Abstract
Skeletal muscle is endowed with a remarkable capacity for regeneration, primarily due to the reserve pool of muscle resident satellite cells. The satellite cell is the physiologically quiescent muscle stem cell that resides beneath the basal lamina and adjacent to the sarcolemma. The anatomic location of satellite cells is in close proximity to vasculature where they interact with other muscle resident stem/stromal cells (e.g., mesenchymal stem cells and pericytes) through paracrine mechanisms. This mini-review describes the components of the muscle stem cell niche, as well as the influence of exercise and aging on the muscle stem cell niche. Although exercise promotes ECM reorganization and stem cell accumulation, aging is associated with dense ECM deposition and loss of stem cell function resulting in reduced regenerative capacity and strength. An improved understanding of the niche elements will be valuable to inform the development of therapeutic interventions aimed at improving skeletal muscle regeneration and adaptation over the life span.
Keywords: aging, exercise, extracellular matrix, niche, stem cells
SKELETAL MUSCLE EXTRACELLULAR MATRIX
The extracellular matrix (ECM) in skeletal muscle plays an integral role in tissue development, structural support, and force transmission (78). The ECM accounts for up to 10% of muscle weight and is organized into three layers: the endomysium that surrounds individual muscle fibers, the perimysium that divides the muscle into fascicles, and the epimysium that provides external support to the entire muscle (47). Intramuscular connective tissue is dominated by type I collagen, a fibrous ECM protein that can vary widely in content and alignment between layers and different muscle types to accommodate function. Whereas collagen fibrils are more loosely oriented near the individual fiber to allow for contraction, collagen is densely layered and highly oriented in the perimysium to optimally transmit force to the tendon.
The basement membrane is a specialized layer of ECM that exists between the sarcolemma of each muscle fiber and the surrounding endomysium. It is subdivided into 1) the outer reticular lamina, composed mainly of collagenous fibrils (type I, III, and VI collagens) and fibronectin and 2) the inner basal lamina, composed of nonfibrillar collagen (collagen type IV) and laminin (47). Collagen IV forms a flexible network throughout the basal lamina and connects with laminins near the sarcolemma. As a cross-shaped, heterotrimeric glycoprotein consisting of α-, β-, and γ-subunits, laminin serves as a primary ligand for two sarcolemmal-associated receptors located at the costamere of Z bands, the dystrophin-associated glycoprotein complex (DGC) and the α7β1 integrin (35). Thus the collagen IV/laminin/transmembrane receptor complex provides a critical scaffold necessary for lateral transfer of mechanical force from the myofiber to the surrounding fibrous connective tissue (inside-outside force transmission) during contraction. Diminished expression of any of the structures in the basal lamina (type IV collagen, laminin, transmembrane receptors) can severely impair sarcolemmal and myofiber cytoskeletal integrity, providing the basis for different forms of muscular dystrophy (6, 58). The ECM appears to regulate transmembrane linkage protein expression in a complex manner at the level of gene transcription, because α7β1 integrin expression is severely limited in patients with laminin α2 chain congenital dystrophy, as well as mice that do not synthesize the laminin α2 chain (dy/dy) (45).
SKELETAL MUSCLE STEM CELL NICHE
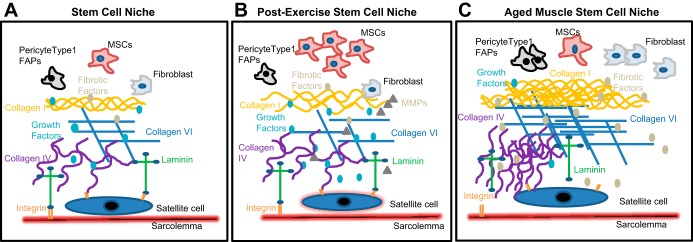
A wide variety of mononuclear cell types reside in skeletal muscle that are essential for tissue repair and maintenance, including stem cells, fibroblasts, immune cells (Fig. 1A). In response to cues provided by damage or mechanical strain, quiescent and unipotent satellite cells (Pax7+) localized outside the sarcolemma and beneath the basal lamina become activated [express myogenic regulatory factors (MRF) Myf5 and MyoD and proliferate] and form myoblasts that can fuse together to regenerate lost tissue or fuse with existing fibers to allow for myofiber repair (14). Pax7− stem/stromal cells specifically residing in the perivascular niche within the interstitium, including pericytes (NG2+CD146+PDGFRβ+), fibroadipogenic progenitor cells (FAPs, PDGFRα+), and muscle-derived mesenchymal stem cells (mMSCs, Sca-1+), may also directly or indirectly assist in fiber repair, yet redundant cell surface marker expression among apparent subpopulations limits our ability to assess their relative contribution at this time (8). Regardless of this gap in knowledge, it is evident that satellite cell and perivascular stem/stromal cell migration, activation, proliferation, and/or function are dependent on cues provided by the niche, including ECM composition, stiffness, topography, and porosity.
Fig. 1.
Schematic representation of the skeletal muscle stem cell niche (A) and its alteration postexercise (B) and aging (C). Exercise results in increased mesenchymal stem cell (MSC) accumulation and ECM reorganization facilitated by matrix metalloproteinases (MMPs). In contrast, aging is associated with increased ECM deposition and reduced growth factor concentration resulting in stem cell dysfunction. [Modified with permission from Taylor & Francis Ltd. (http://www.tandfonline.com) (78).]
Satellite cells and myoblasts express the α7β1 integrin and are highly reliant on the presence of laminin in the basal lamina for multiple activities, including proliferation, adhesion, migration, and differentiation within skeletal muscle (15, 22, 34, 75, 89). Laminins exist as multiple isoforms in a variety of tissues, and at least four are expressed in muscle during development, including laminin-111 (α1β1γ1; LM-111, formerly laminin-1), LM-211 (formerly laminin-2, also known as merosin), LM-121 (formerly laminin-3), and LM-221 (formerly laminin-4) (35). LM-111 is only present in skeletal muscle during early development, and LM-211/LM-221 are the preferential integrin binding isoforms present during adulthood (18). Loss of regenerative capacity in laminin-deficient (dy/dy) mice, as well as improved satellite cell expansion and activity with exogenous LM-111 injection in vivo, suggest a vital role for this ECM protein in the regulation of stem cell function postinjury (45, 71, 93). The influence of other ECM proteins on stem cell activity is also evident from recent studies. For example, fibronectin binding to syndecan-4 can stimulate Wnt7a-dependent expansion of satellite cells (5) and lack of collagen VI in Col6a−/− mice can impair satellite cell self-renewal and repair after injury (7, 81). A recent study also suggests a role for tenascin C in the regulation of satellite cell expansion (79). In vitro studies, although limited in translational capacity, also can delineate the stem cell response to ECM proteins. By direct comparison, laminin can promote muscle stem cell proliferation and differentiation to a greater extent than collagen type I, fibronectin, and gelatin in culture (86). ECM influence on stem cells is clearly not restricted to satellite cells, because our recent work suggests collagen type I can suppress mMSC function (19) and other studies implicate collagen in the initiation of fibroblast-mediated fibrosis (positive feedback loop) (67).
ECM components not only influence stem cell behavior, but interactions are reciprocal such that stem cells also dictate niche composition. This reciprocal relationship is highlighted by studies that demonstrate reconstitution of laminin in dy/dy mice upon myoblast and CD90+ (mMSC) transplantation (25, 84, 85) and accumulation of collagen in wild-type mouse skeletal muscle upon conditional ablation of satellite cells (23, 64). Therefore, although fibroblasts are considered the main contributor to the ECM composition in skeletal muscle, satellite cells and perivascular stem/stromal cells also synthesize a wide variety of ECM components that are necessary for tissue remodeling, including collagens, laminins, fibronectin, and matrix metalloproteinases (MMPs) (5, 19, 36, 63, 74). Interestingly, the ECM gene signature of satellite cells suggests that several ECM components are preferentially expressed in the quiescent state (laminin α2 and γ1), and fibronectin gene and protein expression are uniquely upregulated during proliferation and differentiation (5). Thus it is clear the ECM does not simply serve as a supportive scaffold for skeletal muscle, but dynamically regulates mononuclear cell behavior in a manner that can dictate tissue development, repair, remodeling, and overall function.
EXERCISE-MEDIATED REGULATION OF THE STEM CELL NICHE
Physical activity and mechanical loading provide a strong stimulus for ECM production and degradation in skeletal muscle (Fig. 1B) (42, 43, 46, 47, 56). Collagen type I, III, and IV gene expression, their posttranslational modifying enzymes, and the concentration of hydroxyproline are increased in response to an acute bout of downhill running (39, 48, 49). Collagen type I, III, and IV gene expression is slowly elevated and is sustained in human muscle several weeks after an acute bout of eccentric exercise (46). Damage does not appear to be necessary for ECM synthesis, as an acute bout of nondamaging kicking exercise can increase collagen protein synthesis 3.5 times its resting value by 6 h (61). Collagen synthesis after acute exercise is transient as levels peak at 24 h and gradually return to baseline by 72 h. Similar increases in collagen protein synthesis are observed after heavy resistance exercise (4-fold at 8 h postexercise), regardless of contraction mode (62). MMP-2 and MMP-9, zinc- and calcium-dependent proteolytic enzymes that target collagen type IV and laminin in the basal lamina are also increased in response to both endurance and resistance exercise training (11, 66, 72, 73). Conversely, there is evidence to suggest that lack of loading in the form of immobilization can suppress both protein synthesis and gene expression of collagen type III and IV in skeletal muscle (40, 82).
ECM turnover during and postexercise likely alters the satellite cell niche in a manner that can accommodate participation, all events leading to repair, including proliferation, migration, and differentiation. Pax7+ satellite cells accumulate in skeletal muscle after acute and repeated bouts of strength training (4, 69), with preferential localization observed in the niche surrounding type II fibers after an acute bout of eccentric exercise (13). The fact that high-intensity eccentric exercise selectively recruits type II fibers that incur more damage than type I suggests preferential changes in the type II fiber niche that allow for satellite cell expansion. Accordingly, laminin-211 is expressed to a greater extent in predominantly type II rectus femoris muscle compared with predominantly type I soleus muscle in rats (50). Prominent localization of intracellular MMP-2 expression and gelatinolytic activity in type II fibers compared with type I fibers suggest that remodeling can occur in a muscle fiber type-specific or spatially discrete manner (11, 38). Degradation of the ECM by MMP-2 can facilitate satellite cell migration through the interstitium, as well as release hepatocyte growth factor (HGF) tethered to the matrix (88), an event that can facilitate satellite cell migration and proliferation. Interestingly, MMP-2 is not only necessary for regeneration postinjury (52) and activity-induced angiogenesis (37), but also overload-induced muscle growth (91). Although adult myofiber growth may not be dependent on satellite cell activation and/or fusion (59), it is clear that satellite cells prevent fibroblast-mediated collagen deposition and are necessary for maintenance of growth with long-term chronic loading in mice (23). In addition, our recent data suggest that perivascular stem/stromal cells positively contribute to eccentric exercise-induced gains in muscle fiber size and function in mice (94). Thus ECM remodeling of the basal lamina postexercise may not only facilitate myofiber repair when necessary, but may promote healthy expansion of the interstitial connective tissue environment to accommodate myofiber growth.
AGING AND ECM
Structural changes in the muscle ECM are associated with a decline in mechanical properties and muscle strength with age (51). Collagen deposition has been shown to increase with age using several quantitative and semiquantitative techniques such as hydroxyproline content measurement, picosirius red, or Masson's trichrome staining (54, 70). In-depth evaluation of aged muscle demonstrates that the proportion of collagen subtypes is altered such that levels of collagen type I increase and collagen type III decrease (44). Collagen type IV concentration is enhanced in the basal lamina of slow twitch muscles, whereas laminin concentration decreases with age (50). Similar to immobilization, age can dramatically decrease extracellular gene expression in rat skeletal muscle, including procollagen type I (68), suggesting that accumulation reflects decreased degradation rather than increased synthesis. In support of this hypothesis, aged skeletal muscle exhibits lower resting levels of MMP-2 (20) and baseline MMP-2 gene expression strongly correlates with gains in strength and muscle size posttraining in older adults (21). Interestingly, transplantation of fibroblasts modified to secrete MMP-9 and placenta growth factor diminished collagen and fat deposition in aged dystrophic skeletal muscle (28), suggesting the capacity to reverse ECM dysfunction with disease.
The lack of ECM turnover in aged skeletal muscle may increase susceptibility to posttranslational modifications. In fact, enzymatically mediated crosslinks and advanced glycation end products (AGE) are increased in muscle with age (41, 87). The Young's modulus of skeletal muscle is increased with age, and increased stiffness is associated with excessive accumulation of crosslinked collagen (54, 87).
Alterations in ECM composition, density, and biophysical properties (stiffness) with age can negatively influence satellite cell function. With regard to ECM composition and density, increased thickening of the basal lamina due to increased collagen deposition (Fig. 1C) can influence the physical association between satellite cells and the myofiber (76). Increased accumulation of proteolytic fragments derived from the impaired degradation of ECM proteins such fibronectin, elastin, and laminin may further limit the interaction of satellite cells with neighboring cells and muscle fibers (53). A reduction in the activity of the primary pathways responsible for the removal of damaged or modified proteins (e.g., proteasome) in aged skeletal muscle exacerbates the problem (16). In addition, ECM stiffness alone can impact the overall proliferation and differentiation capability of the satellite cell (31). By utilizing stiffness-tunable hydrogels, it was shown that satellite cells were more likely to proliferate on a hydrogel with a Young's modulus of 2 kPa (80). However, when stiffness was increased to 18 kPa (similarly observed in damaged aged myofibers), satellite cells were more likely to undergo differentiation (54). Therefore, changes in both ECM composition and mechanical properties may play an important role in reducing the regenerative capacity of the skeletal muscle with age.
Brack et al. (9) recently provided a comprehensive review regarding the impact of age on satellite cell senescence and factors that limit the ability to retain quiescence. Aging is a complex and insidious process, and whether deficiencies in satellite cell function occur as a result of structural aberrations in the niche or whether intrinsic satellite cell dysfunction provides the basis for structural modifications is not clear. Lifelong reduction of muscle satellite cells does not appear to accelerate or exacerbate atrophy or further restrict mechanical load-induced growth in aged mice. However, long-term satellite cell depletion does increase collagen content under sedentary conditions compared with age-matched control mice (24, 55), and in vitro studies support the concept that satellite cells secrete factor that prevents fibrosis. Thus deficiencies in the ability for the satellite cell to directly or indirectly modify the ECM may provide the underlying basis for age-related deficits in growth and strength.
THERAPEUTIC APPROACHES TOWARD IMPROVING THE AGED SKELETAL MUSCLE STEM CELL NICHE
Exercise training can reverse the effects of aging on skeletal muscle in both rodents (77) and humans (60). Exercise training can positively impact the passive viscoelastic properties of the skeletal muscle by reducing collagen cross-linking levels in both young (12) and aged (32, 92) rats. Lower levels of ECM crosslinking reduce the stiffness of skeletal muscle, resulting in improved mechanical properties and mechanotransduction to the resident stem cells. Resistance training is known to reduce fat infiltration in aging skeletal muscle and is an effective strategy to maintain skeletal muscle mass and cross-sectional area with age (57, 90). Unfortunately, disabilities due to injury or disease can reduce physical activity and full engagement may not be possible. Thus alternative clinical approaches must be explored.
TGF-β1 signaling promotes fibroblast-mediated collagen deposition and inhibits satellite cell activation and differentiation (27). A recent study demonstrated that administration of losartan, an angiotensin II type 1 receptor blocker, inhibits TGF-β1 signaling and improves muscle regeneration and function after cardiotoxin injury in aged mice. Losartan also enhanced muscle mass and myofiber cross-sectional area in aged, immobilized mice (10). In another study, inhibition of myostatin (a member of the TGF-β1 super-family) using an injectable propeptide resulted in increased muscle mass and muscle fiber size in aged mice (1). Thus manipulation of TGF-β1 signaling may provide a therapeutic approach to aging.
Satellite cell transplantation fails to produce any beneficial effects in mouse or human skeletal muscle due to early cell death, limited proliferation, and migration (65). Several studies have shown that decellularized ECM scaffolds (in which the ECM is preserved) can guide stem cell migration and differentiation and positively influence regenerative outcomes when implanted into injured tissues (2, 3). ECM-based biomaterials engineered to mimic the mechanical properties of healthy young muscle have been effective in rejuvenating aged muscle stem cells. It has been shown that culturing aged satellite cells on soft hydrogels (mimicking physiological stiffness of young skeletal muscle) can restore their regenerative capacity when transplanted into injured muscles of aged mice (17). In a similar study, the myogenic and angiogenic capacity of aged pericytes was improved when they were cultured on fibrinogen-based hydrogels that mimicked the stiffness and mechanical cues of young muscle ECM (26).
LM-111 supplementation has demonstrated remarkable regenerative capacity in several models of disease (33, 71, 83) and injury (93), primarily by upregulating α7 integrin protein expression in the myofiber and influencing satellite cell activity. Laminin α1 chain has also been reported to substitute for the missing endogenous laminin α2 chains and improve the overall health and longevity of mice with congenital muscular dystrophy (29, 30). We speculate that age-related collagen accumulation inhibits the physical interaction of laminin with the α7 integrin within the sarcolemma, as well as integrin expressed by satellite cells, and that exogenous LM-111 or LM-211/221 possess the potential to restore binding and overall muscle function in the context of aging.
CONCLUSION
Exercise training can potently stimulate stem cell activation and positively influence skeletal muscle ECM remodeling in a manner that suggests both factors are important and perhaps codependent in their ability to improve and/or maintain muscle structure and function following a physiological stimulus. Although most investigators acknowledge and appreciate the importance of the ECM in the maintenance of muscle health across the life span, detailed information regarding the impact of age on ECM composition, density, and posttranslational modifications and the subsequent influence on stem cell activation and muscle health is limited. Incorporation of large scale technologies in combination with in vivo and in vitro analyses are necessary to elucidate the complex interaction between the ECM and stem cells, as well as the interaction between the niche and myofiber. The work will undoubtedly be challenging, but will be necessary to develop new paradigms that can effectively treat age-related disabilities.
Present address of K. Garg: Saint Louis University, Department of Biomedical Engineering, 3507 Lindell Blvd., Suite 2011, St. Louis, MO 63103.
GRANTS
This work was partially supported by a grant from the National Institutes of Health (NIH R21 AR065578 to M.D.B.).
AUTHOR CONTRIBUTIONS
K.G. and M.D.B. prepared figures; K.G. and M.D.B. drafted manuscript; K.G. and M.D.B. edited and revised manuscript; K.G. and M.D.B. approved final version of manuscript.
REFERENCES
- 1.Arounleut P, Bialek P, Liang LF, Upadhyay S, Fulzele S, Johnson M, Elsalanty M, Isales CM, Hamrick MW. A myostatin inhibitor (propeptide-Fc) increases muscle mass and muscle fiber size in aged mice but does not increase bone density or bone strength. Exp Gerontol 48: 898–904, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badylak SF. Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: factors that influence the host response. Ann Biomed Eng 42: 1517–1527, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Barnes CA, Brison J, Michel R, Brown BN, Castner DG, Badylak SF, Ratner BD. The surface molecular functionality of decellularized extracellular matrices. Biomaterials 32: 137–143, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellamy LM, Joanisse S, Grubb A, Mitchell CJ, McKay BR, Phillips SM, Baker S, Parise G. The acute satellite cell response and skeletal muscle hypertrophy following resistance training. PLoS One 9: e109739, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, Rudnicki MA. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell 12: 75–87, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonilla E, Samitt CE, Miranda AF, Hays AP, Salviati G, DiMauro S, Kunkel LM, Hoffman EP, Rowland LP. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell 54: 447–452, 1988. [DOI] [PubMed] [Google Scholar]
- 7.Bönnemann CG, Laing NG. Myopathies resulting from mutations in sarcomeric proteins. Curr Opin Neurol 17: 529–537, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Boppart MD, De Lisio M, Zou K, Huntsman HD. Defining a role for non-satellite stem cells in the regulation of muscle repair following exercise. Front Physiol 4: 310, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brack AS, Muñoz-Cánoves P. The ins and outs of muscle stem cell aging. Skelet Muscle 6: 1, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burks TN, Andres-Mateos E, Marx R, Mejias R, Van Erp C, Simmers JL, Walston JD, Ward CW, Cohn RD. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci Transl Med 3: 82ra37, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmeli E, Moas M, Lennon S, Powers SK. High intensity exercise increases expression of matrix metalloproteinases in fast skeletal muscle fibres. Exp Physiol 90: 613–619, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Carroll CC, Martineau K, Arthur KA, Huynh RT, Volper BD, Broderick TL. The effect of chronic treadmill exercise and acetaminophen on collagen and cross-linking in rat skeletal muscle and heart. Am J Physiol Regul Integr Comp Physiol 308: R294–R299, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Cermak NM, Snijders T, McKay BR, Parise G, Verdijk LB, Tarnopolsky MA, Gibala MJ, Van Loon LJ. Eccentric exercise increases satellite cell content in type II muscle fibers. Med Sci Sports Exerc 45: 230–237, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Chargé SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev 84: 209–238, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Clark P, Coles D, Peckham M. Preferential adhesion to and survival on patterned laminin organizes myogenesis in vitro. Exp Cell Res 230: 275–283, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Combaret L, Dardevet D, Béchet D, Taillandier D, Mosoni L, Attaix D. Skeletal muscle proteolysis in aging. Curr Opin Clin Nutr Metab Care 12: 37–41, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, Llewellyn ME, Delp SL, Blau HM. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med 20: 255–264, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crawley S, Farrell EM, Wang W, Gu M, Huang HY, Huynh V, Hodges BL, Cooper DN, Kaufman SJ. The alpha7beta1 integrin mediates adhesion and migration of skeletal myoblasts on laminin. Exp Cell Res 235: 274–286, 1997. [DOI] [PubMed] [Google Scholar]
- 19.De Lisio M, Jensen T, Sukiennik RA, Huntsman HD, Boppart MD. Substrate and strain alter the muscle-derived mesenchymal stem cell secretome to promote myogenesis. Stem Cell Res Ther 5: 74, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dennis RA, Przybyla B, Gurley C, Kortebein PM, Simpson P, Sullivan DH, Peterson CA. Aging alters gene expression of growth and remodeling factors in human skeletal muscle both at rest and in response to acute resistance exercise. Physiol Genomics 32: 393–400, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dennis RA, Zhu H, Kortebein PM, Bush HM, Harvey JF, Sullivan DH, Peterson CA. Muscle expression of genes associated with inflammation, growth, and remodeling is strongly correlated in older adults with resistance training outcomes. Physiol Genomics 38: 169–175, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster RF, Thompson JM, Kaufman SJ. A laminin substrate promotes myogenesis in rat skeletal muscle cultures: analysis of replication and development using antidesmin and anti-BrdUrd monoclonal antibodies. Dev Biol 122: 11–20, 1987. [DOI] [PubMed] [Google Scholar]
- 23.Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu H, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 28: 1654–1665, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med 21: 76–80, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukada S, Yamamoto Y, Segawa M, Sakamoto K, Nakajima M, Sato M, Morikawa D, Uezumi A, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Yamamoto H. CD90-positive cells, an additional cell population, produce laminin alpha2 upon transplantation to dy(3k)/dy(3k) mice. Exp Cell Res 314: 193–203, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Fuoco C, Sangalli E, Vono R, Testa S, Sacchetti B, Latronico MV, Bernardini S, Madeddu P, Cesareni G, Seliktar D, Rizzi R, Bearzi C, Cannata SM, Spinetti G, Gargioli C. 3D hydrogel environment rejuvenates aged pericytes for skeletal muscle tissue engineering. Front Physiol 5: 203, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg K, Corona BT, Walters TJ. Therapeutic strategies for preventing skeletal muscle fibrosis after injury. Front Pharmacol 6: 87, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gargioli C, Coletta M, De Grandis F, Cannata SM, Cossu G. PlGF-MMP-9-expressing cells restore microcirculation and efficacy of cell therapy in aged dystrophic muscle. Nat Med 14: 973–978, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Gawlik K, Miyagoe-Suzuki Y, Ekblom P, Takeda S, Durbeej M. Laminin alpha1 chain reduces muscular dystrophy in laminin alpha2 chain deficient mice. Hum Mol Genet 13: 1775–1784, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Gawlik KI, Durbeej M. Transgenic overexpression of laminin alpha1 chain in laminin alpha2 chain-deficient mice rescues the disease throughout the lifespan. Muscle Nerve 42: 30–37, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329: 1078–1081, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gosselin LE, Adams C, Cotter TA, McCormick RJ, Thomas DP. Effect of exercise training on passive stiffness in locomotor skeletal muscle: role of extracellular matrix. J Appl Physiol (1985) 85: 1011–1016, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Goudenege S, Lamarre Y, Dumont N, Rousseau J, Frenette J, Skuk D, Tremblay JP. Laminin-111: a potential therapeutic agent for Duchenne muscular dystrophy. Mol Ther 18: 2155–2163, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grefte S, Vullinghs S, Kuijpers-Jagtman AM, Torensma R, Von den Hoff JW. Matrigel, but not collagen I, maintains the differentiation capacity of muscle derived cells in vitro. Biomed Mater 7: 055004, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Grounds MD, Sorokin L, White J. Strength at the extracellular matrix-muscle interface. Scand J Med Sci Sports 15: 381–391, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Guérin CW, Holland PC. Synthesis and secretion of matrix-degrading metalloproteases by human skeletal muscle satellite cells. Dev Dyn 202: 91–99, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Haas TL, Milkiewicz M, Davis SJ, Zhou AL, Egginton S , Brown MD, Madri JA, Hudlicka O. Matrix metalloproteinase activity is required for activity-induced angiogenesis in rat skeletal muscle. Am J Physiol Heart Circ Physiol 279: H1540–H1547, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Hadler-Olsen E, Solli AI, Hafstad A, Winberg JO, Uhlin-Hansen L. Intracellular MMP-2 activity in skeletal muscle is associated with type II fibers. J Cell Physiol 230: 160–169, 2015. [DOI] [PubMed] [Google Scholar]
- 39.Han XY, Wang W, Komulainen J, Koskinen SO, Kovanen V, Vihko V, Trackman PC, Takala TE. Increased mRNAs for procollagens and key regulating enzymes in rat skeletal muscle following downhill running. Pflugers Arch 437: 857–864, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Han XY, Wang W, Myllyla R, Virtanen P, Karpakka J, Takala TE. mRNA levels for alpha-subunit of prolyl 4-hydroxylase and fibrillar collagens in immobilized rat skeletal muscle. J Appl Physiol (1985) 87: 90–96, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol (1985) 103: 2068–2076, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Heinemeier KM, Olesen JL, Haddad F, Langberg H, Kjaer M, Baldwin KM, Schjerling P. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol 582: 1303–1316, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinemeier KM, Olesen JL, Haddad F, Schjerling P, Baldwin KM, Kjaer M. Effect of unloading followed by reloading on expression of collagen and related growth factors in rat tendon and muscle. J Appl Physiol (1985) 106: 178–186, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Hindle AG, Horning M, Mellish JA, Lawler JM. Diving into old age: muscular senescence in a large-bodied, long-lived mammal, the Weddell seal (Leptonychotes weddellii). J Exp Biol 212: 790–796, 2009. [DOI] [PubMed] [Google Scholar]
- 45.Hodges BL, Hayashi YK, Nonaka I, Wang W, Arahata K, Kaufman SJ. Altered expression of the alpha7beta1 integrin in human and murine muscular dystrophies. J Cell Sci 110: 2873–2881, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Hyldahl RD, Nelson B, Xin L, Welling T, Groscost L, Hubal MJ, Chipkin S, Clarkson PM, Parcell AC. Extracellular matrix remodeling and its contribution to protective adaptation following lengthening contractions in human muscle. FASEB J 29: 2894–2904, 2015. [DOI] [PubMed] [Google Scholar]
- 47.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84: 649–698, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Kjaer M, Magnusson P, Krogsgaard M, Boysen Møller J, Olesen J, Heinemeier K, Hansen M, Haraldsson B, Koskinen S, Esmarck B, Langberg H. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat 208: 445–450, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koskinen SO, Wang W, Ahtikoski AM, Kjaer M, Han XY, Komulainen J, Kovanen V, Takala TE. Acute exercise induced changes in rat skeletal muscle mRNAs and proteins regulating type IV collagen content. Am J Physiol Regul Integr Comp Physiol 280: R1292–R1300, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Kovanen V, Suominen H, Risteli J, Risteli L. Type IV collagen and laminin in slow and fast skeletal muscle in rats–effects of age and life-time endurance training. Coll Relat Res 8: 145–153, 1988. [DOI] [PubMed] [Google Scholar]
- 51.Kragstrup TW, Kjaer M, Mackey AL. Structural, biochemical, cellular, and functional changes in skeletal muscle extracellular matrix with aging. Scand J Med Sci Sports 21: 749–757, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Kuang W, Xu H, Vilquin JT, Engvall E. Activation of the lama2 gene in muscle regeneration: abortive regeneration in laminin alpha2-deficiency. Lab Invest 79: 1601–1613, 1999. [PubMed] [Google Scholar]
- 53.Labat-Robert J. Age-dependent remodeling of connective tissue: role of fibronectin and laminin. Pathol Biol (Paris) 51: 563–568, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Lacraz G, Rouleau AJ, Couture V, Söllrald T, Drouin G, Veillette N, Grandbois M, Grenier G. Increased stiffness in aged skeletal muscle impairs muscle progenitor cell proliferative activity. PLoS One 10: e0136217, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee JD, Fry CS, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Aged muscle demonstrates fiber-type adaptations in response to mechanical overload, in the absence of myofiber hypertrophy, independent of satellite cell abundance. J Gerontol A Biol Sci Med Sci 71: 461–467, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mackey AL, Brandstetter S, Schjerling P, Bojsen-Moller J, Qvortrup K, Pedersen MM, Doessing S, Kjaer M, Magnusson SP, Langberg H. Sequenced response of extracellular matrix deadhesion and fibrotic regulators after muscle damage is involved in protection against future injury in human skeletal muscle. FASEB J 25: 1943–1959, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marcus RL, Addison O, Kidde JP, Dibble LE, Lastayo PC. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging 14: 362–366, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mayer U, Saher G, Fässler R, Bornemann A, Echtermeyer F, von der Mark H., Miosge N., Pöschl E., von der Mark K. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet 17: 318–323, 1997. [DOI] [PubMed] [Google Scholar]
- 59.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657–3666, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melov S, Tarnopolsky MA, Beckman K, Felkey K, Hubbard A. Resistance exercise reverses aging in human skeletal muscle. PLoS One 2: e465, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller BF, Olesen JL, Hansen M, Døssing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 567: 1021–1033, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab 288: E1153–E1159, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Motohashi N, Uezumi A, Yada E, Fukada S, Fukushima K, Imaizumi K, Miyagoe-Suzuki Y, Takeda S. Muscle CD31(-) CD45(-) side population cells promote muscle regeneration by stimulating proliferation and migration of myoblasts. Am J Pathol 173: 781–791, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138: 3625–3637, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Negroni E, Vallese D, Vilquin JT, Butler-Browne G, Mouly V, Trollet C. Current advances in cell therapy strategies for muscular dystrophies. Expert Opin Biol Ther 11: 157–176, 2011. [DOI] [PubMed] [Google Scholar]
- 66.Ogasawara R, Nakazato K, Sato K, Boppart MD, Fujita S. Resistance exercise increases active MMP and β1-integrin protein expression in skeletal muscle. Physiol Rep 2: 2, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parker MW, Rossi D, Peterson M, Smith K, Sikström K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest 124: 1622–1635, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pattison JS, Folk LC, Madsen RW, Childs TE, Booth FW. Transcriptional profiling identifies extensive downregulation of extracellular matrix gene expression in sarcopenic rat soleus muscle. Physiol Genomics 15: 34–43, 2003. [DOI] [PubMed] [Google Scholar]
- 69.Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol (1985) 104: 1736–1742, 2008. [DOI] [PubMed] [Google Scholar]
- 70.Ramaswamy KS, Palmer ML, van der Meulen JH, Renoux A, Kostrominova TY, Michele DE, Faulkner JA. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J Physiol 589: 1195–1208, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rooney JE, Gurpur PB, Burkin DJ. Laminin-111 protein therapy prevents muscle disease in the mdx mouse model for Duchenne muscular dystrophy. Proc Natl Acad Sci USA 106: 7991–7996, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rullman E, Norrbom J, Stromberg A, Wagsater D, Rundqvist H, Haas T, Gustafsson T. Endurance exercise activates matrix metalloproteinases in human skeletal muscle. J Appl Physiol (1985) 106: 804–812, 2009. [DOI] [PubMed] [Google Scholar]
- 73.Rullman E, Rundqvist H, Wagsater D, Fischer H, Eriksson P, Sundberg CJ, Jansson E, Gustafsson T. A single bout of exercise activates matrix metalloproteinase in human skeletal muscle. J Appl Physiol (1985) 102: 2346–2351, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Schuler F, Sorokin LM. Expression of laminin isoforms in mouse myogenic cells in vitro and in vivo. J Cell Sci 108: 3795–3805, 1995. [DOI] [PubMed] [Google Scholar]
- 75.Siegel AL, Atchison K, Fisher KE, Davis GE, Cornelison DD. 3D timelapse analysis of muscle satellite cell motility. Stem Cells 27: 2527–2538, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Snow MH. The effects of aging on satellite cells in skeletal muscles of mice and rats. Cell Tissue Res 185: 399–408, 1977. [DOI] [PubMed] [Google Scholar]
- 77.Song W, Kwak HB, Lawler JM. Exercise training attenuates age-induced changes in apoptotic signaling in rat skeletal muscle. Antioxid Redox Signal 8: 517–528, 2006. [DOI] [PubMed] [Google Scholar]
- 78.Thomas K, Engler AJ, Meyer GA. Extracellular matrix regulation in the muscle satellite cell niche. Connect Tissue Res 56: 1–8, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tierney MT, Gromova A, Sesillo FB, Sala D, Spenlé C, Orend G, Sacco A. autonomous extracellular matrix remodeling controls a progressive adaptation in muscle stem cell regenerative capacity during development. Cell Reports 14: 1940–1952, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trensz F, Lucien F, Couture V, Söllrald T, Drouin G, Rouleau AJ, Grandbois M, Lacraz G, Grenier G. Increased microenvironment stiffness in damaged myofibers promotes myogenic progenitor cell proliferation. Skelet Muscle 5: 5, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, Montemurro F, Tedesco FS, Blaauw B, Cossu G, Vozzi G, Rando TA, Bonaldo P. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun 4: 1964, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Urso ML, Scrimgeour AG, Chen YW, Thompson PD, Clarkson PM. Analysis of human skeletal muscle after 48 h immobilization reveals alterations in mRNA and protein for extracellular matrix components. J Appl Physiol (1985) 101: 1136–1148, 2006. [DOI] [PubMed] [Google Scholar]
- 83.Van Ry PM, Minogue P, Hodges BL, Burkin DJ. Laminin-111 improves muscle repair in a mouse model of merosin-deficient congenital muscular dystrophy. Hum Mol Genet 23: 383–396, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vilquin JT, Guérette B, Puymirat J, Yaffe D, Tomé FM, Fardeau M, Fiszman M, Schwartz K, Tremblay JP. Myoblast transplantations lead to the expression of the laminin alpha 2 chain in normal and dystrophic (dy/dy) mouse muscles. Gene Ther 6: 792–800, 1999. [DOI] [PubMed] [Google Scholar]
- 85.Vilquin JT, Kinoshita I, Roy B, Goulet M, Engvall E, Tomé F, Fardeau M, Tremblay JP. Partial laminin alpha2 chain restoration in alpha2 chain-deficient dy/dy mouse by primary muscle cell culture transplantation. J Cell Biol 133: 185–197, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilschut KJ, Haagsman HP, Roelen BA. Extracellular matrix components direct porcine muscle stem cell behavior. Exp Cell Res 316: 341–352, 2010. [DOI] [PubMed] [Google Scholar]
- 87.Wood LK, Kayupov E, Gumucio JP, Mendias CL, Claflin DR, Brooks SV. Intrinsic stiffness of extracellular matrix increases with age in skeletal muscles of mice. J Appl Physiol (1985) 117: 363–369, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamada M, Sankoda Y, Tatsumi R, Mizunoya W, Ikeuchi Y, Sunagawa K, Allen RE. Matrix metalloproteinase-2 mediates stretch-induced activation of skeletal muscle satellite cells in a nitric oxide-dependent manner. Int J Biochem Cell Biol 40: 2183–2191, 2008. [DOI] [PubMed] [Google Scholar]
- 89.Yao CC, Ziober BL, Sutherland AE, Mendrick DL, Kramer RH. Laminins promote the locomotion of skeletal myoblasts via the alpha 7 integrin receptor. J Cell Sci 109: 3139–3150, 1996. [DOI] [PubMed] [Google Scholar]
- 90.Zanandrea V, Giua R, Costanzo L, Vellas B, Zamboni M, Cesari M. Interventions against sarcopenia in older persons. Curr Pharm Des 20: 5983–6006, 2014. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Q, Joshi SK, Lovett DH, Zhang B, Bodine S, Kim HT, Liu X. Matrix metalloproteinase-2 plays a critical role in overload induced skeletal muscle hypertrophy. Muscles Ligaments Tendons J 4: 446–454, 2015. [PMC free article] [PubMed] [Google Scholar]
- 92.Zimmerman SD, McCormick RJ, Vadlamudi RK, Thomas DP. Age and training alter collagen characteristics in fast- and slow-twitch rat limb muscle. J Appl Physiol (1985) 75: 1670–1674, 1993. [DOI] [PubMed] [Google Scholar]
- 93.Zou K, De Lisio M, Huntsman HD, Pincu Y, Mahmassani Z, Miller M, Olatunbosun D, Jensen T, Boppart MD. Laminin-111 improves skeletal muscle stem cell quantity and function following eccentric exercise. Stem Cells Transl Med 3: 1013–1022, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zou K, Huntsman HD, Carmen Valero M, Adams J, Skelton J, De Lisio M, Jensen T, Boppart MD. Mesenchymal stem cells augment the adaptive response to eccentric exercise. Med Sci Sports Exerc 47: 315–325, 2015. [DOI] [PubMed] [Google Scholar]