This is the first study using gas exchange during exercise to assess the response to pulmonary arterial hypertension drug therapy in patients with chronic thromboembolic pulmonary hypertension. We also for the first time compare patients with operable and inoperable chronic thromboembolic pulmonary hypertension.
Keywords: chronic thromboembolic pulmonary hypertension, pulmonary arterial hypertension drug therapy, cardiopulmonary exercise testing
Abstract
We tested the hypothesis that patients with chronic thromboembolic pulmonary hypertension (CTEPH) that was deemed to be inoperable were more likely to respond to drugs for treating pulmonary arterial hypertension (PAH) by using cardiopulmonary exercise (CPX) testing than those with CTEPH that was deemed to be operable. We analyzed CPX testing data of all patients with CTEPH who were treated with PAH drugs and had undergone CPX testing before and after treatment at a single pulmonary hypertension center between February 2009 and March 2013. Suitability for pulmonary endarterectomy (PEA) was decided by experts in PEA who were associated with a treatment center. The group with inoperable CTEPH included 16 patients, the operable group included 26 patients. There were no differences in demographics and baseline hemodynamic data between the groups. Unlike patients in the operable group, after drug treatment patients with inoperable CTEPH had a significantly higher peak V̇o2 (P < 0.001), work load (P = 0.002), and oxygen pulse (P < 0.001). In terms of gas exchange, there was an overall net trend toward improved V̇e/V̇co2 in the group with inoperable CTEPH, with an increased PaCO2 (P = 0.01), suggesting reduced hyperventilation. No changes were observed in patients with operable CTEPH. In conclusion, treatment with PAH drug therapy reveals important pathophysiological differences between inoperable and operable CTEPH, with significant pulmonary vascular and cardiac responses in inoperable disease. Drug effects on exercise function observed in inoperable CTEPH cannot be translated to all forms of CTEPH.
NEW & NOTEWORTHY
This is the first study using gas exchange during exercise to assess the response to pulmonary arterial hypertension drug therapy in patients with chronic thromboembolic pulmonary hypertension. We also for the first time compare patients with operable and inoperable chronic thromboembolic pulmonary hypertension.
chronic thromboembolic pulmonary hypertension (CTEPH) is a rare disease caused by obstruction of the pulmonary vasculature by organized chronic thromboemboli resulting in an increase in pulmonary vascular resistance (PVR) and mean pulmonary artery pressure (mPAP), ultimately causing right ventricular failure (12). Moser and Braunwald (14) originally proposed that small-vessel arteriopathy akin to that observed in pulmonary arterial hypertension (PAH) may exist in “open” vessels and not in “closed” vessels unexposed to high pulmonary artery pressures, thus creating a two-compartment model. Histologic examination of biopsy and autopsy samples from patients with CTEPH have subsequently demonstrated features indistinguishable from PAH, including plexogenic lesions (13). Further studies have suggested that this process can occur distal to obstructed vessels (7, 11). The presence of this PAH-like pathology has led to the hypothesis that PAH-targeted therapies may be efficacious in patients with CTEPH. Trials in patients with inoperable CTEPH have demonstrated contradictory results regarding the response of patients' exercise capacity (6, 9, 17). Likewise, in patients with CTEPH that has been deemed to be operable, PAH drugs used as a bridge to pulmonary endarterectomy (PEA) have shown different effects on the functional status of patients (15, 16).
In the current study, we sought to assess the response to PAH drugs of patients who were deemed to have operable or inoperable CTEPH using cardiopulmonary exercise (CPX) testing. Because inoperable CTEPH resembles PAH more than operable CTEPH does, we hypothesized that patients with inoperable CTEPH might have a better response to PAH drugs.
METHODS
Patients.
Consecutive patients attending a single-specialist pulmonary hypertension center between February 2009 and March 2013 were studied. We included patients who were diagnosed with CTEPH, began taking PAH-specific drugs, and had attended at least two incremental CPX sessions, one immediately before starting treatment and one at their subsequent visit, usually between 3 and 6 mo later. Patients were investigated according to a standard guideline-based protocol (5). CTEPH was defined in accordance with guidelines at the time by the presence of precapillary pulmonary hypertension, whereby mPAP ≥25 mmHg, pulmonary artery wedge pressure ≤15 mmHg, and PVR >2 Wood units in patients with multiple, chronic/organized occlusive thrombi in elastic pulmonary arteries (5). In our center we routinely use both ventilation/perfusion (V/Q) scan and computed tomography (CT) pulmonary angiogram to diagnose CTEPH. We excluded patients with severe lung disease identified clinically via spirometry and high-resolution CT scan, because PAH drugs are contraindicated in these patients (5).
Treatment protocol.
Patients were treated in accordance with a nationally agreed policy that had been drafted by the National Pulmonary Hypertension Service Physicians' Committee and National Health Service Specialized Commissioners (18). In accordance with this policy, PAH-targeted therapy was offered at diagnosis and prior to the assessment of operability in patients with World Health Organization functional class III/IV symptoms of PAH. Medical treatment was continued after assessment of operability if patients were deemed either to have inoperable CTEPH or, if the disease was ultimately deemed to be operable, it was continued in an attempt to improve symptoms while patients were on the waiting list for pulmonary endarterectomy surgery. Patients were offered treatment as a choice, and not all opted to receive it. Patients were not offered treatment as an alternative to surgery, and surgery was not delayed to assess the response to medical therapy. First-line treatment included a phosphodiesterase type 5 inhibitor (PDE5i); second-line treatment included an endothelin receptor antagonist (ERA). Prostacyclins could not be used in accordance with national policy at the time.
Assessment of operability.
All patients were referred to Papworth Hospital, the national PEA center, where an experienced, multidisciplinary CTEPH team adjudicated whether a patient's disease was operable. Decisions on operability were based mainly on the degree of pulmonary artery obstruction as determined by imaging, and the degree to which PVR, measured at right heart catheterization, was in proportion to this, with the CTEPH team deciding whether the majority of the vascular resistance could be explained by obstructive disease that could be removed by endarterectomy surgery. In view of the association between high PVR and operative mortality, most experienced staff in PEA surgical centers agree that mortality risk with PEA increases when the PVR is >1,200 dyn/cm/s−5 (3). For patients with this degree of hemodynamic impairment, PEA was still offered as long as the patient had at least 6-7 occluded segments. The final decision was based on the ratio of risk and benefit for the individual patient. There are few absolute contraindications to PEA, although most clinicians agree that patients with severe parenchymal lung disease and very poor ventilation will not benefit from improved perfusion.
Cardiopulmonary exercise testing.
CPX testing was performed on an upright cycle ergometer using a breath-by-breath system (Master Screen CPX; Jaeger, Hoechberg, Germany) according to the American Thoracic Society/American Society of Chest Physicians Statement on Cardiopulmonary Exercise Testing (2) and is described in the appendix. We compared data from CPX testing that patients undertook before they started therapy with the first available test after treatment. The interval between pretreatment CPX and cardiac catheterization was 1 day in the majority of our patients. All measurements were made by an experienced physiologist who was unaware of the patient's operable/inoperable status.
Statistical analysis.
Continuous variables were expressed either as means ± standard deviation or as a median value (with interquartile ranges) when they did not follow a normal distribution. Categorical variables were expressed as frequency and percentages. A paired Student's t-test was applied to compare pre- and posttreatment CPX testing variables within the groups and an unpaired test was applied for between-group comparisons. A χ2 test was used to assess differences in categorical values between groups. We performed a univariate linear regression analysis of CPX variables and hemodynamic (independent variables) status to examine their correlation with post/pre peak V̇o2 ratio (dependent variable). All statistical analyses were performed using SPSS (version 20). The study was approved by the National Research Ethics Service Committee - London South East (13/LO/0695).
RESULTS
Patient characteristics.
Between February 2009 and March 2013, 42 patients received PAH drug therapy and had pre- and post-drug CPX testing information available for analysis. A further 17 patients who were started on therapy prior to surgery underwent surgery prior to repeat testing and were not included. A further five patients could not undergo testing because they were unable to complete a test due to injury, deterioration, or administrative error. Of the 42 patients with paired CPX testing data, 16 were deemed to have inoperable CTEPH and 26 were deemed to have operable CTEPH. The baseline characteristics of the two groups are shown in Table 1. There were no significant differences among patient demographics, 6-min walk test distance, or hemodynamic data at baseline.
Table 1.
Baseline characteristics
| Inoperable CTEPH, n = 16 | Operable CTEPH, n = 26 | P | |
|---|---|---|---|
| Age, yr | 61 ± 17 | 61 ± 15 | 0.82 |
| Women, n (%) | 9 (56%) | 15 (58%) | 0.75 |
| WHO functional class | 0.82 | ||
| II | 0 (0%) | 1 (4%) | |
| III | 15 (94%) | 24 (92%) | |
| IV | 1 (6%) | 1 (4%) | |
| 6-min WT distance, m | 313 ± 103 | 274 ± 122 | 0.31 |
| mPAP, mmHg | 47 ± 11 | 47 ± 12 | 0.95 |
| PAWP, mmHg | 12 ± 3 | 12 ± 3 | 0.89 |
| LVEDP, mmHg | 11 ± 4 | 12 ± 4 | 0.66 |
| mRAP, mmHg | 10 ± 4 | 10 ± 4 | 0.82 |
| RVEDP, mmHg | 11 ± 5 | 11 ± 4 | 0.71 |
| Cardiac index, l/min/m2 | 2.1 ± 0.7 | 2.2 ± 0.6 | 0.60 |
| PVR, Wood units | 11 ± 7 | 10 ± 6 | 0.59 |
Data are presented as n (%) or mean values ± SD. CTEPH, chronic thromboembolic pulmonary hypertension; LVEDP, left ventricular end-diastolic pressure; mPAP, mean pulmonary artery pressure; mRAP, mean right atrial pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RVEDP, right ventricular end-diastolic pressure; WHO, World Health Organization; WT, walk test.
The median time interval between start of PAH therapy and posttreatment CPX testing was 6 (interquartile range 3–7.5) mo in patients with inoperable CTEPH and 5.5 (4–12) mo in the patients with operable CTEPH (P = 0.23). In the group with inoperable CTEPH, 11 patients (69%) were treated with PDE5i and 5 (31%) were treated with ERA, whereas in the group with operable CTEPH, 23 patients (88.5%) were prescribed PDE5i and 3 (11.5%) were prescribed an ERA (P = 0.09). The most common form of PDE5i was 25 mg of sildenafil to be taken three times a day.
Exercise performance improves in patients with inoperable, but not operable CTEPH, with PAH therapy.
Patients with inoperable CTEPH achieved a significantly higher peak V̇o2 (P < 0.001) and work load (P = 0.002) after starting treatment (Table 2), whereas there was no difference among patients with operable CTEPH (Table 3). There was also an increase in peak lactate (P = 0.01) and O2 pulse (P < 0.001) in patients with inoperable CTEPH; therefore, to account for any difference in effort, we measured O2 pulse at the same work rate (isowork O2 pulse) in the pre- and posttreatment tests. We chose the peak work rate in either test, whichever was the lower, to capture the highest work rate available for analysis in both tests and showed that isowork O2 pulse was higher posttreatment (P < 0.001) in patients with inoperable CTEPH. PaCO2 and physiologic dead space at peak exercise (VD/VTphys) were significantly increased posttreatment in patients with inoperable CTEPH (P = 0.01 and 0.03, respectively), but the overall effect led to a trend toward greater ventilatory efficiency (V̇e/V̇co2 slope decreased, P = 0.1) following treatment. No changes were noticed in the group of patients with operable CTEPH after treatment. No differences were observed according to type of drug therapy used (data not shown). When data from patients treated with PDE5i alone were analyzed, improvements in peak V̇o2, O2 pulse, isowork O2 pulse, peak work load, and PaCO2 remained significantly different in patients with inoperable CTEPH (P = 0.04, 0.03, 0.03, <0.001, and 0.004, respectively), with no changes observed among patients with operable CTEPH.
Table 2.
Comparison of cardiopulmonary exercise variables before and after PAH therapy in patients deemed to have inoperable CTEPH
| Variable, n = 16 | Pretreatment | Posttreatment | P |
|---|---|---|---|
| V̇o2 at anaerobic threshold | 717 ± 238 | 752 ± 211 | 0.19 |
| Peak V̇o2, ml·kg−1·min−1 | 11.7 ± 2.6 | 12.9 ± 2.5 | <0.001 |
| Respiratory exchange ratio | 1.04 ± 0.09 | 1.05 ± 0.09 | 0.70 |
| Lactate, mmol/l | 4.4 ± 1.7 | 5.3 ± 1.9 | 0.01 |
| Heart rate reserve, bpm | 22 ± 15 | 21 ± 14 | 0.48 |
| V̇e/V̇co2 | 55 ± 13 | 50 ± 10 | 0.12 |
| Eqco2 | 50 ± 9 | 48 ± 6 | 0.19 |
| O2 pulse, ml/beat | 6.4 ± 2.4 | 7.1 ± 2.2 | <0.001 |
| Isowork O2 pulse, ml/beat | 6.4 ± 2.4 | 6.9 ± 2.4 | 0.008 |
| VD/VTphys, % | 41 ± 5 | 46 ± 3 | 0.03 |
| Work load, W | 56 ± 29 | 66 ± 32 | 0.002 |
| Work load, % | 53 ± 17 | 60 ± 19 | 0.02 |
| Breathing reserve, l/min | 25 ± 20 | 24 ± 21 | 0.68 |
| Tidal volume, liter | 1.7 ± 0.7 | 1.7 ± 0.6 | 0.85 |
| Breathing frequency, breaths/min | 33 ± 8 | 34 ± 8 | 0.20 |
| PaCO2, mmHg | 31 ± 4 | 33 ± 3 | 0.01 |
Data are presented as means ± SD. bpm, beats per minute; Eqco2, ventilatory equivalent of carbon dioxide; PaCO2, partial carbon dioxide arterial pressure; PAH, pulmonary arterial hypertension; V̇co2, carbon dioxide production; VD/VTphys, physiologic dead space; V̇e, ventilation; V̇o2, oxygen consumption.
Table 3.
Comparison of cardiopulmonary exercise variables before and after PAH therapy in patients deemed to have operable CTEPH
| Variable, n = 26 | Pretreatment | Posttreatment | P |
|---|---|---|---|
| V̇o2 at anaerobic threshold | 774 ± 260 | 760 ± 237 | 0.80 |
| Peak V̇o2, ml·kg−1·min−1 | 12.4 ± 3.0 | 12.6 ± 3.2 | 0.52 |
| Respiratory exchange ratio | 1.01 ± 0.08 | 0.99 ± 0.07 | 0.14 |
| Lactate, mmol/l | 4.8 ± 1.8 | 4.4 ± 1.8 | 0.71 |
| Heart rate reserve, bpm | 31 ± 18 | 30 ± 20 | 0.78 |
| V̇e/V̇co2 | 57 ± 18 | 56 ± 16 | 0.68 |
| Eqco2 | 50 ± 13 | 49 ± 10 | 0.91 |
| O2 pulse, ml/beat | 7.5 ± 2.1 | 7.6 ± 2.0 | 0.70 |
| VD/VTphys, % | 48 ± 7 | 47 ± 8 | 0.91 |
| Work load, W | 61 ± 26 | 64 ± 31 | 0.12 |
| Work load, % | 59 ± 26 | 61 ± 26 | 0.24 |
| Breathing reserve, l/min | 20 ± 17 | 23 ± 15 | 0.37 |
| Tidal volume, liter | 1.6 ± 0.5 | 1.7 ± 0.6 | 0.36 |
| Breathing frequency, breaths/min | 37 ± 7 | 36 ± 7 | 0.36 |
| PaCO2, mmHg | 31 ± 6 | 30 ± 6 | 0.27 |
Data are presented as means ± SD.
PEA surgery improves physiological outcomes in patients with operable CTEPH.
Not all patients with operable CTEPH received PEA surgery; 1 had comorbid coronary artery disease and was surgical intervention was deemed to be too risky, 8 refused surgery, and 1 was on the waiting list. Those patients who underwent surgery exhibited significant improvements in functional class, 6-min walk distance, hemodynamic data, and exercise performance (Tables 4 and 5).
Table 4.
Hemodynamic data before and after PEA in 15 patients with operable CTEPH at 3 mo
| Patient Data, n = 15 | Pre-PEA | Post-PEA | P |
|---|---|---|---|
| WHO functional class | <0.001 | ||
| I | 0 (0%) | 4 (27%) | |
| II | 0 (0%) | 10 (67%) | |
| III | 14 (93%) | 1 (6%) | |
| IV | 1 (7%) | 0 (0%) | |
| 6-min WT distance, m | 290 ± 109 | 366 ± 86 | 0.04 |
| mPAP, mmHg | 47 ± 13 | 26 ± 7 | <0.001 |
| PAWP, mmHg | 13 ± 3 | 10 ± 2 | 0.02 |
| mRAP, mmHg | 11 ± 4 | 7 ± 2 | 0.006 |
| RVEDP, mmHg | 11 ± 5 | 9 ± 3 | 0.13 |
| Cardiac index, l/min/m2 | 2.1 ± 0.5 | 2.4 ± 0.4 | 0.07 |
| PVR, Wood units | 11 ± 6 | 4 ± 2 | 0.006 |
Data are presented as n (%) or means ± SD. PEA, pulmonary endarterectomy.
Table 5.
Comparison of cardiopulmonary exercise variables before and after PEA in 14 patients with operable CTEPH
| Patients Data, n = 14 | Pre-PEA | Post-PEA | P |
|---|---|---|---|
| V̇o2 at anaerobic threshold | 733 ± 111 | 865 ± 172 | 0.04 |
| Peak V̇o2, ml·kg−1·min−1 | 12.4 ± 2.7 | 15.6 ± 4.5 | 0.008 |
| Respiratory exchange ratio | 1.04 ± 0.08 | 1.04 ± 0.10 | 0.40 |
| Lactate, mmol/l | 5.3 ± 2.0 | 6.4 ± 2.4 | 0.28 |
| Heart rate reserve, % | 16 ± 11 | 16 ± 14 | 0.99 |
| Heart rate reserve, bpm | 28 ± 20 | 29 ± 25 | 0.89 |
| V̇e/V̇co2 | 58 ± 20 | 41 ± 6 | 0.005 |
| Eqco2 | 50 ± 15 | 40 ± 6 | 0.01 |
| O2 pulse, ml/beat | 6.9 ± 1.2 | 8.9 ± 2 | <0.001 |
| Isowork O2 pulse, ml/beat | 6.9 ± 1.2 | 8.3 ± 1.9 | 0.001 |
| VD/VTphys, % | 47 ± 7 | 37 ± 6 | 0.002 |
| Workload, W | 61 ± 19 | 80 ± 33 | 0.01 |
| Workload, % | 59 ± 27 | 72 ± 25 | 0.004 |
| Breathing reserve, l/min | 15 ± 16 | 19 ± 12 | 0.46 |
| Tidal volume, liter | 1.6 ± 0.5 | 1.6 ± 0.5 | 0.92 |
| Breathing frequency, breaths/min | 38 ± 7 | 36 ± 6 | 0.37 |
| PaCO2, mmHg | 31 ± 7 | 34 ± 5 | 0.04 |
Data are presented as means ± SD.
DISCUSSION
This is the first study using gas exchange during exercise to assess the response to PAH drug therapy in patients with CTEPH. It is also the first study to assess patients with operable and inoperable CTEPH. This is an observational study based on a standardized departmental clinical treatment protocol. Not only do we show that patients with inoperable CTEPH improve their peak exercise performance 6 mo after starting treatment whereas patients with operable CTEPH do not, but this is supported by significant effort-independent changes, much less vulnerable to bias, in cardiopulmonary physiology in the group with inoperable CTEPH, whereas no such effects exist in the group with operable CTEPH. Following treatment in the group of patients with inoperable CTEPH, gas exchange changed significantly with an increase in peak VD/VTphys, suggesting significant pulmonary vascular effects of therapy, and PaCO2 rose in keeping with decreased hyperventilation, a feature associated with severity of heart failure. In all of these respects, operable CTEPH was starkly unaffected by drug therapy and, as expected, showed significant improvements in hemodynamic and exercise physiology following PEA surgery.
The cardinal features of CTEPH upon exercise are impaired oxygen delivery and ventilatory inefficiency (Fig. 1A). Impaired oxygen delivery manifests as a reduced peak V̇o2 and an early anaerobic threshold as a result of a reduced stroke volume. Effective treatment in pulmonary hypertension would be expected to increase stroke volume through right ventricular afterload reduction and thus increase peak V̇o2 and the V̇o2 at anaerobic threshold (Fig. 1B). In support of this, in patients with inoperable CTEPH we observed an increase in peak V̇o2, a trend toward an increase in V̇o2 at anaerobic threshold and a significant increase in O2 pulse, suggesting an increase in stroke volume (Table 2).
Fig. 1.
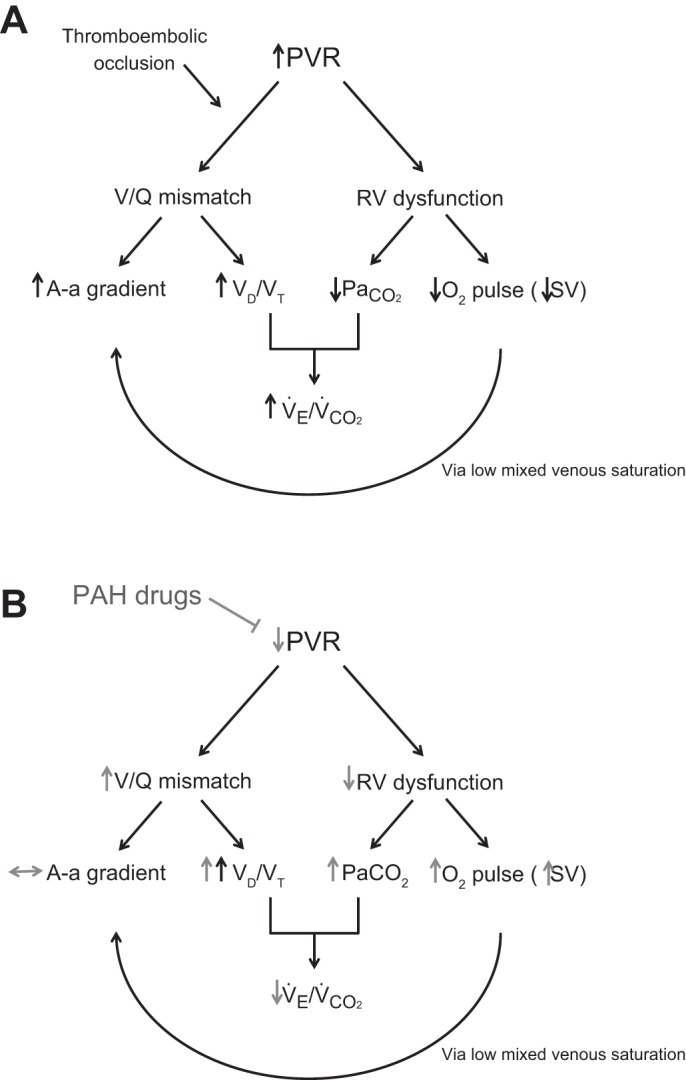
Pathophysiological mechanisms in chronic thromboembolic pulmonary hypertension (CTEPH; A) and the effect of pulmonary arterial hypertension (PAH; B) therapy. A-a gradient, alveolar-arterial gradient; PaCO2, partial carbon dioxide arterial pressure; PVR, pulmonary vascular resistance; RV, right ventricular; V̇co2, carbon dioxide production; VD/VTphys, physiologic dead space; V̇e, ventilation; V/Q mismatch, ventilation/perfusion mismatch.
Ventilatory inefficiency results from a combination of increased VD/VTphys due to pulmonary vasculopathy/vascular thrombotic occlusion and hypocapnia, as illustrated by the modified Bohr equation:
It is well documented that patients with pulmonary hypertension develop hypocapnia as a result of hyperventilation due to increased chemosensitivity associated with heart failure, and that this correlates with impairment of cardiac function and mortality (8). With effective reduction in right ventricular afterload by pulmonary vasodilators, we would expect an increase in PaCO2 and thus an improvement in ventilatory inefficiency. This has not been demonstrated before except following PEA surgery, when PaCO2 increased from 31 to 34 mmHg at 3 mo (20), which is identical to results in our study. Here, we also show a significant increase in PaCO2 to a similar degree in patients with inoperable CTEPH but not in those with operable CTEPH following treatment with PAH therapy, which is compatible with improved cardiac function and a trend toward lower V̇e/V̇co2 (−8.5%) and EqCO2 (−5.0%).
Hyperventilation itself has been proposed to result in increased VD/VTphys (4), but in our study, VD/VTphys increased in patients with inoperable CTEPH following treatment despite an increase in PaCO2, suggesting a real increase in ventilation-perfusion mismatch (Fig. 1B). This will have offset the increase in PaCO2 attenuating the benefit on improving ventilatory inefficiency as seen from the modified Bohr equation. This is the first time the effect of treatment on VD/VTphys has been reported in CTEPH. We have not assessed the mechanism of this worsening of VD/VTphys because this would require the use of the multiple inert gas-elimination technique, but propose that this results from increased perfusion to low-ventilation-perfusion areas of lung. It has previously been suggested that any intervention that lowers pulmonary vascular tone causes a deterioration in ventilation-perfusion relationships (1, 10).
The lack of any discernible change in any of the gas exchange parameters in patients with operable CTEPH compared with those with inoperable CTEPH suggests that drug therapy is unmasking different pathophysiological characteristics between the two phenotypes, perhaps with increased pulmonary vascular tone being far less relevant in operable disease. It also further supports the view that PAH therapy has little effect on operable CTEPH with surgical intervention being the treatment of choice.
In previous trials using sildenafil and bosentan, improvement in hemodynamics has not been matched by an improvement in 6-min walk test distance in patients with inoperable CTEPH and persistent pulmonary hypertension after PEA surgery (9, 17). We previously hypothesized that the worse ventilatory inefficiency during walking may account for the disconnection between improvement in 6-min walk and hemodynamics, if patients are running out of breathing reserve before cardiac reserve (19). Data from the CHEST-1 study of the soluble guanylate cyclase stimulator riociguat in patients with inoperable CTEPH show no such disparity (6). The effects of riociguat during CPX have not yet been assessed in inoperable CTEPH. One explanation may be that it has a more favorable effect on VD/VTphys than other PAH therapies, translating hemodynamic benefits into improved exercise function. An alternative explanation is that compared with previous studies, our study population was a purer population with distal disease due to better patient selection. Operability in CTEPH is subjective and depends on the experience of the PEA surgeon(s) within the adjudication committee and their ability to reach more distal segmental obstruction. With growing expertise, hospital centers are operating on more distal disease, thus the definition of inoperability may become more precise with time, with the result that patients deemed to have inoperable CTEPH are more “PAH-like” and thus more likely to respond to medical therapies.
This study was not intended to evaluate the efficacy of PAH therapies; rather, it was intended to address how different disease phenotypes physiologically respond to drug intervention, which is akin to looking for potential responders and nonresponders within a heterogeneous group of patients. Hence we compare patients with their baseline measurements rather than perform any statistical analysis between groups. Although patients were not blinded to their treatment, we showed that effort-dependent measurements were tracked by changes in effort-independent measurements, supporting the validity of the peak data. Measures such as PaCO2 and lactate are not open to bias. Peak V̇o2 and O2 pulse are reported directly by the exercise system software, and VD/VTphys is directly calculated from end-of-test data and cannot be manipulated. We also used a measure of isowork O2 pulse to guard against an effort effect. The only measures open to user input in our study are V̇e/V̇co2 slope and the anaerobic threshold; however, the physiologist taking the measurements was unaware of patient operative status. We acknowledge that the open-label nature of the study could still have affected the absolute levels of effort-dependent change, such as peak V̇o2 and work rate. Furthermore, the peak respiratory exchange ratio measures are lower than expected for a maximal cardiovascular effort, suggesting that maximal capacity may not have been assessed. Thus although we can state with confidence that the two subgroups of patients with CTEPH respond differentially to treatment, the true magnitude of these two effort-dependent effects remains uncertain. To evaluate efficacy/magnitude is a different question and would have required a different design, including blinding and a placebo arm.
A greater proportion of patients received PDE5i than ERA, although no differences in response were noted according to drug treatment and the findings were unchanged when analyzing the data of only those who received PDE5i. We nonetheless chose to include both therapy classes because neither is currently licensed and both have been shown to cause a reduction in pulmonary vascular resistance by similar amounts in inoperable CTEPH (9, 17). We have not evaluated the effect of riociguat.
Conclusions.
We have demonstrated that the responses to PDE5i/ERA in inoperable and operable CTEPH are different, suggesting that these two forms of CTEPH demonstrate distinct pathophysiological features. These therapies produce significant alterations in cardiac function and gas exchange in patients with CTEPH that was deemed to be inoperable; however, the treatments used in our cohort are not licensed for use in treating CTEPH because they have not shown efficacy in phase III clinical trials. This study shows the value of CPX in unraveling the reasons behind exercise limitation and eliciting differences in pathophysiology between CTEPH subtypes. We illustrate the importance of an expert CTEPH team in determining operability, because this will lead to the best outcome for patients. Finally, with the advent of new drugs shown to be effective in inoperable CTEPH (6), our study reinforces the recommendation of PEA as the treatment of choice for patients with operable CTEPH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.C. and L.S.H. conception and design of research; K.M. performed experiments; A.C. and L.S.H. analyzed data; K.M. and L.S.H. interpreted results of experiments; A.C. and L.S.H. prepared figures; A.C. and L.S.H. drafted manuscript; A.C., J.S.R.G., R.J.D., W.G.S., K.M., K.K.S., J.P.-Z., D.P.J., and L.S.H. edited and revised manuscript; A.C., J.S.R.G., R.J.D., W.G.-S., K.M., K.K.S., J.P.-Z., D.P.J., and L.S.H. approved final version of manuscript.
APPENDIX
Cardiopulmonary Exercise Protocol
Each test was performed in three stages: 3 min of rest, 3 min of unloaded pedaling, and a progressive ramp increase in work load to maximum exercise, with the ramp rate estimated to result in a work phase of 8 to 12 min. Patients either wore a face mask or breathed via a mouth piece while wearing a nose clip.
Systemic blood pressure was measured with a sphygmomanometer, finger oxygen saturation was measured with a pulse oximeter, and a 12-lead electrocardiogram was continuously recorded. Breath-by-breath measurements included oxygen uptake (V̇o2), carbon dioxide production (V̇co2), and ventilation (V̇e).
An arterial blood sample was drawn just after termination of exercise for measurement of arterial partial pressure of O2 (PaO2), arterial partial pressure of CO2 (PaCO2) and lactate level.
The best estimate of anaerobic threshold (AT) was calculated manually using a combination of the V-slope method and ventilatory equivalents for oxygen. V̇e/V̇co2 slope was obtained by linear regression analysis of the relationship between V̇e and V̇co2 during exercise prior to the respiratory compensation point. The ventilatory equivalent for CO2 (EqCO2) was measured at its lowest value over a 30-s average, reflecting the highest degree of ventilatory efficiency for each patient.
Equations
Breathing reserve (BR) was calculated as MVV − peak ventilation, where MVV is maximal voluntary ventilation, and was calculated as 35 × FEV1.
Heart rate reserve (HRR) was defined as maximum predicted heart rate − maximum heart rate.
Physiologic dead space was (VD/VTphys) was measured using the Bohr equation:
where PeCO2 is the measured mixed expired partial pressure of CO2.
REFERENCES
- 1.Agusti AG, Rodriguez-Roisin R. Effect of pulmonary hypertension on gas exchange. Eur Respir J 6: 1371–1377, 1993. [PubMed] [Google Scholar]
- 2.American Thoracic Society; American College of Chest Physicians. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 167: 211–277, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Dartevelle P, Fadel E, Mussot S, Chapelier A, Hervé P, de Perrot M, Cerrina J, Ladurie FL, Lehouerou D, Humbert M, Sitbon O, Simonneau G. Chronic thromboembolic pulmonary hypertension. Eur Respir J 23: 637–648, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Delcroix M, Vonk Noordegraaf A, Fadel E, Lang I, Simonneau G, Naeije R. Vascular and right ventricular remodeling in chronic thromboembolic pulmonary hypertension. Eur Respir J 41: 224–232, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 30: 2493–2537, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Ghofrani HA, D'Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins M, Fritsch A, Neuser D, Weimann G, Wang C; CHEST1 Study Group. Riociguat for the treatment of chronic thromboebolic pulmonary hypertension. N Engl J Med 369: 319–329, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch AM, Moser KM, Anger WR, Channick RN, Fedullo PF. Unilateral pulmonary artery thrombotic occlusion: is distal arteriopathy a consequence? Am J Respir Crit Care Med 152: 491–496, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Hoeper MM, Pletz MW, Golpon H, Welte T. Prognostic value of blood gas analyses in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 29: 944–950, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Jaïs X, D'Armini AM, Jansa P, Torbicki A, Delcroix M, Ghofrani HA, Hoeper MM, Lang IM, Mayer E, Pepke-Zaba J, Perchenet L, Morganti A, Simonneau G, Rubin LJ. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 52: 2127–2134, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Mélot C, Naeije R, Mols P, Vandenbossche JL, Denolin H. Effects of nifedipine on ventilation/perfusion matching in primary pulmonary hypertension. Chest 83: 203–207, 1983. [DOI] [PubMed] [Google Scholar]
- 11.Michel RP, Hu F, Meyrick BO. Myoendothelial junctional complexes in post obstructive pulmonary vasculopathy: a quantitative electron microscopic study. Exp Lung Res 21: 437–452, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Moser KM, Auger WR, Fedullo PF. Chronic major-vessel thromboembolic pulmonary hypertension. Circulation 81: 1735–1743, 1990. [DOI] [PubMed] [Google Scholar]
- 13.Moser KM, Bloor CM. Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest 103: 685–692, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Moser KM, Braunwald NS. Successful surgical intervention in severe chronic thromboembolic pulmonary hypertension. Chest 64: 29–35, 1973. [DOI] [PubMed] [Google Scholar]
- 15.Nagaya N, Sasaki N, Ando M, Ogino H, Sakamaki F, Kyotani S, Nakanishi N. Prostacyclin therapy before pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension. Chest 123: 338–343, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Reesink HJ, Surie S, Kloek JJ, Tan HL, Tepaske R, Fedullo PF, Bresser P. Bosentan as a bridge to pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg 139: 85–91, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Suntharalingam J, Treacy CM, Doughty NJ, Goldsmith K, Soon E, Toshner MR, Sheares KK, Hughes R, Morrell NW, Pepke-Zaba J. Long term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Chest 134: 229–236, 2008. [DOI] [PubMed] [Google Scholar]
- 18.National Specialised Commissioning Group. Targeted therapies for the treatment of pulmonary hypertension in adults (Online). London, UK: National Health Service; http://www.emscg.nhs.uk/Library/P009V1NationalSpecialisedCommissioningGroupPolicy2.pdf Accessed on 30 January 2016. [Google Scholar]
- 19.Zhai Z, Murphy K, Tighe H, Wang C, Wilkins MR, Gibbs JS, Howard LS. Differences in ventilatory inefficiency between pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Chest 140: 1284–1291, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Zoia MC, D'Armini AM, Beccaria M, Corsico A, Fulgoni P, Klersy C, Piovella F, Viganò M, Cerveri I; Pavia Thromboendarterectomy Group. Mid-term effects of pulmonary thromboendarterectomy on clinical and cardiopulmonary function status. Thorax 57: 608–612, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


