significant increases in IL-1β have been detected in the serum of these patients (43). Since the sources of bladder inflammation and pain are likely to vary between patients, identification of a common mediator such as NLRP3 could lead to an effective therapeutic strategy.
BPH and prostatitis.
Studies are just beginning to explore inflammasomes in the prostate. Although no study has systematically examined the repertoire present, expression of NLRP1, NLRP3, and AIM2 have been reported (13, 56, 78). Nonneoplastic prostatic pathologies include BPH and prostatitis. While two distinct entities, there is significant clinical overlap, and large studies such as Medical Therapy Of Prostatic Symptoms have implicated a critical inflammatory component in both (87). The National Institutes of Health consensus on prostatitis (55) lists four distinct types: I. acute bacterial, II. chronic bacterial, III. inflammatory/noninflammatory chronic, and IV. asymptomatic inflammatory. Given their responsiveness to PAMPs, it is relatively easy to envision the activation of inflammasomes in the prostate by bacteria (types I and II prostatitis), although this has not been explored. However, two recent reports have examined sterile prostatitis (type III) induced by chemical injection (carrageenan or formalin), and both detect inflammasome activation, although they implicate NLRP1 over NLRP3 (13, 49). These studies found elevated NLRP1, caspase-1, and IL-1β levels but not NLRP3. Moreover, Kashyap et al. (49) found IL-18 increased relative to IL-1β. In a related paper, Hamakawa et al. (30) performed a cDNA microarray analysis on 31,100 genes in BPH patients and found 7 inflammation-related genes upregulated (>2-fold), with IL-18 increased 11.09-fold but no change in IL-1β. These results suggest that in sterile prostatitis, NLRP1 production of IL-18 predominates, with little or no involvement by NLRP3 or IL-1β.
Finally, one study documented AIM2 (which detects DNA) expression in the normal prostate with an increase in BPH tissue (78). Although the type of BPH was not defined, AIM2 could be activated in prostate cell lines, suggesting the possible involvement of this inflammasome in prostatic disease.
Role of Inflammasomes in Urinary Tract Infections
Infectious cystitis.
The majority of inflammasome-forming PPRs we have found to be present in the urothelia (36) have pathogen-associated ligands, suggesting that they evolved primarily to respond to infectious agents. Infectious cystitis, the second most common type of human infection (9, 33), is caused by viral, mycobacterial, chlamydial, fungal, and schistosomal agents although uropathogenic Escherichia coli (UPEC) are responsible for up 80% of community acquired infections (23, 88). UPEC contain many prototypical PAMPs that activate NLRs such as LPS and flagellin. Moreover, IL-1β is among the first cytokines detectable in urine following infection (1), suggesting early activation of inflammasomes during urinary tract infections (UTIs). Despite the seemingly obvious connection, little is known about the role of inflammasomes in the host defense against infection.
NLRP1, which we have found to reside in the urothelium and detrusor (36), recognizes muramyl dipeptide (21). Muramyl dipeptide is a common peptidoglycan of both gram-negative and gram-positive bacteria, but to date no studies have investigated a role for this PAMP or NLRP1 in UTIs. NLRP3, also expressed in the urothelia, responds to hemolysin in addition to LPS (66, 71). Hemolysin is a virulence factor for UPEC, and its expression correlates with the pathogen's ability to evade host defenses and infect the upper urinary tracts (85). Hemolysin has also been shown to induce pyroptosis in human urothelial cells in part via the activation of the NLRP3 inflammasome through the noncanonical activation pathway (72). Recently, Symington et al. (101) showed in mice that UPEC taken up in macrophages activates NLRP3 and results in protection from infection through an IL-1β-dependent mechanism. Interestingly, such protection appears to be inversely regulated by a key autophagy protein, ATG16L1 (101, 110, 112). Schaale et al. (91) also studied the interaction of UPEC with human and mouse macrophages. Although only certain strains of UPEC activated inflammasomes and pyroptosis (91), the responses differed depending on the species. In mouse macrophages, the responses were completely NLRP3 dependent and largely α-hemolysin dependent, while in human macrophages NLRP-3-independent and α-hemolysin-independent pathways were activated as well.
Rat urothelia also express NLRP6 (36), which has no known ligand but is critical in regulating the gut microbiota and mucin production (8, 14, 20). Studies are just beginning to appreciate the importance of the bladder microbiota and its role in UTIs (11, 34, 118), whereas dysfunctional mucosal barriers have been implicated in many bladder disorders including UTIs and interstitial cystitis (77). Consequently, the role of NLRP6 in UTIs may prove to be an important subject for future studies. Rat urothelia also express NLRP7 (36), which responds to microbial acylated lipopeptides and appears to block intracellular replication of bacteria in human macrophages (52). Since progression from acute UTI to chronic cystitis depends on the establishment of intracellular bacterial communities, (4, 5, 31), targeting this PRR may prove useful for preventing recurrent UTIs. NLRC4 is also expressed and can be activated in the urothelia (36, 37) and is best known for responding to flagellin (24, 68), a critical virulence factor that allows UPEC to migrate upstream in the urinary tract (74). NLRC4 also responds to the type III secretion system (12). UPEC typically express these secretion systems and thus potentially engage NLRC4 in a second way. Finally, urothelia express AIM2, whose ligand is double stranded DNA. AIM2 may therefore engage bacteria that release DNA into the cytoplasm (for example, through bacteriolysis) (90, 116) as well as sensing viral DNA in the case of viral cystitis.
Infectious pyelonephritis.
Uropathogen migration into the upper tracts may cause life-threatening acute pyelonephritis (APN) or acute lobar nephronia (ALN). Chen et al. (16) recently examined single-nucleotide polymorphisms (SNPs) in 360 pediatric patients and found that genetic polymorphisms in NLRP3 were related to the patients' susceptibility to APN and ALN (16). This is an important link between mutations in the inflammasome pathways and sensitivity to UTIs. However, the mechanisms by which inflammasomes coordinate the innate immune response to infection in the upper tracts has yet to be formally studied.
Conclusion
Although inflammasomes have only recently been discovered (64), there is considerable evidence that they play important roles in almost all inflammatory diseases, and the urinary tract is no exception. Understanding their role will provide significant insight into the importance of inflammation in urological and renal pathology and potentially identify a central therapeutic target for treating and controlling multiple disorders.
GRANTS
Research in the Purves Laboratory is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK103534. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.T.P. and F.M.H. provided conception and design of research; J.T.P. and F.M.H. interpreted results of experiments; J.T.P. and F.M.H. prepared figures; J.T.P. and F.M.H. drafted manuscript; J.T.P. and F.M.H. edited and revised manuscript; J.T.P. and F.M.H. approved final version of manuscript.
REFERENCES
- 1.Abraham SN, Miao Y. The nature of immune responses to urinary tract infections. Nat Rev Immunol 15: 655–663, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyama A, Stein PC, Houshiar A, Parsons CL. Urothelial cytoprotective activity of Tamm-Horsfall protein isolated from the urine of healthy subjects and patients with interstitial cystitis. Int J Urol 7: 176–183, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Anders HJ, Schaefer L. Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol 25: 1387–1400, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson GG, Dodson KW, Hooton TM, Hultgren SJ. Intracellular bacterial communities of uropathogenic Escherichia coli in urinary tract pathogenesis. Trends Microbiol 12: 424–430, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Anderson GG, Martin SM, Hultgren SJ. Host subversion by formation of intracellular bacterial communities in the urinary tract. Microbes Infect 6: 1094–1101, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Arcidiacono T, Mingione A, Macrina L, Pivari F, Soldati L, Vezzoli G. Idiopathic calcium nephrolithiasis: a review of pathogenic mechanisms in the light of genetic studies. Am J Nephrol 40: 499–506, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Balasubramaniam G, Almond M, Dasgupta B. Improved renal function in diabetic patients with acute gout treated with anakinra. Kidney Int 88: 195–196, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Barbe F, Douglas T, Saleh M. Advances in Nod-like receptors (NLR) biology. Cytokine Growth Factor Rev 25: 681–697, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Barnett BJ, Stephens DS. Urinary tract infection: an overview. Am J Med Sci 314: 245–249, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183: 787–791, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brubaker L, Wolfe AJ. The new world of the urinary microbiota in women. Am J Obstet Gynecol 213: 644–649, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buttner D. Protein export according to schedule: architecture, assembly, and regulation of type III secretion systems from plant- and animal-pathogenic bacteria. Microbiol Mol Biol Rev 76: 262–310, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CS, Chang PJ, Lin WY, Huang YC, Ho DR. Evidences of the inflammasome pathway in chronic prostatitis and chronic pelvic pain syndrome in an animal model. Prostate 73: 391–397, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Chen GY. Role of Nlrp6 and Nlrp12 in the maintenance of intestinal homeostasis. Eur J Immunol 44: 321–327, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen K, Zhang J, Zhang W, Zhang J, Yang J, Li K, He Y. ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. Int J Biochem Cell Biol 45: 932–943, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Cheng CH, Lee YS, Chang CJ, Lin JC, Lin TY. Genetic polymorphisms in inflammasome-dependent innate immunity among pediatric patients with severe renal parenchymal infections. PLoS One 10: e0140128, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidovich P, Kearney CJ, Martin SJ. Inflammatory outcomes of apoptosis, necrosis and necroptosis. Biol Chem 395: 1163–1171, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Deveaud CM, Macarak EJ, Kucich U, Ewalt DH, Abrams WR, Howard PS. Molecular analysis of collagens in bladder fibrosis. J Urol 160: 1518–1527, 1998. [PubMed] [Google Scholar]
- 19.Eddy AA, Lopez-Guisa JM, Okamura DM, Yamaguchi I. Investigating mechanisms of chronic kidney disease in mouse models. Pediatr Nephrol 27: 1233–1247, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145: 745–757, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell 25: 713–724, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Fougeray S, Pallet N. Mechanisms and biological functions of autophagy in diseased and ageing kidneys. Nat Rev Nephrol 11: 34–45, 2015. [DOI] [PubMed] [Google Scholar]
- 23.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 113, Suppl 1A: 5S–13S, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Nunez G. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol 7: 576–582, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Ghaemi-Oskouie F, Shi Y. The role of uric acid as an endogenous danger signal in immunity and inflammation. Curr Rheumatol Rep 13: 160–166, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomes TN, Santos CC, Souza-Filho MV, Cunha FQ, Ribeiro RA. Participation of TNF-alpha and IL-1 in the pathogenesis of cyclophosphamide-induced hemorrhagic cystitis. Braz J Med Biol Res 28: 1103–1108, 1995. [PubMed] [Google Scholar]
- 27.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21: 677–687, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haldar S, Dru C, Bhowmick NA. Mechanisms of hemorrhagic cystitis. Am J Clin Exp Urol 2: 199–208, 2014. [PMC free article] [PubMed] [Google Scholar]
- 29.Haldar S, Dru C, Choudhury D, Mishra R, Fernandez A, Biondi S, Liu Z, Shimada K, Arditi M, Bhowmick NA. Inflammation and pyroptosis mediate muscle expansion in an interleukin-1beta (IL-1beta)-dependent manner. J Biol Chem 290: 6574–6583, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamakawa T, Sasaki S, Shibata Y, Imura M, Kubota Y, Kojima Y, Kohri K. Interleukin-18 may lead to benign prostatic hyperplasia via thrombospondin-1 production in prostatic smooth muscle cells. Prostate 74: 590–601, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Hannan TJ, Totsika M, Mansfield KJ, Moore KH, Schembri MA, Hultgren SJ. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol Rev 36: 616–648, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res 25: 1285–1298, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am 11: 551–581, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Horwitz D, McCue T, Mapes AC, Ajami NJ, Petrosino JF, Ramig RF, Trautner BW. Decreased microbiota diversity associated with urinary tract infection in a trial of bacterial interference. J Infect 71: 358–367, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsieh LT, Nastase MV, Zeng-Brouwers J, Iozzo RV, Schaefer L. Soluble biglycan as a biomarker of inflammatory renal diseases. Int J Biochem Cell Biol 54: 223–235, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes FM Jr, Turner DP, Purves JT. The potential repertoire of the innate immune system in the bladder: expression of pattern recognition receptors in the rat bladder and a rat urothelial cell line (MYP3 cells). Int Urol Nephrol 47: 1953–1964, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hughes FM Jr, Vivar NP, Kennis JG, Pratt-Thomas JD, Lowe DW, Shaner BE, Nietert PJ, Spruill LS, Purves JT. Inflammasomes are important mediators of cyclophosphamide-induced bladder inflammation. Am J Physiol Renal Physiol 306: F299–F308, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hughes FM Jr, Hill HM, Wood CM, Edmondson AT, Dumas A, Oelsen JM, Rac G, Purves JT. The NLRP3 inflammasome mediates inflammation produced by bladder outlet obstruction. J Urol 195: 1598–1605, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hung SC, Choi CH, Said-Sadier N, Johnson L, Atanasova KR, Sellami H, Yilmaz O, Ojcius DM. P2X4 assembles with P2X7 and pannexin-1 in gingival epithelial cells and modulates ATP-induced reactive oxygen species production and inflammasome activation. PLoS One 8: e70210, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hurst RE, Roy JB, Min KW, Veltri RW, Marley G, Patton K, Shackelford DL, Stein P, Parsons CL. A deficit of chondroitin sulfate proteoglycans on the bladder uroepithelium in interstitial cystitis. Urology 48: 817–821, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, Sutterwala FS. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci USA 106: 20388–20393, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaenike JR. The renal response to ureteral obstruction: a model for the study of factors which influence glomerular filtration pressure. J Lab Clin Med 76: 373–382, 1970. [PubMed] [Google Scholar]
- 43.Jiang YH, Peng CH, Liu HT, Kuo HC. Increased pro-inflammatory cytokines, C-reactive protein and nerve growth factor expressions in serum of patients with interstitial cystitis/bladder pain syndrome. PLoS One 8: e76779, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jimenez Fernandez D, Lamkanfi M. Inflammatory caspases: key regulators of inflammation and cell death. Biol Chem 396: 193–203, 2015. [DOI] [PubMed] [Google Scholar]
- 45.Jock M, Leggett RE, Schuler C, Callaghan C, Levin RM. Effect of partial bladder outlet obstruction and reversal on rabbit bladder physiology and biochemistry: duration of recovery period and severity of function. BJU Int 114: 946–954, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Joshi S, Wang W, Peck AB, Khan SR. Activation of the NLRP3 inflammasome in association with calcium oxalate crystal induced reactive oxygen species in kidneys. J Urol 193: 1684–1691, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri ES. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem 287: 36617–36622, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanno Y, Mitsui T, Kitta T, Moriya K, Tsukiyama T, Hatakeyama S, Nonomura K. The inflammatory cytokine IL-1beta is involved in bladder remodeling after bladder outlet obstruction in mice. Neurourol Urodyn 35: 377–381, 2016. [DOI] [PubMed] [Google Scholar]
- 49.Kashyap M, Pore S, Wang Z, Gingrich J, Yoshimura N, Tyagi P. Inflammasomes are important mediators of prostatic inflammation associated with BPH. J Inflamm (Lond) 12: 37, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526: 666–671, 2015. [DOI] [PubMed] [Google Scholar]
- 51.Keay SK, Birder LA, Chai TC. Evidence for bladder urothelial pathophysiology in functional bladder disorders. BioMed Res Int 2014: 865463, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khare S, Dorfleutner A, Bryan NB, Yun C, Radian AD, de Almeida L, Rojanasakul Y, Stehlik C. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity 36: 464–476, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim SM, Lee SH, Kim YG, Kim SY, Seo JW, Choi YW, Kim DJ, Jeong KH, Lee TW, Ihm CG, Won KY, Moon JY. Hyperuricemia-induced NLRP3 activation of macrophages contributes to the progression of diabetic nephropathy. Am J Physiol Renal Physiol 308: F993–F1003, 2015. [DOI] [PubMed] [Google Scholar]
- 54.Knauf F, Asplin JR, Granja I, Schmidt IM, Moeckel GW, David RJ, Flavell RA, Aronson PS. NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int 84: 895–901, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krieger JN, Nyberg L Jr, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA 282: 236–237, 1999. [DOI] [PubMed] [Google Scholar]
- 56.Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R, Tschopp J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem 55: 443–452, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell 157: 1013–1022, 2014. [DOI] [PubMed] [Google Scholar]
- 58.Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol 187: 61–70, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leite CA, Alencar VT, Melo DL, Mota JM, Melo PH, Mourao LT, Wong DV, Magalhaes PJ, Santos AA, Brito GA, Lima-Junior RC, Cunha FQ, Ribeiro RA. Target inhibition of IL-1 receptor prevents ifosfamide induced hemorrhagic cystitis in mice. J Urol 194: 1777–1786, 2015. [DOI] [PubMed] [Google Scholar]
- 60.Li C, Wang W, Kwon TH, Isikay L, Wen JG, Marples D, Djurhuus JC, Stockwell A, Knepper MA, Nielsen S, Frokiaer J. Downregulation of AQP1, -2, and -3 after ureteral obstruction is associated with a long-term urine-concentrating defect. Am J Physiol Renal Physiol 281: F163–F171, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Liu D, Xu M, Ding LH, Lv LL, Liu H, Ma KL, Zhang AH, Crowley SD, Liu BC. Activation of the Nlrp3 inflammasome by mitochondrial reactive oxygen species: a novel mechanism of albumin-induced tubulointerstitial inflammation. Int J Biochem Cell Biol 57: 7–19, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lorenz G, Darisipudi MN, Anders HJ. Canonical and non-canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrol Dial Transplant 29: 41–48, 2014. [DOI] [PubMed] [Google Scholar]
- 63.Luna-Gomes T, Santana PT, Coutinho-Silva R. Silica-induced inflammasome activation in macrophages: role of ATP and P2X7 receptor. Immunobiology 220: 1101–1106, 2015. [DOI] [PubMed] [Google Scholar]
- 64.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10: 417–426, 2002. [DOI] [PubMed] [Google Scholar]
- 65.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241, 2006. [DOI] [PubMed] [Google Scholar]
- 66.McCoy AJ, Koizumi Y, Toma C, Higa N, Dixit V, Taniguchi S, Tschopp J, Suzuki T. Cytotoxins of the human pathogen Aeromonas hydrophila trigger, via the NLRP3 inflammasome, caspase-1 activation in macrophages. Eur J Immunol 40: 2797–2803, 2010. [DOI] [PubMed] [Google Scholar]
- 67.Metcalfe PD, Wang J, Jiao H, Huang Y, Hori K, Moore RB, Tredget EE. Bladder outlet obstruction: progression from inflammation to fibrosis. BJU Int 106: 1686–1694, 2010. [DOI] [PubMed] [Google Scholar]
- 68.Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, Aderem A. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci USA 107: 3076–3080, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mulay SR, Evan A, Anders HJ. Molecular mechanisms of crystal-related kidney inflammation and injury. Implications for cholesterol embolism, crystalline nephropathies and kidney stone disease. Nephrol Dial Transplant 29: 507–514, 2014. [DOI] [PubMed] [Google Scholar]
- 70.Mulay SR, Kulkarni OP, Rupanagudi KV, Migliorini A, Darisipudi MN, Vilaysane A, Muruve D, Shi Y, Munro F, Liapis H, Anders HJ. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1beta secretion. J Clin Invest 123: 236–246, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Munoz-Planillo R, Franchi L, Miller LS, Nunez G. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J Immunol 183: 3942–3948, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nagamatsu K, Hannan TJ, Guest RL, Kostakioti M, Hadjifrangiskou M, Binkley J, Dodson K, Raivio TL, Hultgren SJ. Dysregulation of Escherichia coli alpha-hemolysin expression alters the course of acute and persistent urinary tract infection. Proc Natl Acad Sci USA 112: E871–E880, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nastase MV, Young MF, Schaefer L. Biglycan: a multivalent proteoglycan providing structure and signals. J Histochem Cytochem 60: 963–975, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nielubowicz GR, Mobley HL. Host-pathogen interactions in urinary tract infection. Nat Rev Urol 7: 430–441, 2010. [DOI] [PubMed] [Google Scholar]
- 75.Oka M, Fukui T, Ueda M, Tagaya M, Oyama T, Tanaka M. Suppression of bladder oxidative stress and inflammation by a phytotherapeutic agent in a rat model of partial bladder outlet obstruction. J Urol 182: 382–390, 2009. [DOI] [PubMed] [Google Scholar]
- 76.Olsen SM, Stover JD, Nagatomi J. Examining the role of mechanosensitive ion channels in pressure mechanotransduction in rat bladder urothelial cells. Ann Biomed Eng 39: 688–697, 2011. [DOI] [PubMed] [Google Scholar]
- 77.Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology 69: 9–16, 2007. [DOI] [PubMed] [Google Scholar]
- 78.Ponomareva L, Liu H, Duan X, Dickerson E, Shen H, Panchanathan R, Choubey D. AIM2, an IFN-inducible cytosolic DNA sensor, in the development of benign prostate hyperplasia and prostate cancer. Mol Cancer Res 11: 1193–1202, 2013. [DOI] [PubMed] [Google Scholar]
- 79.Prencipe G, Caiello I, Cherqui S, Whisenant T, Petrini S, Emma F, De Benedetti F. Inflammasome activation by cystine crystals: implications for the pathogenesis of cystinosis. J Am Soc Nephrol 25: 1163–1169, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pulskens WP, Butter LM, Teske GJ, Claessen N, Dessing MC, Flavell RA, Sutterwala FS, Florquin S, Leemans JC. Nlrp3 prevents early renal interstitial edema and vascular permeability in unilateral ureteral obstruction. PLoS One 9: e85775, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell 49: 331–338, 2013. [DOI] [PubMed] [Google Scholar]
- 82.Rabadi MM, Ghaly T, Goligorksy MS, Ratliff BB. HMGB1 in renal ischemic injury. Am J Physiol Renal Physiol 303: F873–F885, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ribeiro RA, Freitas HC, Campos MC, Santos CC, Figueiredo FC, Brito GA, Cunha FQ. Tumor necrosis factor-alpha and interleukin-1beta mediate the production of nitric oxide involved in the pathogenesis of ifosfamide induced hemorrhagic cystitis in mice. J Urol 167: 2229–2234, 2002. [PubMed] [Google Scholar]
- 84.Rissiek B, Haag F, Boyer O, Koch-Nolte F, Adriouch S. P2X7 on mouse T cells: one channel, many functions. Front Immunol 6: 204, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ristow LC, Welch RA. Hemolysin of uropathogenic Escherichia coli: a cloak or a dagger? Biochim Biophys Acta 1858: 538–545, 2016. [DOI] [PubMed] [Google Scholar]
- 86.Roberts JS, Yilmaz. Dangerous liaisons: caspase-11 and reactive oxygen species crosstalk in pathogen elimination. Int J Mol Sci 16: 23337–23354, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roehrborn CG. BPH progression: concept and key learning from MTOPS, ALTESS, COMBAT, and ALF-ONE. BJU Int 101, Suppl 3: 17–21, 2008. [DOI] [PubMed] [Google Scholar]
- 88.Russo TA, Johnson JR. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect 5: 449–456, 2003. [DOI] [PubMed] [Google Scholar]
- 89.Saigal CS, Joyce G. Economic costs of benign prostatic hyperplasia in the private sector. J Urol 173: 1309–1313, 2005. [DOI] [PubMed] [Google Scholar]
- 90.Sauer JD, Witte CE, Zemansky J, Hanson B, Lauer P, Portnoy DA. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe 7: 412–419, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schaale K, Peters KM, Murthy AM, Fritzsche AK, Phan MD, Totsika M, Robertson AA, Nichols KB, Cooper MA, Stacey KJ, Ulett GC, Schroder K, Schembri MA, Sweet MJ. Strain- and host species-specific inflammasome activation, IL-1beta release, and cell death in macrophages infected with uropathogenic Escherichia coli. Mucosal Immunol 9: 124–136, 2016. [DOI] [PubMed] [Google Scholar]
- 92.Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem 289: 35237–35245, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schaefer L, Macakova K, Raslik I, Micegova M, Grone HJ, Schonherr E, Robenek H, Echtermeyer FG, Grassel S, Bruckner P, Schaefer RM, Iozzo RV, Kresse H. Absence of decorin adversely influences tubulointerstitial fibrosis of the obstructed kidney by enhanced apoptosis and increased inflammatory reaction. Am J Pathol 160: 1181–1191, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schrepf A, O'Donnell M, Luo Y, Bradley CS, Kreder K, Lutgendorf S; Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network. Inflammation and inflammatory control in interstitial cystitis/bladder pain syndrome: Associations with painful symptoms. Pain 155: 1755–1761, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526: 660–665, 2015. [DOI] [PubMed] [Google Scholar]
- 96.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514: 187–192, 2014. [DOI] [PubMed] [Google Scholar]
- 97.Shigeoka AA, Mueller JL, Kambo A, Mathison JC, King AJ, Hall WF, Correia Jda S, Ulevitch RJ, Hoffman HM, McKay DB. An inflammasome-independent role for epithelial-expressed Nlrp3 in renal ischemia-reperfusion injury. J Immunol 185: 6277–6285, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smaldone MC, Vodovotz Y, Tyagi V, Barclay D, Philips BJ, Yoshimura N, Chancellor MB, Tyagi P. Multiplex analysis of urinary cytokine levels in rat model of cyclophosphamide-induced cystitis. Urology 73: 421–426, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Solini A, Iacobini C, Ricci C, Chiozzi P, Amadio L, Pricci F, Di Mario U, Di Virgilio F, Pugliese G. Purinergic modulation of mesangial extracellular matrix production: role in diabetic and other glomerular diseases. Kidney Int 67: 875–885, 2005. [DOI] [PubMed] [Google Scholar]
- 100.Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann NY Acad Sci 1319: 82–95, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Symington JW, Wang C, Twentyman J, Owusu-Boaitey N, Schwendener R, Nunez G, Schilling JD, Mysorekar IU. ATG16L1 deficiency in macrophages drives clearance of uropathogenic E. coli in an IL-1beta-dependent manner. Mucosal Immunol 8: 1388–1399, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang X, Lieske JC. Acute and chronic kidney injury in nephrolithiasis. Curr Opin Nephrol Hypertens 23: 385–390, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tasic V, Gucev Z. Nephrolithiasis and nephrocalcinosis in children—metabolic and genetic factors. Pediatr Endocrinol Rev 13: 468–476, 2015. [PubMed] [Google Scholar]
- 104.Thompson DB, Siref LE, Feloney MP, Hauke RJ, Agrawal DK. Immunological basis in the pathogenesis and treatment of bladder cancer. Expert Rev Clin Immunol 11: 265–279, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tian S, Zhang L, Tang J, Guo X, Dong K, Chen SY. HMGB1 exacerbates renal tubulointerstitial fibrosis through facilitating M1 macrophage phenotype at the early stage of obstructive injury. Am J Physiol Renal Physiol 308: F69–F75, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ucero AC, Benito-Martin A, Izquierdo MC, Sanchez-Nino MD, Sanz AB, Ramos AM, Berzal S, Ruiz-Ortega M, Egido J, Ortiz A. Unilateral ureteral obstruction: beyond obstruction. Int Urol Nephrol 46: 765–776, 2014. [DOI] [PubMed] [Google Scholar]
- 107.Vigano E, Mortellaro A. Caspase-11: the driving factor for noncanonical inflammasomes. Eur J Immunol 43: 2240–2245, 2013. [DOI] [PubMed] [Google Scholar]
- 108.Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, Li Y, Clark SA, Tschopp J, Trpkov K, Hemmelgarn BR, Beck PL, Muruve DA. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol 21: 1732–1744, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wada J, Makino H. Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol 12: 13–26, 2016. [DOI] [PubMed] [Google Scholar]
- 110.Wang C, Mendonsa GR, Symington JW, Zhang Q, Cadwell K, Virgin HW, Mysorekar IU. Atg16L1 deficiency confers protection from uropathogenic Escherichia coli infection in vivo. Proc Natl Acad Sci USA 109: 11008–11013, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang C, Pan Y, Zhang QY, Wang FM, Kong LD. Quercetin and allopurinol ameliorate kidney injury in STZ-treated rats with regulation of renal NLRP3 inflammasome activation and lipid accumulation. PLoS One 7: e38285, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang C, Symington JW, Mysorekar IU. ATG16L1 and pathogenesis of urinary tract infections. Autophagy 8: 1693–1694, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang J, Wen Y, Lv LL, Liu H, Tang RN, Ma KL, Liu BC. Involvement of endoplasmic reticulum stress in angiotensin II-induced NLRP3 inflammasome activation in human renal proximal tubular cells in vitro. Acta Pharmacol Sin 36: 821–830, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang W, Luo R, Lin Y, Wang F, Zheng P, Levi M, Yang T, Li C. Aliskiren restores renal AQP2 expression during unilateral ureteral obstruction by inhibiting the inflammasome. Am J Physiol Renal Physiol 308: F910–F922, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang W, Wang X, Chun J, Vilaysane A, Clark S, French G, Bracey NA, Trpkov K, Bonni S, Duff HJ, Beck PL, Muruve DA. Inflammasome-independent NLRP3 augments TGF-beta signaling in kidney epithelium. J Immunol 190: 1239–1249, 2013. [DOI] [PubMed] [Google Scholar]
- 116.Warren SE, Armstrong A, Hamilton MK, Mao DP, Leaf IA, Miao EA, Aderem A. Cutting edge: Cytosolic bacterial DNA activates the inflammasome via Aim2. J Immunol 185: 818–821, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wen H, Miao EA, Ting JP. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity 39: 432–441, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Whiteside SA, Razvi H, Dave S, Reid G, Burton JP. The microbiome of the urinary tract–a role beyond infection. Nat Rev Urol 12: 81–90, 2015. [DOI] [PubMed] [Google Scholar]
- 119.Wu H, Ma J, Wang P, Corpuz TM, Panchapakesan U, Wyburn KR, Chadban SJ. HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol 21: 1878–1890, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yamagishi H, Yokoo T, Imasawa T, Mitarai T, Kawamura T, Utsunomiya Y. Genetically modified bone marrow-derived vehicle cells site specifically deliver an anti-inflammatory cytokine to inflamed interstitium of obstructive nephropathy. J Immunol 166: 609–616, 2001. [DOI] [PubMed] [Google Scholar]
- 121.Yamaguchi O, Nomiya M, Andersson KE. Functional consequences of chronic bladder ischemia. Neurourol Urodyn 33: 54–58, 2014. [DOI] [PubMed] [Google Scholar]
- 122.Yang H, Wang H, Chavan SS, Andersson U. High Mobility Group Box Protein 1 (HMGB1): the prototypical endogenous danger molecule. Mol Med 21, Suppl 1: S6–S12, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang SM, Ka SM, Wu HL, Yeh YC, Kuo CH, Hua KF, Shi GY, Hung YJ, Hsiao FC, Yang SS, Shieh YS, Lin SH, Wei CW, Lee JS, Yang CY, Chen A. Thrombomodulin domain 1 ameliorates diabetic nephropathy in mice via anti-NF-kappaB/NLRP3 inflammasome-mediated inflammation, enhancement of NRF2 antioxidant activity and inhibition of apoptosis. Diabetologia 57: 424–434, 2014. [DOI] [PubMed] [Google Scholar]
- 124.Zhuang Y, Hu C, Ding G, Zhang Y, Huang S, Jia Z, Zhang A. Albumin impairs renal tubular tight junctions via targeting the NLRP3 inflammasome. Am J Physiol Renal Physiol 308: F1012–F1019, 2015. [DOI] [PubMed] [Google Scholar]
- 125.Zoppini G, Targher G, Chonchol M, Ortalda V, Abaterusso C, Pichiri I, Negri C, Bonora E. Serum uric acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabetes Care 35: 99–104, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]