The novel and noteworthy findings are 1) in humans, the vasodilator response to infusion of exogenous ATP does not wane and 2) the magnitude of change in hyperemic responses to exercise during the ATP infusion is unaffected. The ability of ATP to evoke prolonged vasodilation shows it meets a key criterion of any putative dilator substance. The latter observation suggests a disconnect between the usual matching of blood flow and oxygen delivery during contractions.
Keywords: vasodilation, adenosine triphosphate, blood flow, exercise hyperemia, tachyphylaxis
Abstract
In humans, intra-arterial ATP infusion in limbs mimics many features of exercise hyperemia. However, it remains unknown whether ATP can evoke the prolonged vasodilation seen during exercise. Therefore, we addressed two questions during a continuous 3-h brachial artery infusion of ATP [20 μg·100 ml forearm volume (FAV)−1·min−1]: 1) would skeletal muscle blood flow remain robust or wane over time (tachyphylaxis); and 2) would the hyperemic response to moderate-intensity exercise performed during the ATP administration be blunted compared with that during control (saline) infusion. Nine participants (25 ± 1 yr) performed one trial consisting of seven bouts of rhythmic handgrip exercise (20 contractions/min at 20% of maximum), two bouts during saline (control), and five bouts during 180 min of continuous ATP infusion. Five minutes of ATP infusion resulted in a 710% increase in forearm vascular conductance (FVC) from control (4.8 ± 0.77 vs. 35.0 ± 5.7 ml·min−1·100 mmHg−1·dl FAV−1, P < 0.05). Contrary to our expectations, FVC did not wane over time with values of 35.0 ± 5.7 and 36.0 ± 7.7 ml·min−1·100 mmHg−1·dl FAV−1 (P > 0.05), seen prior to the exercise bouts at 5 vs. 150 min, respectively. During superimposed exercise, FVC increased from 35.0 ± 5.7 to 49.6 ± 5.4 ml·min−1·100 mmHg−1·dl FAV−1 at 5 min and 36.0 ± 7.7 to 54.5 ± 5.0 at 150 min (P < 0.05). Our findings demonstrate ATP vasodilation is prolonged over time without tachyphylaxis; however, exercise hyperemia responses remain intact. Our results challenge the metabolic theory of exercise hyperemia, suggesting a disconnect between matching of blood flow and metabolic demand.
NEW & NOTEWORTHY
The novel and noteworthy findings are 1) in humans, the vasodilator response to infusion of exogenous ATP does not wane and 2) the magnitude of change in hyperemic responses to exercise during the ATP infusion is unaffected. The ability of ATP to evoke prolonged vasodilation shows it meets a key criterion of any putative dilator substance. The latter observation suggests a disconnect between the usual matching of blood flow and oxygen delivery during contractions.
during exercise, skeletal muscle blood flow increases via local vasodilation to match the metabolic demands of the contracting muscles (13, 26, 27). One underlying idea that might explain exercise hyperemia is that a substance (or substances) released by or near the contracting muscles in proportion to the level of muscular activity is responsible for matching blood flow to metabolism (13). The contribution of several potential locally derived substances have been studied over many years, and no one substance has proven to be obligatory or account for a majority of the dilation (13, 27).
In the 1950s, brachial artery infusion of ATP was shown to evoke significant vasodilation in the human forearm (8, 21). Over the past 15 years, there has been renewed enthusiasm for its role in exercise hyperemia. Newer studies have supported a role for ATP as a vasodilating metabolite that contributes to exercise hyperemia largely because exogenous ATP administration at rest can evoke levels of limb vasodilation similar to those seen during heavy exercise. Additionally, exogenous ATP mimics the functional sympatholysis seen during exercise (3, 10, 16, 19, 25). There are multiple sources of ATP that might cause exercise hyperemia, including release from endothelial cells, deoxygenating and/or mechanically deformed red blood cells, and the contracting muscles themselves (15). Any ATP released would then bind to purinergic P2Y receptors on the nearby endothelium and vascular smooth muscle to cause vasodilation (15, 20).
Because exercise hyperemia can occur for many hours (13), a major unanswered question is whether the vasodilation evoked by ATP can be prolonged over time. If ATP plays a key role in regulating exercise hyperemia, then an infusion of ATP for hours should also evoke prolonged vasodilation. However, assessing the role of ATP as a mediator of exercise hyperemia in humans is challenging due to the lack of a direct receptor antagonist approved for human use. Therefore, to overcome the lack of an antagonist, we used an indirect experimental approach and adapted the experimental design used by Hester et al., who in 1982, studied the role of adenosine in exercise hyperemia in dogs (11). They reported tachyphylaxis and a progressive blunting of the dilator response to prolonged administration of adenosine. However, even when the dilator response to exogenous adenosine was no longer present, the hyperemic responses to electrically induced contractions were unaffected. On the basis of these observations, Casey and Joyner (1, 12) concluded that adenosine was not obligatory for exercise hyperemia. Work from our laboratory has supported this conclusion.
Hence, our rationale for the current study was that if ATP were a major and/or obligatory vasodilating metabolite, then an exogenous ATP infusion should be able to evoke a prolonged forearm vasodilator response at rest equivalent to that observed during moderate-intensity workload. Additionally, we speculated how the dilator responses to contractions might be blunted when performed during an ongoing infusion of ATP.
With this information as background, the primary aim of the present investigation was to determine whether the vasodilation caused by a prolonged infusion of exogenous ATP would remain robust or wane over time. Our secondary aim was to determine whether the vasodilator response to moderate-intensity exercise [20% of maximal voluntary contraction (MVC)] (24, 31) performed during prolonged continuous infusion of exogenous ATP would be reduced.
METHODS
Overview of Experimental Strategy
A major challenge in studying the role of ATP is the absence of a direct P2Y receptor antagonist that can be used in humans (13). Therefore, to circumvent the challenge of studying the role of ATP in the absence of a direct P2Y receptor antagonist, our experimental strategy was to administer exogenous ATP at a dose that would evoke robust forearm vasodilation at rest similar to that observed during moderate intensity (20% MVC) exercise (24, 31). The participants then performed moderate-intensity exercise bouts during the prolonged continuous ATP infusion. We reasoned that this strategy would allow us to evaluate whether the forearm vasodilator responses to moderate-intensity exercise during prolonged ATP infusion would be reduced. To execute this strategy, we evaluated changes only in the human forearm vascular bed and infused a dose of ATP via the brachial artery to cause equivalent levels of vasodilation in the forearm at rest compared with moderate-intensity (20% MVC) forearm exercise. The dose of ATP that we administered [20 μg·100 ml forearm volume (FAV)−1·min−1] has previously been shown to cause a robust forearm dilator response without causing any systemic hemodynamic changes (24, 31).
Subjects.
A total of nine young healthy recreationally active subjects (5 men/4 women) volunteered to participate in the study after providing written informed consent. All participants were free of acute and chronic cardiovascular or respiratory disease, not taking any medication or supplements, and all nonsmoking and nonobese [body mass index (BMI) <30 kg/m2]. All participants refrained from exercise, alcohol, and caffeine for at least 24 h and fasted for 12 h before the start of the study. To control the effects of the reproductive hormones on cardiovascular function, all female subjects were studied during the early follicular phase of the menstrual cycle or the placebo phase of oral contraceptives, and all had a negative pregnancy test the morning of the study day (18). Women with an interuterine device were excluded. All study procedures were approved by the Institutional Review Board of the Mayo Clinic and performed according to the Declaration of Helsinki.
Experimental Protocol
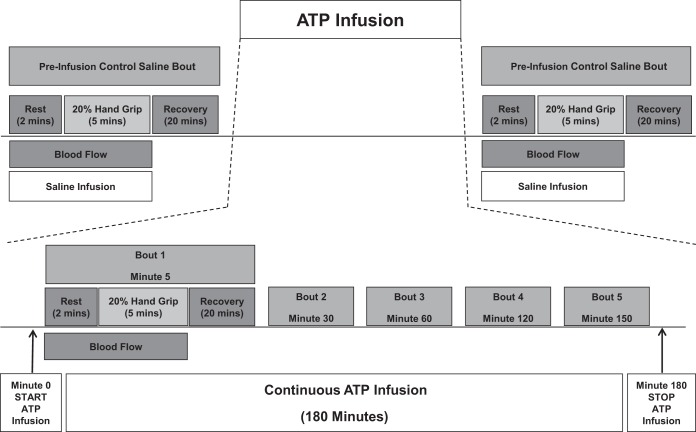
The experimental protocol is shown in Fig. 1. The study was performed during one experimental session in the Clinical Research and Trials Unit (CRTU) at the Mayo Clinic with an ambient temperature maintained between 22 and 24°C. On arrival to the laboratory, demographics, pregnancy test (women), forearm volume, and maximal voluntary contraction were obtained. All subjects then underwent placement of brachial arterial catheter and electrocardiogram (ECG) leads followed by a 20-min rest period.
Fig. 1.
Overview of the experimental timeline. ATP, adenosine triphosphate. Schematic showing overview of experimental timeline. Each bout consists of a 2-min rest period, 5 min of dynamic forearm hand gripping exercise, and 2 min of recovery. Continuous blood flow measurement by Doppler ultrasound was performed throughout the experiment.
Saline (control) bout.
Following a rest period, the trial started with a control saline infusion at an individualized rate (1.8-3.2 ml/min), based on each subject's forearm volume and response to maximal voluntary contraction. Blood flow velocities and brachial artery diameters were obtained continuously using Duplex ultrasound (see Fig. 2). Two minutes of baseline values were recorded followed by 5 min of rhythmic handgrip exercise, and 2 min of recovery.
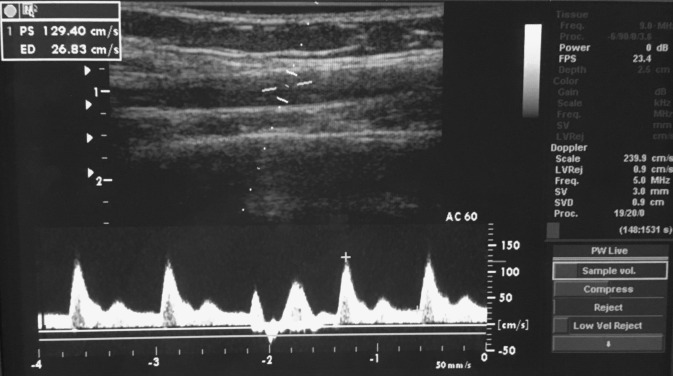
Fig. 2.
Duplex ultrasound methods image showing brachial artery blood velocity tracing during forearm handgrip exercise of 1-s contraction and 2-s relaxation.
Drug (ATP) bouts.
Following a 20-min rest period after completion of the saline trail, preinfusion Doppler measurements were obtained to confirm that blood flow had returned to baseline flows. A continuous ATP infusion was then started for a total of 180 min. Subjects were asked to perform five rhythmic handgrip exercise bouts identical to the saline bout at 5, 30, 60, 120, and 150 min into the ATP infusion. Five minutes after the end of the ATP infusion, an additional control saline exercise bout was performed [data not shown], which marked the end of the trial. Of note, our previous work has shown that the vasodilator responses to exercise over time are consistent, and blood flow rapidly returns to resting values within 20 min (2).
Subject Monitoring
Brachial artery catheterization and blood pressure and heart rate.
In all subjects a 20-gauge, 5-cm (model RA-04020; Arrow International, Reading, PA) catheter for measurement of arterial pressure and drug infusion was placed into the brachial arterial of the exercising nondominant arm under aseptic conditions after local anesthesia (2% lidocaine). Attached to the catheter was a three-way port system connector in series and transducer (model PX600F, Edwards Lifescience, Irvine, CA), permitting simultaneous measurement of beat-by-beat brachial arterial pressure and administration of ATP, as previously described in detail (7). Heart rate was recorded via a continuous three-lead electrocardiogram.
Drug administered: ATP
ATP (A7699; Sigma, St. Louis, MO) was dissolved in isotonic saline (1 mg/ml) prepared by the Mayo Clinic Research Pharmacy to a concentration of 80 μg/ml. Dosage adjustments were made for each participant's forearm volume to keep a constant infusion rate of 20 μg·100 ml FAV−1·min−1 intra-arterially for a continuous 180 min using a Harvard infusion syringe pump. The same infusion rates were used during saline control infusions.
Forearm Exercise Bouts
Each bout consisted of 5 min of rhythmic (20 contractions per minute) forearm exercise with a handgrip dynamometer at a moderate intensity of exercise (20% MVC). This level of exercise was selected on the basis of our previous studies showing it evokes stable blood flow responses over time and can be performed repeatedly without fatigue in healthy subjects. This exercise level also limits the contribution of systemic hemodynamics and reflex activation of the sympathetic nervous system on exercise hyperemia (2, 23). The weight was lifted 4 to 5 cm over a pulley at a duty cycle of 1-s contraction and 2-s relaxation using a metronome. Each bout was monitored by laboratory personnel to ensure proper timing of contractions.
Forearm Blood Flow
Brachial artery mean blood velocity (MBV) and brachial artery diameter were determined with a 12-MHz linear-array Doppler probe (model M12L, Vivid 7, General Electric, Milwaukee, WI). Brachial artery blood velocity was measured throughout each condition with a probe insonation angle previously calibrated to 60°. Brachial artery diameter measurements were obtained at end diastole between contractions during steady-state conditions (Fig. 2). This method has been previously used for several other studies in our laboratory (2, 22). Forearm blood flow (FBF) was calculated as FBF = MBV × π × (brachial artery diameter/2)2 × 60 with MBV (cm/s) and brachial artery cross-sectional area (cm2) and expressed as milliliters per minute (ml/min).
Forearm Vascular Conductance
Forearm vascular conductance (FVC) was calculated as (FBF/mean arterial pressure) × 100 and expressed as ml·min−1·100 mmHg−1·dl FAV−1, so that FVC will be quantitatively similar to the standard units of FBF (Fig. 3B). To calculate the change for FBF and FVC, the saline baseline condition served as a control. We utilized calculated conductance as an index of vasodilation because it is linearly related to blood flow, accounts for small changes in blood pressure, and allowed us to compare responses in participants with substantial differences in FAV along with conditions of elevated baseline flow during the ATP infusion (22, 29).
Fig. 3.
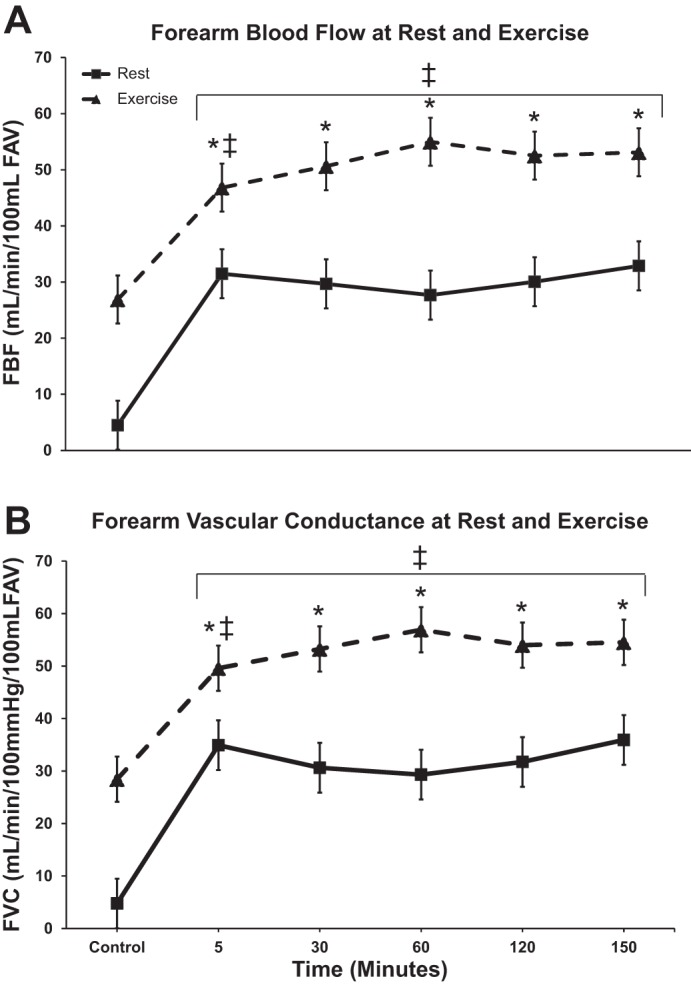
Forearm blood flow (FBF) and forearm vascular conductance (FVC) responses. FBF (A) and FVC (B) during the ATP infusion over time, showing rest and exercise responses. Values are expressed as means ± SE for all nine subjects. *P < 0.05 vs. control rest. ‡P < 0.05 vs. resting measurement. Note “control” occurs during the saline infusion, while time points 5–150 min occur during the ATP infusion.
Data Analysis
Data were collected at 250 Hz, stored on a computer, and analyzed off-line with signal processing software (WinDaq; DATAq Instruments, Akron, OH and Powerlab; ADInstruments, Sydney, Australia). Heart rate (HR) and mean arterial pressure (MAP) were analyzed from the electrocardiogram and the brachial artery pressure waveform, respectively. Mean blood velocity, HR, and MAP were determined by averaging the last 60 s of each baseline and exercise bout during control and continuous ATP infusion. Arterial diameters were measured at baseline and in the last 15 s of the exercise bouts. Percent changes were calculated as [(Bout exercise FBF − bout baseline FBF)/bout baseline FBF]·100 and [(Bout exercise FVC − bout baseline FVC)/bout baseline FVC]·100.
Two-way repeated-measures ANOVAs were performed to test significance between and within bouts. Following a significant F test, pair-wise differences were identified using Tukey's post hoc test procedure. The significant level was set at P < 0.05, and all data values are presented as means ± SE, unless stated otherwise.
RESULTS
All nine participants completed the protocol. The mean age was 30 ± 1 years, body weight 81 ± 4 kg, height 179 ± 3 cm, and BMI 25 ± 1 kg/m2. Mean forearm volume was 1,026 ± 62 ml (range = 17-1,290 ml).
Systemic Hemodynamic Variables
The group data for systemic hemodynamic variables are presented in Table 1. There were no significant systemic differences in MAP or HR between the saline control and ATP bouts (P > 0.05).
Table 1.
Participant characteristics
| Variable | Value |
|---|---|
| Systolic blood pressure, mmHg | 120 ± 4 |
| Diastolic blood pressure, mmHg | 71 ± 2 |
| Heart rate, bpm | 64 ± 4 |
| 20% Maximum value contraction, kg | 9.5 ± 0.5 |
| Forearm volume, ml | 1026 ± 62 |
| Infusion flow rate, ml/min | 2.57 ± 0.2 |
| Volume delivered, ml | 461 ± 28 |
| Total dose, mg | 37 ± 2 |
Values are expressed as means ± SE for all nine subjects. BPM, beats per minute.
Effect of ATP Infusion on Resting Forearm Blood Flow and Forearm Vascular Conductance
The group data for MAP, HR, FBF, and FVC during ATP infusion are presented in Table 2. Five minutes of ATP infusion resulted in a significant 710% increase in FVC (4.8 ± 0.77 vs. 35.0 ± 5.7 ml·min−1·100 mmHg−1·dl FAV−1, P < 0.05). FVC during ATP infusion was similar at minutes 5 and 150 (35.0 ± 5.7 and 36 ± 7.7 ml·min−1·100 mmHg−1, P > 0.05).
Table 2.
Rest and exercise vascular hemodynamics
| Variable | Saline (Control) | Bout 1 | Bout 2 | Bout 3 | Bout 4 | Bout 5 |
|---|---|---|---|---|---|---|
| Time, min | 5 | 30 | 60 | 120 | 150 | |
| Heart rate, beats/min | ||||||
| Rest | 59 ± 2 | 59 ± 2 | 59 ± 2 | 61 ± 2 | 59 ± 2 | 59 ± 2 |
| Exercise | 63 ± 2 | 62 ± 2 | 63 ± 2 | 62 ± 2 | 64 ± 2 | 65 ± 2 |
| Mean arterial pressure, mmHg | ||||||
| Rest | 93 ± 2 | 92 ± 2 | 95 ± 2 | 97 ± 2 | 96 ± 2 | 96 ± 2 |
| Exercise | 98 ± 2 | 95 ± 2 | 96 ± 2 | 97 ± 2 | 100 ± 2 | 101 ± 2 |
| % FBF from Control | ||||||
| Rest | 716 ± 58 | 676 ± 58 | 574 ± 47 | 615 ± 49 | 669 ± 54 | |
| Δ FBF from Rest, ml·min−1·100 ml FAV−1 | ||||||
| Exercise | 24.9 ± 1.8 | 17.2 ± 1.9 | 23.3 ± 2.0 | 30.4 ± 2.0 | 25.0 ± 1.8 | 22.5 ± 1.9 |
| % FVC from Control | ||||||
| Rest | 710 ± 62 | 593 ± 65 | 526 ± 44 | 574 ± 46 | 675 ± 56 | |
| Δ FVC from rest, ml·min−1·100 mmHg−1·dl FAV−1 | ||||||
| Exercise | 26.3 ± 1.9 | 16.4 ± 2.1 | 25.1 ± 2.1 | 30.7 ± 1.8 | 24.7 ± 1.9 | 20.7 ± 1.8 |
Values are expressed as means ± SE for all nine subjects. *P < 0.05 from control. FBF, forearm blood flow; Percent change was calculated as [(Bout exercise FBF − bout baseline FBF)/bout baseline FBF]·100. FVC, forearm vascular conductance. Percent change was calculated as [(Bout exercise FVC − bout baseline FVC)/bout baseline FVC]·100. Δ FVC from control was calculated as (exercise bout − rest bout). FAV, forearm volume.
Effect of Exercise Superimposed on Saline and ATP Infusions on Forearm Blood Flow and Forearm Vascular Conductance
The group data for MAP, HR, FBF, and FVC during exercise bouts are presented in Table 2. FVC during exercise under control (saline) condition increased from 4.8 ± 0.77 to 28.4 ± 3.1 ml·min−1·100 mmHg−1·dl FAV−1, P < 0.05. When exercise was superimposed during the ATP infusion, FVC further increased from 35.0 ± 5.7 to 49.6 ± 5.4 ml·min−1·100 mmHg−1·dl FAV−1, at 5 min (P < 0.05). The response of FVC evoked by exercise superimposed during the ATP infusion did not wane over time with equivalent values between bout 1 of exercise vs. bout 5 (49.6 ± 5.4 vs. 54.5 ± 5.0 ml·min−1·100 mmHg−1·dl FAV−1, P > 0.05, respectively). Figure 3 shows the absolute mean FBF and FVC responses. The change in FVC during exercise under control (saline) conditions were 26.3 ± 3.2 ml·min−1·100 mmHg−1 vs. exercise during ATP infusion at minute 5 and minute 150 [16.4 ± 4.6 and 20.7 ± 4.7 ml·min−1·100 mmHg−1·dl FAV−1, respectively (P > 0.05)].
DISCUSSION
The main findings of our study are 1) the vasodilator responses to exogenous ATP did not wane over 180 min of continuous infusion, and 2) the magnitude of change (Δ) in the hyperemic responses to exercise during the ATP infusion were similar to those observed during the saline infusion.
Prolonged Vasodilation to Exogenous ATP
Exogenous ATP infusion at rest can evoke levels of blood flow similar to those seen during moderate-intensity exercise (3, 9, 25). We administered ATP at a dose (20 μg·100 ml FAV−1·min−1) designed to evoke a robust forearm vasodilator response (24, 25, 31) equivalent to that observed during moderate-intensity exercise. The forearm model permitted us to use this dose of ATP without systemic hemodynamic changes (see Table 2).
Our results show that the vasodilator responses to exogenous ATP mimic the ability of exercise to evoke prolonged vasodilation in human skeletal muscle. To our knowledge, there are no other studies that have used prolonged infusions of putative vasodilators to study issues related to exercise hyperemia in humans and shown that they mimic the normal physiological responses to exercise over an extended period of time. Thus, ATP meets an additional key criterion for any putative vasodilator substance thought to be critically involved in exercise hyperemia in humans (13).
Hester et al. (11) studied the role of adenosine in exercise hyperemia in an isolated dog gracilis muscle preparation and concluded that adenosine was not obligatory to generate a normal vasodilator response to contractions. They infused adenosine at ∼1,000 times the normal resting venous level and showed tachyphylaxis occurred over the 150 min of infusion. This suggested that the skeletal muscle blood vessels had become desensitized to the vasodilator effects of adenosine. By contrast, in the present study, prolonged ATP infusion did not result in a tachyphylaxis. There are two possible explanations for these divergent observations. First, we did not give a sufficient dose of exogenous ATP to fully engage the ATP dilator system and elicit desensitization similar to that observed for adenosine in the Hester study. However, the dose of ATP that we administered (20 μg·100 ml FAV−1·min−1) was 30-fold higher compared with Rosenmeier et al. (25) without observing any systemic hemodynamic changes, which they reported at a lower dose during infusions into the leg (24, 31). Second, the ectonucleotidedases thought to terminate ATP signaling may cause breakdown of ATP into dephosphorylated vasoactive metabolites, including adenosine, ADP, and AMP. However, it has been shown that adenosine acts on different nucleoside-selective P1 receptors and, thus, the prolonged vasodilator effects of exogenous ATP that we observed are most likely mediated via ATP itself through stimulation of P2Y receptors.
Exercise Hyperemia during Prolonged ATP Infusion
The general approach of infusing a vasodilator agent and performing a moderate-intensity exercise is an extension of the experimental model developed by Patterson and Shepherd (21), who showed that intra-arterial infusion of ATP at a rate sufficient to more than double the resting flow through the forearm had no effect on the blood-debt repayment seen after a brief period of exercise. In humans, one of the major challenges to study the role of ATP in exercise hyperemia is that at present, no pharmacological antagonist is available to block ATP purinergic (P2Y) receptors on the vascular endothelium and smooth muscle cells. To circumvent this challenge our strategy was to administer exogenous ATP at a dose designed to evoke a robust forearm vasodilator response at rest matching that of moderate-intensity exercise blood flow values (24, 25, 31).
In this context, ATP infusion alone in our study caused baseline blood flow to rise equivalently to the levels reached during moderate-intensity rhythmic handgrip exercise (∼710% increase from rest). Importantly, the magnitude of the hyperemic responses to exercise during the ATP infusion was similar to that seen during saline, and the responses to contraction did not change over time. Thus, although we found no evidence that the blood vessels became desensitized to ATP over time in contrast to the Hester et al. (11) observation for adenosine, we did see substantial additional vasodilation when exercise was performed during the ATP infusion.
There are several possible explanations for how exercise performed during exogenous ATP infusion might evoke additional vasodilation. The first explanation is that other substances are responsible for the dilation that we observed. For example, K+-mediated hyperpolarization in conjunction with nitric oxide and prostaglandins appears to be an essential component of the rapid dilator response seen at the onset of exercise and also contributes to the dilator responses seen during 5-min bouts of exercise (4–6). Thus, these dilator systems might still be available to act independently of any ATP-mediated responses. They might also be independent of oxygen delivery compared with “metabolic” signals.
Second, it is also possible that additional endogenous ATP was released during exercise and caused further vasodilation. Rosenmeier et al. (25) have previously shown that a higher dose of ATP infused via the femoral artery is required to evoke a maximum leg blood flow response to exogenous ATP, so it is possible that despite matching exercise blood flow levels, additional amounts of endogenous ATP released may have contributed in the normal dilator response. There are multiple sources of intravascular ATP, including release from endothelial cells and deoxygenating and/or mechanically deformed red blood cells. Red blood cells, in particular, contain a high intracellular ATP concentration and release ATP in response to hypoxia. The mechanism by which the oxygen unloading stimulates ATP release remains uncertain, but it has been suggested the desaturation of hemoglobin triggers ATP release (15).
In this context, it remains possible that increased metabolic demand in the exercising muscle caused additional ATP to be released with oxygen unloading in the microcirculation near the contracting muscles, thereby contributing to a normal vasodilator response to exercise. While theoretically possible, this seems unlikely given the extremely high levels of blood flow evoked by the ATP infusion. It may also be possible that the mechanical effects of contraction on blood flow were amplified when the vessels were dilated by ATP or that deformation of red cells by contraction caused additional ATP to be released. Given that only very modest increases in flow are generated via mechanical compression of the forearm under control conditions, it seems unlikely that a mechanically mediated rise in flow would be increased 5- to 10-fold by the infusion of a vasodilator (14, 28, 30). While our results clearly show that the dilator response to exercise remains normal when baseline blood flow is elevated via an exogenous infusion of ATP, at this time, the mechanism(s) responsible remain obscure. Whatever the mechanism for the increases in blood flow and vasodilation when contractions were performed “on top” of the ongoing ATP infusion, it is important to note that we dissociated blood flow from metabolic demand using this approach. This suggests that at least some components of the exercise hyperemia response can be relatively insensitive to oxygen delivery.
Experimental Considerations
There are several limitations to our study. First, we did not perform a full saline control for 180 min rather only before and after ATP administration. However, previous studies have shown that the dilator responses to repeated bouts of exercise are consistent over time (2). Second, we did not directly measure blood gases for endogenous or exogenous plasma ATP levels. However, given the very high blood flow values evoked by the ATP infusions, we doubt large changes in venous ATP would have been seen during concurrent exercise. Third, we could not measure the membrane-bound and soluble nucleotidases and ectonucleotidase activity, which degrades ATP rapidly (3). However, because the vasodilator responses did not wane over time, our dose was sufficient to have caused robust and continual stimulation of the P2Y receptors. It is also possible that the exogenous ATP may not have dilated the resistance vessels in the forearm, which are most affected by exercise, as seen when exogenous ATP is given to the maximally exercising leg at high altitude (17). Although this seems unlikely, given the moderate level of exercise employed in the present study, the dose of the ATP we used, and importantly, that it was continuously infused during the exercise bouts, it is still not possible to make definitive statements about how the distribution of flow in the forearm might have differed among our experimental conditions.
Summary
In humans, prolonged infusions of exogenous ATP can evoke consistently high and prolonged levels of forearm blood flow and vascular conductance, similar to those seen during moderate intensity exercise. The additional dilation observed when exercise was performed during the ATP infusion suggests that additional vasodilating mechanisms are available and remain intact in spite of a level of blood flow far in excess of metabolic demand. The latter observation demonstrates that under some circumstances, it is possible to disconnect the usual matching of blood flow and oxygen delivery during exercise, challenging the metabolic theory of exercise hyperemia.
GRANTS
This research was supported by National Institutes of Health Research Grants HL-119337 (to M. J. Joyner and F. A. Dinenno) and by the Clinical and Translational Science Award UL1-TR-000135. The Caywood Professorship via the Mayo Foundation also supported this research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.R.S., M.J.J., F.A.D., T.B.C., and S.M.R. conception and design of research; J.R.S., M.J.J., T.B.C., and S.M.R. performed experiments; J.R.S. and S.M.R. analyzed data; J.R.S., M.J.J., F.A.D., and S.M.R. interpreted results of experiments; J.R.S. prepared figures; J.R.S. drafted manuscript; J.R.S., M.J.J., F.A.D., T.B.C., and S.M.R. edited and revised manuscript; J.R.S., M.J.J., F.A.D., T.B.C., and S.M.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to the study volunteers for participation. We thank D. Schroeder and R. Carter for statistical consultation. We also thank M. Mozer, S. Roberts, S. Wolhart, M. Johnson, N. Meyer, C. Johnson, R. Harvey, G. Dillon, S Baker, L. Newhouse, H. Petersen-Jones, A. Eugene, J. Limberg, and W. Holbein for technical assistance.
REFERENCES
- 1.Casey DP, Joyner MJ. Skeletal muscle blood flow responses to hypoperfusion at rest and during rhythmic exercise in humans. J Appl Physiol (1985) 107: 429–437, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casey DP, Ranadive SM, Joyner MJ. Aging is associated with altered vasodilator kinetics in dynamically contracting muscle: role of nitric oxide. J Appl Physiol (1985) 119: 232–241, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crecelius AR, Kirby BS, Dinenno FA. Intravascular ATP and the regulation of blood flow and oxygen delivery in humans. Exerc Sport Sci Rev 43: 5–13, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Crecelius AR, Kirby BS, Hearon CM Jr, Luckasen GJ, Larson DG, Dinenno FA. Contracting human skeletal muscle maintains the ability to blunt alpha1 -adrenergic vasoconstriction during KIR channel and Na+/K+-ATPase inhibition. J Physiol 593: 2735–2751, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. Mechanisms of rapid vasodilation after a brief contraction in human skeletal muscle. Am J Physiol Heart Circ Physiol 305: H29–H40, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crecelius AR, Luckasen GJ, Larson DG, Dinenno FA. KIR channel activation contributes to onset and steady-state exercise hyperemia in humans. Am J Physiol Heart Circ Physiol 307: H782–H791, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol 480: 361–368, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duff F, Patterson GC, Shepherd JT. A quantitative study of the response to adenosine triphosphate of the blood vessels of the human hand and forearm. J Physiol 125: 581–589, 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R, Secher NH, Dawson EA, Dufour SP. Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: insight into the role of muscle mechanisms on cardiovascular function. J Physiol 586: 2405–2417, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 91: 1046–1055, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Hester RL, Guyton AC, Barber BJ. Reactive and exercise hyperemia during high levels of adenosine infusion. Am J Physiol Heart Circ Physiol 243: H181–H186, 1982. [DOI] [PubMed] [Google Scholar]
- 12.Joyner MJ, Casey DP. Muscle blood flow, hypoxia, and hypoperfusion. J Appl Physiol (1985) 116: 852–857, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joyner MJ, Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95: 549–601, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol 583: 861–874, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirby BS, Crecelius AR, Richards JC, Dinenno FA. Sources of intravascular ATP during exercise in humans: critical role for skeletal muscle perfusion. Exp Physiol 98: 988–998, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirby BS, Voyles WF, Carlson RE, Dinenno FA. Graded sympatholytic effect of exogenous ATP on postjunctional α-adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol 586: 4305–4316, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lundby C, Boushel R, Robach P, Moller K, Saltin B, Calbet JA. During hypoxic exercise some vasoconstriction is needed to match O2 delivery with O2 demand at the microcirculatory level. J Physiol 586: 123–130, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Mortensen SP, Gonzalez-Alonso J, Bune LT, Saltin B, Pilegaard H, Hellsten Y. ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol Regul Integr Comp Physiol 296: R1140–R1148, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Mortensen SP, Saltin B. Regulation of the skeletal muscle blood flow in humans. Exp Physiol 99: 1552–1558, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Patterson GC, Shepherd JT. The effects of continuous infusions into the brachial artery of adenosine triphosphate, histamine and acetylcholine on the amount and rate of blood debt repayment following rhythmic exercise of the forearm muscles. Clin Sci (Lond) 13: 85–91, 1954. [PubMed] [Google Scholar]
- 22.Ranadive SM, Joyner MJ, Walker BG, Taylor JL, Casey DP. Effect of vitamin C on hyperoxia-induced vasoconstriction in exercising skeletal muscle. J Appl Physiol (1985) 117: 1207–1211, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards JC, Crecelius AR, Kirby BS, Larson DG, Dinenno FA. Muscle contraction duration and fibre recruitment influence blood flow and oxygen consumption independent of contractile work during steady-state exercise in humans. Exp Physiol 97: 750–761, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rongen GA, Smits P, Thien T. Characterization of ATP-induced vasodilation in the human forearm vascular bed. Circulation 90: 1891–1898, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Rosenmeier JB, Hansen J, Gonzalez-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol 558: 351–365, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand 162: 421–436, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Shepherd J. Circulation to skeletal muscle. In: Handbook of Physiology:The Cardiovascular System, Peripheral Circulation and Organ Blood Flow. Bethesda, MD: American Physiological Society, sect. 2, vol. 3, part 1, 1983, p. 219–370. [Google Scholar]
- 28.Tschakovsky ME, Sheriff DD. Immediate exercise hyperemia: contributions of the muscle pump vs. rapid vasodilation. J Appl Physiol (1985) 97: 739–747, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol 541: 623–635, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol 541: 623–635, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Ginneken EE, Meijer P, Verkaik N, Smits P, Rongen GA. ATP-induced vasodilation in human skeletal muscle. Br J Pharmacol 141: 842–850, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]