Short sampling durations are used commonly to assess baseline muscle sympathetic nerve activity (MSNA) before a stress to quantify sympathetic responsiveness. The validity and reliability of these measures have not been tested. Our analyses demonstrate that MSNA, sampled from 2 and 1 min and 30 and 15 s epochs, possesses high agreement and no fixed bias compared with a standard 5-min control but consistent evidence of a proportional bias that would confound interindividual comparisons.
Keywords: muscle sympathetic nerve activity, reliability, reproducibility
Abstract
Resting muscle sympathetic nerve activity (MSNA) demonstrates high intraindividual reproducibility when sampled over 5–30 min epochs, although shorter sampling durations are commonly used before and during a stress to quantify sympathetic responsiveness. The purpose of the present study was to examine the intratest validity and reliability of MSNA sampled over 2 and 1 min and 30 and 15 s epoch durations. We retrospectively analyzed 68 resting fibular nerve microneurographic recordings obtained from 53 young, healthy participants (37 men; 23 ± 6 yr of age). From a stable 7-min resting baseline, MSNA (burst frequency and incidence, normalized mean burst amplitude, total burst area) was compared among each epoch duration and a standard 5-min control. Bland-Altman plots were used to determine agreement and bias. Three sequential MSNA measurements were collected using each sampling duration to calculate absolute and relative reliability (coefficients of variation and intraclass correlation coefficients). MSNA values were similar among each sampling duration and the 5-min control (all P > 0.05), highly correlated (r = 0.69–0.93; all P < 0.001), and demonstrated no evidence of fixed bias (all P > 0.05). A consistent proportional bias (P < 0.05) was present for MSNA burst frequency (all sampling durations) and incidence (1 min and 30 and 15 s), such that participants with low and high average MSNA underestimated and overestimated the true value, respectively. Reliability decreased progressively using the 30- and 15-s sampling durations. In conclusion, short 2 and 1 min and 30 s sampling durations can provide valid and reliable measures of MSNA, although increased sample size may be required for epochs ≤30 s, due to poorer reliability.
NEW & NOTEWORTHY
Short sampling durations are used commonly to assess baseline muscle sympathetic nerve activity (MSNA) before a stress to quantify sympathetic responsiveness. The validity and reliability of these measures have not been tested. Our analyses demonstrate that MSNA, sampled from 2 and 1 min and 30 and 15 s epochs, possesses high agreement and no fixed bias compared with a standard 5-min control but consistent evidence of a proportional bias that would confound interindividual comparisons.
the sympathetic nervous system is critical for the regulation of arterial pressure at rest and in response to a stress, such as exercise (20, 30). In humans, sympathetic activity is quantified, most commonly, using measurements of plasma norepinephrine, radiolabeled norepinephrine spillover, or microneurographic recordings of muscle (or skin) sympathetic nerve activity (MSNA) (10, 19, 30, 39). Unlike discrete measurements of norepinephrine concentrations, microneurography permits continuous data collection, making it ideal for assessing regional vasoconstrictor responses to psychophysiological stressors, such as exercise, temperature, or pain (4, 8, 34). Unfortunately, the temporal resolution of MSNA recordings is limited by the spontaneous, albeit rhythmic and pulse synchronous, nature of muscle sympathetic discharges, which require that bursts of activity be sampled over specific epochs of time, commonly minutes (6, 19, 39). Such measurements, expressed as bursts per minute (burst frequency), or to account for differences in heart rate, bursts per 100 heartbeats (burst incidence), are highly reproducible within an individual over the short (minutes to hours) (13, 15, 39a), intermediate (days to weeks) (13, 15, 23), and long (months to years) (11, 12) term, when sampled from epochs ranging between 5 and 30 min in duration. Whether MSNA, measured over shorter sampling durations, exhibits similarly high intraindividual reliability, thus allowing for greater resolution, is unknown.
In healthy individuals, beat-to-beat oscillations in spontaneous peripheral sympathetic activity are the integrated product of rhythmic central generation and afferent feedback from stretch-sensitive lung receptors, chemoreceptors, and baroreceptors (18, 29, 30). This fluctuation in vasomotor sympathetic outflow results in cyclic, 0.1 Hz changes in arterial pressure, termed Mayer waves (33). Inherently, the rhythmic occurrence of muscle sympathetic bursts would be expected to increase measurement variability when sampling durations are short (e.g., seconds). Surprisingly, no study has examined the impact of sampling duration on the validity (accuracy to the “true value”) or reliability (consistency over time) of MSNA. This is critical, as a growing number of studies have assessed MSNA over 30 s, 1 min, or 2 min sampling durations (9, 17, 25, 26, 34, 36), primarily during a baseline period before exercise. As these measures are frequently used to calculate the absolute or percent change from baseline to quantify sympathetic responsiveness (9, 17, 25, 26, 34, 36), poor validity or reliability would increase the measurement error and the risk of spurious conclusions. Sampling durations, lasting 10–30 s, have also been used during exercise (5, 14, 16, 26), although the accuracy of these measurements at rest has never been established.
Therefore, the purpose of the present study was to examine the impact of sampling duration on the validity and reliability of resting MSNA in a large cohort of healthy, young participants. Specifically, we sought to compare MSNA burst frequency, burst incidence, normalized mean burst amplitude, and total MSNA burst area calculated from a standard 5-min control epoch (true value) with values sampled from 2 and 1 min and 30 and 15 s durations. In addition, we examined three sequential MSNA measurements for each sampling duration to determine intratest reproducibility. We hypothesized that 1) mean values of MSNA would be similar between the 5-min control and all sampling durations; 2) the absolute reliability of repeated MSNA measurements would be lowest in the 15-s epoch (i.e., highest coefficients of variation); and 3) the relative reliability (intraclass correlation coefficient) of repeated MSNA measurements would be good to excellent [r > 0.60 (7)] for each epoch duration, validating its use in past and future work.
METHODS
Participants.
The data used in this study were analyzed retrospectively from 68 previous microneurographic studies conducted in our laboratory. All studies were approved by the University of Guelph Research Ethics Board and occurred following completion of informed, written consent by each participant. Data were collected from 53 healthy, young, asymptomatic participants (37 men; 23 ± 6 yr), who were all normotensive, nonsmoking, in sinus rhythm, free of known cardiovascular or metabolic disease, and not taking any acute or chronic medications. Before each study visit, participants abstained from caffeine, alcohol, and strenuous exercise for 12–24 h.
Muscle sympathetic nerve activity.
In each study, participants underwent instrumentation and a 10-min rest period, followed by a 7-min baseline MSNA recording in a temperature-controlled (22°C) and quiet laboratory. Efferent postganglionic, multiunit MSNA was measured from the common peroneal (fibular) nerve, as previously described (31, 32, 34). A 2-mΩ tungsten microelectrode (FHC, Bowdoin, ME) was inserted percutaneously into a motor fascicle and adjusted until bursts of spontaneous, multiunit muscle sympathetic activity were detected. A ground electrode was placed ~2 cm away. The MSNA signal was amplified (75,000×), band-pass filtered (0.7–2.0 kHz), rectified, and integrated using a 0.1-s time constant to obtain the mean voltage neurogram (Nerve Traffic Analyzer, Model 662C-4; University of Iowa, Iowa City, IA). Muscle sympathetic activity was confirmed by observing reflexive increases in response to an end-expiration apnea and a lack of responsiveness to unexpected clapping. The neural signal was monitored both visually and audibly to ensure no alteration in microelectrode position throughout each recording.
MSNA was analyzed using a semiautomated custom LabVIEW program (National Instruments, Austin, TX) (31, 32). Determination of a sympathetic burst was made based on a 3:1 signal:noise ratio and alignment with the time-shifted cardiac cycle. From the integrated neurogram, MSNA burst frequency (bursts/minute), burst incidence (bursts/100 heartbeats), mean burst amplitude normalized to the tallest burst (percentage) in the 7-min recording, and total MSNA burst area (arbitrary units/minute) were calculated.
Data analysis.
Our first analysis was to investigate the effects of sampling duration on the validity of MSNA measurements. From the 7-min stable microneurography recording, we computed MSNA values over five epoch durations: 5 (true value control), 2, and 1 min and 30 and 15 s. The start point for each interval was randomly determined using an online random number generator (Random.org, https://www.random.org/; Randomness and Integrity Services, Dublin, Ireland), with the requirement that each interval could not exceed the 7-min window. For example, to determine the 5-min epoch start, a random number between 0 and 120 s was generated for each recording. All random numbers were rounded to the nearest 5 s. Next, we investigated the intratest reliability of three sequential MSNA measurements using each short sampling duration (2 and 1 min and 30 and 15 s). Again from the 7-min microneurography recording, three sequential (nonoverlapping) time points (T1, T2, T3) were obtained for each sampling duration. The start point for each interval was determined randomly using the methods above and again, fulfilled the requirement that each interval could not exceed the 7-min window (e.g., 2 min epoch duration started between 0 and 60 s).
Statistical analysis.
Analyses were performed using IBM SPSS Statistics 23 (Armonk, NY) and GraphPad Prism (GraphPad Software, La Jolla, CA). We examined the effects of sampling duration on MSNA agreement using a one-way repeated-measures ANOVA. Linear regression analyses were conducted and Bland-Altman plots created to compare each sampling duration (2 and 1 min and 30 and 15 s) against the 5-min control. Bland-Altman plots were used to determine the fixed bias among sampling durations, defined as the mean difference between the 5-min control MSNA value and each of the shorter epoch durations. A fixed bias was considered to be present if the mean difference was significantly different from zero using a one-sample t-test or if the 95% confidence intervals of the mean difference did not include zero (28). To rule out the existence of proportional bias as average MSNA values increase or decrease, we tested whether the slope of the linear regression line, fit to the Bland-Altman plot, differed significantly from zero using a one-sample t-test or whether the 95% confidence intervals did not include zero (28). One-way repeated-measures ANOVAs were used to investigate agreement among the three sequential time points (T1, T2, T3) during each sampling duration. To evaluate intratest absolute and relative reliability, we calculated the coefficients of variation and intraclass correlation coefficients for each sampling duration, respectively. Significance was considered P < 0.05, and data are presented as means ± SD.
RESULTS
Participants were young [23 ± 6 yr (range: 18–51)], predominantly men (n = 37), with a normal body mass index [23 ± 3 kg/m2 (range: 17–29)], heart rate [63 ± 9 beats/min (range: 48–84)], and blood pressure [106 ± 9 mmHg (range: 85–123)/64 ± 6 mmHg (range: 50–77)]. High-quality microneurographic recordings were obtained in all participants. Characteristic of MSNA in healthy adults (6, 21, 39), measures of burst frequency [24 ± 6 bursts/min (range: 9–41)] and burst incidence [38 ± 11 bursts/100 heartbeats (range: 16–75)] exhibited large interindividual variability.
Effects of sampling duration on the validity of MSNA measures.
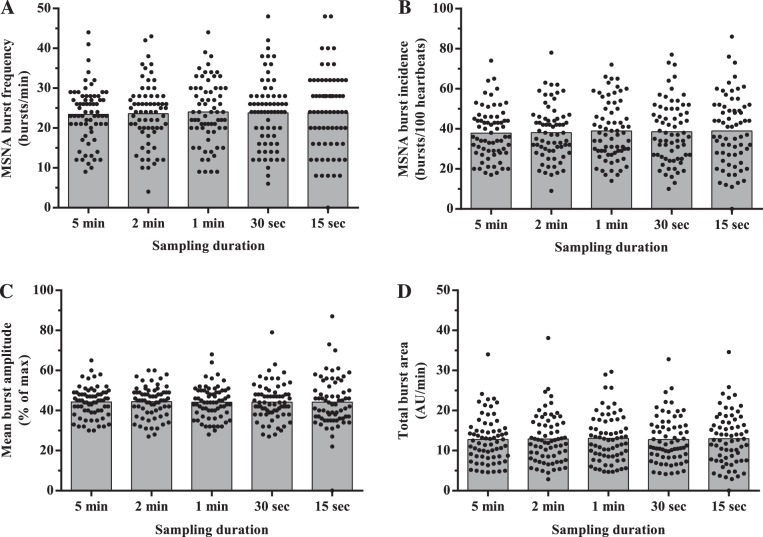
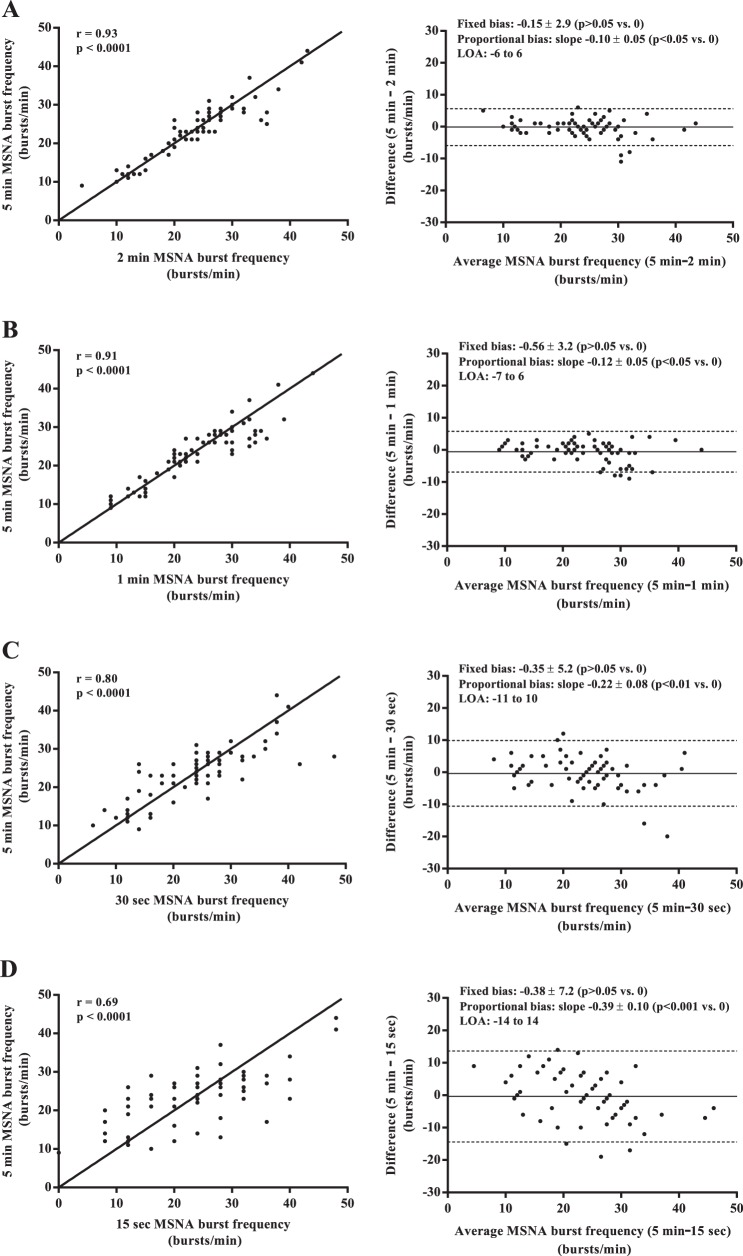
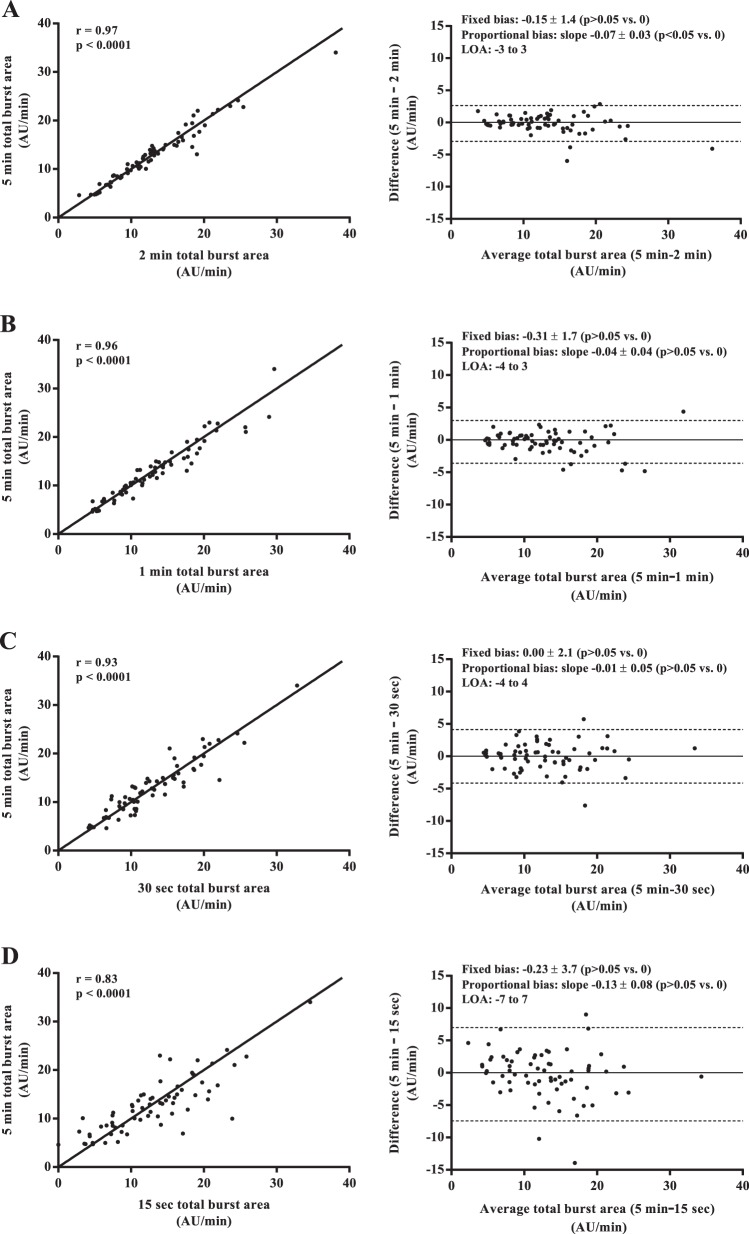
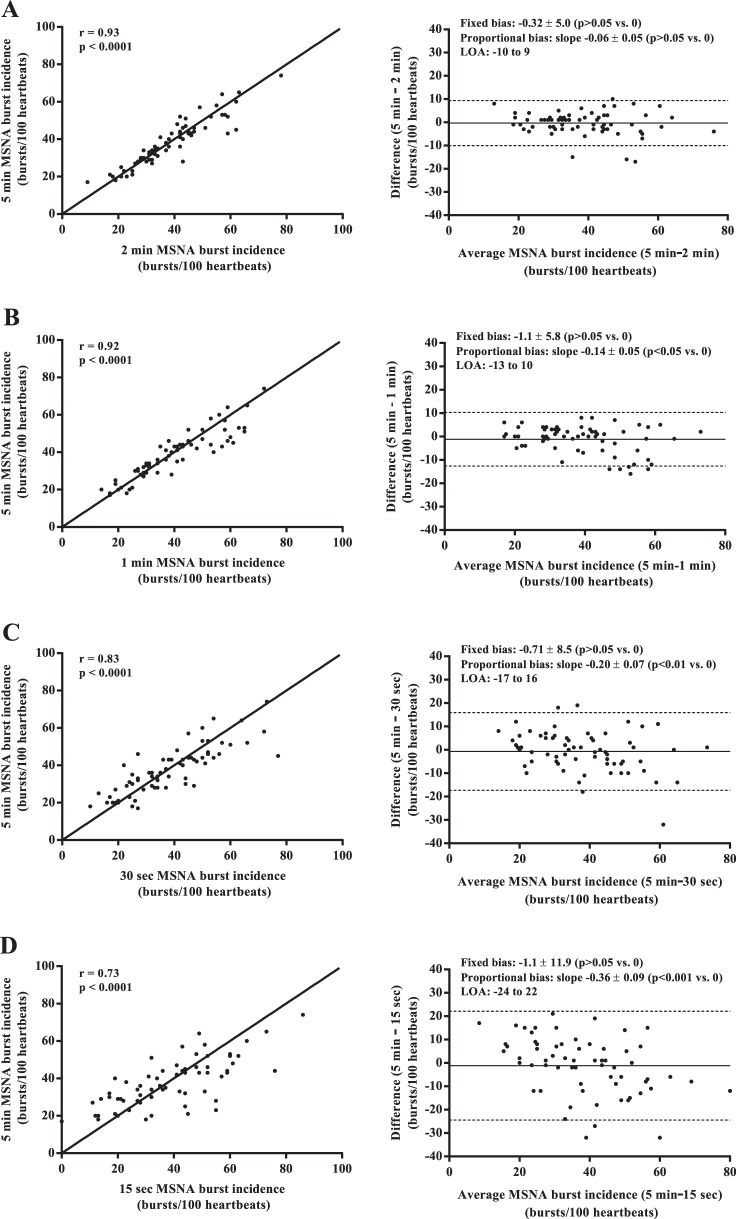
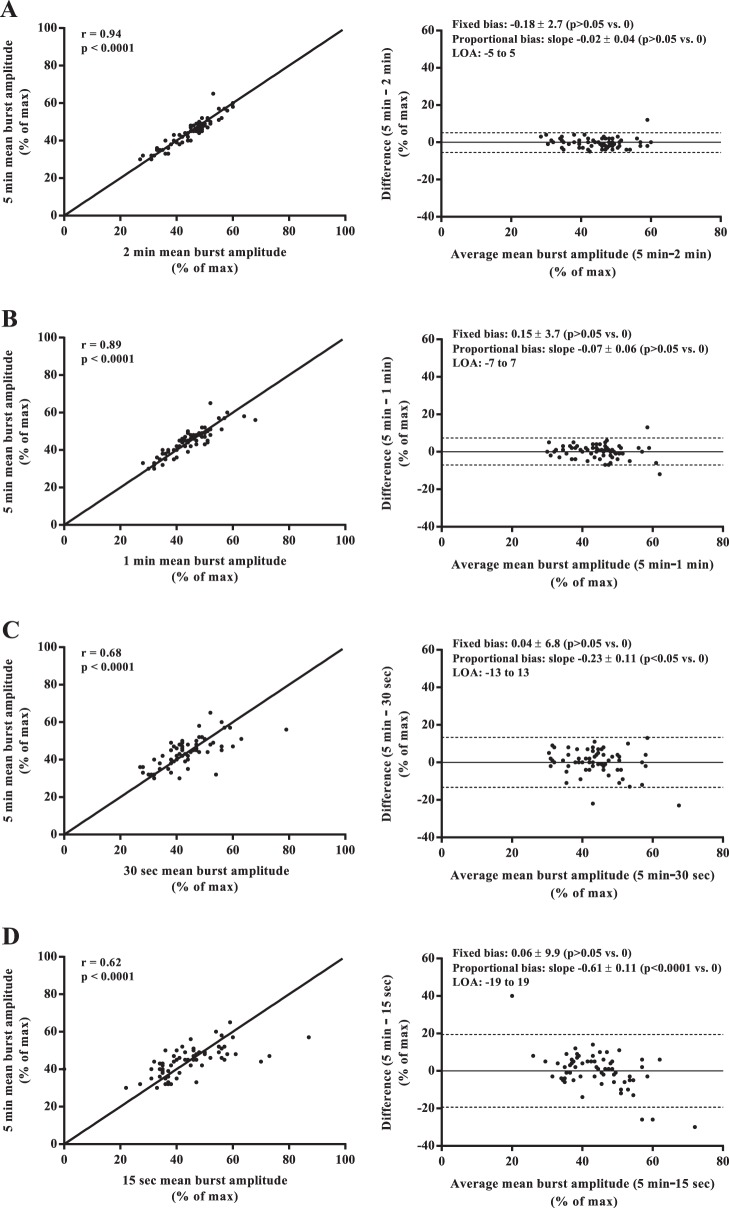
Mean values of MSNA burst frequency, burst incidence, normalized mean burst amplitude, and total MSNA burst area were similar among all sampling durations (all P > 0.05; Fig. 1). Regression analyses demonstrated significant moderate to very strong relationships between the 5-min control epoch and each of the shorter sampling durations (r = 0.62–0.97; all P < 0.0001; Figs. 2–5). Each of the Bland-Altman plots (Figs. 2–5) demonstrated no evidence of fixed bias (all P > 0.05) and thus good agreement between the 5-min control epoch and each of the shorter sampling durations. In contrast, a negative slope significantly different from zero (proportional bias) was detected for MSNA burst frequency (all sampling durations), burst incidence (1 min and 30 and 15 s sampling durations), mean burst amplitude (30 and 15 s sampling durations), and total MSNA burst area (2 min sampling duration; all P < 0.05).
Fig. 1.
Effect of sampling duration on mean and individual measurements of muscle sympathetic nerve activity (MSNA) burst frequency (A), burst incidence (B), mean burst amplitude (C), and total MSNA burst area (D).
Fig. 2.
Regression analyses (left column) and Bland-Altman plots (right column) comparing muscle sympathetic nerve activity (MSNA) burst frequency measured from the 5-min control and the 2-min (A), 1-min (B), 30-s (C), and 15-s (D) sampling durations. The solid lines in the regression analyses represent the line of identity. LOA, limits of agreement.
Fig. 5.
Regression analyses (left column) and Bland-Altman plots (right column) comparing total muscle sympathetic nerve activity (MSNA) burst area measured from the 5-min control and the 2-min (A), 1-min (B), 30-s (C), and 15-s (D) sampling durations. The solid lines in the regression analyses represent the line of identity.
Fig. 3.
Regression analyses (left column) and Bland-Altman plots (right column) comparing muscle sympathetic nerve activity (MSNA) burst incidence measured from the 5-min control and the 2-min (A), 1-min (B), 30-s (C), and 15-s (D) sampling durations. The solid lines in the regression analyses represent the line of identity.
Fig. 4.
Regression analyses (left column) and Bland-Altman plots (right column) comparing muscle sympathetic nerve activity (MSNA) mean burst amplitude measured from the 5-min control and the 2-min (A), 1-min (B), 30-s (C), and 15-s (D) sampling durations. The solid lines in the regression analyses represent the line of identity.
Effects of sampling duration on the intratest reproducibility of MSNA measures.
As shown in Table 1, no significant differences in MSNA were detected among sequential measurements (all P > 0.05). For all MSNA variables, the coefficients of variation were significantly higher in the 15-s epoch duration compared with all other intervals (all P < 0.05; Table 1). With the exception of normalized mean burst amplitude, the coefficients of variation were also significantly higher in the 30-s epoch compared with the 2- and 1-min durations (all P < 0.05). Good to excellent intraclass correlation coefficients (7) were found for MSNA variables in 2 and 1 min and 30 s epoch durations (r = 0.58–0.94; all P < 0.0001; Table 1). With the exception of total MSNA burst area, the 15-s epoch duration exhibited only fair to good intraclass correlation coefficients (r = 0.33–0.54; all P < 0.0001).
Table 1.
Effects of sampling duration on the reproducibility of sequentially sampled measurements of muscle sympathetic nerve activity (MSNA)
| T1 | T2 | T3 | ANOVA, P | CV, % | ICC | |
|---|---|---|---|---|---|---|
| 2 min | ||||||
| Burst frequency, bursts/min | 23.5 ± 7.4 | 23.9 ± 7.7 | 23.3 ± 7.6 | 0.36 | 11 ± 8 | 0.88 |
| Burst incidence, bursts/100 heartbeats | 38.0 ± 13.2 | 38.6 ± 13.2 | 37.5 ± 13.4 | 0.40 | 11 ± 9 | 0.89 |
| Mean burst amplitude, % of max | 44.1 ± 7.7 | 44.0 ± 7.1 | 44.4 ± 7.4 | 0.83 | 6 ± 5 | 0.81 |
| Total MSNA, arbitrary units/min | 12.7 ± 5.9 | 12.9 ± 5.9 | 12.7 ± 5.9 | 0.64 | 10 ± 8 | 0.94 |
| 1 min | ||||||
| Burst frequency, bursts/min | 23.1 ± 8.3 | 23.7 ± 7.3 | 23.8 ± 8.2 | 0.38 | 13 ± 8 | 0.84 |
| Burst incidence, bursts/100 heartbeats | 37.2 ± 13.6 | 38.3 ± 12.5 | 38.0 ± 14.3 | 0.51 | 15 ± 9 | 0.81 |
| Mean burst amplitude, % of max | 45.0 ± 8.5 | 44.3 ± 7.3 | 44.0 ± 7.7 | 0.30 | 7 ± 5 | 0.74 |
| Total MSNA, arbitrary units/min | 12.7 ± 5.8 | 12.9 ± 5.7 | 12.9 ± 6.4 | 0.71 | 12 ± 7 | 0.92 |
| 30 s | ||||||
| Burst frequency, bursts/min | 22.3 ± 8.9 | 22.8 ± 8.2 | 23.0 ± 7.5 | 0.69 | 20 ± 13*† | 0.65 |
| Burst incidence, bursts/100 heartbeats | 35.5 ± 14.6 | 36.9 ± 14.8 | 37.1 ± 12.9 | 0.48 | 21 ± 13*† | 0.65 |
| Mean burst amplitude, % of max | 44.8 ± 8.7 | 44.2 ± 8.4 | 44.1 ± 8.5 | 0.76 | 11 ± 6* | 0.58 |
| Total MSNA, arbitrary units/min | 12.1 ± 6.5 | 12.4 ± 6.0 | 12.6 ± 6.1 | 0.48 | 19 ± 12*† | 0.83 |
| 15 s | ||||||
| Burst frequency, bursts/min | 23.4 ± 10.9 | 22.8 ± 9.7 | 23.2 ± 10.1 | 0.85 | 30 ± 19*†‡ | 0.52 |
| Burst incidence, bursts/100 heartbeats | 37.3 ± 17.6 | 36.7 ± 16.9 | 37.6 ± 18.0 | 0.90 | 31 ± 20*†‡ | 0.54 |
| Mean burst amplitude, % of max | 44.1 ± 12.5 | 44.6 ± 13.9 | 45.4 ± 11.3 | 0.75 | 18 ± 16*†‡ | 0.33 |
| Total MSNA, arbitrary units//min | 12.1 ± 6.7 | 12.4 ± 6.5 | 12.7 ± 6.9 | 0.63 | 28 ± 19*†‡ | 0.73 |
Means ± SD. T1–T3, time points 1–3; CV, coefficient of variation; ICC, intraclass correlation coefficient.
P < 0.05 vs. 2 min;
P < 0.05 vs. 1 min;
P < 0.05 vs. 30 s.
DISCUSSION
Proper interpretation of scientific research relies on the basic assumption that the methods used demonstrate high reliability and reproducibility (1). Although technically challenging (30), microneurography is considered the gold standard for measuring sympathetic outflow to skeletal muscle in humans (2). Measurements of MSNA have consistently been shown to exhibit high intraindividual reliability (11–13, 15, 23, 39a); however, these analyses were all conducted using sampling durations lasting 5–30 min. Given the growing number of studies using shorter epoch lengths (i.e., <2 min) (9, 17, 25, 26, 34, 36), the present analysis sought to examine comprehensively the effect of sampling duration on the validity and reliability of resting MSNA. The primary novel finding was that MSNA measurements obtained from sampling durations as short as 30 s demonstrated high agreement and no fixed bias with control values but consistent evidence of a proportional bias that may confound the use of short sampling durations for interindividual comparisons in participants with highly variable MSNA. Furthermore, intratest reliability declined sharply as sampling duration decreased, suggesting that caution is warranted in drawing conclusions from studies relying on short epochs for MSNA analysis without appropriate adjustment for sample-size requirements. Collectively, these results provide much needed clarification regarding the measurement error associated with the analysis of MSNA.
Prior investigations have used shorter sampling durations (<2 min) to quantify MSNA at baseline or during a stress (9, 16, 17, 25, 26, 34, 36), but to our knowledge, the validity and reliability of these measurement epochs have never been established. Without proper knowledge of the underlying measurement error associated with these sampling durations, a priori calculation of the sample size required to detect a significant difference (should one exist) would be confounded. Furthermore, the use of these potentially variable baseline values will contribute error to the calculation of change scores frequently used to characterize sympathetic responsiveness to a stress. Our results support the hypothesis that compared with a standard 5-min control epoch, shorter sampling durations demonstrate no evidence of disagreement or fixed bias among MSNA measurements. However, a drawback of shorter sampling durations is that MSNA exhibits poorer absolute and relative reliability (i.e., coefficient of variation and intraclass correlation coefficients), thereby reducing statistical power. A reduction in the intraclass correlation coefficient from r = 0.9 to r = 0.7 has been estimated to require a 22% increase in sample size to detect a significant difference (35). This information is highly relevant for future study design, particularly given that studies measuring MSNA typically recruit <15–20 participants per group (16, 25, 26, 31, 32, 34). One factor likely contributing to the decreased reliability of MSNA obtained from short sampling durations is the reporting of values in bursts/minute or bursts/100 heartbeats, which would multiply the magnitude of the error. This can be seen in Fig. 1, where the 15-s sampling duration produces banding in MSNA measurements. As a result, the use of short measurement epochs (<30 s) at rest (or during exercise) may be better suited to report absolute burst count.
A novel observation of our analysis was the existence of a consistent proportional bias, most evident for measures of MSNA burst frequency and burst incidence. As the slopes of all significant regression lines were negative, a low average MSNA underestimated true values, while a high average MSNA overestimated true values. This finding has important implications given the large interindividual variability of resting MSNA in healthy adults (6, 21, 39), as it would add systematic measurement error among participants. Furthermore, sampling from short epochs may overestimate the true difference in MSNA reactivity between two populations with contrasting levels of sympathetic activity (e.g., healthy controls vs. heart failure or hypertension patients) (27, 34, 40). If short sampling durations are used for assessing baseline or changes in MSNA, studies should confirm findings as a percent change or by controlling for baseline MSNA.
In addition to the more commonly reported measures of MSNA burst frequency or incidence, normalized mean burst amplitude, a measure of the strength of a sympathetic discharge (23, 38), demonstrated the lowest coefficient of variation during each of the sampling durations. This finding is consistent with prior literature demonstrating that mean burst amplitude exhibits excellent intraindividual reproducibility at rest (38) and between-day reproducibility at rest and in response to head-up tilt (23). The assessment of burst amplitude is important given that the regulation of burst strength and occurrence is thought to occur independently (22), and different conclusions on neural reflex control can be made based on which measure of muscle sympathetic activity is selected as the dependent variable (24). It is important to acknowledge that absolute MSNA burst amplitude is dependent on the proximity of the recording microelectrode to the discharging fibers limiting interindividual or between-day comparisons (38), although normalization attempts to circumvent this methodological issue by calculating relative burst amplitude changes (23, 38).
Limitations.
The present analysis examined resting MSNA in young, healthy adults and may not be generalizable to older or clinical populations. However, we would hypothesize, based on the knowledge that MSNA increases both with age (37, 39) and many cardiovascular disease states (27, 34, 40), that measurement agreement for short sampling durations may be improved, owing to a decrease in periods of neural silence (i.e., higher MSNA burst incidence). We selected sampling duration based on previously published work (9, 16, 17, 25, 26, 34, 36); however, the balance between temporal resolution and high validity and reliability likely differs within each individual, based on the prevailing resting heart rate and sympathetic activation. Finally, although resting MSNA exhibits high test-retest reproducibility when sampled from 5 min epochs (13, 23), our results may not extend to control values obtained from longer sampling durations.
In conclusion, the present study provides the first evidence supporting the validity and reliability of measuring MSNA using short sampling durations. Such analysis is already conducted (9, 16, 17, 25, 26, 34, 36), although whether the risk of increased measurement error may obscure the physiological interpretation has not been studied. We provide strong support for the use of 2 and 1 min or 30 s sampling durations and suggest that caution is warranted with epochs of 15 s or shorter. The existence of a proportional bias across shorter sampling durations reveals an unrecognized limitation of using such measures to compare MSNA among participants or groups that should be addressed in future studies.
GRANTS
Support for this research was provided by a Natural Science and Engineering Research Council (NSERC) of Canada Discovery Grant (#1256447-2015; to P. J. Millar), a University of Guelph-Humber Research Fund Grant (to P. J. Millar), and the Canada Foundation for Innovation (#34379; to P. J. Millar). Support for A. V. Incognito was provided by a Canadian Institutes of Health Research Fredrick Banting and Charles Best Canada Graduate Scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.J.M. conception and design of research; K.N., A.V.I., C.J.D., M.J.B., and P.J.M. performed experiments; K.N., J.D.S., A.V.I., C.J.D., and M.J.B. analyzed data; K.N., J.D.S., A.V.I., M.N., M.J.B., and P.J.M. interpreted results of experiments; K.N., J.D.S., and M.N. prepared figures; K.N., J.D.S., A.V.I., M.N., and P.J.M. drafted manuscript; K.N., J.D.S., A.V.I., C.J.D., M.N., M.J.B., and P.J.M. edited and revised manuscript; K.N., J.D.S., A.V.I., C.J.D., M.N., M.J.B., and P.J.M. approved final version of manuscript.
REFERENCES
- 1.Begley CG, Ioannidis JPA. Reproducibility in science: improving the standard for basic and preclinical research. Circ Res 116: 116–126, 2015. doi: 10.1161/CIRCRESAHA.114.303819. [DOI] [PubMed] [Google Scholar]
- 2.Bertisch SM, Taylor JA. Caveat utilitor: take measure of your marker. J Physiol 589: 5341, 2011. doi: 10.1113/jphysiol.2011.221770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton AR, Birznieks I, Bolton PS, Henderson LA, Macefield VG. Effects of deep and superficial experimentally induced acute pain on muscle sympathetic nerve activity in human subjects. J Physiol 587: 183–193, 2009. doi: 10.1113/jphysiol.2008.162230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callister R, Suwarno NO, Seals DR. Sympathetic activity is influenced by task difficulty and stress perception during mental challenge in humans. J Physiol 454: 373–387, 1992. doi: 10.1113/jphysiol.1992.sp019269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charkoudian N, Wallin BG. Sympathetic neural activity to the cardiovascular system: integrator of systemic physiology and interindividual characteristics. Compr Physiol 4: 825–850, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Chinn S. Statistics in respiratory medicine. 2. Repeatability and method comparison. Thorax 46: 454–456, 1991. doi: 10.1136/thx.46.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crandall CG, Etzel RA, Farr DB. Cardiopulmonary baroreceptor control of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol 277: H2348–H2352, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 299: H1318–H1327, 2010. doi: 10.1152/ajpheart.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esler M, Jennings G, Korner P, Blombery P, Sacharias N, Leonard P. Measurement of total and organ-specific norepinephrine kinetics in humans. Am J Physiol 247: E21–E28, 1984. [DOI] [PubMed] [Google Scholar]
- 11.Fagius J, Wallin BG. Long-term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clin Auton Res 3: 201–205, 1993. doi: 10.1007/BF01826234. [DOI] [PubMed] [Google Scholar]
- 12.Floras JS, Hara K. Sympathoneural and haemodynamic characteristics of young subjects with mild essential hypertension. J Hypertens 11: 647–655, 1993. doi: 10.1097/00004872-199306000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Fonkoue IT, Carter JR. Sympathetic neural reactivity to mental stress in humans: test-retest reproducibility. Am J Physiol Regul Integr Comp Physiol 309: R1380–R1386, 2015. doi: 10.1152/ajpregu.00344.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Q, Verheyden B, Wieling W, Levine BD. Cardiac output and sympathetic vasoconstrictor responses during upright tilt to presyncope in healthy humans. J Physiol 590: 1839–1848, 2012. doi: 10.1113/jphysiol.2011.224998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grassi G, Bolla G, Seravalle G, Turri C, Lanfranchi A, Mancia G. Comparison between reproducibility and sensitivity of muscle sympathetic nerve traffic and plasma noradrenaline in man. Clin Sci (Lond) 92: 285–289, 1997. doi: 10.1042/cs0920285. [DOI] [PubMed] [Google Scholar]
- 16.Greaney JL, Edwards DG, Fadel PJ, Farquhar WB. Rapid onset pressor and sympathetic responses to static handgrip in older hypertensive adults. J Hum Hypertens 29: 402–408, 2015. doi: 10.1038/jhh.2014.106. [DOI] [PubMed] [Google Scholar]
- 17.Greaney JL, Schwartz CE, Edwards DG, Fadel PJ, Farquhar WB. The neural interaction between the arterial baroreflex and muscle metaboreflex is preserved in older men. Exp Physiol 98: 1422–1431, 2013. doi: 10.1113/expphysiol.2013.073189. [DOI] [PubMed] [Google Scholar]
- 18.Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol 4: 1511–1562, 2014. doi: 10.1002/cphy.c140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagbarth KE, Vallbo ÅB. Pulse and respiratory grouping of sympathetic impulses in human muscle-nerves. Acta Physiol Scand 74: 96–108, 1968. doi: 10.1111/j.1365-201X.1968.tb10904.x. [DOI] [PubMed] [Google Scholar]
- 20.Joyner MJ, Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95: 549–601, 2015. doi: 10.1152/physrev.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joyner MJ, Charkoudian N, Wallin BG. Sympathetic nervous system and blood pressure in humans: individualized patterns of regulation and their implications. Hypertension 56: 10–16, 2010. doi: 10.1161/HYPERTENSIONAHA.109.140186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kienbaum P, Karlsson T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531: 861–869, 2001. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimmerly DS, O’Leary DD, Shoemaker JK. Test-retest repeatability of muscle sympathetic nerve activity: influence of data analysis and head-up tilt. Auton Neurosci 114: 61–71, 2004. doi: 10.1016/j.autneu.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Kimmerly DS, Shoemaker JK. Hypovolemia and MSNA discharge patterns: assessing and interpreting sympathetic responses. Am J Physiol Heart Circ Physiol 284: H1198–H1204, 2003. doi: 10.1152/ajpheart.00229.2002. [DOI] [PubMed] [Google Scholar]
- 25.Lalande S, Barron CC, Shoemaker JK. Sex difference in the influence of central blood volume mobilization on the exercise pressor response. Eur J Appl Physiol 115: 2653–2660, 2015. doi: 10.1007/s00421-015-3272-z. [DOI] [PubMed] [Google Scholar]
- 26.Lalande S, Sawicki CP, Baker JR, Shoemaker JK. Effect of age on the hemodynamic and sympathetic responses at the onset of isometric handgrip exercise. J Appl Physiol (1985) 116: 222–227, 2014. doi: 10.1152/japplphysiol.01022.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leimbach WN Jr, Wallin BG, Victor RG, Aylward PE, Sundlöf G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation 73: 913–919, 1986. doi: 10.1161/01.CIR.73.5.913. [DOI] [PubMed] [Google Scholar]
- 28.Ludbrook J. Statistical techniques for comparing measurers and methods of measurement: a critical review. Clin Exp Pharmacol Physiol 29: 527–536, 2002. doi: 10.1046/j.1440-1681.2002.03686.x. [DOI] [PubMed] [Google Scholar]
- 29.Malpas SC. Neural influences on cardiovascular variability: possibilities and pitfalls. Am J Physiol Heart Circ Physiol 282: H6–H20, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 90: 513–557, 2010. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 31.Millar PJ, Murai H, Floras JS. Paradoxical muscle sympathetic reflex activation in human heart failure. Circulation 131: 459–468, 2015. doi: 10.1161/CIRCULATIONAHA.114.010765. [DOI] [PubMed] [Google Scholar]
- 32.Millar PJ, Murai H, Morris BL, Floras JS. Microneurographic evidence in healthy middle-aged humans for a sympathoexcitatory reflex activated by atrial pressure. Am J Physiol Heart Circ Physiol 305: H931–H938, 2013. doi: 10.1152/ajpheart.00375.2013. [DOI] [PubMed] [Google Scholar]
- 33.Myers CW, Cohen MA, Eckberg DL, Taylor JA. A model for the genesis of arterial pressure Mayer waves from heart rate and sympathetic activity. Auton Neurosci 91: 62–75, 2001. doi: 10.1016/S1566-0702(01)00289-2. [DOI] [PubMed] [Google Scholar]
- 34.Notarius CF, Millar PJ, Murai H, Morris BL, Marzolini S, Oh P, Floras JS. Divergent muscle sympathetic responses to dynamic leg exercise in heart failure and age-matched healthy subjects. J Physiol 593: 715–722, 2015. doi: 10.1113/jphysiol.2014.281873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perkins DO, Wyatt RJ, Bartko JJ. Penny-wise and pound-foolish: the impact of measurement error on sample size requirements in clinical trials. Biol Psychiatry 47: 762–766, 2000. doi: 10.1016/S0006-3223(00)00837-4. [DOI] [PubMed] [Google Scholar]
- 36.Saito M, Iwase S, Hachiya T. Resistance exercise training enhances sympathetic nerve activity during fatigue-inducing isometric handgrip trials. Eur J Appl Physiol 105: 225–234, 2009. doi: 10.1007/s00421-008-0893-5. [DOI] [PubMed] [Google Scholar]
- 37.Sundlöf G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sverrisdóttir YB, Rundqvist B, Elam M. Relative burst amplitude in human muscle sympathetic nerve activity: a sensitive indicator of altered sympathetic traffic. Clin Auton Res 8: 95–100, 1998. doi: 10.1007/BF02267819. [DOI] [PubMed] [Google Scholar]
- 39.Vallbo ÅB, Hagbarth KE, Wallin BG. Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. J Appl Physiol (1985) 96: 1262–1269, 2004. doi: 10.1152/japplphysiol.00470.2003. [DOI] [PubMed] [Google Scholar]
- 39a.van de Borne P, Montano N, Zimmerman B, Pagani M, Somers VK. Relationship between repeated measures of hemodynamics, muscle sympathetic nerve activity, and their spectral oscillations. Circulation 96: 4326–4332, 1997. doi: 10.1161/01.CIR.96.12.4326. [DOI] [PubMed] [Google Scholar]
- 40.Wallin BG, Delius W, Hagbarth KE. Comparison of sympathetic nerve activity in normotensive and hypertensive subjects. Circ Res 33: 9–21, 1973. doi: 10.1161/01.RES.33.1.9. [DOI] [PubMed] [Google Scholar]