Abstract
Exercise is an effective therapy against the metabolic syndrome. However, the molecular pathways underlying the advantageous effects of exercise are elusive. Glucagon receptor signaling is essential for exercise benefits, and recent evidence indicates that a downstream effector of glucagon, fibroblast growth factor 21 (FGF21), is implicated in this response. Therefore, we tested the hypothesis that FGF21 action is necessary in mediating metabolic effects of exercise. We utilized acute exhaustive treadmill exercise in Wistar rats to identify a putative, concomitant increase in plasma glucagon and FGF21 with the increase in glucose and lactate following exercise. To test the necessity of FGF21 action in the exercise response, we exposed FGF21 congenitally deficient mice (Fgf21−/−) and their wild-type (Wt) littermates to chronic high-fat (HF) feeding and inoperable (sedentary) or operable (exercise) voluntary running wheels. Physiological tests were performed to assess the role of FGF21 in the beneficial effect of exercise on glucose metabolism. Wt and Fgf21−/− littermates exhibited similar running behavior, and exercise was effective in suppressing weight and fat mass gain and dyslipidemia independently of genotype. However, exercise failed to positively affect hepatic triglyceride content and glucose tolerance in HF diet-fed Fgf21−/− mice. Furthermore, Fgf21−/− mice exhibited an impaired adaptation to exercise training, including reduced AMP-activated protein kinase activity in skeletal muscle. This study demonstrates that FGF21 action is necessary to achieve the full metabolic benefits of exercise during chronic HF feeding.
Keywords: exercise, glucose tolerance, insulin action, lipid homeostasis, fibroblast growth factor 21, AMP-activated protein kinase
increasing worldwide obesity has been accompanied by an unprecedented increase in metabolic disturbances including dyslipidemia, type 2 diabetes mellitus, and nonalcoholic fatty liver disease (61). Although exercise alone may not stimulate weight loss (40, 57), it is identified as an effective means for body weight maintenance or prevention of weight gain (11) and is recognized as an effective behavioral intervention for the metabolic syndrome (4, 10, 31). Exercise regulates a host of adaptions that are crucial to its overall metabolic benefits, including improvements in whole body glucose disposal (47), insulin sensitivity (39, 48), and lipid homeostasis (12, 16). However, the metabolic regulators upstream of these beneficial effects are not fully known. Therefore, we set out to investigate the hormonal adaptations that mediate the pleiotropic metabolic effects of exercise.
The pancreatic hormone glucagon is well appreciated as a component of the exercise response, yet its role has been relegated to the prevention of hypoglycemia during exercise (33, 55). However, glucagon may have a broader role in the exercise response. Glucagon receptor (GcgR) signaling is necessary for the metabolic benefits of exercise training (32), including amelioration of diet-induced fatty liver (4). Intriguingly, GcgR activation during chronic exercise induces hepatic expression of fibroblast growth factor 21 (Fgf21) (4), suggesting that FGF21 is downstream of glucagon action. Furthermore, it is possible that FGF21 may be integral to the metabolic impact of glucagon action in the responses to exercise. Our group recently showed that FGF21 mediates multiple components of glucagon action, including regulation of energy balance, glucose, and lipid metabolism (19). However, the role of GcgR-FGF21 signaling during exercise remains unclear.
In support of a role for FGF21 in the exercise response, FGF21 (like exercise) has pleiotropic effects on glucose and lipid homeostasis. Administration of FGF21 to obese diabetic rodents and rhesus monkeys enhances whole body insulin sensitivity, ameliorates hypertriglyceridemia, and reduces hepatic lipogenesis (8, 28, 56). Furthermore, emerging evidence suggests that the liver contributes to FGF21 plasma levels during exercise in humans, and this secretion is regulated by the glucagon:insulin ratio (21). On the basis of these findings, we aimed to investigate the role of FGF21 action in the benefits of exercise on metabolic homeostasis. Because preexisting obesity is a metabolic state already associated with FGF21 resistance (13), we studied the role of this hormone in mice exposed to high-fat (HF) feeding upon initiation of exercise.
The results described herein suggest that FGF21 is not required for exercise regulation of body weight and fat mass but is necessary for the gluco- and lipo-regulatory benefits of exercise during HF feeding. Furthermore, we illustrate that FGF21-mediated regulation of the exercise response is, at least in part, mediated through AMP-activated protein kinase (AMPK) action in skeletal muscle.
METHODS
Rodents.
All studies were conducted on a single cohort of rats and a single cohort of mice as detailed below. Rodents were maintained in our facilities (12-h:12-h light/dark cycle; 25 ± 1°C; 50–60% humidity) and provided ad libitum access to water and pelleted standard chow diet (7012, LM-485; 25% protein, 58% carbohydrate, and 17% fat, 3.1 kcal/g AFE; Harlan Teklad, Madison, WI). Where noted, HF diet (HFD) (D-12492; 60 kCal % fat; Research Diets, New Brunswick, NJ) was provided to induce obesity and defects in glucose metabolism. The Institutional Animal Care and Use Committees at the University of Cincinnati and the University of Alabama at Birmingham approved all experimental protocols. Male, age-matched, Wistar rats (400–550 g) were obtained from Harlan Laboratories (Madison, WI). Age-matched wild-type (Wt) and congenital FGF21 knockout (Fgf21−/−) male littermates were generated as previously described (19, 25) and maintained in our facilities.
Acute forced treadmill exercise in Wt rats.
Throughout the study, all rats were provided ad libitum access to water and standard chow diet. Rats (n = 5) were utilized, as they allow for a larger and more rapid blood collection, thus providing a more accurate sampling of humoral factors. All rats were acclimated on a motor-driven treadmill (Columbus Instruments, Columbus, OH) by running 5 min/day on a 5% incline for five consecutive days as detailed in Table 1 with electrical stimulation used to encourage running. Following a 4-day rest period, ad libitum rats began forced running at 10 m/min, and treadmill speed was increased 1 m/min every 3 min until exhaustion, as indicated by 4 s of electrical stimulation. The average maximum velocity achieved was 21.4 ± 1.63 m/min, and the average time to exhaustion was 38.1 ± 2.73 min. Tail vein blood was collected immediately before the exercise period, at the time of exhaustion, and then 4 h following exhaustion.
Table 1.
Rat treadmill acclimatization
| Day | Speed, m/min |
|---|---|
| 1 | 10 |
| 2 | 10 |
| 3 | 11 |
| 4 | 13 |
| 5 | 15 |
Five-day acclimation protocol was utilized before single-bout exhaustive exercise study.
Voluntary running wheel exercise training in FGF21-deficient and Wt littermates.
At 10 wk of age, all mice (n = 9 Wt and 18 Fgf21−/−) were individually housed and acclimated for 1 wk in cages with blocked running wheels. Following acclimation, all mice were provided ad libitum access to HFD, and running wheels were released in the exercise (EX) groups (n = 5 Wt and 8 Fgf21−/−). Voluntary running activity was recorded every 10 min via a computerized system (Computerized Animal Wheel Monitoring System; Lafayette Instrument, Lafayette, IN).
Energy balance and body composition.
Food intake and body weight of all mice were measured weekly, and body composition was measured using noninvasive nuclear magnetic resonance spectroscopy (EchoMRI; Echo Medical Systems, Houston, TX).
Glucose and insulin tolerance tests.
Intraperitoneal (IP) glucose (1.5 g/kg, 20% wt/vol d-glucose in 0.9% wt/vol saline; Sigma-Aldrich, St. Louis, MO) and insulin (0.75 units/kg insulin Humalog in 0.9% wt/vol saline; Eli Lilly, Indianapolis, IN) tolerance tests (ITT) were conducted in 5-h-fasted mice as previously published (30). Tail vein blood glucose was assessed using a glucometer immediately before and 15, 30, 45, 60, 90, and 120 min after injection (TheraSense Freestyle glucometer; Abbott Laboratories, Abbott Park, IL). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as previously described (5).
Tissue isolation.
Following 12 wk of running wheel exercise, mice were removed from running wheel-equipped cages at 0400 (Zeitgeber time 22) and fasted for 5 h. Mice were briefly sedated with pentobarbital and decapitated. Trunk blood was collected, and liver, gastrocnemius, and extensor digitorum longus (EDL) tissues were harvested and stored at −80°C until analysis.
Plasma hormone, glucose, lactate, and lipid analyses.
Glucose and lactate levels in plasma were determined using an Analox GM7 analyzer (Analox Instruments USA, Lunenburg, MA). Plasma insulin and FGF21 were determined via ELISA assay according to the manufacturer's instructions (Crystal Chem, Downers Grove, IL and Millipore, Billerica, MA). Plasma glucagon was determined using Meso Scale Discovery MULTI-ARRAY Assay System (Meso Scale Discovery, Gaithersburg, MD) as previously described (20). Plasma and liver cholesterol, triglyceride (TG), and nonesterified fatty acids were analyzed by enzymatic assay (Pointe Scientific, Canton, MI and Wako Diagnostic, Richmond, VA). All analyses were measured per the manufacturer's instructions. Analytical variation of assays was assumed as reported by the manufacturer.
Quantitative real-time PCR and Western analysis.
Tissue RNA was isolated using the RNeasy Lipid Mini-Kit according to the manufacturer's instructions (Qiagen, Valencia, CA), and cDNA was synthesized by reverse transcription PCR using SuperScriptIII, DNase treatment, and anti-RNase treatment according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Quantitative real-time PCR was performed using TaqMan Universal Master Mix and 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). TaqMan Gene Expression Assays were performed for the following primer/probe sets purchased from Applied Biosystems: glucose transporter 1 (Glut 1; Mm00441480_m1), Glut 2 (Mm00446229_m1), Glut 4 (Mm01245502_m1), carnitine palmitoyltransferase α (Cpt1α; Mm01231183_m1), Cpt1β (Mm00487191_g1), phosphoenolpyruvate carboxykinase 1 (Pck1; Mm01247058_m1), glucagon receptor (Gcgr; Mm00433546_m1), AMP-activated protein kinase α2 (AMPKα2; Mm01264789_m1), glycogen synthase kinase 3β (Gsk3β; Mm00444911_m1), peroxisome proliferator-activated receptor α (Pparα; Mm00440939), fibroblast growth factor 21 (Fgf21; Mm00840165_g1), sterol regulatory element-binding transcription factor 1 (Srebf1; Mm00550338_m1), fatty acid synthase (Fasn; Mm00662319), acetyl-CoA carboxylase 1 (Acc1; Mm01304257_m1), stearoyl-CoA desaturase 1 (Scd1; Mm00772290_m1), and HMG-CoA reductase (Hmgcr; Mm01282499_m1). TaqMan data were normalized to the levels of hprt (liver) or rp18s (skeletal muscle) mRNA using the ΔΔCT calculation. Western blot analyses were performed using total protein extracted using RIPA lysis buffer containing Halt protease inhibitor cocktail (Thermo Scientific, Waltham, MA). Immunoblot analysis of AMPK phosphorylation was conducted with anti-AMP-activated protein kinase α (anti-AMPKα) and anti-phospho-AMPKα (Thr172) (Cell Signaling, Beverly, MA). Total and phospho-AMPK signals were normalized to β-tubulin (Cell Signaling) as a loading control. Densitometry analysis was completed using ImageJ.
Statistics.
All data are represented as means ± SE. Statistical significance was assigned when P < 0.05. Unpaired Student's t-tests were utilized for comparison of two groups. One-way ANOVA with Tukey's posttest was utilized for the analysis of rat humoral components. Two-way ANOVA with Bonferroni posttest was implemented for analysis of the final parameters, whereas two-way repeated ANOVA with Sidak posttest was utilized for measurements over time in the mouse exercise study. Statistics were completed using GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA).
RESULTS
Glucagon and FGF21 secretion is elevated during exhaustive exercise in rats.
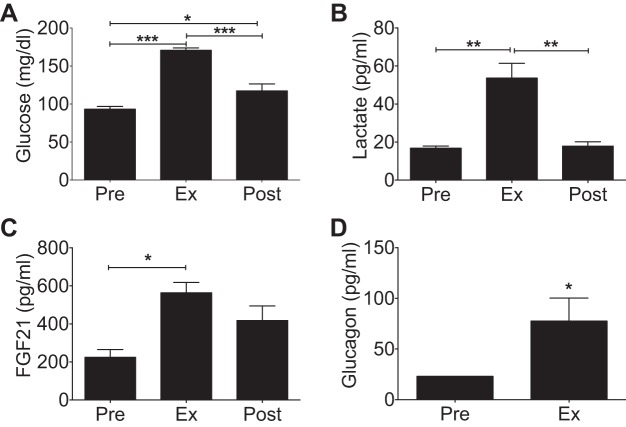
Glucagon action is an integral component of the exercise response (55); however, the downstream signaling molecules mediating these effects are unknown. We first investigated whether exercise activated a concomitant elevation of glucagon and a downstream effector of glucagon action, FGF21, during exhaustive exercise. With confirmation that exhaustion was achieved, plasma glucose and lactate were significantly elevated postexercise in both groups (Fig. 1, A and B) (46). We also found that plasma glucagon and FGF21 were increased immediately following exhaustion (Fig. 1, C and D), which is consistent with their individual contribution to the exercise response (3, 4, 29). Furthermore, FGF21 levels remained elevated 4 h after exhaustive exercise bout, providing evidence for a role for FGF21 in the recovery phase of exercise.
Fig. 1.
Acute exhaustive treadmill running in rats. Plasma levels of glucose (n = 5) (A), lactate (n = 4) (B), and fibroblast growth factor 21 (FGF21) (n = 3) (C) preexercise, at exhaustion, and 4 h postexercise. D: plasma glucagon (n = 5) before an acute exercise bout and at exhaustion. Average maximum velocity achieved was 21.4 ± 1.63 m/min, and mean time to exhaustion was 38.1 ± 2.73 min. All data are represented as means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001.
Voluntary running wheel behavior is FGF21 independent.
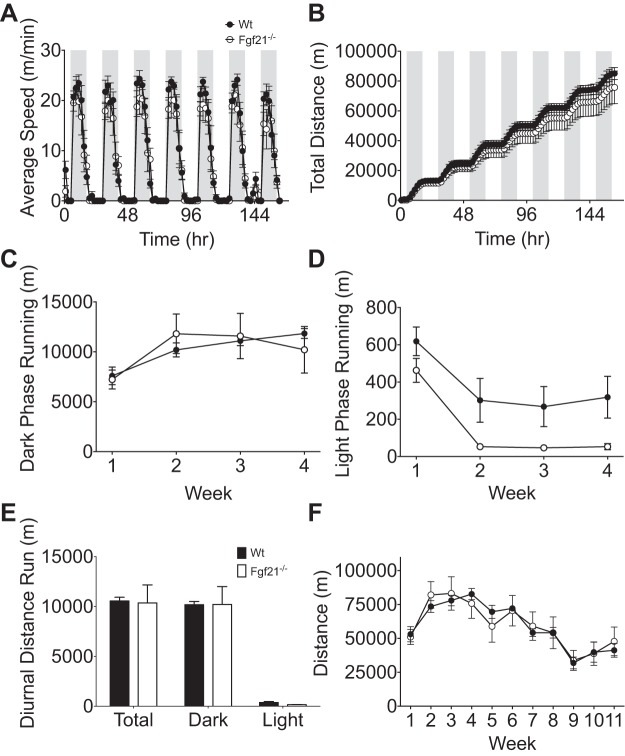
We analyzed the average speed (m/min) during light and dark phases and the cumulative distance run throughout week 4 of exercise. Week 4 was chosen for this analysis to limit the effects of novelty/learning induced during the first week and stresses of physiological experiments experienced during the later weeks. We found that Fgf21−/− mice and Wt littermates exhibited similar average running velocity (m/min) during light and dark (active) phases (Fig. 2A) and that cumulative distance run throughout the week was slightly but not significantly reduced in Fgf21−/− mice (Fig. 2B). In light of recent evidence suggesting a role for FGF21 in circadian regulation of exercise (6), we also calculated the average distance run during the light and dark phases of weeks 1–4. Although we found no difference in the amount of running during the dark phase (Fig. 2C), we observed a reduced distance of running during the light phase in Fgf21−/− mice compared with Wt controls (Fig. 2D). This effect did not, however, translate to changes in total diurnal running distance (Fig. 2E). We also quantified the average weekly distance run and observed a similar pattern in the weekly distance between groups (Fig. 2F). We noted that, following week 4, the amount of weekly distance run decreased independent of genotype, which we attributed to stress induced from physiological tests. Taken together, these data suggest that FGF21 is not necessary for the behavioral drive to engage in voluntary exercise, at least during the active phase.
Fig. 2.
Running wheel activity in wild-type (Wt) and FGF21-deficient (Fgf21−/−) mice. A: average speed (m/min). B: total cumulative distance (m) during week 4. C: average dark phase distance (m) during weeks 1–4. D: average light phase running (m) during weeks 1–4. E: diurnal distance run (m). F: average weekly distance (m). Wt mice are represented by ●, n = 5; Fgf21−/− mice are represented by ○, n = 6. Shaded area indicates dark phase. All values are represented as means ± SE.
Exercise regulation of energy balance is FGF21 independent.
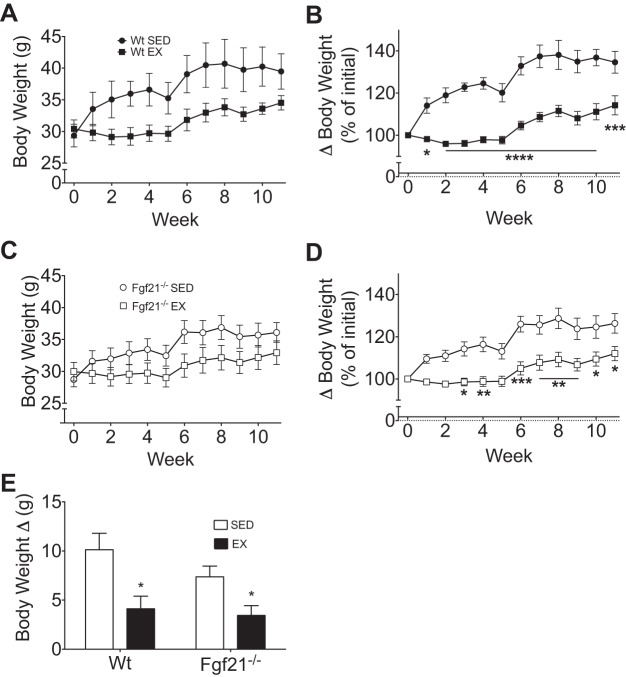
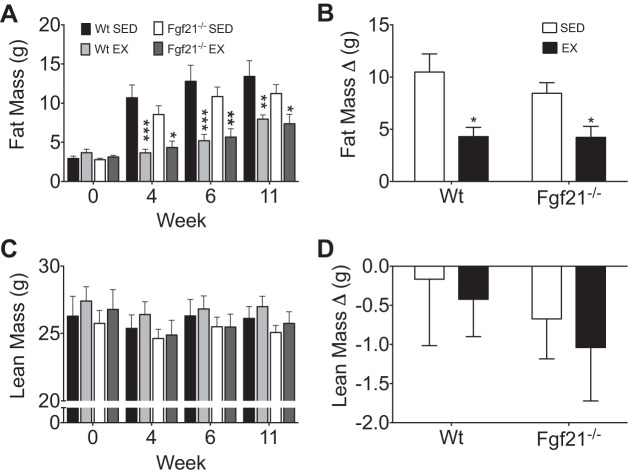
Under sedentary conditions, Wt mice gained an average of 10.1 g (Fig. 3, A and E), which equates to a 34.6% gain in weight over baseline (Fig. 3B). With exercise, Wt mice gained about 4.1 g of body wt (Fig. 3, A and E), which equates to a 14.2% increase over baseline (Fig. 3B). Similarly, sedentary Fgf21−/− mice gained 7.4 g (Fig. 3, C and E) or 26.3% over baseline (Fig. 3D), whereas trained Fgf21−/− mice gained 3.4 g (Fig. 3, C and E) or 12.0% over baseline (Fig. 3D). Thus, independent of genotype, exercise blunted HFD-induced obesity (Fig. 3, B and D). Likewise, when we quantified the change (Δ) in body weight week 1 to week 11, there was a significant reduction in HFD-induced body weight gain with exercise in both Wt and Fgf21−/− mice (Fig. 3E). Body composition analysis identified a significant reduction in fat mass accumulation in Wt and Fgf21−/− littermates following 4, 6, and 11 wk of exercise (Fig. 4A). The change (Δ) in fat mass from baseline was to that observed in body weight. Specifically, sedentary Wt mice gained ∼10.0 g of fat mass (Fig. 4B), whereas Fgf21−/− mice accumulated 8.4 g of fat mass in response to HF feeding (Fig. 4B). In both genotypes, this fat mass accrual accounted for the entirety of the body mass gain. As was also observed in body mass accumulation, we observed a significant reduction in fat mass gain with exercise independent of genotype (Fig. 4B). In contrast, there was no impact of exercise or genotype on absolute or the change in lean mass (Fig. 4, C and D).
Fig. 3.
Exercise regulation of energy balance in Wt and Fgf21−/− mice. Weekly average body weight measurements of Wt (exercised, EX, ■, n = 5; sedentary, SED, ●, n = 4) (A) and Fgf21−/− (EX, □, n = 8; SED, ○, n = 10) (C) are shown. Percent body weight gain from initial in Wt (B) and Fgf21−/− (D) mice is shown. E: change in body weight (final-initial). All data are represented as means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with sedentary control.
Fig. 4.
Accumulated body mass in exercised and sedentary Wt and Fgf21−/− mice. Body composition analysis at 0, 4, 6, and 11 weeks. A: fat mass. C: lean mass. B: change in fat mass (final-baseline). D: change in lean mass (final-baseline). Wt (EX, n = 5; SED, n = 4), Fgf21−/− (EX, n = 8; SED, n = 10). Data are represented as means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001 compared with sedentary control.
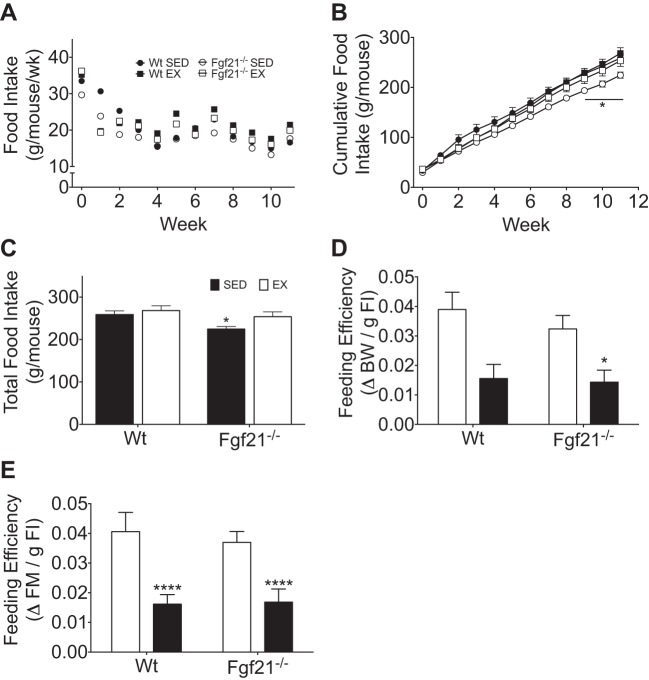
We observed similar average weekly food intake (g/wk per mouse) among Wt and Fgf21−/− mice, independent of exercise status (Fig. 5A). Cumulative food intake (g/mouse) was similar in sedentary and exercised Wt mice throughout the study (Fig. 5B); however, by week 9, sedentary Fgf21−/− mice exhibited reduced cumulative (Fig. 5B) and total food intake (Fig. 5C) compared with their exercised Fgf21−/− mice. Despite this, when we analyzed the efficiency with which food contributed to body weight gain (change in body weight per gram of food consumed) and fat mass gain, we observed a protective effect of exercise in Wt and Fgf21−/− mice (Fig. 5, D and E). Overall, the energy balance data suggest that the beneficial effects of exercise on feeding efficiency, body weight, and fat mass gain during chronic HF feeding occur in an FGF21-independent manner.
Fig. 5.
Food intake during exercise training and under sedentary conditions in Wt and Fgf21−/− mice. A: average weekly food intake (g/wk per mouse). B: cumulative food intake (g/mouse). C: total food intake (g/mouse). D: feeding efficiency (g of body mass/g food intake). E: feeding efficiency (g fat mass/g of food intake). Wt (EX, n = 5; SED, n = 4), Fgf21−/− (EX, n = 8; SED, n = 10). All data are represented as means ± SE. *P < 0.05 and ****P < 0.0001 compared with sedentary control.
Exercise requires FGF21 to improve HFD-induced glucose intolerance.
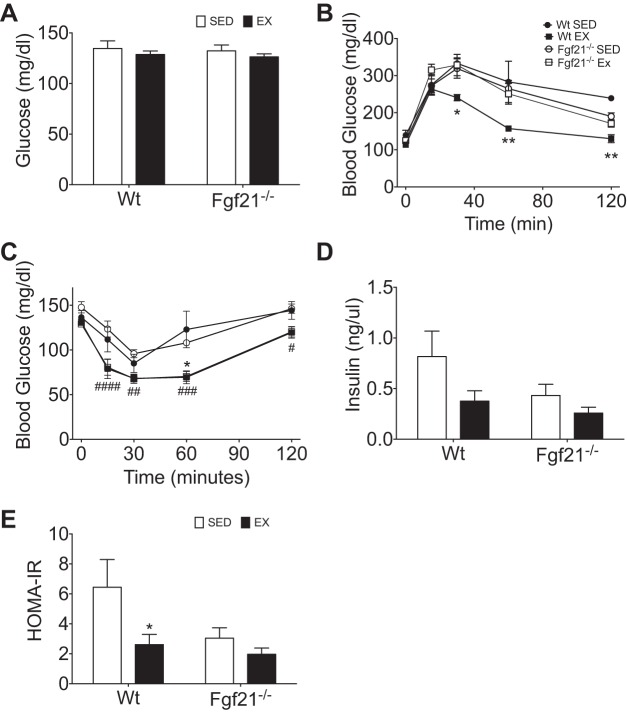
Exercise is known to impact glucose homeostasis in part by improving fasting glycemia as well as glucose tolerance (35). In our study, there was not a significant exercise-induced reduction in fasting glycemia in Wt or Fgf21−/− littermates when measured at 5, 6, or 7 wk of exercise (Fig. 6A). However, exercise did significantly enhance glucose tolerance in Wt mice compared with their sedentary littermates. However, in stark contrast, this beneficial effect of exercise was lost in Fgf21−/− littermates (Fig. 6B).
Fig. 6.
Exercise regulation of glucose tolerance and insulin sensitivity in exercised and sedentary Wt and Fgf21−/− mice. A: average fasting blood glucose in weeks 5–7. B: blood glucose excursion during intraperitoneal glucose tolerance test (IP-GTT). C: blood glucose excursion during intraperitoneal insulin tolerance test (IP-ITT). D: average fasting blood insulin in weeks 5–6. E: homeostasis model assessment of insulin resistance (HOMA-IR) calculated from fasting glycemia and insulin represented in C and E. Wt (EX ■, n = 4; SED ●, n = 4), Fgf21−/− (EX □, n = 8; SED ○, n = 10). All data are represented as means ± SE. *P < 0.05, **P < 0.01 exercise effect within Wt only; #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 exercise effect within both genotypes.
Following these intriguing results, we performed intraperitoneal ITT to interrogate insulin action in Wt and Fgf21−/− littermates following chronic exercise training or sedentary conditions. Surprisingly, we observed that exercise enhanced insulin action independent of genotype (Fig. 6C). However, when we quantified fasting plasma insulin levels and calculated HOMA-IR, we observed a trend for a reduction in fasting insulin and a significant reduction in HOMA-IR with exercise in Wt mice (Fig. 6, D and E). This exercise effect was lacking in Fgf21−/− mice and rather was associated with a significant reduction in fasting plasma insulin that was independent of exercise status (Fig. 6E). Together with the impaired glucose tolerance, these results may suggest that β-cell function may be compromised in Fgf21−/− mice.
Gluco-regulatory gene expression during exercise occurs independently of FGF21.
Contrary to our hypothesis, gluco-regulatory gene expression was altered by exercise but in an FGF21-independent manner. Specifically, in the liver, neither exercise nor genotype significantly altered the mRNA levels of Glut 2 or Pck1 (Table 1). In contrast, exercise increased the hepatic expression of Ampkα2, Gcgr, and Gsk3β regardless of genotype (Table 1). Intriguingly, exercise reduced hepatic mRNA of Fgf21 in Wt mice and was predictably undetectable in Fgf21−/− mice (Table 1). In skeletal muscle (EDL), the mRNA expression of Glut1, Glut4, Ampkα2, and Gsk3β was not regulated by running wheel exercise or genotype, and Fgf21 mRNA at this time point was undetectable in both genotypes (Table 1).
FGF21 deficiency impairs exercise-induced AMPK activation in skeletal muscle.
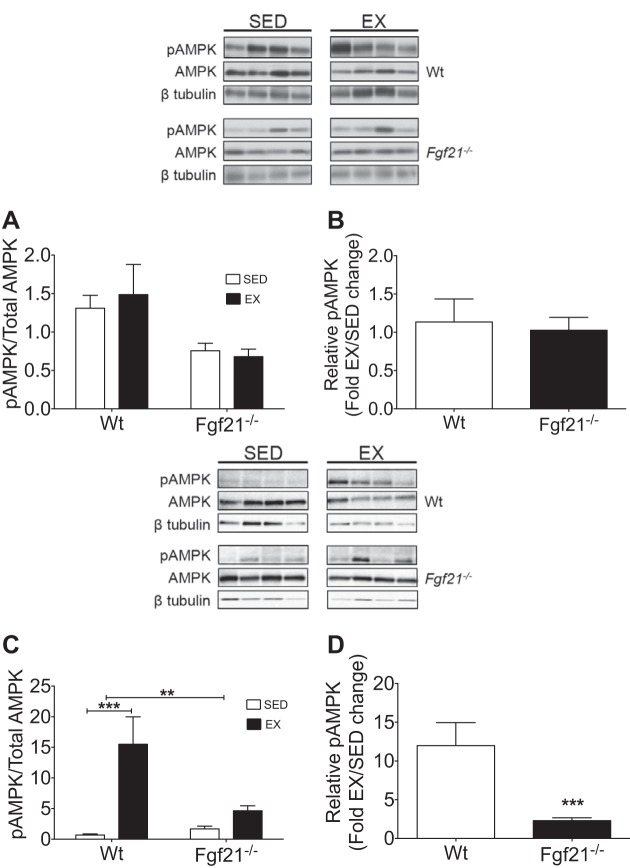
Total and phosphorylated hepatic AMPK protein was similar in all mice and unchanged following exercise regardless of genotype (Fig. 7, A and B). However, in skeletal muscle (gastrocnemius), we identified a marked exercise-induced increase in phospho-AMPK levels in Wt mice compared with their sedentary controls (Fig. 7C). Conversely, we observed only a slight increase in AMPK phosphorylation in Fgf21−/− mice compared with their controls (Fig. 7C). Wt mice exhibited a 12-fold increase in gastrocnemius AMPK phosphorylation with exercise compared with their sedentary controls, whereas Fgf21−/− littermates had a 2-fold increase in AMPK phosphorylation with exercise (Fig. 7D). Consistent with the loss of exercise-enhanced glucose tolerance in Fgf21−/− mice, these results implicate a muscle-specific FGF21-dependent regulation of AMPK in the beneficial effects of exercise on glucose tolerance.
Fig. 7.
Hepatic and skeletal muscle AMP-activated protein kinase (AMPK) analysis in exercise-trained and sedentary Wt and Fgf21−/− mice. Hepatic analysis is in A and B. A: ratio of phospho-AMPK (Thr172) normalized to β-tubulin and total AMPK normalized to β-tubulin. B: phosphorylation of AMPK represented as fold change compared with sedentary. Gastrocnemius analysis is in C and D. C: ratio of phospho-AMPK (Thr172) normalized to β-tubulin and total AMPK normalized to β-tubulin. D: effect of exercise on phosphorylation of AMPK represented as fold change compared with sedentary Wt (EX, n = 5; SED, n = 4), Fgf21−/− (EX, n = 8; SED, n = 8). All data are represented as means ± SE. **P < 0.01, ***P < 0.001 compared with sedentary control or Wt exercise group.
Two key adaptations to long-term exercise mediated by AMPK include increasing oxidative capacity and energy storage capacity in skeletal muscle (1, 17, 50). Thus we also analyzed mRNA levels of key lipogenic genes in muscle implicated in intramuscular lipid accrual in response to exercise (42, 51). On the basis of our evidence that the skeletal muscle response to chronic exercise was substantially reduced in Fgf21−/− mice, we hypothesized that the effect of exercise on mRNA expression of skeletal muscle lipogenic genes is greater in Wt mice than their Fgf21−/− littermates. In support of this hypothesis, we observed a significant elevation in Fasn (a central regulator of de novo lipogenesis) mRNA levels in exercise Wt mice only (Table 1). We also quantified expression of Srebf1 (a major transcription factor involved in lipid biosynthesis) and observed an increase in expression with exercise independent of genotype. Furthermore, when we quantified mRNA levels of a downstream target of SREBF1, Acc (the rate-limiting enzyme for de novo lipogenesis), we identified a trend for increased expression in exercised Wt mice (Table 1). Acc mRNA levels remained unchanged by exercise in Fgf21−/− mice (Table 1). Finally, we analyzed skeletal muscle mRNA levels of Scd1 (a central mediator for synthesizing monounsaturated fatty acids). However, Scd1 expression was unaffected by exercise in Wt or Fgf21−/− mice (Table 1).
FGF21 contributes to exercise-induced regulation of hepatic lipid metabolism.
Lipid analysis indicated that exercise reduced plasma cholesterol levels in Wt and Fgf21−/− littermates, whereas plasma TG and free fatty acids were unchanged by exercise independent of genotype (Table 2). Consistent with its known protective and therapeutic effects on hepatic lipid metabolism, exercise reduced liver lobe mass, hepatic TG, and cholesterol in Wt mice (Table 2). Although exercise significantly reduced liver lobe mass, it did not change liver TG or cholesterol levels in Fgf21−/− mice (Table 2). Liver cholesterol was reduced in sedentary Fgf21−/− mice compared with sedentary Wt mice (Table 2), which might be a result of congenital loss of FGF21 in these mice.
Table 2.
Liver and skeletal muscle gene expression (a.u.)
|
Wt |
Fgf21−/− |
|||
|---|---|---|---|---|
| Sedentary | Exercised | Sedentary | Exercised | |
| Liver | ||||
| >Glut 2 | 1 ± 0.10 | 1.06 ± 0.14 | 0.81 ± 0.05 | 1.04 ± 0.10 |
| Pck1 | 1 ± 0.22 | 1.15 ± 0.19 | 0.79 ± 0.11 | 1.03 ± 0.16 |
| Gsk3β | 1 ± 0.07 | 1.30 ± 0.22* | 0.85 ± 0.05 | 1.14 ± 0.13* |
| Gcgr | 1 ± 0.15 | 1.64 ± 0.70* | 1.22 ± 0.24 | 2.03 ± 0.40* |
| Ampkα2 | 1 ± 0.09 | 1.51 ± 0.28* | 1.02 ± 0.10 | 1.31 ± 0.13* |
| Cpt1α | 1 ± 0.21 | 1.10 ± 0.18 | 0.81 ± 0.07 | 1.16 ± 0.17 |
| Pparα | 1 ± 0.17 | 1.06 ± 0.11 | 0.83 ± 0.07 | 0.98 ± 0.10 |
| Srebf1 | 1 ± 0.15 | 1.12 ± 0.10 | 0.99 ± 0.07 | 1.27 ± 0.10* |
| Fasn | 1 ± 0.23 | 1.00 ± 0.11 | 0.56 ± 0.10† | 0.56 ± 0.06† |
| Acc1 | 1 ± 0.10 | 1.25 ± 0.16 | 0.91 ± 0.13 | 1.00 ± 0.20 |
| Scd1 | 1 ± 0.21 | 0.37 ± 0.11* | 0.50 ± 0.14† | 0.52 ± 0.11† |
| Hmgcr | 1 ± 0.37 | 1.14 ± 0.22 | 0.46 ± 0.06† | 0.68 ± 0.12† |
| Fgf21 | 1 ± 0.24 | 0.35 ± 0.11* | ND | ND |
| Skeletal Muscle | ||||
| Glut 1 | 1 ± 0.12 | 1.54 ± 0.36 | 1.17 ± 0.19 | 1.30 ± 0.27 |
| Glut 4 | 1 ± 0.14 | 1.34 ± 0.15 | 0.82 ± 0.16 | 1.04 ± 0.16 |
| Gsk3β | 1 ± 0.08 | 0.98 ± 0.15 | 0.76 ± 0.11 | 0.94 ± 0.14 |
| Ampkα2 | 1 ± 0.07 | 1.03 ± 0.11 | 0.85 ± 0.10 | 0.94 ± 0.14 |
| Cpt1β | 1 ± 0.11 | 1.39 ± 0.33 | 1.19 ± 0.08 | 1.17 ± 0.13 |
| Srebf1 | 1 ± 0.10 | 1.93 ± 0.38* | 1.18 ± 0.12 | 1.40 ± 0.29* |
| Acc1 | 1 ± 0.09 | 1.78 ± 0.33 | 1.36 ± 0.17 | 1.43 ± 0.15 |
| Fasn | 1 ± 0.25 | 2.1 ± 0.17* | 0.99 ± 0.10† | 1.01 ± 0.21† |
| Scd1 | 1 ± 0.28 | 1.29 ± 0.25 | 0.86 ± 0.16 | 0.50 ± 0.12 |
| Fgf21 | ND | ND | ND | ND |
Values are means ± SE normalized to Hprt (liver) and Rp18s (extensor digitorum longus, EDL). Quantitative PCR analysis of liver and EDL of exercised (EX) and sedentary (SED) wild-type (Wt) and fibroblast growth factor 21-deficient (Fgf21−/−) mice. Real-time PCR analysis of liver and skeletal muscle: Wt (EX, n = 4; SED, n = 4), Fgf21−/− (EX, n = 7; SED, n = 10).
P < 0.05 compared with sedentary control.
P < 0.05 compared with Wt control. ND, not detected.
Scd1 was significantly decreased by exercise in Wt mice compared their sedentary littermates and compared with Fgf21−/− mice (Table 1). In contrast, exercise had no impact on Scd1 in Fgf21−/− mice (Table 1). Exercise did not significantly increase Cpt1α or Pparα mRNA levels in Wt or Fgf21−/− littermates (Table 1). Whereas exercise did not change Srebf1, Fasn, and Acc mRNA in Wt mice (Table 1), exercise significantly increased Srebf1, but not Fasn or Acc, expression in Fgf21−/− mice (Table 1). Additionally, Fasn mRNA levels were reduced in Fgf21−/− mice compared with sedentary Wt littermates (Table 1). Finally, exercise did not alter Hmgcr expression in either genotype (Table 1), but, consistent with the reduced hepatic cholesterol levels, there was a significant reduction Hmgcr in Fgf21−/− mice compared with sedentary Wt (Tables 2 and 3).
Table 3.
Plasma and liver values (final)
|
Wt |
Fgf21−/− |
|||
|---|---|---|---|---|
| Sedentary | Exercised | Sedentary | Exercised | |
| Plasma glucose, mg/dl | 136.5 ± 0.97 | 131.0 ± 0.56 | 147.0 ± 0.45 | 129.1 ± 0.35 |
| Plasma insulin, ng/ml | 0.35 ± 0.09 | 0.23 ± 0.07 | 0.24 ± 0.04§ | 0.19 ± 0.03§ |
| Plasma FGF21, ng/ml | 770.9 ± 207.1 | 426.1 ± 129.9 | 57.9 ± 3.8 | 61.4 ± 3.8 |
| Plasma triglycerides, mg/dl | 62.6 ± 1.5 | 57.4 ± 3.9 | 77.2 ± 5.7 | 66.2 ± 2.9 |
| Plasma cholesterol, mg/dl | 80.4 ± 5.2 | 51.5 ± 4.5† | 93.2 ± 3.2 | 71.8 ± 0.7‡ |
| Plasma free fatty acid, mEq/l | 0.18 ± 0.06 | 0.20 ± 0.05 | 0.38 ± 0.05 | 0.18 ± 0.02 |
| Liver lobe mass, mg | 569.9 ± 2.92 | 484.0 ± 1.32* | 429.7 ± 0.8 | 383.75 ± 1.13* |
| Liver triglycerides, mg/g liver | 71.9 ± 0.98 | 33.8 ± 0.50* | 55.15 ± 0.70 | 39.13 ± 0.40 |
| Liver cholesterol, mg/g liver | 53.23 ± 8.95 | 34.39 ± 5.54* | 26.9 ± 2.87† | 30.21 ± 1.76† |
Values are means ± SE. Plasma and liver analysis in EX and SED Wt and Fgf21−/− mice. Plasma analysis: Wt (EX, n = 4; SED, n = 4), Fgf21−/− (EX, n = 7; SED, n = 10).
P < 0.05,
P < 0.01,
P < 0.001 compared with sedentary control.
P < 0.05 compared with Wt control.
DISCUSSION
Herein, we present results supporting that FGF21 is not required for exercise prevention of diet-induced obesity but is necessary for exercise-mediated prevention of diet-induced glucose intolerance. Our evidence indicates that a physiological adaptation to chronic voluntary exercise involves FGF21-AMPK signaling in skeletal muscle, which may contribute to exercise regulation of glucose homeostasis. Furthermore, we also show that FGF21 action is necessary for exercise-induced reduction in hepatic TG content. These findings implicate that FGF21 action is essential to reap some of the gluco- and lipo-regulatory rewards of exercise and that these effects are likely independent of changes in body weight or may represent the metabolic benefits observed before weight loss in therapies with an exercise component.
FGF21 in the response to acute and chronic exercise.
Reports in rodents and human subjects suggest that acute (29) and chronic (4, 9) exercise increases FGF21. In healthy human subjects, plasma FGF21 increases from ∼180 ng/l at rest to ∼300 ng/l after exercise (21). Interestingly, FGF21 levels are also elevated in the elderly (54) and in obesity (>250 ng/l) (60); however, these likely represent FGF21-resistant states. Consistent with this hypothesis, elderly subjects who participated in a 5-week endurance exercise program were characterized by a reduction in circulating FGF21 (54).
FGF21 secretion can be blocked via somatostatin treatment, suggesting that it is downstream of glucagon, insulin, or growth hormone signaling (22). Consistent with this observation, evidence published during the drafting of this manuscript identified the splanchnic insulin:glucagon ratio as a regulator of circulating FGF21 in human subjects (21). Importantly this system is a critical determinant of hepatic FGF21 production in response to exercise (21). In agreement with this work, we found that FGF21 increases after a single bout of exhaustive exercise. In contrast, voluntary running in conjunction with HF feeding elicited a reduction in hepatic mRNA levels of Fgf21 and FGF21 plasma levels. Our findings agree with prior evidence presented by Fletcher et al. (14), illustrating that chronic voluntary running wheel exercise of obese rodents significantly reduces hepatic Fgf21 mRNA and protein levels as well as plasma FGF21 compared with sedentary controls. This reduction in circulating FGF21 following endurance exercise training was also recently reported in human subjects (54). However, these results are in contrast with those of Berglund et al. (4), who showed that hepatic Fgf21 mRNA is elevated in diet-induced obese rodents following 10 wk of running wheel activity. We speculate that our results may differ from those of Berglund et al. for several reasons. First, in their study, mice were given access to HFD for 6 wk before starting the exercise program, whereas our mice were provided concomitant access to HFD and exercise. Second, the time of tissue and plasma harvest could have played a role in our discordant results. Our samples were harvested from mice that were removed from running wheels and fasted for 5 h before euthanasia (to maintain continuity with the fasting period utilized before glucose metabolism in vivo experiments), which is consistent with the killing protocol utilized by Fletcher et al (13). Moreover, the reduced hepatic Fgf21 mRNA expression following chronic exercise in mice on HFD may be the result of increased FGF21 sensitivity. A well-appreciated phenomenon associated with dietary obesity is an elevation in FGF21 expression and secretion and the development of FGF21 resistance (13). Much like the observed reduction of insulin in states of increased insulin sensitivity, a reduction in hepatic Fgf21 expression with exercise in our study may reflect increased FGF21 sensitivity. Future investigations will need to examine a putative enhancement in FGF21 signaling following exercise in HFD-fed mice, as well as dissect the function of FGF21 during acute and chronic exercise.
Secretion of glucagon and FGF21 in response to acute exhaustive exercise.
Previous work has indicated that a significant interaction exists between glucagon and FGF21 (19); however, it is unclear whether this interaction occurs in the physiological context of exercise. To investigate this, we subjected rats to an acute bout of exhaustive treadmill exercise. Rats were chosen for this acute exercise study to allow for rapid and adequate tail vein blood collection and paired statistical analysis.
We showed that, in response to acute exhaustive exercise, there is a concomitant increase in plasma glucagon and FGF21, which supports a role for both hormones in the exercise response. Importantly, in the context of our previous work whereby FGF21 appears to mediate specific glucagon effects (19), the results of the present study suggest that glucagon action increases plasma FGF21 during exercise. This hypothesis is supported by evidence from Berglund et al. (4) showing that loss of glucagon receptor (Gcgr−/−) action prevents an exercise-induced increase in hepatic Fgf21 expression. Our observation that FGF21 secretion is increased in response to acute exercise suggests that FGF21 may have an important role in the early responses to exercise. Furthermore, we also observed a sustained elevation of plasma FGF21 following the exhaustive exercise bout, suggesting that FGF21 may be involved the metabolic changes associated with the recovery phase of exercise. Overall, FGF21 action in the exercise response may be broad, encompassing short- and long-term effects on metabolism as described in the following sections.
Physiological adaptations to exercise training in Wt and Fgf21−/− mice: Energy balance.
A major aim of this study was to determine the requirement of FGF21 in exercise-induced regulation of body and fat mass. We found that FGF21 is not necessary for development of diet-induced obesity, as Wt and Fgf21−/− littermates similarly gained weight and fat mass under sedentary conditions. Furthermore, FGF21 is not necessary for exercise-mediated prevention of diet-induced obesity during HF feeding because exercise was effective in reducing body and fat mass accrual independent of genotype. These results might be surprising in light of evidence illustrating that exogenous FGF21 alone causes a reduction in body mass and adiposity in diabetic and obese rodent models (8, 58). However, it is important to note that the metabolic effects of FGF21 at physiological levels (such as during exercise) and at pharmacological doses appear to be quite different (15).
We also analyzed running wheel behavior to determine whether FGF21 is involved in volitional running during HF feeding. Although running velocity and distance were similar between Wt and Fgf21−/− mice, we did observe that Fgf21−/− mice had reduced running wheel activity during the light phase. In response to recently published evidence illustrating that central FGF21 action regulates circadian running wheel behavior (6), it is possible that our observation might be attributable to the loss of FGF21 signaling in the central nervous system. However, in contrast to the findings by Bookout and colleagues (6), we did not see an increase in running wheel behavior when FGF21 action was lost. We propose that this may be due to differences in experimental design. Specifically, Bookout et al. (6) utilized a transgenic Fgf21 overexpression mouse model and mouse models with tissue-specific knockout of the obligatory FGF21 receptor β-Klotho, whereas our study was completed using a mouse model globally deficient for the FGF21 ligand. Moreover, our study was not specifically designed to assess circadian biology.
Lipid metabolism.
This study also aimed to determine whether FGF21 contributes to exercise regulation of lipid metabolism attributable to the role of FGF21 in regulation of lipid homeostasis. We observed similar exercise-induced prevention of fat mass gain in Wt and Fgf21−/− littermates. However, suppression of ectopic TG accumulation in the liver and hepatic mRNA expression of Scd1 [a lipogenic gene associated with hepatic diet-induced insulin resistance (18) and known to be beneficially reduced in expression with exercise (34)] in response to exercise was diminished in Fgf21−/− mice. Furthermore, we did not observe an exercise-induced reduction in hepatic cholesterol content or mRNA expression of lipogenic genes Fasn and Hmgcr in Fgf21−/− mice, which lends further support to the notion that exercise requires FGF21 action to beneficially regulate components of liver lipid metabolism.
Because FGF21 deficiency caused a reduction in parameters of hepatic lipid metabolism, it is possible that the genotype effect (possibly as a result of congenital deletion and lifelong lack of FGF21 action) is masking a similar exercise-mediated impact on lipid homeostasis in Fgf21−/− mice. Alternatively, another possibility is that FGF21 is involved in the exercise-induced benefits on hepatic lipid metabolism via activation of FGFR-β-Klotho complexes expressed in tissues other than the liver, such as expressed by adipocytes (59). Furthermore, in light of recent evidence that FGFR-β-Klotho is expressed in specific hypothalamic and brainstem nuclei (6), it is also conceivable that endocrine FGF21 signaling in the CNS regulates hepatic lipid metabolism during exercise.
Glucose metabolism.
On the basis of the similar effects of exercise (26, 47) and FGF21 (27, 58) on glucose homeostasis, we postulated that exercise may enhance glucose metabolism via FGF21 action. Intriguingly, our studies elucidated a considerable disparity in the benefits of exercise on glucose tolerance in Wt and Fgf21−/− mice during HF feeding.
Importantly, this disparity in exercise effects on glucose tolerance exists despite similar running wheel activity, fat mass, insulin action, and expression of gluco-regulatory gene expression in liver (Glut2, Cpt1α, Pck1) and skeletal muscle (Glut1, Glut4, Ampkα2, Cpt1β, Gsk3β) in Wt mice and Fgf21−/− littermates. We observed a reduction in fasting insulin levels in Fgf21−/− mice during chronic HF feeding, which might indicate that islet β-cell function is impaired in these mice. Reduced β-cell function is a plausible explanation considering findings by Wente et al. (56) illustrating that FGF21 enhances β-cell survival and function (56). Impaired insulin secretion in Fgf21−/− mice may contribute to enhanced insulin sensitivity (41) and thereby overshadow a difference in the beneficial impact of exercise on insulin action in Fgf21−/− mice compared with Wt littermates. Moreover, these effects may be mediated in part via a change in the well-known incretin response, specifically through glucagon-like peptide 1 (GLP1), which is elevated during exercise (36). However, it should be noted that intraperitoneal glucose challenge does not stimulate the incretin response, and thus GLP1 action is likely not responsible for the genotypic differences in intraperitoneal glucose tolerance in these mice (45). Therefore, the possibility that FGF21 is positively affecting peripheral tissue insulin signaling (and thus systemic insulin action) in response to exercise warrants further investigation.
Additionally, FGF21 may regulate glucose homeostasis in exercise via mechanisms that do not directly involve insulin action. AMPK is recognized for its central role in maintaining cellular energy during exercise (2, 24, 43, 44), including stimulation of insulin-independent glucose uptake (24). Therefore, we analyzed AMPK activity in liver and muscle in sedentary and exercised Wt and Fgf21−/− littermates to identify a putative role of FGF21 in regulation of AMPK in the exercise response. Analysis of posttranslational AMPK phosphorylation of residue Threonine-172 (23) indicated that exercise did not increase AMPK activation in the liver of Wt or Fgf21−/− littermates. This was somewhat surprising in the context of previous work (4, 52). However, our experimental design was unique from these published studies as outlined above. Thus we surmise that the regulation of hepatic AMPK described by Berglund et al. (4) is likely present in our mice, but its detection is simply masked by the differences in experimentation.
However, when we analyzed skeletal muscle AMPK activation responses to exercise in Wt and Fgf21−/− littermates, we observed a profound AMPK response to exercise in Wt mice, increasing 12-fold. Importantly, we observed a much smaller (2-fold) increase in skeletal muscle AMPK activation in Fgf21−/− mice. We propose that, on the basis of these findings, FGF21-mediated activation of skeletal muscle AMPK is a potential mechanism by which exercise modulates glucose homeostasis and that loss of FGF21 signaling prevents enhanced glucose uptake in muscle in response to exercise. This effect may contribute to the failure of exercise to prevent diet-induced glucose intolerance in Fgf21−/− mice.
FGF21 stimulation of AMPK activity has previously been reported in adipocytes (7); however, this is, to the best of our knowledge, the first evidence of an FGF21-AMPK signaling axis in skeletal muscle. Importantly, activation of AMPK in the contracting skeletal muscle stimulates a profound enhancement of muscle glucose metabolism (38), ultimately enhancing whole body glucose and lipid homeostasis (53). Thus our evidence that FGF21 action is upstream of AMPK activity in skeletal muscle following exercise may suggest that this axis may contribute to insulin-independent glucose metabolism.
In addition to enhancing glucose uptake during exercise, AMPK stimulation also increases myofibrillar oxidative capacity by stimulating mitochondrial biogenesis (1) and a shift toward greater abundance of oxidative muscle fibers (50). An important consequence of increased oxidative capacity is an elevation in intramuscular energy storage, including TG synthesis (17). Our results indicating a greater increase in expression of lipogenic genes associated with intramuscular lipid storage in Wt mice following exercise training compared with Fgf21−/− littermates support that FGF21 action contributes to beneficial adaptations in skeletal muscle in response to exercise.
The present study did not specifically investigate the location of an FGF21-AMPK signaling axis. Future studies will need to aim to address tissue-specific signaling of FGF21 in the responses to exercise. However, historical evidence can provide some insight. It is possible that FGF21 stimulation of skeletal muscle AMPK may result from FGFR1-β-Klotho complex activation within the skeletal muscle (49) or via this interaction within the CNS (6). Insight into this regulation can be taken from recent work by Mashili et al. (37), showing that exposure of isolated mouse EDL to FGF21 enhances insulin-stimulated glucose uptake in the isolated muscle. However, this uptake was independent of AMPK activation (37), which may suggest a neuroendocrine mode of regulation in our system. These data, in conjunction with our findings, may also suggest that other unknown humoral factors may be mediating the activation of AMPK in skeletal muscle.
In summary, although exercise does not require FGF21 action to prevent weight gain and dyslipidemia, we report a mechanism linking two key antiobesigenic and antidiabetic targets, FGF21 and AMPK, to the beneficial effects of exercise on glucose metabolism. Our discovery sheds new light on the mechanisms regulating the response to exercise, including a divergent regulation of energy balance and glucose homeostasis.
GRANTS
This work is supported by NIH DRC P30 DK079626 and DK098319 (K. Habegger), American Diabetes Association Junior Faculty Grant 1-13-JF-21 (K. Habegger), and University of Alabama at Birmingham Center for Exercise Medicine no. 1T32 HD071866 (C. Loyd).
DISCLOSURES
D. Sandoval has research support from Ethicon Endo-Surgery, Novo Nordisk, and Boerhinger Ingelheim. No other conflicts, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
CL and KMH were responsible for study conception and design, data analyses and interpretation, and drafting the article; CL, IJM, MH, RK, and SB generated experimental data; NI, DS, SO, and DP-T advised study concept and critical revision of the article. KMH is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Adhihetty PJ, Irrcher I, Joseph AM, Ljubicic V, Hood DA. Plasticity of skeletal muscle mitochondria in response to contractile activity. Exp Physiol 88: 99–107, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Bergeron R, Russell RR 3rd, Young LH, Ren JM, Marcucci M, Lee A, Shulman GI. Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am J Physiol Endocrinol Metab 276: E938–E944, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Berglund ED, Kang L, Lee-Young RS, Hasenour CM, Lustig DG, Lynes SE, Donahue EP, Swift LL, Charron MJ, Wasserman DH. Glucagon and lipid interactions in the regulation of hepatic AMPK signaling and expression of PPARα and FGF21 transcripts in vivo. Am J Physiol Endocrinol Metab 299: E607–E614, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Berglund ED, Lustig DG, Baheza RA, Hasenour CM, Lee-Young RS, Donahue EP, Lynes SE, Swift LL, Charron MJ, Damon BM, Wasserman DH. Hepatic glucagon action is essential for exercise-induced reversal of mouse fatty liver. Diabetes 60: 2720–2729, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 23: 57–63, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, Kliewer SA. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med 19: 1147–1152, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc Natl Acad Sci USA 107: 12553–12558, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149: 6018–6027, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Cuevas-Ramos D, Almeda-Valdes P, Meza-Arana CE, Brito-Cordova G, Gomez-Perez FJ, Mehta R, Oseguera-Moguel J, Aguilar-Salinas CA. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS One 7: e38022, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dengel DR, Hagberg JM, Pratley RE, Rogus EM, Goldberg AP. Improvements in blood pressure, glucose metabolism, and lipoprotein lipids after aerobic exercise plus weight loss in obese, hypertensive middle-aged men. Metabolism 47: 1075–1082, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK; American College of Sports Medicine. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 41: 459–471, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Finucane FM, Sharp SJ, Purslow LR, Horton K, Horton J, Savage DB, Brage S, Besson H, De Lucia Rolfe E, Sleigh A, Martin HJ, Aihie Sayer A, Cooper C, Ekelund U, Griffin SJ, Wareham NJ. The effects of aerobic exercise on metabolic risk, insulin sensitivity and intrahepatic lipid in healthy older people from the Hertfordshire Cohort Study: A randomised controlled trial. Diabetologia 53: 624–631, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, Maratos-Flier E. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 59: 2781–2789, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher JA, Meers GM, Laughlin MH, Ibdah JA, Thyfault JP, Rector RS. Modulating fibroblast growth factor 21 in hyperphagic OLETF rats with daily exercise and caloric restriction. Appl Physiol Nutr Metab 37: 1054–1062, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gimeno RE, Moller DE. FGF21-based pharmacotherapy–potential utility for metabolic disorders. Trends Endocrinol Metab 25: 303–311, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg L, Elliot DL. The effect of exercise on lipid metabolism in men and women. Sports Med 4: 307–321, 1987. [DOI] [PubMed] [Google Scholar]
- 17.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: Evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86: 5755–5761, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez-Juarez R, Pocai A, Mulas C, Ono H, Bhanot S, Monia BP, Rossetti L. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J Clin Invest 116: 1686–1695, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habegger KM, Stemmer K, Cheng C, Muller TD, Heppner KM, Ottaway N, Holland J, Hembree JL, Smiley D, Gelfanov V, Krishna R, Arafat AM, Konkar A, Belli S, Kapps M, Woods SC, Hofmann SM, D'Alessio D, Pfluger PT, Perez-Tilve D, Seeley RJ, Konishi M, Itoh N, Kharitonenkov A, Spranger J, DiMarchi RD, Tschop MH. Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes 62: 1453–1463, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habegger KM, Stemmer K, Cheng C, Muller TD, Heppner KM, Ottaway N, Holland J, Hembree JL, Smiley D, Gelfanov V, Krishna R, Arafat AM, Konkar A, Belli S, Kapps M, Woods SC, Hofmann SM, D'Alessio D, Pfluger PT, Perez-Tilve D, Seeley RJ, Konishi M, Itoh N, Kharitonenkov A, Spranger J, Dimarchi RD, Tschop MH. Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes 62: 1453–1463, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen JS, Clemmesen JO, Secher NH, Hoene M, Drescher A, Weigert C, Pedersen BK, Plomgaard P. Glucagon-to-insulin ratio is pivotal for splanchnic regulation of FGF-21 in humans. Mol Metab 4: 551–560, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen JS, Pedersen BK, Xu G, Lehmann R, Weigert C, Plomgaard P. Exercise-induced secretion of FGF21 and follistatin are blocked by pancreatic clamp and impaired in type 2 diabetes. J Clin Endocrinol Metab 101: 2816–2825, 2016. [DOI] [PubMed] [Google Scholar]
- 23.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 271: 27879–27887, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 47: 1369–1373, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Hotta Y, Nakamura H, Konishi M, Murata Y, Takagi H, Matsumura S, Inoue K, Fushiki T, Itoh N. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology 150: 4625–4633, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Joslin ER, White HF, Marble P. The Treatment of Diabetes Mellitus. Boston, MA: Joslin Diabetes Center, 1917. [Google Scholar]
- 27.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest 115: 1627–1635, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148: 774–781, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Kim KH, Kim SH, Min YK, Yang HM, Lee JB, Lee MS. Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS One 8: e63517, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirchner H, Hofmann SM, Fischer-Rosinsky A, Hembree J, Abplanalp W, Ottaway N, Donelan E, Krishna R, Woods SC, Muller TD, Spranger J, Perez-Tilve D, Pfluger PT, Tschop MH, Habegger KM. Caloric restriction chronically impairs metabolic programming in mice. Diabetes 61: 2734–2742, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krawczewski Carhuatanta KA, Demuro G, Tschop MH, Pfluger PT, Benoit SC, Obici S. Voluntary exercise improves high-fat diet-induced leptin resistance independent of adiposity. Endocrinology 152: 2655–2664, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishna MG, Coker RH, Lacy DB, Zinker BA, Halseth AE, Wasserman DH. Glucagon response to exercise is critical for accelerated hepatic glutamine metabolism and nitrogen disposal. Am J Physiol Endocrinol Metab 279: E638–E645, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Leclair E, Liggins RT, Peckett AJ, Teich T, Coy DH, Vranic M, Riddell MC. Glucagon responses to exercise-induced hypoglycaemia are improved by somatostatin receptor type 2 antagonism in a rat model of diabetes. Diabetologia 59: 1724–1731, 2016. [DOI] [PubMed] [Google Scholar]
- 34.Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: Role of stearoyl-CoA desaturase. J Biol Chem 284: 5637–5644, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manders RJ, Van Dijk JW, van Loon LJ. Low-intensity exercise reduces the prevalence of hyperglycemia in type 2 diabetes. Med Sci Sports Exerc 42: 219–225, 2010. [DOI] [PubMed] [Google Scholar]
- 36.Martins C, Morgan LM, Bloom SR, Robertson MD. Effects of exercise on gut peptides, energy intake and appetite. J Endocrinol 193: 251–258, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Mashili FL, Austin RL, Deshmukh AS, Fritz T, Caidahl K, Bergdahl K, Zierath JR, Chibalin AV, Moller DE, Kharitonenkov A, Krook A. Direct effects of FGF21 on glucose uptake in human skeletal muscle: Implications for type 2 diabetes and obesity. Diabetes Metab Res Rev 27: 286–297, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol Endocrinol Metab 273: E1107–E1112, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol Endocrinol Metab 254: E248–E259, 1988. [DOI] [PubMed] [Google Scholar]
- 40.Miller WC, Koceja DM, Hamilton EJ. A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes Relat Metab Disord 21: 941–947, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Minami A, Iseki M, Kishi K, Wang M, Ogura M, Furukawa N, Hayashi S, Yamada M, Obata T, Takeshita Y, Nakaya Y, Bando Y, Izumi K, Moodie SA, Kajiura F, Matsumoto M, Takatsu K, Takaki S, Ebina Y. Increased insulin sensitivity and hypoinsulinemia in APS knockout mice. Diabetes 52: 2657–2665, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Nadeau KJ, Ehlers LB, Aguirre LE, Moore RL, Jew KN, Ortmeyer HK, Hansen BC, Reusch JE, Draznin B. Exercise training and calorie restriction increase SREBP-1 expression and intramuscular triglyceride in skeletal muscle. Am J Physiol Endocrinol Metab 291: E90–E98, 2006. [DOI] [PubMed] [Google Scholar]
- 43.O'Neill HM, Holloway GP, Steinberg GR. AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: Implications for obesity. Mol Cell Endocrinol 366: 135–151, 2013. [DOI] [PubMed] [Google Scholar]
- 44.O'Neill HM, Maarbjerg SJ, Crane JD, Jeppesen J, Jorgensen SB, Schertzer JD, Shyroka O, Kiens B, van Denderen BJ, Tarnopolsky MA, Kemp BE, Richter EA, Steinberg GR. AMP-activated protein kinase (AMPK) beta1beta2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc Natl Acad Sci USA 108: 16092–16097, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh TJ. In vivo models for incretin research: From the intestine to the whole body. Endocrinol Metab (Seoul) 31: 45–51, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olsen GN, Weiman DS. Assessment of exercise oxygen consumption. Ann Thorac Surg 46: 483–484, 1988. [DOI] [PubMed] [Google Scholar]
- 47.Pruett ED, Oseid S. Effect of exercise on glucose and insulin response to glucose infusion. Scand J Clin Lab Invest 26: 277–285, 1970. [DOI] [PubMed] [Google Scholar]
- 48.Richter EA, Mikines KJ, Galbo H, Kiens B. Effect of exercise on insulin action in human skeletal muscle. J Appl Physiol 66: 876–885, 1989. [DOI] [PubMed] [Google Scholar]
- 49.Riuzzi F, Sorci G, Beccafico S, Donato R. S100B engages RAGE or bFGF/FGFR1 in myoblasts depending on its own concentration and myoblast density. Implications for muscle regeneration. PLoS One 7: e28700, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rockl KS, Hirshman MF, Brandauer J, Fujii N, Witters LA, Goodyear LJ. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes 56: 2062–2069, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Rogowski MP, Flowers MT, Stamatikos AD, Ntambi JM, Paton CM. SCD1 activity in muscle increases triglyceride PUFA content, exercise capacity, and PPARdelta expression in mice. J Lipid Res 54: 2636–2646, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruderman NB, Park H, Kaushik VK, Dean D, Constant S, Prentki M, Saha AK. AMPK as a metabolic switch in rat muscle, liver and adipose tissue after exercise. Acta Physiol Scand 178: 435–442, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414: 799–806, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Taniguchi H, Tanisawa K, Sun X, Kubo T, Higuchi M. Endurance exercise reduces hepatic fat content and serum fibroblast growth factor 21 levels in elderly men. J Clin Endocrinol Metab 101: 191–198, 2016. [DOI] [PubMed] [Google Scholar]
- 55.Wasserman DH, Lickley HL, Vranic M. Important role of glucagon during exercise in diabetic dogs. J Appl Physiol 59: 1272–1281, 1985. [DOI] [PubMed] [Google Scholar]
- 56.Wente W, Efanov AM, Brenner M, Kharitonenkov A, Koster A, Sandusky GE, Sewing S, Treinies I, Zitzer H, Gromada J. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes 55: 2470–2478, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Wing RR. Physical activity in the treatment of the adulthood overweight and obesity: Current evidence and research issues. Med Sci Sports Exerc 31: S547–S552, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Veniant MM. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58: 250–259, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang C, Wang C, Ye M, Jin C, He W, Wang F, McKeehan WL, Luo Y. Control of lipid metabolism by adipocyte FGFR1-mediated adipohepatic communication during hepatic stress. Nutr Metab (Lond) 9: 94, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, Wong RL, Chow WS, Tso AW, Lam KS, Xu A. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 57: 1246–1253, 2008. [DOI] [PubMed] [Google Scholar]
- 61.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 414: 782–787, 2001. [DOI] [PubMed] [Google Scholar]