Chronic heart failure (CHF) impairs skeletal muscle vascular control and tolerance to exercise. Dietary nitrate supplementation elevates skeletal muscle blood flow and improves exercise performance in some populations. Here we demonstrate that nitrate supplementation via beetroot juice elevates skeletal muscle blood flow during exercise in rats with moderate CHF.
Keywords: blood flow, myocardial infarction, left ventricular end-diastolic pressure
Abstract
Chronic heart failure (CHF) results in central and peripheral derangements that ultimately reduce skeletal muscle O2 delivery and impair exercise tolerance. Dietary nitrate (NO3−) supplementation improves skeletal muscle vascular function and tolerance to exercise. We tested the hypothesis that NO3− supplementation would elevate exercising skeletal muscle blood flow (BF) and vascular conductance (VC) in CHF rats. Myocardial infarction (MI) was induced (coronary artery ligation) in young adult male rats. After 21 days of recovery, rats randomly received 5 days of NO3−-rich beetroot juice (CHF + BR, n = 10) or a placebo (CHF, n = 10). Mean arterial pressure (carotid artery catheter) and skeletal muscle BF (radiolabeled microspheres) were measured during treadmill exercise (20 m/min, 5% grade). CHF-induced dysfunction, as determined by myocardial infarction size (29 ± 3% and 33 ± 4% in CHF and CHF + BR, respectively) and left ventricular end-diastolic pressure (18 ± 2 and 18 ± 2 mmHg in CHF and CHF + BR, respectively), and exercising mean arterial pressure (131 ± 3 and 128 ± 4 mmHg in CHF and CHF + BR, respectively) were not different (P > 0.05) between groups. Total exercising hindlimb skeletal muscle BF (95 ± 5 and 116 ± 9 ml·min−1·100 g−1 in CHF and CHF + BR, respectively) and VC (0.75 ± 0.05 and 0.90 ± 0.05 ml·min−1·100 g−1·mmHg−1 in CHF and CHF + BR, respectively) were 22% and 20% greater in BR-supplemented rats, respectively (P < 0.05). During exercise, BF in 9 and VC in 10 hindlimb muscles and muscle portions were significantly greater in the CHF + BR group. These results provide strong evidence that dietary NO3− supplementation improves skeletal muscle vascular function during exercise in rats with CHF and, thus, support the use of BR as a novel therapeutic modality for the treatment of CHF.
NEW & NOTEWORTHY
Chronic heart failure (CHF) impairs skeletal muscle vascular control and tolerance to exercise. Dietary nitrate supplementation elevates skeletal muscle blood flow and improves exercise performance in some populations. Here we demonstrate that nitrate supplementation via beetroot juice elevates skeletal muscle blood flow during exercise in rats with moderate CHF.
chronic heart failure (CHF) results in severe exercise intolerance, consequent to the impaired pumping capacity of the heart and reduced skeletal muscle vascular and metabolic function. At the onset of exercise, an increase in O2 delivery (Q̇o2) is mandated to meet the rapidly rising O2 demand (V̇o2) of the skeletal muscle. This is accomplished by a complex array of central cardiac and local humoral components that ultimately become dysfunctional in CHF (reviewed in Ref. 54). Of the local humoral components, nitric oxide (NO), a primary controller of skeletal muscle blood flow (BF), is greatly diminished in CHF due, in part, to impaired NO synthase (NOS) function (12, 31). Thus the ability to elevate Q̇o2 relative to V̇o2 is severely compromised, increasing the reliance on glycolytic metabolism, which ultimately contributes to the premature fatigue evident in CHF.
Although once believed to be inert end products of NO metabolism, it is now well understood that nitrate (NO3−) and nitrite (NO2−) can be recycled to produce NO via a step-wise, NOS-independent mechanism, whereby NO3− is reduced to NO2− and, finally, to NO. Crucially, the reduction of NO2− to NO is facilitated in the low-Po2/pH environments common in systemic diseases, making the NO3−-NO2−-NO pathway a prime candidate for a therapy aimed at pathologically induced local and systemic hypoxia (reviewed in Refs. 45 and 46). Our laboratory recently showed that, in healthy rats, direct infusion of NO2− reduces mean arterial pressure (MAP) and improves skeletal muscle vascular control during exercise in the face of NOS blockade via NG-nitro-l-arginine methyl ester (l-NAME) (17). l-NAME presents the most extreme model of NOS inhibition and reduced NOS-related NO bioavailability. Additionally, other investigations utilizing murine models have revealed that NO2− reverses aging-induced vascular endothelial dysfunction (56) and renal injury in l-NAME-induced hypertension (34).
In humans, ingestion of NO3−-rich foodstuffs, such as beetroot juice (BR), also increases circulating NO2− concentration ([NO2−]), reduces the O2 cost of submaximal exercise (41, 42), and can result in improved tolerance to exercise in healthy (reviewed by Refs. 32 and 48) and patient (36, 61) populations. Of particular relevance, Coggan et al. (11) recently demonstrated that dietary NO3− supplementation via BR improves muscle contractile function in patients with heart failure. Further mechanistic insight gained from animal models reveals that BR increases exercising skeletal muscle BF and raises the Q̇o2-to-V̇o2 ratio [thereby increasing microvascular Po2 (Po2mv), the pressure head required for blood-myocyte O2 flux] within fast-twitch, but not slow-twitch, skeletal muscles (19-21). This phenomenon further substantiates the therapeutic use of dietary NO3−, especially when considering that phosphocreatine and glycogen depletion and, thus, fatigue are greater in fast- than slow-twitch muscles (25).
Given that CHF reduces BF primarily in slow-twitch (type I) muscles (53), which is expected to increase reliance on fast-twitch (type II) muscles during submaximal exercise and the lower fatigue resistance evident in type II than type I muscles (25), dietary NO3− may ameliorate the vascular dysfunction caused by CHF. Consequently, the purpose of the present investigation was to test the hypothesis that 5 days of dietary NO3− supplementation via BR would elevate skeletal muscle BF and vascular conductance (VC) during submaximal treadmill exercise in rats with moderate CHF. We anticipated that BR would elevate skeletal muscle BF and VC and that the elevations would occur preferentially in muscle with a high proportion of type IIb + IId/IIx fibers.
METHODS
Ethical approval.
Young (∼3-mo-old) adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were maintained at accredited animal facilities at Kansas State University on a 12:12-h light-dark cycle with food and water provided ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of Kansas State University and conducted according to the National Research Council Guide for the Care and Use of Laboratory Animals.
Myocardial infarction protocol and treadmill exercise acclimatization.
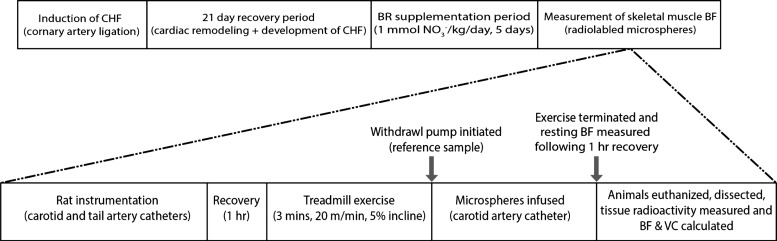
The overall experimental protocol is outlined in Fig. 1. Myocardial infarction (MI) was induced in rats (n = 20) by surgical ligation of the left main coronary artery (53). Briefly, rats were initially anesthetized with a 5% isoflurane-O2 mixture (Butler Animal Health Supply, Elk Grove Village, IL; Linweld, Dallas, TX) and maintained on an ∼2.5% isoflurane-O2 mixture and then intubated and mechanically ventilated with a rodent respirator (model 680, Harvard Instruments, Holliston, MA) for the duration of the surgical procedure. A left thoracotomy was performed to expose the heart through the fifth intercostal space, and the left main coronary artery was ligated 1–2 mm distal to the edge of the left atrium with a 6-0 braided polyester suture. The thorax was then closed with 2-0 gut and the skin with 3-0 silk. Bupivacaine (1.5 mg/kg sc), ampicillin (50 mg/kg im), and buprenorphine (∼0.03 mg/kg im) were administered to alleviate pain and reduce the risk of infection. After the rats were removed from mechanical ventilation and anesthesia, they were monitored closely for ≥6 h postsurgery. After ≥21 days of recovery for complete remodeling of necrotic myocardial tissue and development of compensated CHF (23, 53), all rats were familiarized with running on a custom-built, motor-driven treadmill for ∼5 min daily for 5 consecutive days. All rats ran at a speed of 20 m/min up a 5% incline (∼65% maximal V̇o2) (49).
Fig. 1.
Top: experimental protocol for rats with chronic heart failure (CHF) and rats with CHF treated with beetroot juice (CHF + BR). Rats were supplemented with NO3−-free (placebo) or NO3−-rich BR juice (1 mmol·kg−1·day−1) for 5 days. During the supplementation period, rats were familiarized with treadmill running for ∼5 min/day. BF, blood flow. Bottom: detailed timeline for measurement of skeletal muscle BF and vascular conductance (VC) during exercise in CHF and CHF + BR rats. A ∼0.3-ml blood sample was taken from the carotid artery catheter prior to termination of exercise, and resting BF was measured following ∼1 h of recovery from exercise prior to euthanasia (not shown).
BR supplementation.
Rats were assigned randomly to receive 5 days of NO3−-rich (NO3− dose = 1 mmol·kg−1·day−1; Beet it Sport, James White Drinks, Ipswich UK; CHF + BR, n = 10) or NO3−-depleted (Placebo Beet it Sport, James White Drinks; CHF, n = 10) BR. Daily doses of the placebo and NO3−-rich BR were diluted in 50 ml of tap water. This dose has been used by our laboratory and has been demonstrated to elicit significant physiological effects (18–21). In addition, this dose is also based on that used in humans by Jones et al. (3, 4, 57, 58, 60), accounting for the higher resting metabolic rate of the rat (∼7 times greater than humans) (49), and results in substantial elevations of blood plasma NO3− concentration ([NO3−]) and [NO2−] of ∼120 μM and ∼600 nM, respectively (20). We also ensured that the tap water did not provide any substantial [NO3−] (3.7 μM) or [NO2−] (∼100 nM).
Surgical instrumentation.
On the day of the experiment, rats were anesthetized with a 5% isoflurane-O2 mixture and maintained subsequently on ∼3% isoflurane-O2. The right carotid artery was isolated and cannulated for the advancement of a 2-Fr catheter-tipped pressure transducer (Millar Instruments, Houston, TX) into the left ventricle (LV) for measurement of systolic and diastolic pressures, LV end-diastolic pressure (LVEDP), and the rate of LV pressure rise over time (LV dP/dt). The 2-Fr pressure transducer was removed, and the artery was recannulated with a catheter (PE-10 connected to PE-50, Intra-Medic polyethylene tubing, Clay Adams, Becton, Dickinson, Sparks, MD) for the measurement of MAP and heart rate (HR) and the infusion of radiolabeled microspheres (model 200, DigiMed BPA, Louisville, KY; see below). A second catheter was placed in the caudal (tail) artery as described previously (53) for arterial blood sampling. Both catheters were tunneled subcutaneously through the dorsal aspect of the cervical region and exteriorized via a puncture wound in the skin. The incisions were then closed, anesthesia was terminated, and the rats were given ≥60 min to recover (24). After recovery, the rats were placed on the motor-driven treadmill, and the carotid artery catheter was connected to a pressure transducer (model P23ID, Gould Statham, Valley View, OH) maintained at the same height as the animal. Rats were allowed to stabilize for ∼15 min before the final experimental protocol was initiated while HR and MAP were monitored continuously using the carotid artery catheter.
Measurement of hindlimb skeletal muscle BF during exercise.
After the stabilization period, the caudal artery catheter was connected to a 1-ml syringe chambered in an infusion/withdrawal pump (model 907, Harvard Instruments). Exercise was then initiated up a 5% incline with speed progressing to 20 m/min within the first 30 s. The rat continued at this speed for another 2.5 min until a total time of 3 min was reached. During this time, radiolabeled microspheres (57Co or 85Sr in random order; Perkin Elmer, Waltham, MA) were thoroughly mixed by a vortex agitator (Fisher Scientific, Hampton, NH). At the 3-min exercise mark, the carotid artery catheter was disconnected from the pressure transducer, and 0.5–0.6 × 106 radiolabeled microspheres (15 μm diameter, ∼0.10 ml) were infused into the aortic arch. Simultaneously, the pump connected to the caudal artery catheter was activated and blood withdrawal was initiated at a rate of 0.25 ml/min. Blood withdrawal was terminated 30 s following the microsphere infusion, and ∼0.3 ml of blood was sampled from the carotid artery catheter for the determination of blood lactate concentration ([lactate]), pH, Pco2, Po2, %O2 saturation, and hematocrit (Nova Stat Profile M, Nova Biomedical, Waltham, MA). Exercise was then terminated.
After ≥30 min of recovery, a second microsphere infusion was performed (radiolabeled differently from the first microsphere infusion) while the rat sat quietly on the treadmill for the determination of resting BF, HR, and MAP. This experimental strategy, whereby exercise measurements were made before rest, mitigates the potential influence of the preexercise anticipatory response on resting skeletal muscle BF and MAP measurements (2).
Determination of BF and VC.
After the resting microsphere protocol had been completed, rats were euthanized via pentobarbital sodium overdose (≥50 mg/kg administered into the carotid artery catheter). The thorax of each rat was opened, and accurate placement of the carotid artery catheter was confirmed before the heart, lungs, select internal organs, and 28 individual muscles and muscle parts of the hindlimb were excised. Upon removal, tissues were blotted, weighed, and placed promptly into counting vials. The radioactivity of each tissue was determined with a gamma scintillation counter (model 5230, Auto Gamma Spectrometer, Packard, Downers Grove, IL). Tissue BF was then calculated using the reference sample method (53) and expressed as ml·min−1·100 g tissue−1. Adequate mixing of the microspheres was verified for each microsphere infusion as demonstrated by a <15% difference in BF to the right and left kidneys or to the right and left hindlimb musculature. VC was then calculated by normalizing BF to MAP measured at the time of microsphere infusion and expressed as ml·min−1·100 g tissue−1·mmHg−1. The LV, right ventricle (RV), and lungs were normalized to body weight, and MI size of the LV was measured via planimetry as described previously by our laboratory (22). The necrotic zone caused by ischemia was determined as the actual infarct size. Specifically, the LV was excised and opened from base to apex and flattened onto a wax sheet using pins. Under transillumination, the remodeled region appears white and distinct from the healthy cardiac muscle. Color photographs were taken, and the area infarcted as a percentage of the total (i.e., healthy tissue + infarcted) area was measured via planimetry by cutting out the respective sections and weighing the paper.
Blood sampling and measurement of plasma [NO3−] and [NO2−].
A blood sample was collected from CHF and CHF + BR rats for the measurement of plasma [NO3−] and [NO2−]. These samples were collected following instrumentation and prior to regional BF measurements. Approximately 0.8 ml of blood was drawn from the caudal artery catheter and quickly (i.e., <2 min) centrifuged at 5,000 g at 4°C for 6 min. Plasma was subsequently extracted and frozen at −80°C for later analysis of [NO3−] and [NO2−].
All measurements of plasma NO3− and NO2− were performed within 30 min of thawing via chemiluminescence with an Ionic/Sievers NO analyzer (NOA 280i, Sievers Instruments, Boulder, CO). To obtain plasma NO2− levels and avoid potential reduction of NO3−, potassium iodide in acetic acid was used as a reductant. This reductant possesses the ability to reduce NO2− to NO but is incapable of reducing higher oxides of nitrogen (i.e., NO3−), thus increasing the specificity for NO2−. Plasma NO3− concentrations were then obtained using the same apparatus with the stronger reductant vanadium chloride in hydrochloric acid at 95°C. This stronger reductant reduces the sum of all nitrogen oxides with an oxidation state of +2 or higher [predominantly NO3− (μM)] but also includes NO2− and nitrosothiols (nM).
Statistical analyses.
Indexes of CHF were compared using unpaired Student's t-tests. All other results were compared within (rest vs. exercise) and among (CHF vs. CHF + BR) groups using mixed two-way ANOVAs and Student-Newman-Keuls post hoc tests where appropriate. Pearson's product-moment correlations and linear regressions were used to determine relationships among variables. Muscle fiber type composition was based on the percentage of type I, IIa, IIb, and IId/IIx fibers in the individual muscles and muscle parts of the rat hindlimb according to Delp and Duan (15). Values are means ± SE; significance was set at P < 0.05.
RESULTS
Indexes of CHF.
The average body mass of the 20 CHF rats [n = 10 (CHF) and n = 10 (CHF + BR)] that completed the investigation was 483 ± 11 g, with no significant between-group differences (P > 0.05). There were also no between-group differences in MI size (29 ± 3% and 33 ± 4% of LV area for CHF and CHF + BR, respectively, P > 0.05), LVEDP (18 ± 2 and 18 ± 2 mmHg for CHF and CHF + BR, respectively, P > 0.05), LV dP/dt (6,311 ± 259 and 6,438 ± 436 mmHg/s for CHF and CHF + BR, respectively, P > 0.05), lung weight-to-body mass ratio (5.43 ± 1.55 and 5.35 ± 0.80 mg/g for CHF and CHF + BR, respectively, P > 0.05), or RV-to-body mass ratio (0.66 ± 0.03 and 0.75 ± 0.06 mg/g for CHF and CHF + BR, respectively, P > 0.05). All these values are consistent with moderate CHF (16).
Effect of BR on plasma [NO2−].
Plasma [NO2−] was significantly greater in CHF + BR than CHF rats (345 ± 59 vs. 569 ± 81 nM, P < 0.05).
Hemodynamic responses.
There were no significant between-group differences in MAP at rest (125 ± 5 and 118 ± 3 mmHg for CHF and CHF + BR, respectively, P > 0.05) or during exercise (131 ± 3 and 128 ± 4 mmHg for CHF and CHF + BR, respectively, P > 0.05). In addition, there were no significant between-group differences in HR at rest (409 ± 12 and 413 ± 9 beats/min for CHF and CHF + BR, respectively, P > 0.05) or during exercise (518 ± 10 and 507 ± 5 beats/min for CHF and CHF + BR, respectively, P > 0.05). No between-group differences in arterial Pco2, Po2, %O2 saturation, or hematocrit were present (P > 0.05 for all), and there were no between-group differences in exercising blood [lactate] (2.1 ± 0.2 and 2.1 ± 0.2 mM for CHF and CHF + BR, respectively, P > 0.05).
Impact of BR on skeletal muscle BF and VC at rest and during exercise.
There were no effects of BR on total resting hindlimb skeletal muscle BF or VC (P > 0.05; Fig. 2), nor were there any significant differences in resting BF or VC in any of the 28 hindlimb muscles or muscle portions (Table 1). However, during exercise, total hindlimb skeletal muscle BF and VC were significantly greater in CHF + BR than CHF rats (Fig. 2). Specifically, BR resulted in significantly greater BF in 9 and VC in 10 of the 28 individual hindlimb muscles or muscle parts (Table 2). Significant differences in BF were found in muscles comprised of 9–100% type IIb + IId/IIx fibers, whereas changes in VC were found in muscles comprised of 35–100% type IIb + IId/IIx fibers. There were no significant correlations between the BR-induced changes in BF and VC and muscle fiber type composition (P > 0.05 for both BF and VC); however, spatial distribution of BF increase among muscles was directed preferentially toward ankle and knee extensors (Fig. 3).
Fig. 2.
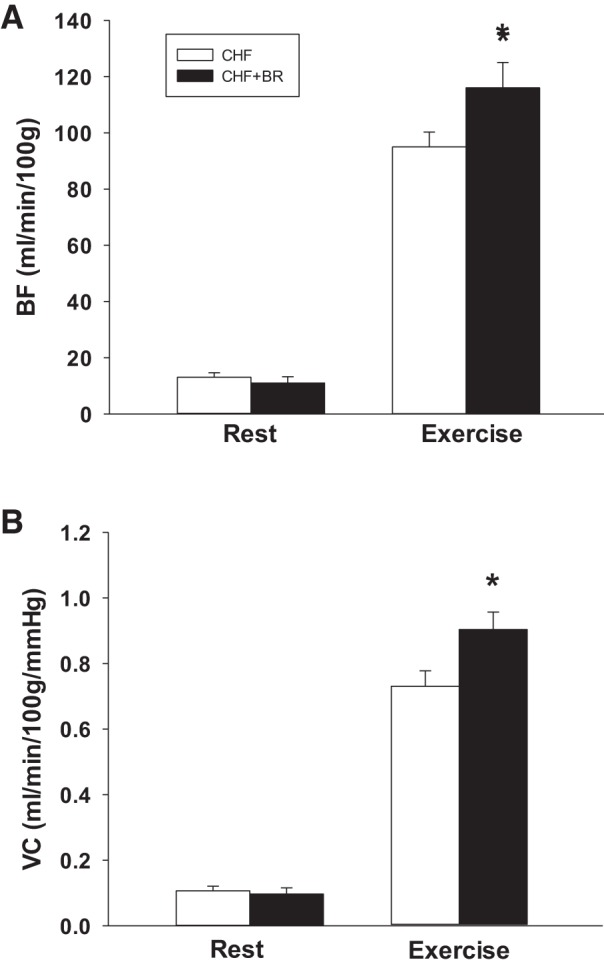
Effects of 5 days of dietary NO3− supplementation via BR on total hindlimb skeletal muscle blood flow (BF) and vascular conductance (VC) at rest and during treadmill exercise in rats with CHF. *P < 0.05.
Table 1.
Effects of BR supplementation on resting hindlimb skeletal muscle BF and VC in CHF and CHF + BR rats
| BF, ml·min−1·100 g−1 |
VC, ml·min−1·100 g−1·mmHg−1 |
|||
|---|---|---|---|---|
| CHF | CHF + BR | CHF | CHF + BR | |
| Ankle extensors | ||||
| Soleus (9%) | 102 ± 19 | 62 ± 17 | 0.85 ± 0.17 | 0.54 ± 0.16 |
| Plantaris (80%) | 9 ± 1 | 13 ± 1 | 0.07 ± 0.01 | 0.11 ± 0.04 |
| Gastrocnemius | ||||
| Red (14%) | 44 ± 10 | 32 ± 9 | 0.36 ± 0.08 | 0.27 ± 0.09 |
| White (100%) | 7 ± 1 | 7 ± 2 | 0.05 ± 0.01 | 0.06 ± 0.01 |
| Mixed (91%) | 13 ± 2 | 12 ± 3 | 0.10 ± 0.02 | 0.10 ± 0.03 |
| Tibialis posterior (73%) | 9 ± 1 | 20 ± 1 | 0.08 ± 0.01 | 0.17 ± 0.07 |
| Flexor digitorum longus (68%) | 13 ± 5 | 26 ± 10 | 0.11 ± 0.04 | 0.22 ± 0.08 |
| Flexor halicus longus (71%) | 9 ± 2 | 9 ± 2 | 0.07 ± 0.01 | 0.08 ± 0.02 |
| Ankle flexors | ||||
| Tibialis anterior | ||||
| Red (63%) | 15 ± 6 | 8 ± 1 | 0.12 ± 0.05 | 0.06 ± 0.01 |
| White (80%) | 13 ± 2 | 11 ± 3 | 0.10 ± 0.02 | 0.09 ± 0.02 |
| Extensor digitorum longus (76%) | 7 ± 3 | 12 ± 5 | 0.06 ± 0.02 | 0.10 ± 0.04 |
| Peroneals (67%) | 12 ± 3 | 9 ± 2 | 0.10 ± 0.02 | 0.07 ± 0.02 |
| Knee extensors | ||||
| Vastus intermedius (4%) | 67 ± 16 | 48 ± 12 | 0.57 ± 0.15 | 0.41 ± 0.10 |
| Vastus medialis (82%) | 15 ± 3 | 9 ± 3 | 0.13 ± 0.03 | 0.08 ± 0.02 |
| Vastus lateralis | ||||
| Red (35%) | 38 ± 10 | 31 ± 8 | 0.32 ± 0.09 | 0.26 ± 0.07 |
| White (100%) | 7 ± 1 | 8 ± 3 | 0.06 ± 0.01 | 0.06 ± 0.02 |
| Mixed (89%) | 10 ± 2 | 10 ± 3 | 0.08 ± 0.02 | 0.08 ± 0.02 |
| Rectus femoris | ||||
| Red (66%) | 16 ± 5 | 11 ± 2 | 0.14 ± 0.05 | 0.09 ± 0.02 |
| White (100%) | 8 ± 1 | 7 ± 2 | 0.06 ± 0.01 | 0.07 ± 0.02 |
| Knee flexors | ||||
| Biceps femoris | ||||
| Anterior (100%) | 6 ± 1 | 7 ± 2 | 0.05 ± 0.01 | 0.06 ± 0.02 |
| Posterior (92%) | 7 ± 1 | 10 ± 2 | 0.06 ± 0.01 | 0.08 ± 0.02 |
| Semitendinosus (83%) | 10 ± 2 | 11 ± 2 | 0.08 ± 0.01 | 0.09 ± 0.02 |
| Semimembranosus | ||||
| Red (72%) | 9 ± 1 | 13 ± 3 | 0.07 ± 0.01 | 0.11 ± 0.03 |
| White (100%) | 7 ± 1 | 8 ± 2 | 0.06 ± 0.08 | 0.07 ± 0.02 |
| Thigh adductors | ||||
| Adductor longus (5%) | 126 ± 14 | 109 ± 19 | 1.03 ± 0.13 | 0.93 ± 0.17 |
| Adductor magnus and brevis (89%) | 10 ± 1 | 9 ± 2 | 0.08 ± 0.01 | 0.07 ± 0.01 |
| Gracilis (77%) | 12 ± 2 | 26 ± 13 | 0.10 ± 0.02 | 0.23 ± 0.11 |
| Pectineus (69%) | 23 ± 4 | 21 ± 4 | 0.19 ± 0.04 | 0.18 ± 0.04 |
Values are means ± SE; n = 10 in each group. CHF, congestive heart failure; BF, blood flow; VC, vascular conductance. Values in parentheses indicate %type IIb + IId/IIx according to Delp and Duan (15). BR, beetroot juice.
Table 2.
Effects of BR supplementation on exercising hindlimb skeletal muscle BF and VC in CHF and CHF + BR rats
| BF, ml·min−1·100 g−1 |
VC, ml·min−1·100 g−1·mmHg−1 |
|||
|---|---|---|---|---|
| CHF | CHF + BR | CHF | CHF + BR | |
| Ankle extensors | ||||
| Soleus (9%) | 262 ± 36 | 337 ± 28* | 2.05 ± 0.32 | 2.62 ± 0.16 |
| Plantaris (80%) | 155 ± 11 | 204 ± 20* | 1.18 ± 0.08 | 1.58 ± 0.14* |
| Gastrocnemius | ||||
| Red (14%) | 442 ± 49 | 407 ± 60 | 3.45 ± 0.49 | 3.14 ± 0.40 |
| White (100%) | 28 ± 3 | 40 ± 6* | 0.21 ± 0.02 | 0.31 ± 0.05* |
| Mixed (91%) | 131 ± 9 | 156 ± 10* | 1.00 ± 0.07 | 1.21 ± 0.05* |
| Tibialis posterior (73%) | 97 ± 13 | 114 ± 17 | 0.75 ± 0.10 | 0.90 ± 0.13 |
| Flexor digitorum longus (68%) | 52 ± 9 | 61 ± 13 | 0.39 ± 0.06 | 1.24 ± 0.77 |
| Flexor halicus longus (71%) | 47 ± 4 | 80 ± 16* | 0.36 ± 0.03 | 0.62 ± 0.12* |
| Ankle flexors | ||||
| Tibialis anterior | ||||
| Red (63%) | 299 ± 22 | 319 ± 33 | 2.29 ± 0.18 | 2.50 ± 0.26 |
| White (80%) | 93 ± 11 | 102 ± 14 | 0.71 ± 0.07 | 0.79 ± 0.10 |
| Extensor digitorum longus (76%) | 61 ± 26 | 98 ± 44 | 0.45 ± 0.18 | 0.78 ± 0.35 |
| Peroneals (67%) | 120 ± 8 | 138 ± 24 | 0.92 ± 0.07 | 1.07 ± 0.18 |
| Knee extensors | ||||
| Vastus intermedius (4%) | 360 ± 52 | 415 ± 40 | 2.79 ± 0.42 | 3.22 ± 0.24 |
| Vastus medialis (82%) | 153 ± 13 | 170 ± 15 | 1.18 ± 0.12 | 1.30 ± 0.10 |
| Vastus lateralis | ||||
| Red (35%) | 340 ± 20 | 411 ± 45 | 2.62 ± 0.19 | 3.18 ± 0.27* |
| White (100%) | 18 ± 3 | 24 ± 5 | 0.14 ± 0.02 | 0.19 ± 0.04 |
| Mixed (89%) | 136 ± 9 | 160 ± 11* | 1.05 ± 0.10 | 1.24 ± 0.07* |
| Rectus femoris | ||||
| Red (66%) | 232 ± 18 | 279 ± 33 | 1.80 ± 0.17 | 2.16 ± 0.23 |
| White (100%) | 91 ± 5 | 108 ± 9* | 0.70 ± 0.04 | 0.85 ± 0.06* |
| Knee flexors | ||||
| Biceps femoris | ||||
| Anterior (100%) | 31 ± 5 | 50 ± 9* | 0.23 ± 0.04 | 0.39 ± 0.06* |
| Posterior (92%) | 77 ± 5 | 95 ± 8* | 0.59 ± 0.04 | 0.74 ± 0.06* |
| Semitendinosus (83%) | 43 ± 4 | 43 ± 7 | 0.33 ± 0.03 | 0.34 ± 0.05 |
| Semimembranosus | ||||
| Red (72%) | 107 ± 10 | 124 ± 9 | 0.82 ± 0.08 | 0.98 ± 0.07 |
| White (100%) | 24 ± 3 | 33 ± 5 | 0.18 ± 0.02 | 0.26 ± 0.04* |
| Thigh adductors | ||||
| Adductor longus (5%) | 264 ± 23 | 298 ± 48 | 2.03 ± 0.19 | 2.29 ± 0.33 |
| Adductor magnus & brevis (89%) | 61 ± 5 | 73 ± 11 | 0.46 ± 0.03 | 0.57 ± 0.09 |
| Gracilis (77%) | 33 ± 5 | 36 ± 8 | 0.25 ± 0.03 | 0.28 ± 0.06 |
| Pectineus (69%) | 42 ± 10 | 53 ± 10 | 0.31 ± 0.08 | 0.42 ± 0.08 |
Values are means ± SE; n = 10 in each group. Values in parentheses indicate %type IIb + IId/IIx according to Delp and Duan (15).
P < 0.05 vs. control (CHF).
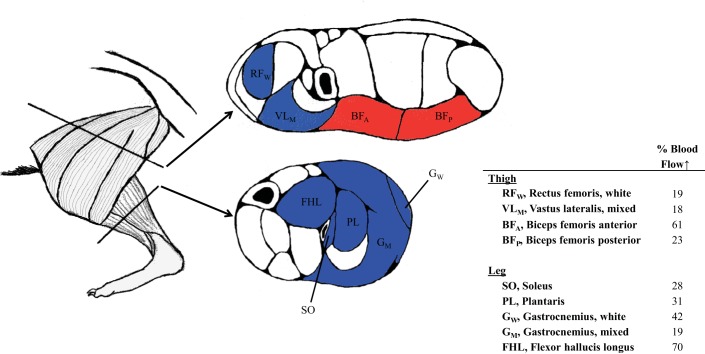
Fig. 3.
Effect of chronic BR supplementation on distribution of skeletal muscle blood flow (BF) among hindlimb muscles in exercising CHF rats. Left: lateral view of rat hindlimb. Middle: cross section of thigh and leg displaying distribution of BF increase. Coloring of muscles corresponds to their action: blue and red represent extensors and flexors, respectively. Right: percent increase in BF to individual muscles after BR compared with placebo.
Effects of BR on renal and splanchnic BF and VC at rest and during exercise.
Renal and splanchnic BF and VC values are presented in Table 3. At rest, BR-supplemented rats had significantly lower BF and VC in the large intestines (P < 0.05); during exercise, only BF was lower in the large intestines of CHF + BR rats (P < 0.05).
Table 3.
Effects of BR supplementation on resting and exercising BF and VC in kidneys and organs of the splanchnic region in CHF and CHF + BR rats.
| Rest |
Exercise |
|||||||
|---|---|---|---|---|---|---|---|---|
| BF |
VC |
BF |
VC |
|||||
| CHF | CHF + BR | CHF | CHF + BR | CHF | CHF + BR | CHF | CHF + BR | |
| Kidney | 541 ± 25 | 436 ± 57 | 4.41 ± 0.28 | 3.64 ± 0.43 | 419 ± 34† | 501 ± 38 | 3.19 ± 0.24† | 3.91 ± 0.22 |
| Stomach | 103 ± 21 | 86 ± 18 | 0.87 ± 0.19 | 0.72 ± 0.14 | 74 ± 13 | 64 ± 5 | 0.57 ± 0.11 | 0.51 ± 0.04 |
| Adrenals | 595 ± 103 | 578 ± 82 | 4.71 ± 0.75 | 4.83 ± 0.65 | 311 ± 53† | 430 ± 41 | 2.35 ± 0.37† | 3.38 ± 0.33 |
| Spleen | 323 ± 21 | 305 ± 38 | 2.57 ± 0.13 | 2.55 ± 0.29 | 91 ± 19† | 101 ± 17† | 0.68 ± 0.14† | 0.79 ± 0.13† |
| Pancreas | 140 ± 17 | 121 ± 18 | 1.15 ± 0.16 | 1.02 ± 0.15 | 137 ± 24 | 155 ± 15 | 1.05 ± 0.18 | 1.22 ± 0.11 |
| Intestine | ||||||||
| Small | 373 ± 48 | 324 ± 59 | 3.04 ± 0.41 | 2.72 ± 0.47 | 274 ± 33 | 299 ± 29 | 2.09 ± 0.23 | 2.35 ± 0.22 |
| Large | 289 ± 46 | 156 ± 30* | 2.38 ± 0.43 | 1.31 ± 0.24* | 245 ± 43 | 134 ± 17* | 1.86 ± 0.31 | 1.05 ± 0.13 |
| Liver (arterial, not portal) | 33 ± 4 | 32 ± 6 | 0.26 ± 0.03 | 0.26 ± 0.04 | 22 ± 3 | 31 ± 5 | 0.17 ± 0.03 | 0.24 ± 0.03 |
Values (means ± SE) are expressed in ml·min−1·100 g−1 (BF) or ml·min−1·100 g−1·mmHg−1 (VC).
P < 0.05 vs. CHF.
P < 0.05 vs. rest.
DISCUSSION
The principal original finding of the present investigation was that, in rats with CHF, 5 days of dietary NO3− supplementation via BR resulted in ∼22% greater total hindlimb skeletal muscle BF and VC during exercise than the placebo group. More specifically, BR resulted in elevated BF and VC to >54% of the total hindlimb skeletal muscle mass, with no distinct fiber type effects. Instead, the elevated BF and VC occurred preferentially in the locomotory muscles responsible for propulsion during running. This should reduce the CHF-induced metabolic perturbation within these tissues owing to an elevated O2 availability (improved perfusive and diffusive O2 transport), which, by definition, would be expected to raise intracellular Po2 and reduce the dependence on glycolytic metabolism (30). Considering that the principal predictor of hospital readmission and mortality in CHF is exercise intolerance (35), these data provide compelling evidence to support the use of dietary NO3− as an effective therapeutic aimed at the skeletal muscle peripheral dysfunction caused by this disease.
Impact of BR on skeletal muscle BF during exercise.
The most striking finding of the present investigation was the greater skeletal muscle BF and VC in the CHF + BR than the CHF group. These results complement a recent study from our laboratory whereby BR resulted in a significant increase in skeletal muscle BF and VC during exercise in healthy rats (20). However, to our knowledge, this is the first investigation to show the impacts of dietary NO3− supplementation on vascular control in rats with CHF. A recent clinical investigation by Zamani et al. (61) demonstrated that a single dose of dietary NO3− raised maximum V̇o2 by ∼10% and improved total work capacity by ∼13% in patients with preserved ejection fraction heart failure (HFpEF). The authors speculated that the beneficial effects of BR were likely due to targeted improvements in peripheral abnormalities, as suggested by the ∼25% reduction in systemic vascular resistance during exercise. These findings are corroborated by the present investigation, whereby BR resulted in a significant increase in BF and VC to approximately half (50% and 54% for BF and VC, respectively) of the total hindlimb skeletal muscle mass during exercise. Importantly, it is well appreciated that reduced ejection fraction heart failure (HFrEF, the model of CHF used here) results in temporal and spatial derangements in Po2mv (the exclusive driving force for O2 flux into the myocyte) during transitions in metabolic demand (6, 7, 12, 13, 16, 22, 29, 30), and while deficits in the skeletal muscle Q̇o2-V̇o2 relationship caused by HFpEF are not fully understood, decrements in convective and diffusive O2 transport are thought to occur in HFpEF patients (37) and animal models (59). The augmented skeletal muscle vascular function in the present study and the functional improvements reported by Zamani et al. (61) suggest that dietary NO3− may partially restore physical functionality via improved skeletal muscle vascular and metabolic control in HFrEF and HFpEF.
Contrary to our original hypothesis, dietary NO3− supplementation augmented BF and VC across the full spectrum of muscle fiber types with no significant preferential effects. This could be due, at least in part, to the CHF-induced decrements in BF and VC in slow-twitch, but not fast-twitch, muscles. More specifically, CHF alters the heterogeneity of skeletal muscle Q̇o2, such that BF to highly oxidative muscles is reduced whereas it may be better maintained in their fast-twitch counterparts (31, 47, 53), likely resulting in the faster fall in Po2mv during the rest-contraction transition in slow-twitch, but not fast-twitch, muscles (6). Considering that slow-twitch muscles may require a much greater BF due to their substantially higher V̇o2 and lower fractional O2 extraction than fast-twitch muscles [nearly double for both BF and V̇o2 (8)] and the evidence to suggest that NO2− bioconversion to NO is facilitated in low-Po2/pH environments (14), it is possible that the CHF etiology may have produced an environment well suited for NO2− reduction in muscles that would otherwise, in health, fall above the critical Po2 required to elicit vascular effects [a Po2 approximating 25 mmHg (14)]. This idea is supported by a recent report from our laboratory, whereby direct NO2− infusion resulted in nonspecific elevations in skeletal muscle BF and VC in healthy rats following NOS inhibition via l-NAME (17). To this end, despite no statistically significant correlations, the “targeted effects” of dietary NO3− supplementation proposed by our group (33) as well as others (28, 32, 36, 60) may have afforded vascular effects in some, but not all, slow-twitch muscles due to the CHF-induced derangements in the Q̇o2-to-V̇o2 ratio and lowered Po2mv.
Blood lactate.
We previously demonstrated that NO3− supplementation via BR reduces blood [lactate] during submaximal exercise (20); therefore, the expectation was that this effect would be associated with the enhanced BFs seen here. However, blood lactate was not different between CHF and CHF + BR. It is also notable that, in healthy animals under NOS blockade (l-NAME), blood [lactate] is increased by NO2− infusions during exercise. This occurs despite elevated BF to select muscles (17). The mechanism whereby elevated plasma [NO2−] alters the balance between rates of appearance and disappearance of lactate in the arterial blood remains to be addressed.
Clinical relevance.
Exercise training elicits a full spectrum of beneficial adaptations to the O2 transport system and, thus, represents an extremely effective therapy for treatment of the skeletal muscle dysfunction elicited by CHF (reviewed in Ref. 30). Of particular relevance to the present investigation is the fact that endurance exercise training produces a redistribution of BF within the hindlimb of the rat, such that BF appears to be directed away from fast-twitch glycolytic muscle toward its highly oxidative counterparts (43, 52). This redistribution occurs in the absence of changes in bulk Q̇o2, yet it substantially augments skeletal muscle Q̇o2-V̇o2 matching during transitions in metabolic demand (27, 38, 39), providing the mechanistic basis for improved exercise tolerance and better quality of life (9) for CHF patients. Considering that 5 days of dietary NO3− supplementation via BR, a nonpharmacological intervention, increased bulk skeletal muscle Q̇o2 (elevated BF and VC; Fig. 2), combinations of exercise-based rehabilitation programs with dietary NO3− supplementation and other NO-based therapies [i.e., sildenafil (44)] may represent an especially effective therapeutic strategy for patients with CHF. Indeed, even small increases in physical capacity may lead to an upregulation of endothelial NOS activity and vascular function (1). These data call for further clinical investigations to elucidate the potential for chronic dietary NO3− to improve exercise tolerance and the efficacy of (and adherence to) cardiac rehabilitation programs.
An unexpected observation was the lower BF at rest and during exercise in the large intestine. This effect is contrary to previous findings that the low intestinal perfusion, and thus Po2, of the intestine in CHF is thought to promote the reduction of NO2− to NO, and, thus, increase, rather than decrease, BF to the gastrointestinal tract (40, 46). It has been speculated that the reason for this effect is that BR resulted in a redistribution of BF to a vascular bed that was not measured in the present investigation.
Experimental considerations and future directions.
The techniques used to measure intra- and intermuscular BF during dynamic exercise represent a major strength of the present investigation. While current technologies preclude these measurements in humans because of technical and ethical limitations, these findings complement those of Zamani et al. (61) by providing crucial mechanistic insight into the physiological benefits of dietary NO3− supplementation in patients with CHF. The lack of sham-operated controls could be considered a potential limitation of the present investigation. However, in many comparisons between controls and sham-operated animals, Musch et al. (5, 16, 50, 51, 53) found no differences. In addition, the measurements of skeletal muscle BF and VC in rats receiving the placebo BR are consistent with previous reports from our laboratory in rats with moderate CHF (53, 55), further supporting the results observed here. However, while the elevations in skeletal muscle BF and VC observed in the present study suggest an improvement in microvascular oxygenation (Po2mv), this was not directly measured in the present investigation, nor was exercise tolerance or key parameters of exercise performance (i.e., critical velocity and pulmonary V̇o2 kinetics). To this point, the potential outcomes of dietary NO3− supplementation on these key parameters of physical function remain speculative and, thus, should be the focus of future investigations utilizing murine (for measurements of Po2mv) and patient populations. Notwithstanding these limitations, Fick's law of diffusion dictates that an elevation of the diffusion pressure gradient, controlled by the Q̇o2-V̇o2 relationship (of which Q̇o2 was increased ∼22% in the present study), will increase blood myocyte O2 flux and, thus, enhance oxidative metabolism while reducing fatigue-associated metabolite accumulation (26, 58). Thus this investigation represents an important step in uncovering the mechanistic basis for the benefits of dietary NO3− supplementation reported in CHF (61) and other patient populations [i.e., pulmonary and peripheral artery diseases (10, 36)]. Finally, because of technical limitations, plasma [NO3−] could not be obtained here. However, as NO2− is the key active intermediate between NO3− and NO and the magnitude of the biological consequences most tightly relate to plasma [NO2−], we believe that measurements of this anion provide the crucial mechanistic linkage between BR consumption and the augmented vascular control observed in the present study.
Conclusions.
This is the first investigation to demonstrate the impact of dietary NO3− supplementation on intra- and intermuscular BF and VC at rest and during exercise in rats with CHF. Five days of NO3−-rich BR supplementation resulted in robust increases in total hindlimb skeletal muscle BF and VC, with significant increases in BF and VC in ≥50% of the total hindlimb muscle mass. The finding that no distinct fiber type preferential effects were observed, despite previous investigations demonstrating BR-induced improvements in fast-twitch muscle function (33), suggests that BR improves vascular function in the full spectrum of muscle fiber types in CHF. Collectively, these results demonstrate the efficacy of a relatively short-term dietary intervention on vascular function in CHF and provide evidence to support implementation of BR as an effective addition to exercise-based cardiac rehabilitation programs.
GRANTS
These experiments were funded by a Kansas State University SMILE award to T. I. Musch and American Heart Association Midwest Affiliate Grant 10GRNT4350011 and National Heart, Lung, and Blood Institute Grant HL-108328 to D. C. Poole.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.K.F., J.C.C., A.M.J., J.D.A., T.I.M., and D.C.P. developed the concept and designed the research; S.K.F., C.T.H., T.D.C., J.L.W., A.F., and T.I.M. performed the experiments; S.K.F. analyzed the data; S.K.F., J.C.C., A.M.J., and D.C.P. interpreted the results of the experiments; S.K.F. and J.C.C. prepared the figures; S.K.F. and D.C.P. drafted the manuscript; S.K.F., C.T.H., T.D.C., J.C.C., A.M.J., J.D.A., T.I.M., and D.C.P. edited and revised the manuscript; S.K.F., C.T.H., T.D.C., J.L.W., J.C.C., A.F., A.M.J., J.D.A., T.I.M., and D.C.P. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank K. Sue Hageman for excellent technical assistance.
REFERENCES
- 1.Allen JD, Giordano T, Kevil CG. Nitrite and nitric oxide metabolism in peripheral artery disease. Nitric Oxide Biol Chem 26: 217–222, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong RB, Hayes DA, Delp MD. Blood flow distribution in rat muscles during preexercise anticipatory response. J Appl Physiol 67: 1855–1861, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol 109: 135–148, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol 107: 1144–1155, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Baily RG, Lehman JC, Gubin SS, Musch TI. Non-invasive assessment of ventricular damage in rats with myocardial infarction. Cardiovasc Res 27: 851–855, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Behnke BJ, Delp MD, McDonough P, Spier SA, Poole DC, Musch TI. Effects of chronic heart failure on microvascular oxygen exchange dynamics in muscles of contrasting fiber type. Cardiovasc Res 61: 325–332, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Behnke BJ, Delp MD, Poole DC, Musch TI. Aging potentiates the effect of congestive heart failure on muscle microvascular oxygenation. J Appl Physiol 103: 1757–1763, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Behnke BJ, McDonough P, Padilla DJ, Musch TI, Poole DC. Oxygen exchange profile in rat muscles of contrasting fibre types. J Physiol 549: 597–605, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation 99: 1173–1182, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Berry MJ, Justus NW, Hauser JI, Case AH, Helms CC, Basu S, Rogers Z, Lewis MT, Miller GD. Dietary nitrate supplementation improves exercise performance and decreases blood pressure in COPD patients. Nitric Oxide Biol Chem 48: 22–30, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coggan AR, Leibowitz JL, Spearie CA, Kadkhodayan A, Thomas DP, Ramamurthy S, Mahmood K, Park S, Waller S, Farmer M, Peterson LR. Acute dietary nitrate intake improves muscle contractile function in patients with heart failure: a double-blind, placebo-controlled, randomized trial. Circ Heart Fail 8: 914–920, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Copp SW, Hirai DM, Ferguson SK, Holdsworth CT, Musch TI, Poole DC. Effects of chronic heart failure on neuronal nitric oxide synthase-mediated control of microvascular O2 pressure in contracting rat skeletal muscle. J Physiol 590: 3585–3596, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Copp SW, Hirai DM, Ferreira LF, Poole DC, Musch TI. Progressive chronic heart failure slows the recovery of microvascular O2 pressures after contractions in the rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol 299: H1755–H1761, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498–1505, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 80: 261–270, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Diederich ER, Behnke BJ, McDonough P, Kindig CA, Barstow TJ, Poole DC, Musch TI. Dynamics of microvascular oxygen partial pressure in contracting skeletal muscle of rats with chronic heart failure. Cardiovasc Res 56: 479–486, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson SK, Glean AA, Holdsworth CT, Wright JL, Fees AJ, Colburn TD, Stabler T, Allen JD, Jones AM, Musch TI, Poole DC. Skeletal muscle vascular control during exercise: impact of nitrite infusion during nitric oxide synthase inhibition in healthy rats. J Cardiovasc Pharmacol Ther 21: 201–208, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC. Dose dependent effects of nitrate supplementation on cardiovascular control and microvascular oxygenation dynamics in healthy rats. Nitric Oxide Biol Chem 39: 51–58, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC. Effects of nitrate supplementation via beetroot juice on contracting rat skeletal muscle microvascular oxygen pressure dynamics. Respir Physiol Neurobiol 187: 250–255, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J Physiol 591: 547–557, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson SK, Holdsworth CT, Wright JL, Fees AJ, Allen JD, Jones AM, Musch TI, Poole DC. Microvascular oxygen pressures in muscles comprised of different fiber types: impact of dietary nitrate supplementation. Nitric Oxide Biol Chem 48: 38–43, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira LF, Hageman KS, Hahn SA, Williams J, Padilla DJ, Poole DC, Musch TI. Muscle microvascular oxygenation in chronic heart failure: role of nitric oxide availability. Acta Physiol (Oxf) 188: 3–13, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Fishbein MC, Maclean D, Maroko PR. Experimental myocardial infarction in the rat: qualitative and quantitative changes during pathologic evolution. Am J Pathol 90: 57–70, 1978. [PMC free article] [PubMed] [Google Scholar]
- 24.Flaim SF, Nellis SH, Toggart EJ, Drexler H, Kanda K, Newman ED. Multiple simultaneous determinations of hemodynamics and flow distribution in conscious rat. J Pharmacol Methods 11: 1–39, 1984. [DOI] [PubMed] [Google Scholar]
- 25.Greenhaff PL, Nevill ME, Soderlund K, Bodin K, Boobis LH, Williams C, Hultman E. The metabolic responses of human type I and II muscle fibres during maximal treadmill sprinting. J Physiol 478: 149–155, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haseler LJ, Richardson RS, Videen JS, Hogan MC. Phosphocreatine hydrolysis during submaximal exercise: the effect of FiO2. J Appl Physiol 85: 1457–1463, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Heinonen I, Koga S, Kalliokoski KK, Musch TI, Poole DC. Heterogeneity of muscle blood flow and metabolism: influence of exercise, aging, and disease states. Exerc Sport Sci Rev 43: 117–124, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez A, Schiffer TA, Ivarsson N, Cheng AJ, Bruton JD, Lundberg JO, Weitzberg E, Westerblad H. Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twitch muscle. J Physiol 590: 3575–3583, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirai DM, Copp SW, Holdsworth CT, Ferguson SK, McCullough DJ, Behnke BJ, Musch TI, Poole DC. Skeletal muscle microvascular oxygenation dynamics in heart failure: exercise training and nitric oxide-mediated function. Am J Physiol Heart Circ Physiol 306: H690–H698, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirai DM, Musch TI, Poole DC. Exercise training in chronic heart failure: improving skeletal muscle O2 transport and utilization. Am J Physiol Heart Circ Physiol 309: H1419–H1439, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirai T, Zelis R, Musch TI. Effects of nitric oxide synthase inhibition on the muscle blood flow response to exercise in rats with heart failure. Cardiovasc Res 30: 469–476, 1995. [PubMed] [Google Scholar]
- 32.Jones AM. Dietary nitrate supplementation and exercise performance. Sports Med 44 Suppl 1: S35–S45, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones AM, Ferguson SK, Bailey SJ, Vanhatalo A, Poole DC. Fiber type-specific effects of dietary nitrate. Exerc Sport Sci Rev 44: 53–60, 2016. [DOI] [PubMed] [Google Scholar]
- 34.Kanematsu Y, Yamaguchi K, Ohnishi H, Motobayashi Y, Ishizawa K, Izawa Y, Kawazoe K, Kondo S, Kagami S, Tomita S, Tsuchiya K, Tamaki T. Dietary doses of nitrite restore circulating nitric oxide level and improve renal injury in l-NAME-induced hypertensive rats. Am J Physiol Renal Physiol 295: F1457–F1462, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Kato T, Nakane E, Funasako M, Miyamoto S, Izumi T, Haruna T, Nohara R, Inoko M. A potential linkage between mitochondrial function of the heart and leg muscles in patients with heart failure. Int J Cardiol 188: 67–69, 2015. [DOI] [PubMed] [Google Scholar]
- 36.Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson JL, Vanbruggen M, Privette G, Yim E, Kraus WE, Allen JD. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol 110: 1582–1591, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, Haykowsky M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol 306: H1364–H1370, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koga S, Poole DC, Ferreira LF, Whipp BJ, Kondo N, Saitoh T, Ohmae E, Barstow TJ. Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J Appl Physiol 103: 2049–2056, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Koga S, Rossiter HB, Heinonen I, Musch TI, Poole DC. Dynamic heterogeneity of exercising muscle blood flow and O2 utilization. Med Sci Sports Exerc 46: 860–876, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Krack A, Sharma R, Figulla HR, Anker SD. The importance of the gastrointestinal system in the pathogenesis of heart failure. Eur Heart J 26: 2368–2374, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Gilchrist M, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol 110: 591–600, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 191: 59–66, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol Heart Circ Physiol 243: H296–H306, 1982. [DOI] [PubMed] [Google Scholar]
- 44.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation 116: 1555–1562, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov 14: 623–641, 2015. [DOI] [PubMed] [Google Scholar]
- 46.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7: 156–167, 2008. [DOI] [PubMed] [Google Scholar]
- 47.McAllister RM, Laughlin MH, Musch TI. Effects of chronic heart failure on skeletal muscle vascular transport capacity of rats. Am J Physiol Heart Circ Physiol 264: H689–H691, 1993. [DOI] [PubMed] [Google Scholar]
- 48.Muggeridge DJ, Howe CC, Spendiff O, Pedlar C, James PE, Easton C. A single dose of beetroot juice enhances cycling performance in simulated altitude. Med Sci Sport Exerc 46: 143–150, 2014. [DOI] [PubMed] [Google Scholar]
- 49.Musch TI, Bruno A, Bradford GE, Vayonis A, Moore RL. Measurements of metabolic rate in rats: a comparison of techniques. J Appl Physiol 65: 964–970, 1988. [DOI] [PubMed] [Google Scholar]
- 50.Musch TI, Moore RL, Leathers DJ, Bruno A, Zelis R. Endurance training in rats with chronic heart failure induced by myocardial infarction. Circulation 74: 431–441, 1986. [DOI] [PubMed] [Google Scholar]
- 51.Musch TI, Moore RL, Smaldone PG, Riedy M, Zelis R. Cardiac adaptations to endurance training in rats with a chronic myocardial infarction. J Appl Physiol 66: 712–719, 1989. [DOI] [PubMed] [Google Scholar]
- 52.Musch TI, Nguyen CT, Pham HV, Moore RL. Training effects on the regional blood flow response to exercise in myocardial infarcted rats. Am J Physiol Heart Circ Physiol 262: H1846–H1852, 1992. [DOI] [PubMed] [Google Scholar]
- 53.Musch TI, Terrell JA. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. Am J Physiol Heart Circ Physiol 262: H411–H419, 1992. [DOI] [PubMed] [Google Scholar]
- 54.Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol 302: H1050–H1063, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sims GE, Copp SW, Hirai DM, Ferguson SK, Holdsworth CT, Poole DC, Musch TI. Effects of pentoxifylline on exercising skeletal muscle vascular control in rats with chronic heart failure. J Cardiol Ther 2: 32–44, 2014. [Google Scholar]
- 56.Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 10: 429–437, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG, Jones AM. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol 299: R1121–R1131, 2010. [DOI] [PubMed] [Google Scholar]
- 58.Vanhatalo A, Fulford J, Bailey SJ, Blackwell JR, Winyard PG, Jones AM. Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. J Physiol 589: 5517–5528, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wust RC, Myers DS, Stones R, Benoist D, Robinson PA, Boyle JP, Peers C, White E, Rossiter HB. Regional skeletal muscle remodeling and mitochondrial dysfunction in right ventricular heart failure. Am J Physiol Heart Circ Physiol 302: H402–H411, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wylie LJ, Mohr M, Krustrup P, Jackman SR, Ermiotadis G, Kelly J, Black MI, Bailey SJ, Vanhatalo A, Jones AM. Dietary nitrate supplementation improves team sport-specific intense intermittent exercise performance. Eur J Appl Physiol 113: 1673–1684, 2013. [DOI] [PubMed] [Google Scholar]
- 61.Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, Doulias PT, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC, Chirinos JA. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation 131: 371–380, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]