This study identified sex- and hormone-related differences in Achilles tendon healing, which support the notion that patient sex and hormone status should be considered in future clinical investigations defining optimal treatments for Achilles tendon rupture.
Keywords: Achilles tendon, tendon healing, biomechanics, sex differences, ovariectomy, female athlete
Abstract
Achilles tendon ruptures are common injuries. Sex differences are present in mechanical properties of uninjured Achilles tendon, but it remains unknown if these differences extend to tendon healing. We hypothesized that ovariectomized females (OVX) and males would exhibit inferior postinjury tendon properties compared with females. Male, female, and OVX Sprague-Dawley rats (n = 32/group) underwent acclimation and treadmill training before blunt transection of the Achilles tendon midsubstance. Injured hindlimbs were immobilized for 1 wk, followed by gradual return to activity and assessment of active and passive hindlimb function. Animals were euthanized at 3 or 6 wk postinjury to assess tendon structure, mechanics, and composition. Passive ankle stiffness and range of motion were superior in females at 3 wk; however, by 6 wk, passive and active function were similar in males and females but remained inferior in OVX. At 6 wk, female tendons had greater normalized secant modulus, viscoelastic behavior, and laxity compared with males. Normalized secant modulus, cross-sectional area and tendon glycosaminoglycan composition were inferior in OVX compared with females at 6 wk. Total fatigue cycles until tendon failure were similar among groups. Postinjury muscle fiber size was better preserved in females compared with males, and females had greater collagen III at the tendon injury site compared with males at 6 wk. Despite male and female Achilles tendons withstanding similar durations of fatigue loading, early passive hindlimb function and tendon mechanical properties, including secant modulus, suggest superior healing in females. Ovarian hormone loss was associated with inferior Achilles tendon healing.
NEW & NOTEWORTHY
This study identified sex- and hormone-related differences in Achilles tendon healing, which support the notion that patient sex and hormone status should be considered in future clinical investigations defining optimal treatments for Achilles tendon rupture.
achilles tendon ruptures are common injuries that affect 15 to 55 per 100,000 people each year (21). The majority of Achilles tendon injuries are sports related, which may explain why Achilles tendon rupture is associated with younger age at time of injury compared with other tendons (19, 22, 27, 30). However, despite men and women showing no difference in age or sport participation at time of injury (22), clinical studies demonstrate that as many as 84% of all Achilles tendon ruptures occur in men (46).
To better understand this apparent sex difference in injury risk (16), previous studies have explored the role of hormones and their effect on the Achilles tendon-muscle complex. Estrogen receptors have been identified in lower limb tendons (2, 28), which may provide a mechanism for some of the estrogen-related changes in tendon material properties observed in humans (3). In addition to ovarian hormones, the effect of male sex alone on Achilles tendon properties has also been explored. In one study of male, female, and ovariectomized (OVX) female rats, tendon elastic modulus was inferior in males compared with females; this was thought to explain the increased rate of Achilles tendon rupture observed clinically in men compared with women (31). However, it remained unclear if such sex-related differences in tendon baseline properties could be extended to include differences in Achilles tendon healing (10). Previous studies in other musculoskeletal tissues, including the anterior cruciate ligament, have demonstrated that sex differences in baseline tissue properties (1, 18) and injury risk (24) may, indeed, be accompanied by postinjury differences in tissue mechanics and functional outcomes (23). Therefore, understanding if such sex differences also exist for healing of Achilles tendon would prove necessary for informing a potential need for sex-specific treatment recommendations and outcome expectations after Achilles tendon rupture.
The purpose of this study was to determine the effects of sex and ovarian hormones on Achilles tendon healing after acute tendon rupture. Given previous data in animals (31) and humans (39), we hypothesized that male rats would exhibit inferior Achilles tendon mechanical and biological properties but equivalent limb function compared with females after injury. We further hypothesized that tendons from injured OVX rats would exhibit inferior mechanical properties and limb function compared with non-OVX females. Given the reported role of estrogen in tenocyte proliferation (48), we hypothesized that effects both of sex and of ovarian hormone loss would be more prominent earlier, rather than later, in healing.
MATERIALS AND METHODS
Study design and surgery.
Male, female, and OVX rats at 16 wk of age (n = 32/group; Charles River Laboratories, Malvern, PA) received 2 wk of treadmill training (up to 60 min/day at 10 m/min) (13) in this University of Pennsylvania Institutional Animal Care and Use Committee-approved study (Fig. 1).
Fig. 1.
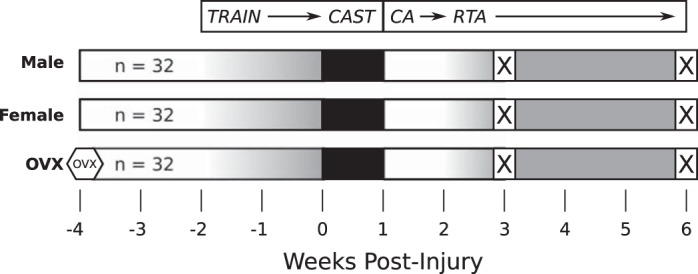
Study design. All animals underwent acclimation and gradual treadmill training (gradient in gray). Surgery was performed to simulate acute Achilles tendon rupture by blunt transection. After surgery, injured hindlimbs were placed in full plantarflexion splint and cast (black). After 1 wk, casts were removed and animals were allowed 1 wk of cage activity (CA) followed by gradual return to treadmill activity (RTA). Half of all animals were euthanized (X) at 3 wk and the remainder at 6 wk postinjury for further study of tendon structure, composition, and mechanics.
Animals were housed in a conventional facility with 12:12-h light/dark cycles and were fed standard chow and provided water ad libitum. Animals were anesthetized (2% isoflurane) and underwent surgery using sterile techniques, including blunt transection of the right Achilles tendon midsubstance and resection of the right central plantaris longus tendon without tendon repair. Analgesia was achieved with preoperative buprenorphine (0.05 mg/kg) and maintained through postoperative day 3 by buprenorphine normal-release (0.05 mg/kg, every 12 h) or sustained-release (1.0 mg/kg, single dose) formulations. After surgery, all animals underwent sequential immobilization (1 wk), cage activity (1 wk), and return to treadmill activity (1–4 wk). Animals were euthanized at 3 wk (n = 16/group) or 6 wk postinjury (n = 16/group).
Postinjury immobilization.
The injured hindlimb was immobilized from below the knee to the toes in a fully plantarflexed position, as described previously (9). Throughout the immobilization period, animals were checked daily to ensure proper hindlimb position and circulation. After 1 wk, immobilization was removed using an oscillating cast saw (HEBU Medical).
Ankle range of motion and stiffness (passive).
Passive ankle joint range of motion (ROM) and stiffness (n = 15–52/group) were quantified using a custom torque cell and orientation device (33) on anesthetized animals before injury and at 3 and 6 wk postinjury (9). Dorsiflexion and plantarflexion torque cutoffs (−8.70 N × mm, +8.35 N × mm) were employed post hoc to ensure that a consistent range of torques was analyzed for all animals. Total ROM was calculated as the average magnitude of combined dorsiflexion and plantarflexion excursion. Regional joint stiffness into dorsiflexion and plantarflexion was calculated by applying a bilinear fit (2 regions: “toe”, “linear”) to torque-angle data (33). The transition point was defined as the angle separating the toe and linear regions of the bilinear fit and was determined by identifying the angle that minimized the combined RMSE of the toe and linear fitted lines. Transition point ROM was calculated as the average magnitude of combined dorsiflexion and plantarflexion transition point excursion.
Quantitative ambulatory assessment (active).
Hindlimb ground reaction forces (lateral, braking, propulsion, vertical) and temporal patterns (stride length, step speed) of the right hindlimb were quantified using an instrumented walkway and digital camera, as described previously (32, 36). Data were collected before surgery (“preinjury”) and at 3 and 6 wk postinjury. Animals were weighed on each day of ambulatory assessment, and ground reaction forces were calculated as percent of animal body weight (%BW).
Tendon sample preparation.
Animals were euthanized at 3 wk [mass (mean ± SD): male 492 ± 33 g; female 268 ± 19 g, OVX 317 ± 18 g] or 6 wk postinjury (male 517 ± 41 g, female 304 ± 21 g, OVX 366 ± 27 g). Tendon-muscle complexes were randomized and blinded before structural assessment and mechanical testing. For mechanical testing, the Achilles tendon-foot complex was removed en bloc and fine dissected to remove nontendinous soft tissue. Tendon midsubstance cross sectional area (CSA) (most central 6 mm) was measured using a laser-based device and LVDT stage (7). Stain dots (Chartpak, Leeds, MA) were applied to the tendon for optical strain analysis. The calcaneus-foot complex was embedded in PMMA, and the free end of tendon was affixed to sandpaper using cyanoacrylate (Loctite 454; Henkel), leaving a gauge length of 12 mm (9).
High-frequency ultrasound assessment of tissue structure.
Tendons (n = 9–12/group) were loaded at 1 N in a 1× PBS bath while sagittal B-mode images were acquired at 0.5-mm increments using a 40 MHz scanner (MS550D; VisualSonics). Three-to-four of the centermost images were further analyzed in MATLAB to determine injury site echogenicity (mean gray scale brightness, range 0 to 255) and collagen fiber disorganization (novel algorithm used to evaluate fiber alignment, reported as circular standard deviation), as described previously (34).
Mechanical testing.
Bone-tendon units were gripped using custom aluminum fixtures so that the foot and Achilles tendon were maintained perpendicular, similar to in vivo physiological loading. The fixtures were attached to a testing frame (Electropuls E3000, Instron, Norwood, MA) using a 250 N load cell, and all specimens remained submerged in a 1× PBS bath (37°C). Tendons were mechanically tested (n = 10/group), including preloading, preconditioning, stress relaxation, frequency sweep, force ramp at 2% strain/s to 35 N (male) or 25 N (female, OVX), and fatigue loading [5–35 N (male) or 5–25 N (female, OVX) at 2 Hz, representing ∼8–43% of ultimate failure load] using a sinusoidal waveform until failure, as described previously (9). Force-displacement data were acquired by WaveMatrix (Instron, Norwood, MA) and analyzed in MATLAB. Achilles tendon percent relaxation, dynamic modulus (frequency sweep stiffness divided by CSA), linear modulus (force ramp stiffness divided by CSA), hysteresis (energy lost during cyclic displacement), laxity (tendon lengthening during fatigue cycling), secant stiffness, secant modulus (stiffness divided by CSA), and total cycles to failure were evaluated. With the exception of linear modulus (assessed optically), all properties were calculated using recorded force (N) and grip-to-grip displacement (mm), at a sampling rate between 100 and 1,000 Hz, with 0% strain established at the 0.1 N preload position (11, 12, 14, 33).
Tendon histology.
Achilles tendons were harvested at time of death and processed using standard paraffin procedures (n = 5–8/group). Sagittal sections (7 μm) were collected and stained with hematoxylin-eosin (H&E) and Safranin-O/Fast Green. Images of the H&E-stained injury site (FOV: 0.87 mm × 0.65 mm) were graded by three blinded investigators for cell density (1, low; 2, moderate; 3, high) and nuclear shape (1, spindle shaped; 2, mixed; 3, rounded). Cell density was further quantified by automated cell counting using the Fiji (37) distribution of ImageJ (38) (NIH, v1.50g). Full-length images of Safranin-O/Fast Green-stained tendon sections were obtained by stitching of several smaller images (FOV: 2.14 × 1.61 mm). Full-length images were cropped to remove the calcaneus and fibrocartilaginous tendon insertion and were thresholded to obtain Safranin-O area stained (red) using Fiji. Sections were also stained for collagen type III using standard immunohistochemical methods. Protein was visualized by DAB staining, where antibody-protein conjugates turn brown (SIGMAFAST, Sigma Aldrich Cat-D4293; St. Louis, MO). Injury site was evaluated for collagen type III from images obtained by stitching of several smaller images (FOV: 2.14 × 1.61 mm). Staining was further quantified as percent area stained using Fiji.
Muscle sample preparation.
Posterior hindlimb muscle tissue was excised at time of death, embedded in optimal cutting temperature (OCT) compound and flash frozen in liquid N2-cooled N-methyl butane, as described previously (31). Before sectioning, distal and proximal portions of muscle tissue were removed to isolate the gastrocnemius-soleus muscles. Muscle samples (n = 5–8 per group) were stored at −80°C before axial cryosectioning at 10 μm.
Muscle immunofluorescence.
Cryosections were stained for laminin and myosin heavy chain (MyHC) types 1, 2a, and 2b, as described previously (31). The anti-MyHC antibodies developed by Stefano Schiaffino were obtained from the Developmental Studies Hybridoma Bank, created by the National Institute of Child Health and Development of the National Institutes of Health and maintained at The University of Iowa, Department of Biology, Iowa City, IA. MyHC2x expression was presumed from unstained fibers. After staining, three images were taken from both the superficial and deep regions of each muscle. Each set of three images was analyzed to determine fiber type fraction and fiber type-specific fiber size (minimum Feret diameter) using the SMASH application (40).
Statistical analysis and data normalization.
Before analysis, all variables of interest (except tendon histology data, cycles to failure) were normalized by group (male, female, OVX) using either preinjury functional data (obtained from this study) or baseline tendon and muscle data [obtained from a previous study (31)]. Data normalization allowed for identification of relative changes in biomechanical properties that reflected true sex- and hormone-related differences in tendon healing, in contrast to mere extensions of baseline differences. Data normality was evaluated with Shapiro-Wilk tests (R v3.2.4; Vienna, Austria) (43). Normally distributed data were evaluated with two-way ANOVAs in R to assess the effects of group (male vs. female; OVX vs. female) and time postinjury (3 vs. 6 wk). Within-group comparisons to preinjury values (3 wk vs. preinjury; 6 wk vs. preinjury) were determined by additional two-way ANOVAs using nonnormalized data, with post hoc testing performed for the effect of time only. Ordinal H&E scoring data were evaluated by Kruskal-Wallis tests at 3 and 6 wk postinjury. For significant effects (P < .05), post hoc pairwise comparisons (two-tailed) were performed with Bonferroni corrections using the R package lsmeans (26); all reported P values are shown after correcting for the number of post hoc comparisons (range: 3 to 7).
RESULTS
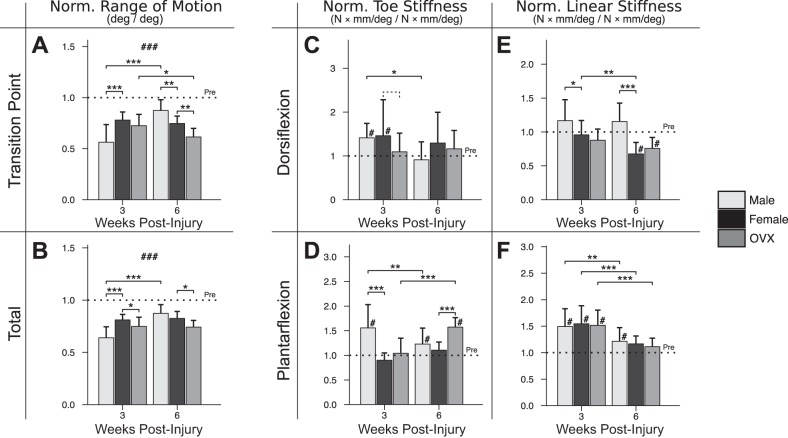
Sex (male vs. female) was a significant determinant of passive ankle function at 3 wk postinjury (Fig. 2). In males, normalized transition point ROM (Fig. 2A), total ROM (Fig. 2B), plantarflexion toe stiffness (Fig. 2D), and dorsiflexion linear stiffness (Fig. 2E) were all farther from preinjury compared with females at 3 wk. However, by 6 wk, stiffness in males had returned closer to preinjury values, and total ROM was not different between sexes. The difference in total ROM in males between 3 and 6 wk was driven by lower dorsiflexion and plantarflexion toe stiffnesses (Fig. 2, C and D) and wider toe regions (Fig. 2A) at 6 wk. Similar to the effect of sex, ovarian hormone loss (female vs. OVX) was also a significant determinant of passive ankle function at 3 wk postinjury, resulting in less normalized total ROM in OVX compared with females. However, unlike male ROM (which was greater by 6 wk), the OVX deficit was maintained at 6 wk, alongside newly elevated plantarflexion toe stiffness.
Fig. 2.
In vivo assessment of passive ankle range of motion (ROM) and stiffness. Effects of male sex and ovarian hormone loss were present for normalized transition point ROM (−21.7% in male vs. female, P < 0.001) (A) and overall ROM (−17.1% in male vs. female, P < 0.001; −6.2% in OVX vs. female, P = 0.018) (B) at 3 wk postinjury. By 6 wk, normalized transition point ROM in males exceeded that of females (+12.6% in male vs. female, P = 0.002), and normalized overall ROM in females exceeded that of OVX (−8.4% in OVX vs. female, P = .028). In addition to ROM, ankle stiffness was also assessed in the toe (C and D) and linear (E and F) regions of dorsiflexion and plantarflexion, respectively. At 3 wk, males had greater plantarflexion toe stiffness (+65.8% in male vs. female, P < 0.001) (D) and dorsiflexion linear stiffness (+21.0% in male vs. female, P = 0.013) (E). By 6 wk, males had relatively greater stiffness in the dorsiflexion linear-region (+47.8% in male vs. female, P < 0.001), despite males and females exhibiting no difference in normalized total ROM. Plantarflexion toe stiffness was elevated in OVX at 6 wk (+46.9% in OVX vs. female, P < 0.001) (D). “Pre” indicates preinjury values. Solid lines indicate significant differences (P < 0.05); dashed lines indicate trends (P < 0.10). #Significant difference from preinjury (P < 0.05); ### is shown if all values differ from preinjury (P < 0.05); *P < 0.05; **P < 0.01; ***P < 0.001. All P values have been corrected for multiple comparisons. Error bars indicate standard deviation.
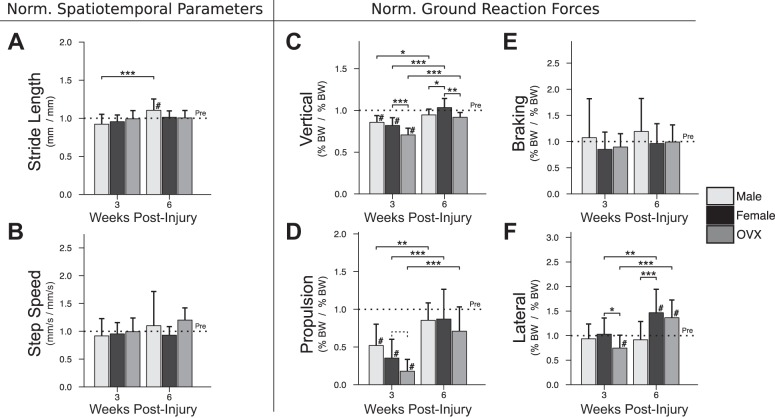
In contrast to passive function, no sex difference was observed for active function at 3 wk postinjury (Fig. 3). By 6 wk, normalized lateral ground reaction (“push-off”) force in males was lower than females but not different from male preinjury values (Fig. 3F). Unlike males, ovarian hormone loss significantly affected active function at 3 wk postinjury, manifested in OVX by lower normalized lateral (Fig. 3F) and vertical (Fig. 3C) ground reaction forces, and a trend toward lower propulsion force (Fig. 3D). By 6 wk, normalized vertical force remained lower in OVX. Stride length and step speed (Fig. 3, A and B) were not significantly different between any groups at either time point.
Fig. 3.
In vivo assessment of active ambulatory function. Normalized spatiotemporal parameters, including stride length (A) and step speed (B) were not different between groups at 3 or 6 wk postinjury. At 3 wk postinjury, normalized vertical (C), propulsion (D), and lateral (F) ground reaction forces were not different between males and females but were relatively lower in OVX (vertical: −11.2% vs. female, P < 0.001; propulsion: −17.4% vs. female, P = 0.082 [trend]; lateral: −28.2% vs. female, P = 0.012). By 6 wk, vertical and propulsion forces returned to preinjury values for all groups, despite a persistent OVX-related difference in vertical force (−11.6% in OVX vs. female, P = 0.003). Braking (E) force was unaffected by injury. In contrast, lateral (F) force at 6 wk was relatively greater than preinjury values in females (+46.6% vs. preinjury, P = 0.001) and OVX (+36.4% vs. preinjury, P = 0.005), but not in males. “Pre” indicates preinjury values. Solid lines indicate significant differences (P < 0.05); dashed lines indicate trends (P < 0.10). #Significant differences from preinjury (P < 0.05); *P < 0.05; **P < 0.01; ***P < 0.001. All P values have been corrected for multiple comparisons. Error bars indicate standard deviation.
HFUS revealed greater collagen disorganization (Fig. 4A) and less echogenicity (Fig. 4B) in all groups at 3 and 6 wk compared with preinjury. Males had greater (farther from preinjury) normalized disorganization at 3 wk but greater (closer to preinjury) normalized echogenicity by 6 wk. HFUS outcomes were not significantly different between females and OVX at either time point. Normalized tendon cross sectional area (CSA) (Fig. 4C) was greater than preinjury levels in all groups at 3 and 6 wk; in OVX, normalized CSA was greater at 6 wk compared with 3 wk and, by 6 wk, was greater than that of females.
Fig. 4.
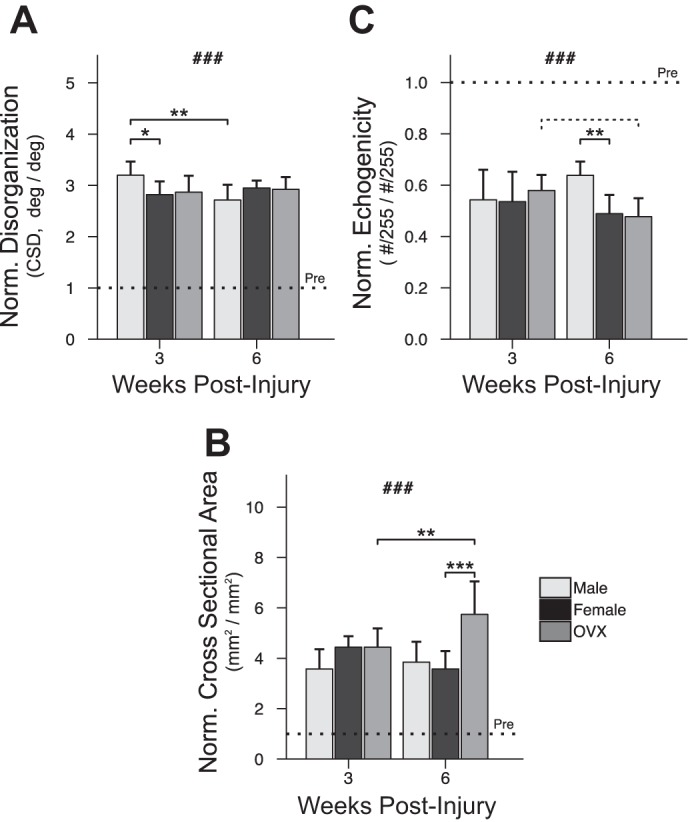
Assessment of tendon structure. Tendon disorganization (A) was higher (P < 0.001) and tendon echogenicity (B) lower (P < 0.001) in all groups after injury. Males were relatively more disorganized than females at 3 wk (+38.1% in male vs. female, P = 0.025). By 6 wk, normalized echogenicity was greater in male tendons (+23.6% in male vs. female, P = 0.004). In contrast to the effect of sex, ovariectomy had no effect on HFUS measures. CSA (C) was greater after injury (P < 0.001) for all groups. Normalized CSA grew significantly in OVX between 3 and 6 wk (+130.1% at 6 vs. 3 wk, P = 0.008) and by 6 wk was greater than females (+216.5% vs. female, P < 0.001). “Pre” indicates preinjury values. Solid lines indicate significant differences (P < 0.05); dashed lines indicate trends (P < 0.10). #Significant differences from preinjury (P < 0.05); ###all values differ from preinjury (P < 0.05); *P < 0.05; **P < 0.01; ***P < 0.001. All P values have been corrected for multiple comparisons. Error bars indicate standard deviation.
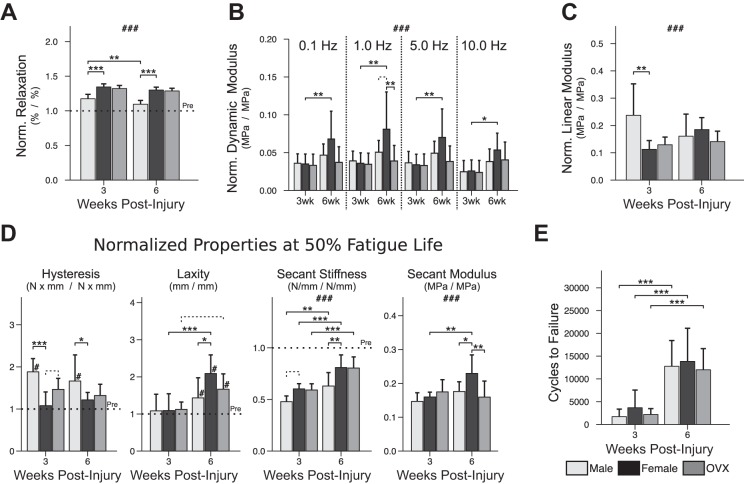
Percent relaxation (Fig. 5A) was greater after injury; in males, relaxation returned significantly closer to preinjury values between 3 and 6 wk and was relatively lower than (closer to preinjury) females at both 3 and 6 wk. Dynamic modulus (Fig. 5B) was not different between groups at either time point, except for greater normalized modulus in females compared with OVX at 1.0 Hz at 6 wk. Normalized linear modulus (Fig. 5C) was slightly greater in males compared with females at 3 wk; by 6 wk, this effect of sex was no longer present. Normalized hysteresis (a measure of energy lost during tendon recoil following stretch) was greater (farther from preinjury) in males compared with females at 50% fatigue life at both 3 and 6 wk (Fig. 5D). Tendon laxity (Fig. 5D) was not different from preinjury in any group at 3 wk, and there was no effect of sex or ovariectomy; by 6 wk, laxity was greater than preinjury for all groups. Male tendons, however, did exhibit lower (closer to preinjury) normalized tendon laxity compared with females at 6 wk. Normalized secant modulus (Fig. 5D) was uniformly lower than preinjury in all groups at 3 wk and remained so at 6 wk. Despite this deficit, normalized modulus of female tendons at 6 wk was still significantly greater (closer to preinjury) than 3 wk females and greater than 6 wk males and OVX. In contrast to secant modulus, normalized secant stiffness at 6 wk was greater (closer to preinjury) compared with 3 wk values for all groups; normalized secant stiffness in females at 6 wk was greater than males but not different from OVX. Finally, overall fatigue resilience—described by the number of fatigue cycles until tendon failure (Fig. 5E)—was also greater at 6 wk postinjury compared with 3 wk for all groups, without an effect of sex or ovariectomy.
Fig. 5.
Postinjury tendon mechanical properties. Normalized percent relaxation (A) was lower in males at 3 wk (−17.0% in male vs. female, P < 0.001) and 6 wk (−20.4% in male vs. female, P < 0.001) postinjury. Viscoelastic testing (B) revealed greater dynamic modulus at 6 wk (vs. 3 wk) that was unique to females; additionally, during cyclic displacement at 1.0 Hz, females had greater normalized modulus at 6 wk (+3.0% vs. male, P = 0.069 [trend]; +4.2% vs. OVX, P = 0.005). Linear modulus (C), measured during the ramp to fatigue, identified relatively greater modulus in males compared with females at 3 wk (+12.5% in male vs. female, P = 0.009). Normalized hysteresis, laxity, secant stiffness, and secant modulus were measured at 50% fatigue life (D). Normalized hysteresis was elevated in males at both 3 wk (+80.3% in male vs. female, P < 0.001) and 6 wk (+44.8% in male vs. female, P = 0.030). Normalized laxity was lower (closer to preinjury) in males at 6 wk compared with females (−65.5% in male vs. female, P = 0.014). In contrast, normalized secant modulus in females was greater at 6 wk (+7.0% at 6 vs. 3 wk, P = 0.001) and by 6 wk was greater than both males (+5.3% in female vs. male, P = 0.023) and OVX (+7.0% in female vs. OVX, P = 0.001). Number of fatigue cycles until tendon failure (E) was greater at 6 wk (vs. 3 wk) for all groups (P < 0.001). “Pre” indicates preinjury values. Solid lines indicate significant differences (P < 0.05); dashed lines indicate trends (P < 0.10). #Significant differences from preinjury (P < 0.05); ###all values differ from preinjury (P < 0.05); *P < 0.05; **P < 0.01; ***P < 0.001. All P values have been corrected for multiple comparisons. Error bars indicate standard deviation.
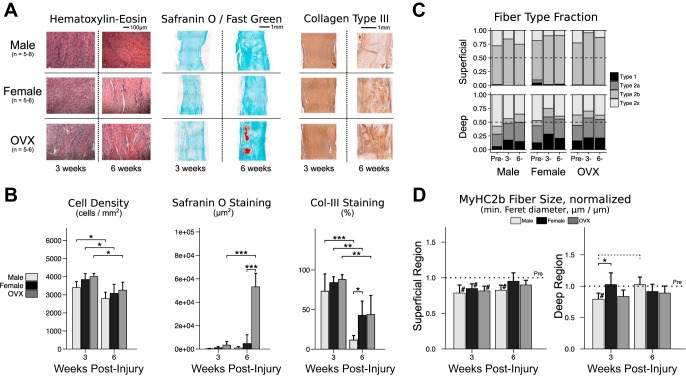
Semiquantitative grading of tendon composition identified no effect of sex or ovariectomy on cell nuclear shape or cell density at 3 or 6 wk. Automated cell counting identified uniformly lower cell density at 6 wk (vs. 3 wk) for all groups. Glycosaminoglycan and proteoglycan (GAG/PG) content—as determined by Safranin-O staining—was uniformly low among all groups at 3 wk. There was, however, a large effect of ovariectomy between 3 and 6 wk, resulting in order-of-magnitude greater GAG/PG staining in OVX at 6 wk (Fig. 6, A and B). Staining for collagen type III was significantly less at 6 wk (vs. 3 wk) postinjury for all groups. Additionally, males at 6 wk exhibited less collagen type III staining compared with females (Fig. 6B).
Fig. 6.
Composition of the Achilles tendon-muscle complex 3 and 6 wk postinjury. Histological staining with hematoxylin-eosin (H&E), Safranin O/Fast Green, and antibody for collagen type III (A) were used to assess tendon composition. On H&E-stained sections, no differences between groups were observed for cell density or nuclear shape by semi-quantitative grading at 3 or 6 wk postinjury (A). Automated cell counting identified a uniformly lower cell density (B) in all groups at 6 wk (vs. 3 wk: male −619 cells/mm2, P = 0.034; female −762 cells/mm2, P = 0.012; OVX −752 cells/mm2, P = 0.014). Greater Safranin O staining was identified in OVX tendons at 6 wk postinjury (+11.3-fold in OVX vs. female, P < 0.001) (B). Type III collagen staining was lower in all groups at 6 wk (vs. 3 wk: male −61.6% area stained, P < 0.001; female −41.4% area stained, P = 0.002; OVX −44.2% area stained, P = 0.001), and by 6 wk there was less type III collagen staining in males compared with females (−31.1% area stained in male vs. female, P = 0.028) (B). Gastrocnemius-soleus muscle composition was also assessed. Fiber type fractions were calculated for deep and superficial regions at “Pre”-injury and at 3 and 6 wk postinjury, which confirmed a predilection of type 1 fibers for deep tissue and type 2b fibers for superficial tissue (C). Compared with preinjury, size of type 2b fibers was lower in all groups in the superficial region at 3 wk postinjury (male −21.5%, P < 0.001; female −15.2%, P = 0.023; OVX −18.5%, P = 0.002) (D); by 6 wk, type 2b fiber size remained below preinjury levels in males (−17.6% in male vs. preinjury, P = 0.001) but not females. In the deep region, normalized type 2b fiber size was lower in males compared with females at 3 wk (−23.4% in male vs. female, P = 0.030); however, by 6 wk, deep type 2b fiber size in males was not different from preinjury levels. “Pre” indicates preinjury values. Solid lines indicate significant differences (P < 0.05); dashed lines indicate trends (P < 0.10). #Significant differences from preinjury (P < 0.05); ###all values differ from preinjury (P < 0.05); *P < 0.05; **P < 0.01; ***P < 0.001. All P values have been corrected for multiple comparisons. Error bars indicate standard deviation.
Postinjury muscle composition was also assessed by fiber size and fiber type in the superficial and deep regions of the gastrocnemius-soleus muscle complex (Fig. 6, C and D). In the superficial region, mean fiber sizes (minimum Feret diameter) were uniformly smaller at 3 wk compared with preinjury for muscle fiber type 2b and type 2x. By 6 wk, the size of superficial type 2b fibers in males remained significantly below preinjury levels; in contrast, type 2x fiber size returned to preinjury levels for all groups. In the deep region, males had smaller normalized type 2b fiber size at 3 wk compared with females. The size of male fiber type 2a (significant) and type 2b (trend) was greater at 6 wk compared with 3 wk, and by 6 wk the male deficit in type 2b fiber size in the deep region was no longer present. Size of fiber types 1 and 2x in the deep region were not different from preinjury for any group at either time point.
DISCUSSION
Achilles tendon ruptures are common injuries. However, evidence supporting the use of specific protocols for treatment (surgical, nonsurgical) and return to activity (early, delayed) has remained “weak” or “inconclusive” (4). Previous work began to address these questions by describing the effects of treatment and return to activity on tendon healing in males after Achilles tendon injury (6, 9). However, given that male and female tendon properties are known to differ even before injury (31), it remained unclear if these insights into male healing could also be applied to females. In this study, we evaluated whether Achilles tendon healing differs among males, females, and ovariectomized females (OVX). By using a rat model of nonoperative treatment of Achilles tendon rupture, we studied the effects of sex and ovarian hormone loss on postinjury limb function, tendon structure, tendon mechanical properties, and composition of the Achilles tendon-muscle complex.
Contrary to our initial hypothesis predicting similar functional outcomes in males and females, we found that passive ankle function was inferior in males at 3 wk postinjury (Fig. 2). Active hindlimb function, however, was not different between sexes at 3 or 6 wk (Fig. 3), despite male deficits in superficial type 2b fiber size (Fig. 6D). Regarding male deficits in fiber size, it is worth noting that the active functional assessment used in this study consisted of simple ambulation as opposed to a more explosive activity such as sprinting or jumping for which fast-twitch type 2b fibers might be more maximally recruited. Therefore, our functional assessment may lack the sensitivity that would be required for detection of male functional deficits related to smaller type 2b fiber size. Additionally, it is interesting that at 6 wk, when relative postinjury tendon stiffness and modulus is greatest in females (and above that of males and OVX), female ankle stiffness is either similar to preinjury or, as is the case for dorsiflexion linear stiffness, actually less than males and less than preinjury levels. This finding reinforces the notion that passive ankle stiffness, as a measure of general joint function, must be considered as separate from the specific mechanical behavior of tendon alone. More generally, the functional outcomes in males and females—slightly in favor of male functional superiority during later healing, despite early male deficits—are supported by a recent observational study in patients 6 and 12 mo after Achilles tendon rupture. That study identified no difference in postinjury symptoms between men and women treated nonsurgically, although men did exhibit greater recovery in heel-rise height at 12 mo postinjury (39). It is also possible that our results perhaps underestimate the postinjury functional performance of males compared with females, given that another human retrospective study recently found superior Achilles tendon rupture score in males at 3 and 12 mo postinjury (17).
In contrast to the effect of sex, ovarian hormone loss was more clearly associated with inferior functional outcomes at 3 and 6 wk—both passive and active—as expected (Figs. 2 and 3). These results could potentially be explained by decreased locomotor activity, which has been associated with ovariectomy (47). A decrease in overall activity, or “physical aptness”, could impair the OVX animal's ability to compensate functionally in the early postinjury period, perhaps resulting in worse functional outcomes (35). Alternatively, the greater ankle stiffness and more restricted ROM in OVX animals 6 wk postinjury may also represent more intrinsic differences in healing related to ovarian hormone loss, such as might be caused by differential deposition of extracellular matrix proteins postinjury (Fig. 6A).
Structural assessment of tendon by HFUS showed that male tissues were relatively more disorganized at 3 wk (Fig. 4A), but by 6 wk had tissue echogenicity superior to females (Fig. 4B). These two phenomena suggest that in males, greater initial disorganization at the tendon injury site may allow for relatively greater (and more normal) tissue echogenicity during later healing.
Superior normalized stress relaxation observed in males at both 3 and 6 wk postinjury (Fig. 5A) indicates that male tendons exhibit greater postinjury elastic behavior in a static, constant strain environment. In contrast, during dynamic fatigue loading, lower (closer to preinjury) normalized hysteresis was observed in female tendons compared with male tendons (Fig. 5D). This suggests that in a dynamic high strain environment (perhaps similar to exercise), female tendons exhibit more elastic and thus more energy-efficient behavior on a cycle-to-cycle basis relative to preinjury values compared with males. And yet, despite the ostensible benefits of a “stronger” (greater normalized modulus) and more “energy efficient” (lower normalized hysteresis) female tendon under dynamic loading conditions at 6 wk, the greater normalized laxity also observed in females further suggests that female tendons undergo greater postinjury lengthening (laxity) during dynamic fatigue loading, relative to lengthening observed preinjury (Fig. 5D). Previously, in uninjured Achilles tendon, greater preinjury laxity was observed in males compared with females (31); therefore, the greater relative laxity observed in females at 6 wk postinjury suggests a tendency for female laxity to become more like males after injury.
Consistent with our hypothesis and similar to some effects of sex, ovarian hormone loss was also associated with inferior changes in tendon structural and mechanical properties at 3 and 6 wk postinjury. The greater CSA observed in OVX tendons at 6 wk (Fig. 4C) may reflect a divergence in injury site composition from that of females. In a previous study, coculture of tenocytes with adipose-derived mesenchymal stromal cells (MSCs) obtained from OVX females resulted in greater gene expression of the proteoglycan decorin; however, this effect was not observed for MSCs obtained from non-OVX females (45). Therefore, differences in the properties of MSCs recruited to the tendon injury site in females and OVX could explain why proteoglycan deposits were identified in OVX, but not in females, at 6 wk postinjury (Fig. 6A). Interestingly, this postinjury finding contrasts with known OVX differences in preinjury gene expression, where ovariectomy has been associated with decreased expression of aggrecan, biglycan, and decorin (20). In addition to postinjury differences in CSA and composition, OVX also exhibited lower normalized modulus compared with females throughout dynamic fatigue loading at 6 wk (Fig. 5D); however, this difference was driven by changes in CSA in OVX, given that secant stiffness was not different between groups.
Previous studies implicated estrogen as a regulator of fibroblast proliferation and collagen synthesis (5, 44, 48). Given these reports, we hypothesized that the greatest sex- and hormone-related effects on tendon mechanical properties would be present during early healing, at a time in which tenocyte proliferation and collagen synthesis would be expected to be greatest. Contrary to our hypothesis, the greatest differences in tendon mechanical properties and composition were noted later in healing, at 6 wk postinjury (Fig. 5D). Interestingly, we also identified greater injury site collagen type III staining in females at 6 wk compared with males (Fig. 6A). Given the known progression from deposition of collagen type III to collagen type I during wound maturation, this finding suggests that female sex could be associated with a more prolonged inflammatory response or, perhaps, an estrogen-related decrease in protein turnover compared with males (15). A prolonged inflammatory response in females could agree with our observation of greater sex-related differences later in healing, if one considers that changes in mechanical properties may result from processes and effects that accumulate over time. Alternatively, the greater postinjury expression of collagen type III observed in females might also be the result of underlying sex differences in baseline gene expression, such as in the case of patellar tendon, in which resting collagen type III gene expression is known to be higher in women compared with men (42).
Despite steps to maximize translatability through the use of clinically paralleled immobilization and return to activity protocols, there are limitations to this animal study. For example, the full plantarflexion splint and cast used in this model may not permit full functional weight bearing of the injured rat hindlimb, which stands in contrast to the functional weight bearing that is recommended clinically in humans (41). Furthermore, our assessment of muscle composition provided analysis of fiber type and size, but failed to include a more global parameter such as muscle volume, which might have provided useful information regarding muscle atrophy and hypertrophy occurring along fibers. Additionally, previous studies of sex hormones in tendon and ligament healing have focused on the role of estrogen. Despite our data indicating worse healing associated with ovarian hormone loss, we are unable to specifically attribute these ovariectomy-related effects to any one hormone in particular, given that ovariectomy results in perturbation of multiple sex hormones that include not only estrogen, but also progesterone (29). Moreover, OVX animals in this study underwent ovariectomy 4 wk before Achilles tendon rupture. This time point was thought appropriate given previous studies in ovariectomized rodents (8, 25); however, it is possible that studying healing longer after ovariectomy might have revealed even greater ovariectomy-related differences in tendon healing. Finally, the animal model employed here was a model of nonrepair treatment of Achilles tendon rupture. Previous data suggest that functional and mechanical outcomes after injury do depend on the decision to repair (or not repair) the ruptured Achilles tendon (9). Therefore, future studies will be needed to determine if these effects of sex and ovarian hormone loss may also extend to include models of surgical repair.
In conclusion, this study begins to define Achilles tendon healing in an animal model that incorporates variation in sex and hormone status. Functional, structural, mechanical, and histological properties of Achilles tendon were studied in male, female, and OVX rats after acute tendon rupture. Male and female limb function were ultimately similar by 6 wk postinjury, and male and female Achilles tendons withstood similar durations of fatigue loading; nevertheless, other important differences related to early postinjury ankle stiffness and ROM, as well as later tendon mechanical properties, do suggest a role for sex-specific treatment of Achilles tendon rupture. Additionally, loss of ovarian hormones was clearly associated with inferior limb function, inferior tendon mechanics, and inferior tendon composition, all of which further highlight the biological importance of sex hormones in musculoskeletal connective tissue function. Future studies are needed to optimize treatment of Achilles tendon rupture in a manner that accounts for variation in baseline characteristics and healing potential, including sex and hormone status.
GRANTS
This study was funded by National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) Grant (R01AR064216S1), the NIAMS supported Penn Center for Musculoskeletal Disorders (P30 AR050950), the National Center for Advancing Translational Sciences (TL1TR000138), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (T32AR007132), and National Science Foundation Graduate Research Fellowship Program.
DISCLOSURES
We have received grants to my university from multiple companies (DJO, Orthofix, Bonti, Marine Polymer Technologies) for various studies, but none of these are related/conflict with the work presented in this study (Soslowsky).
AUTHOR CONTRIBUTIONS
G.W.F., B.R.F., and L.J.S. conception and design of research; G.W.F., B.R.F., C.D.H., N.S.S., A.M.P., and S.N.W. performed experiments; G.W.F. and B.R.F. analyzed data; G.W.F., B.R.F., C.D.H., D.C.F., and L.J.S. interpreted results of experiments; G.W.F. prepared figures; G.W.F. drafted manuscript; G.W.F., B.R.F., C.D.H., N.S.S., A.M.P., S.N.W., D.C.F., and L.J.S. edited and revised manuscript; G.W.F., B.R.F., C.D.H., N.S.S., A.M.P., S.N.W., D.C.F., and L.J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank C.N. Riggin and K.G. Silbernagel for contributions.
REFERENCES
- 1.Bates NA, Nesbitt RJ, Shearn JT, Myer GD, Hewett TE. Sex-based differences in knee ligament biomechanics during robotically simulated athletic tasks. J Biomech 49: 1429–1436, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bridgeman JT, Zhang Y, Donahue H, Wade AM, Juliano PJ. Estrogen receptor expression in posterior tibial tendon dysfunction: a pilot study. Foot Ankle Int 31: 1081–1084, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Bryant AL, Clark RA, Bartold S, Murphy A, Bennell KL, Hohmann E, Marshall-Gradisnik S, Payne C, Crossley KM. Effects of estrogen on the mechanical behavior of the human Achilles tendon in vivo. J Appl Physiol 105: 1035–1043, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Chiodo CP, Glazebrook M, Bluman EM, Cohen BE, Femino JE, Giza E, Watters WC III, Goldberg MJ, Keith M, Haralson RH III, Turkelson CM, Wies JL, Hitchcock K, Raymond L, Anderson S, Boyer K, Sluka P; American Academy of Orthopaedic Surgeons . American Academy of Orthopaedic Surgeons clinical practice guideline on treatment of Achilles tendon rupture. J Bone Joint Surg Am 92: 2466–2468, 2010. [PubMed] [Google Scholar]
- 5.Circi E, Akpinar S, Balcik C, Bacanli D, Guven G, Akgun RC, Tuncay IC. Biomechanical and histological comparison of the influence of oestrogen deficient state on tendon healing potential in rats. Int Orthopaedics 33: 1461–1466, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eliasson P, Andersson T, Aspenberg P. Achilles tendon healing in rats is improved by intermittent mechanical loading during the inflammatory phase. J Orthop Res 30: 274–279, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Favata M. Scarless healing in the fetus: implications and strategies for postnatal tendon repair. In: Bioengineering. Philadelphia: University of Pennsylvania, 2006. [Google Scholar]
- 8.Fisher JS, Kohrt WM, Brown M. Food restriction suppresses muscle growth and augments osteopenia in ovariectomized rats. J Appl Physiol 88: 265–271, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Freedman BR, Gordon JA, Bhatt PB, Pardes AM, Thomas SJ, Sarver JJ, Riggin CN, Tucker JJ, Williams AW, Zanes RC, Hast MW, Farber DC, Silbernagel KG, Soslowsky LJ. Nonsurgical treatment and early return to activity leads to improved Achilles tendon fatigue mechanics and functional outcomes during early healing in an animal model. J Orthop Res, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman BR, Gordon JA, Soslowsky LJ. The Achilles tendon: fundamental properties and mechanisms governing healing. Muscles Ligaments Tendons J 4: 245–255, 2014. [PMC free article] [PubMed] [Google Scholar]
- 11.Freedman BR, Sarver JJ, Buckley MR, Voleti PB, Soslowsky LJ. Biomechanical and structural response of healing Achilles tendon to fatigue loading following acute injury. J Biomech 47: 2028–2034, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman BR, Zuskov A, Sarver JJ, Buckley MR, Soslowsky LJ. Evaluating changes in tendon crimp with fatigue loading as an ex vivo structural assessment of tendon damage. J Orthop Res 33: 904–910, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillis GB, Biewener AA. Hindlimb muscle function in relation to speed and gait: in vivo patterns of strain and activation in a hip and knee extensor of the rat (Rattus norvegicus). J Exp Biol 204: 2717–2731, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Gordon JA, Freedman BR, Zuskov A, Iozzo RV, Birk DE, Soslowsky LJ. Achilles tendons from decorin- and biglycan-null mouse models have inferior mechanical and structural properties predicted by an image-based empirical damage model. J Biomechanics 48: 2110–2115, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen M, Kjaer M. Influence of sex and estrogen on musculotendinous protein turnover at rest and after exercise. Exerc Sport Sci Rev 42: 183–192, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Hansen M, Kjaer M. Sex hormones and tendon. Adv Exp Med Biol 920: 139–149, 2016. [DOI] [PubMed] [Google Scholar]
- 17.Hansen MS, Christensen M, Budolfsen T, Ostergaard TF, Kallemose T, Troelsen A, Barfod KW. Achilles tendon Total Rupture Score at 3 months can predict patients' ability to return to sport 1 year after injury. Knee Surg Sports Traumatol Arthrosc 24: 1365–1371, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Hewett TE, Zazulak BT, Myer GD, Ford KR. A review of electromyographic activation levels, timing differences, and increased anterior cruciate ligament injury incidence in female athletes. Br J Sports Med 39: 347–350, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houshian S, Tscherning T, Riegels-Nielsen P. The epidemiology of Achilles tendon rupture in a Danish county. Injury 29: 651–654, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Huisman ES, Andersson G, Scott A, Reno CR, Hart DA, Thornton GM. Regional molecular and cellular differences in the female rabbit Achilles tendon complex: potential implications for understanding responses to loading. J Anat 224: 538–547, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huttunen TT, Kannus P, Rolf C, Fellander-Tsai L, Mattila VM. Acute Achilles tendon ruptures: incidence of injury and surgery in Sweden between 2001 and 2012. Am J Sports Med 42: 2419–2423, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Jozsa L, Kvist M, Balint BJ, Reffy A, Jarvinen M, Lehto M, Barzo M. The role of recreational sport activity in Achilles tendon rupture. A clinical, pathoanatomical, and sociological study of 292 cases. Am J Sports Med 17: 338–343, 1989. [DOI] [PubMed] [Google Scholar]
- 23.Kiapour AM, Fleming BC, Proffen BL, Murray MM. Sex influences the biomechanical outcomes of anterior cruciate ligament reconstruction in a preclinical large animal model. Am J Sports Med 43: 1623–1631, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiapour AM, Murray MM. Basic science of anterior cruciate ligament injury and repair. Bone Joint Res 3: 20–31, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambers FM, Kuhn G, Schulte FA, Koch K, Muller R. Longitudinal assessment of in vivo bone dynamics in a mouse tail model of postmenopausal osteoporosis. Calcif Tissue Int 90: 108–119, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Lenth RV. Least-Squares Means: The R Package lsmeans. J Stat Software 69: 33, 2016. [Google Scholar]
- 27.Leppilahti J, Puranen J, Orava S. Incidence of Achilles tendon rupture. Acta Orthop Scand 67: 277–279, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Liu SH, al-Shaikh R, Panossian V, Yang RS, Nelson SD, Soleiman N, Finerman GA, Lane JM. Primary immunolocalization of estrogen and progesterone target cells in the human anterior cruciate ligament. J Orthop Res 14: 526–533, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Brazilian J Biol 62: 609–614, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Moller A, Astron M, Westlin N. Increasing incidence of Achilles tendon rupture. Acta Orthop Scand 67: 479–481, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Pardes AM, Freedman BR, Fryhofer GW, Salka NS, Bhatt PR, Soslowsky LJ. Males have inferior Achilles tendon material properties compared to females in a rodent model. Ann Biomed Eng 44: 2901–2910, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pardes AM, Freedman BR, Soslowsky LJ. Ground reaction forces are more sensitive gait measures than temporal parameters in rodents following rotator cuff injury. J Biomech 49: 376–381, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peltz CD, Dourte LM, Kuntz AF, Sarver JJ, Kim SY, Williams GR, Soslowsky LJ. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am 91: 2421–2429, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riggin CN, Sarver JJ, Freedman BR, Thomas SJ, Soslowsky LJ. Analysis of collagen organization in mouse Achilles tendon using high-frequency ultrasound imaging. J Biomech Eng 136: 021029, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandberg OH, Danmark I, Eliasson P, Aspenberg P. Influence of a lower leg brace on traction force in healthy and ruptured Achilles tendons. Muscles Ligaments Tendons 5: 63–67, 2015. [PMC free article] [PubMed] [Google Scholar]
- 36.Sarver JJ, Dishowitz MI, Kim SY, Soslowsky LJ. Transient decreases in forelimb gait and ground reaction forces following rotator cuff injury and repair in a rat model. J Biomech 43: 778–782, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silbernagel KG, Brorsson A, Olsson N, Eriksson BI, Karlsson J, Nilsson-Helander K. Sex differences in outcome after an acute achilles tendon rupture. Orthopaedic J Sports Med 3: 2325967115586768, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith LR, Barton ER. SMASH - semi-automatic muscle analysis using segmentation of histology: a MATLAB application. Skelet Muscle 4: 21, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soroceanu A, Sidhwa F, Aarabi S, Kaufman A, Glazebrook M. Surgical versus nonsurgical treatment of acute Achilles tendon rupture: a meta-analysis of randomized trials. J Bone Joint Surg Am 94: 2136–2143, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sullivan BE, Carroll CC, Jemiolo B, Trappe SW, Magnusson SP, Dossing S, Kjaer M, Trappe TA. Effect of acute resistance exercise and sex on human patellar tendon structural and regulatory mRNA expression. J Appl Physiol 106: 468–475, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2016. [Google Scholar]
- 44.Torricelli P, Veronesi F, Pagani S, Maffulli N, Masiero S, Frizziero A, Fini M. In vitro tenocyte metabolism in aging and oestrogen deficiency. Age 35: 2125–2136, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veronesi F, Della Bella E, Torricelli P, Pagani S, Fini M. Effect of adipose-derived mesenchymal stromal cells on tendon healing in aging and estrogen deficiency: an in vitro co-culture model. Cytotherapy 17: 1536–1544, 2015. [DOI] [PubMed] [Google Scholar]
- 46.Vosseller JT, Ellis SJ, Levine DS, Kennedy JG, Elliott AJ, Deland JT, Roberts MM, O'Malley MJ. Achilles tendon rupture in women. Foot Ankle Int 34: 49–53, 2013. [DOI] [PubMed] [Google Scholar]
- 47.Witte MM, Resuehr D, Chandler AR, Mehle AK, Overton JM. Female mice and rats exhibit species-specific metabolic and behavioral responses to ovariectomy. Gen Comp Endocrinol 166: 520–528, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu WD, Panossian V, Hatch JD, Liu SH, Finerman GA. Combined effects of estrogen and progesterone on the anterior cruciate ligament. Clin Orthopaedics Related Res 268–281, 2001. [DOI] [PubMed] [Google Scholar]