Abstract
Chronic kidney disease (CKD) is a worldwide public health problem that affects millions of people from all racial and ethnic groups. Although CKD is not one specific disease, it is a comprehensive syndrome that includes IgA nephropathy. As reported by the Japanese Society of Nephrology, 13.0 million people have CKD. In Japan, major causes of end-stage kidney disease are type 2 diabetic nephropathy, chronic glomerulonephritis, especially IgA nephropathy, hypertensive nephrosclerosis, and polycystic kidney disease. IgA nephropathy is characterized by polymeric IgA1 with aberrant galactosylation (galactose-deficient IgA1) increased in the blood and deposited in the glomerular mesangial areas, as well as partially in the capillary walls. The tonsils are important as one of the responsible regions in this disease. The clarification of the mechanism of galactose-deficient IgA1 production will pave the way for the development of novel therapies. The results of future research are eagerly awaited. At present, the most important therapeutic goals in patients with IgA nephropathy are the control of hypertension, the decrease of urinary protein excretion, and the inhibition of progression to end-stage kidney disease. Several investigators have reported that renin–angiotensin–aldosterone system inhibitors reduce levels of urinary protein excretion and preserve renal function in patients with IgA nephropathy. In Japan, tonsillectomy and steroid pulse therapy are more effective for patients with IgA nephropathy.
Keywords: Diagnosis, IgA nephropathy, Steroid pulse therapy, Tonsillectomy, Treatment
Introduction
Chronic kidney disease (CKD) is a worldwide public health problem that affects millions of people from all racial and ethnic groups. Although CKD is not one specific disease, it is a comprehensive syndrome. In Japan, major causes of end-stage kidney disease (ESKD) are type 2 diabetic nephropathy, chronic glomerulonephritis, especially IgA nephropathy, hypertensive nephrosclerosis, and polycystic kidney disease. According to the 2014 annual report by the Japanese Society of Dialysis Therapy, the total number of dialysis patients was 320,448. Among those patients, > 95% have had hemodialysis therapy. The leading cause of ESKD has been diabetes (43.5%), instead of chronic glomerulonephritis (17.8%), since 1998 [1]. Therefore, these major diseases are also causal diseases of CKD. In Japan, 40–60% of patients with chronic glomerulonephritis are diagnosed as having IgA nephropathy by immunofluorescence.
In this review, I would like to focus on the diagnosis and treatment of patients with IgA nephropathy. The first part covers the definition of CKD in Japan. The second part focuses on the diagnosis and treatment of patients with IgA nephropathy based on a case presentation.
Definition of CKD in Japan
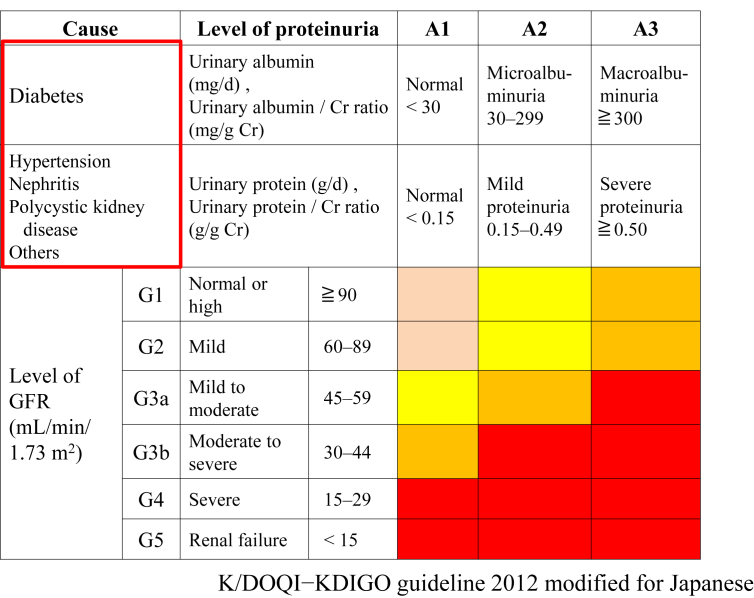
The characteristics of CKD are common, harmful, and treatable. CKD is defined as kidney damage, confirmed by a renal biopsy or damage marker, or a glomerular filtration rate (GFR) of < 60 mL/min/1.73 m2 for > 3 months. Among CKD patients, the stages of the disease were, in the past, based on GFR levels, irrespective of the cause of kidney disease. However, the revised classification of CKD from 2012 is based on cause (C), GFR (G), and albuminuria (A), i.e., the CGA classification in Japan [2]. CKD patients have been shown to have (1) a risk for the loss of kidney function (there are a large number of CKD patients behind the increasing number of people with ESKD); (2) a risk for cardiovascular disease, mortality, and hospitalization; and (3) financial problems.
In Fig. 1, the red areas of CKD stage classification on the heat map show high risks for ESKD, cardiovascular disease, mortality, and hospitalization. First, the causal disease, such as diabetes, hypertension, nephritis, polycystic kidney disease, or an unknown disease, is written in the blank (C). Next, the levels of GFR (mainly estimated GFR [eGFR]) and proteinuria are divided into 6 stages and 3 degrees, respectively. Because the measurement of urinary albumin excretion is covered by the Japanese national insurance system for only diabetes patients, and that of urinary protein excretion is used for nondiabetic patients (Fig. 1).
Figure 1.
New revised CKD (cause) classification in Japan (Japanese Society of Nephrology 2012).
CKD, chronic kidney disease; Cr, creatinine; GFR, glomerular filtration rate.
Case presentation of IgA nephropathy
Case: a 28-year-old business man.
Chief complaint: proteinuria and microscopic hematuria.
Past medical history: unremarkable.
Clinical course/laboratory tests: At age 25 years, proteinuria and microscopic hematuria were first detected during an employment health examination. The patient was hospitalized for a renal biopsy. Urinalysis on hospital admission showed 2+ proteinuria and 2+ hematuria by qualitative examination. Positive qualitative proteinuria quantified at 0.38 g/g Cr. The urinary sediment revealed numerous red blood cells (RBCs)/high power field (HPF) after acute tonsillitis with sore throat. Dysmorphic RBCs were also observed in the urinary sediment, but not marked. However, the patient had no other symptoms, including abdominal pain, arthralgia, purpura, erythema, or fever. Evaluation of renal function showed a serum urea nitrogen level of 15 mg/dL, serum creatinine (sCr) of 0.9 mg/dL, and eGFR of 83.7 mL/min. The markers of tubulointerstitial injury in the urinary samples, i.e., β2-microglobulin (β2MG), N-acetyl-β-d-glucosaminidase, showed normal ranges. In addition, renal ultrasonography revealed no abnormalities. Serological studies showed an elevated serum IgA level of 456 mg/dL (normal range, 110–410 mg/dL), but other immunoglobulin levels and complement activity titers (CH50) were normal. The level of serum C3 was 140 mg/dL (normal range, 86–160 mg/dL). The serum IgA/C3 ratio was 3.26. The serum levels of antinuclear antibody and anti-dsDNA antibody were normal.
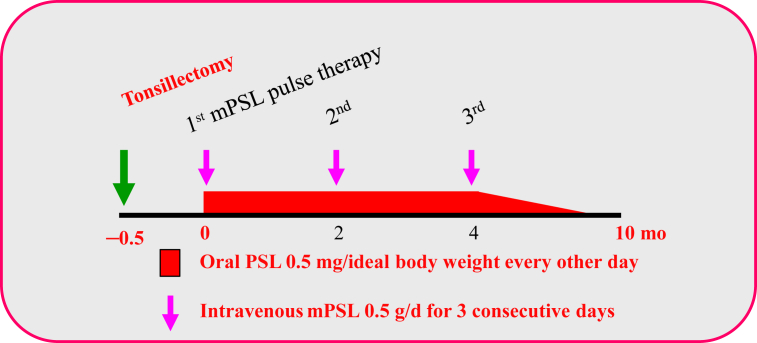
After hospital admission, renal function continued to be normal, but the proteinuria and microscopic hematuria also persisted. After renal biopsy, tonsillectomy and steroid pulse therapy were performed as shown in Fig. 2. About 2 weeks after tonsillectomy, the administration of 0.5 g/d methylprednisolone (steroid pulse therapy) was performed for 3 consecutive days. Oral prednisolone 0.5 mg/ideal body weight every other day was administered between each pulse therapy. After that therapy, the dosages of steroid were gradually tapered and then completed. The proteinuria gradually improved to (±) or (+). Examination of the urinary sediment revealed 1–4 RBCs/HFP. The serum IgA level was 385 mg/dL (normal range, 110–410 mg/dL), and the level of serum C3 was 130 mg/dL (normal range, 86–160 mg/dL). Thus, the serum IgA/C3 ratio was 2.96.
Figure 2.
Protocol of tonsillectomy and steroid pulse therapy for IgA nephropathy patients[19], [20].
mPSL, methylprednisolone; PSL, prednisolone.
Abnormal clinical findings and laboratory data
Clinical findings: chance proteinuria and chance hematuria as well as increase of microscopic hematuria after acute tonsillitis.
Blood tests: serum IgA of 456 mg/dL, serum IgA/C3 ratio of 3.26. The levels of serum IgA and the IgA/C3 ratio were decreased after tonsillectomy and steroid pulse therapy.
Urinalysis: 2+ proteinuria and 2+ hematuria by qualitative examination, proteinuria quantified at 0.38 g/g Cr, numerous RBCs/HPF in urinary sediment after upper respiratory tract infection. After the tonsillectomy and steroid pulse therapy, the proteinuria gradually decreased and improved to (±) or (+). An examination of the urinary sediment revealed 1–4 RBCs/HPF.
Clinical diagnosis by CKD classification: nephritis, G2, A2.
Cause (C): nephritis (suspected of primary chronic glomerulonephritis);
Stage: G2 (eGFR of 83.7 mL/min; CKD G2 stage range, 60–89 mL/min);
Proteinuria: A2 (proteinuria of 0.38 g/gCr; CKD A2 range: 0.15–0.49 g/g Cr);
Clinical (C) grade of IgA nephropathy using the Japanese clinical classification: C-grade I (grade I range, < 0.49 g/g Cr of urinary protein and > 60 mL/min/1.73 m2) [3].
Definite diagnosis: IgA nephropathy
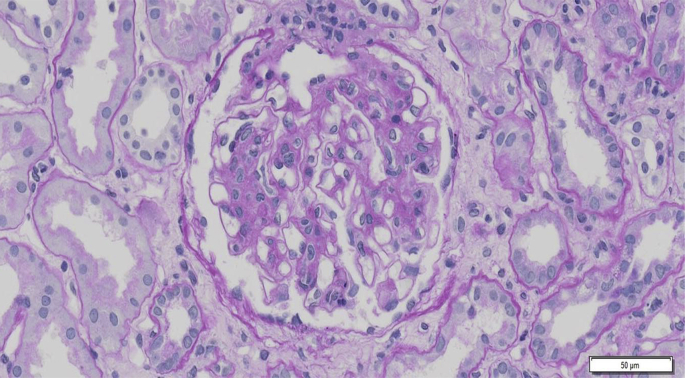
Renal biopsy is required for the definite diagnosis of IgA nephropathy. Granular depositions of IgA and C3 were mainly observed in the glomerular mesangial areas (Fig. 3). Expansion of mesangial matrices with mesangial cell proliferation was observed by periodic acid-Schiff staining (Fig. 4). Electron-dense deposits were mainly observed in the mesangial areas. These findings were consistent with IgA nephropathy.
Figure 3.
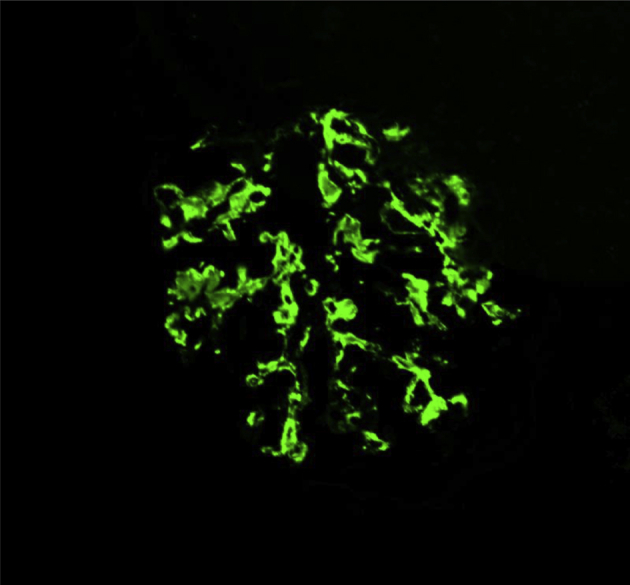
Immunofluorescence of IgA in a glomerulus. Granular deposition of IgA in glomerular mesangial areas (IgA staining) is shown.
Figure 4.
Light microscopic findings of a renal tissue. Expansion of mesangial matrices with mesangial cell proliferation (periodic acid-Schiff staining) is shown.
In Japan, the Japanese classification and the Oxford classification are used for the histological diagnosis of IgA nephropathy patients (Table 1).
-
1.
Japanese histological grading classification (lumped system): H-grade I A/C as shown in Table 1 [4]. Stratification of dialysis induction risk for patients with IgA nephropathy: low risk according to the C-grade I and H-grade I [3], [4].
-
2.
Oxford Classification (split system): M1E1S0T0 as shown in Table 1.
Table 1.
Comparison between Oxford classification and JHGC
| 1. Oxford classification: split system | ||
|---|---|---|
| M | Mesangial hypercellularity | ≤ 0.5 (M0) or ≥ 0.5 (M1) |
| E | Endocapillary hypercellularity | Absent (E0) or present (E1) |
| S | Segmental sclerosis | Absent (S0) or present (S1) |
| T | Tubular atrophy/interstitial fibrosis | < 25% (T0), 26–50% (T1), or > 50% (T2) |
| 2. Japanese histological grading classification: lumped system | ||||
|---|---|---|---|---|
| Histological grade | No of lesions*/total no. of glomeruli | Active lesions only | Active lesion + chronic lesion | Chronic lesion only |
| H-grade I | 0–24.9% | A | A/C | C |
| H-grade II | 25–49.9% | A | A/C | C |
| H-grade III | 50–74.9% | A | A/C | C |
| H-grade IV | 75% | A | A/C | C |
Active lesion (A) indicates cellular crescent and fibrocellular crescent; chronic lesion (C) indicates global sclerosis, segmental sclerosis, fibrous crescent.
JHGC, Japanese Histological Classification.
*Lesions.
Pathogenesis of IgA nephropathy
IgA nephropathy (nephropathy with mesangial IgA and IgG deposits, so-called Berger disease in France) is the most common type of primary chronic glomerulonephritis in the world and was first described by Berger and Hinglais in 1968 [5]. IgA nephropathy has a significant morbidity, culminating in ESKD in about 40% of patients within 20 years of the diagnosis. Histopathologically, IgA nephropathy is characterized by the expansion of glomerular mesangial matrices with mesangial cell proliferation and/or mononuclear cell infiltration. Glomeruli typically contain generalized diffuse granular mesangial deposits of IgA (mainly polymeric IgA1), IgG, and C3. Electron microscopy revealed electron-dense deposits in the glomerular mesangial areas and/or partially in the glomerular basement membrane. Therefore, this disease is generally considered to be an immune complex–mediated glomerulonephritis although the antigenic substances are still unknown. It is postulated that many antigenic substances, i.e., viruses, bacteria, fungi, or food, may stimulate the immune complex formation in patients with IgA nephropathy.
Because the pathogenesis of IgA nephropathy is still obscure, efforts made by many investigators around the world have gradually clarified various aspects of the pathogenesis of IgA nephropathy, as edited recently [3], [4]. There are many progressive factors that contribute to disease progression in patients with IgA nephropathy: (1) complement activation; (2) podocyte injury (podocyte loss: so-called podocytopenia); (3) activation of reactive oxygen species, cytokines, chemokines, and/or blood coagulation; (3) mast cell and/or lymphocyte infiltration in the interstitium; and (4) increase of fibrosis in the interstitium. Predictive factors of this disease in Japan are shown in Table 2.
Table 2.
Summary of the nationwide survey of IgA nephropathy in Japan (Ministry of Health, Labor and Welfare, Japan, 2005)
| Predictive factors after 10 y |
| Male |
| Under 30 y old |
| Diastolic hypertension |
| Heavy proteinuria |
| Mild hematuria |
| Low serum albumin |
| Elevated serum creatinine |
| Impaired renal histopathology |
New biomarkers for diagnosis
Blood samples
Galactose-deficient IgA1
It is necessary to develop noninvasive diagnostic biomarkers before renal biopsy or without biopsy in patients with CKD. We have already reported the importance of 4 clinical markers in the diagnosis of patients with IgA nephropathy or in the differential diagnosis from other types of primary chronic glomerulonephritis: (1) > 5 RBCs/HPF in urinary sediments, (2) persistent proteinuria (urinary protein of > 0.3 g/d), (3) a serum IgA level of > 315 mg/dL, and (4) a serum IgA/C3 ratio of > 3.01. Patients with 3 or 4 clinical markers were easily diagnosed as having IgA nephropathy in our previous reports [6], [7]. Recently, Shimizu et al have measured the serum IgA/C3 ratio and change ratio of the serum IgA/C3 ratio (⊿IgA/C3/y) of IgA nephropathy patients at the time of renal biopsy and on the nearest consultation day in an outpatient clinic of the Juntendo University Hospital. ⊿IgA/C3/y was positively correlated to ⊿IgA/sCr/y. ⊿IgA/C3/y decreased along with the severity of histological grade, clinical grade, and ESKD risk. The patients with tonsillectomy had a smaller ⊿IgA/C3/y than those without tonsillectomy. Thus, it appears that the serum IgA/C3 ratio shows the activity of IgA nephropathy, and the chronological change of the IgA/C3 ratio indicates the efficacy of tonsillectomy for IgA nephropathy (submitted for publication).
However, it is necessary to develop new biomarkers for the early detection of this disease before or without renal biopsy. Several recent studies suggest that aberrant O-glycosylation of circulatory IgA1 is vital in the pathogenesis of IgA nephropathy. The O-linked glycans in the hinge region of IgA1 are generally composed of N-acetylgalactosamine and galactose; sialic acid may be attached to either or both sugars. IgA1-producing cells secrete a mixture of IgA1 O-glycoforms. Studies in the different populations have shown that IgA nephropathy patients have significantly higher levels of circulating IgA1 with galactose-deficient, O-linked, hinge-region glycans. Galactose-deficient IgA1 (Gd-IgA1) is a critical effector molecule in the pathogenesis of IgA nephropathy [8]. Therefore, we examined the prevalence of elevated serum levels of IgA, Gd-IgA1, and glycan-specific IgG and IgA in IgA nephropathy patients and a large cohort of CKD patients to assess the utility of these biomarkers for the noninvasive diagnosis of IgA nephropathy. It was revealed that this panel of biomarkers is helpful in differentiating patients with IgA nephropathy from patients with other glomerular diseases. Yanagawa et al [9] compared the serum levels of IgA, IgG, Gd-IgA1, Gd-IgA1–specific IgG, and Gd-IgA1–specific IgA in 135 IgA nephropathy patients, 79 patients with non–IgA nephropathy CKD, and 106 healthy controls. Serum was collected at the time of renal biopsy from all IgA nephropathy and non–IgA nephropathy CKD patients. Serum levels of Gd-IgA1–specific antibodies are elevated in most IgA nephropathy patients, and their assessment, together with serum levels of Gd-IgA1, improves the specificity of the assays. It appears that a panel of serum biomarkers may be helpful in differentiating IgA nephropathy from other glomerular diseases [9].
Although many researchers have measured serum levels of Gd-IgA1 using a snail helix aspersa agglutinin lectin–based assay, the lectin-dependent assay has some serious problems in robustness. Yasutake et al [10] aimed to establish a more robust and stable enzyme-linked immunosorbent assay (ELISA) method that uses a specific monoclonal antibody to recognize a hinge region in human Gd-IgA1 (Gd-IgA1 ELISA). Levels of serum Gd-IgA1 measured by Gd-IgA1 ELISA in patients with IgA nephropathy were significantly increased compared with those in patients with other renal diseases or nonrenal diseases. The results obtained from Gd-IgA1 by Gd-IgA1 ELISA were positively correlated with those obtained by a helix aspersa agglutinin lectin–based assay (r = 0.75). Recently, an antihuman Gd-IgA1 rat IgG monoclonal antibody (KM55) has been newly released by IBL-Japan in 2016 (http://www.ibl-japan.co.jp). As such, we are now able to measure the levels of serum Gd-IgA1 using Gd-IgA1 ELISA (code no. 27600) commercially.
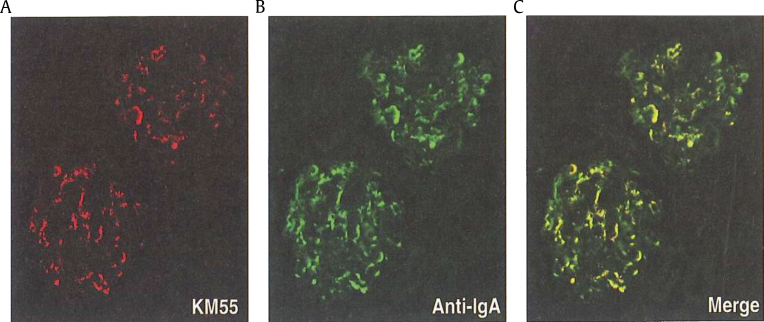
Immunofluorescent staining of glomeruli with KM55 (Gd-IgA1–specific monoclonal antibody) was co-localized with Gd-IgA1 and IgA (Fig. 5). It appears that the novel lectin-independent method with KM55 for the measurement of serum levels of Gd-IgA1 can pave the way for a more convincing diagnosis and activity assessment of IgA nephropathy [10].
Figure 5.
Immunofluorescence of Gd-IgA1 and IgA in glomeruli. Glomerular depositions of Gd-IgA1 (A), IgA (B), and their merge (C). (A) KM55, (B) anti-IgA, and (C) merge [10]. Gd-IgA1 was co-localized with IgA in glomeruli by double immunofluorescence.
Gd-IgA1, galactose-deficient IgA1.
Tumor necrosis factor receptors 1 and 2
IgA nephropathy is characterized by the mesangial deposition of pathogenetic polymeric Gd-IgA1, proliferation of mesangial cells, the increased synthesis of extracellular matrices, and the infiltration of macrophages, monocytes, and T cells. There is a strong correlation between the severity of renal interstitial damage and subsequent renal function decline in IgA nephropathy and diabetic nephropathy. Chan et al [11] have clearly demonstrated that tumor necrosis factor-α released from mesangial cells after IgA deposition activates renal tubular cells and leads to subsequent inflammatory changes in the renal interstitium. Some investigators have reported that the levels of circulating tumor necrosis factor (TNF) pathway–related molecules, such as tumor necrosis factor-α and TNF receptors (TNFRs), are significantly higher in CKD patients. In 2015, Sonoda et al [12] examined whether the levels of TNF receptors 1 and 2 in serum and urine were associated with other markers of kidney injury and renal TNFR expression in IgA nephropathy. Serum TNFR levels were positively correlated with the urinary protein-to-creatinine ratio and 4 tubular damage biomarkers, i.e., N-acetyl-β-d-glucosaminidase, β2MG, liver-type fatty acid–binding protein, and kidney injury molecule-1, and negatively correlated with eGFR. Patients in the highest tertile of serum TNFR levels showed more severe renal interstitial fibrosis than did those in the lowest or second tertiles. Urinary TNFRs were strongly correlated with all 4 tubular damage markers. The tubulointerstitial TNF receptor 2–positive area was significantly correlated with serum levels of TNFRs and eGFR. They concluded that elevated serum TNFR levels were significantly associated with the severity of renal interstitial fibrosis in IgA nephropathy patients [12]. However, they indicated that the source of TNFRs in serum and urine remains unclear.
Urine samples
Primary (primitive) urine after filtration from glomeruli contains albumin and/or other components, such as chemokines, cytokines, complements, transferrin, and others. Those components may stimulate the proximal tubular epithelial cells. After that, the stimulated (injured) epithelial cells may produce the same components and then induce interstitial injury. Thus, it is thought that heavy protein in the primary urine is a result of glomerular and/or vascular damage and also a cause of tubulointerstitial damage.
Podocalyxin has presented on the apical cell membrane of podocytes and is shed in the urine from injured podocytes. It is generally considered that podocyte injury may induce glomerulosclerosis in various glomerular diseases, including IgA nephropathy. Urinary podocalyxin is associated with the severity of active glomerular injury in patients with glomerular diseases. Asao et al [13] examined the relationship between the number of urinary podocytes and the levels of urinary podocalyxin and glomerular injury in adult IgA nephropathy patients. These results showed that the levels of urinary podocalyxin and the number of urinary podocytes are useful biomarkers for predicting histological changes in adult IgA nephropathy patients [13].
Furthermore, glomerular damage in IgA nephropathy is mediated by complement activation via the alternative and lectin pathways. Onda et al [14] reported that urinary membrane attack complex and factor H levels were positively correlated with sCr, urinary N-acetyl-β-d-glucosaminidase, urinary β2MG, urinary protein, interstitial fibrosis, and the percentage of global glomerular sclerosis. They concluded that complement activation occurs in the urinary space in IgA nephropathy, and the measurement of levels of membrane attack complex and factor H in the urine could be a useful indicator for renal injury in patients with IgA nephropathy [14].
Treatment of IgA nephropathy
We have usually used drugs for IgA nephropathy patients according to the recommended grade of the Evidence-based Clinical Practice Guideline for CKD (Japanese Society of Nephrology, 2013, article in Japanese) [2], [3] (Table 3). There are many therapeutic strategies: (1) adequate exercise and a low protein diet (decrease of dietary protein intake according to renal function as long-term dietary restriction is generally considered to reduce the levels of urinary protein and ameliorate glomerular injuries in patients with IgA nephropathy); (2) antiplatelet drugs (dipyridamole, Persantin; dilazep hydrochloride, Comelian): recommendation grade C1; (3) antihypertensive drugs (renin-angiotensin system inhibitor: angiotensin receptor blocker, i.e., olmesartan, Olmetec), urinary protein of > 1.0 g/d and CKD G1–G3b: recommendation grade A; urinary protein of 0.5–1.0 g/d: recommendation grade C1; Ca channel blocker, i.e., benidipine (Coniel): no recommendation; (4) oral adrenocorticosteroid (prednisolone; Predonine), urinary protein of > 1.0 g/d and CKD G1–G2: recommendation grade B; urinary protein of < 1.0 g/d and CKD G1–G2: recommendation grade C; (5) steroid pulse therapy: urinary protein of > 1.0 g/d and CKD G1–G2: recommendation grade B; (6) tonsillectomy with steroid pulse therapy: grade C1; and (7) immunosuppresants: recommendation grade C1.
Table 3.
Rating description of the guideline recommendation (Evidence-Based Clinical Guideline for CKD, JSN, 2013) [3]
| Recommendation grade |
|---|
|
|
|
|
|
CKD, chronic kidney disease; JSN, Japanese Society of Nephrology.
Because macroscopic or microscopic hematuria is observed after upper respiratory tract infections in IgA nephropathy patients, a relationship between tonsillar infection and this disease has been argued. The tonsils are a mucosal lymphatic organ, and polymeric IgA1 is produced on the mucosal membrane. A part of this polymeric IgA1 is considered as a nephritogenic IgA1. The plasma cells, which produce circulating nephritogenic polymeric IgA1, and glomerular deposited immune complexes are formed there. A combined tonsillectomy and steroid pulse therapy has been developed and mainly performed in Japan [15], [16], [17]. The objective of this therapy is to decrease plasma cells producing nephritogenic IgA1 in the tonsils and to prevent the cells from transferring to the bone marrow [18]. Nakata et al [19] indicated the palatine tonsils are probably a major site of Gd-IgA1–producing cells. However, these cells in some patients may propagate to other lymphoid organs, which may partially explain the different responses observed to tonsillectomy alone. In a multicenter randomized controlled trial, Kawamura et al [20] reported that the antiproteinuric effect was significantly greater in the group that received tonsillectomy with steroid pulse therapy in patients with IgA nephropathy. However, the difference was marginal, and its impact on renal functional outcomes remains to be clarified. The effects of tonsillectomy with steroid pulse therapy still need to be determined from multicenter clinical trials in the future.
Conclusion and future perspectives
IgA1 with aberrant galactosylation (Gd-IgA1) is increased in the blood and deposited in the glomerular mesangial areas as well as partially in the capillary walls in patients with IgA nephropathy. The tonsils are important as one of the responsible regions in this disease. The clarification of the mechanism of Gd-IgA1 production will pave the way for the development of novel therapies. The results of future research are eagerly awaited. We are now able to measure the levels of serum Gd-IgA1 using Gd-IgA1 ELISA (code no. 27600) commercially.
Because the pathogenesis of IgA nephropathy is still obscure, the efforts made by many investigators around the world have gradually clarified various aspects of the pathogenesis and treatment of this disease. It is necessary to clarify the pathogenesis of IgA nephropathy and to newly develop treatments for the inhibition of the progression to ESKD in patients with IgA nephropathy. Because the treatment of IgA nephropathy is still controversial internationally, several current topics of etiology and treatment should be discussed among the various societies of nephrology, including the Korean Society of Nephrology.
Conflicts of interest
The author has no conflicts of interest to declare.
Acknowledgments
The author wishes to thank colleagues in the Division of Nephrology, Department of Internal Medicine, Juntendo University Faculty of Medicine, and Medical Corporation SHOWAKAI, Tokyo, Japan.
References
- 1.Dialysis Therapy (JSDT) 2014. Reports from the Japanese Society for Dialysis Therapy (JSDT) [in Japanese] [Google Scholar]
- 2.Japanese Society of Nephrology (JSN) Tokyo Igakusya; Tokyo: 2013. Evidence-based Clinical Practice Guideline for CKD 2013; pp. ix–xiii. [in Japanese] [Google Scholar]
- 3.Katafuchi R. Differences in etiology and treatment in Japan. In: Tomino Y., editor. Pathogenesis and Treatment in IgA Nephropathy: An International Comparison. Springer; Tokyo: 2016. pp. 167–207. [Google Scholar]
- 4.Joh K., McNamara K.M. Differences of histological classification between the Japanese histological grade classification and the Oxford classification. In: Tomino Y., editor. Pathogenesis and Treatment in IgA Nephropathy: An International Comparison. Springer; Tokyo: 2016. pp. 69–87. [Google Scholar]
- 5.Berger J., Hinglais N. Intercapillary deposits of IgA-IgG. J Urol Nephrol (Paris) 1968;74:694–695. [PubMed] [Google Scholar]
- 6.Tomino Y., Suzuki S., Imai H., Saito T., Kawamura T., Yorioka N., Harada T., Yasumoto K., Kida H., Kobayashi Y., Endoh M., Sato H., Saito K. Measurement of serum IgA and C3 may predict the diagnosis of patients with IgA nephropathy prior to renal biopsy. J Clin Lab Anal. 2000;14:220–223. doi: 10.1002/1098-2825(2000)14:5<220::AID-JCLA4>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeda A., Gohda T., Funabiki K., Horikoshi S., Shirato I., Tomino Y. Significance of serum IgA levels and serum IgA/C3 ratio in diagnostic analysis of patients with IgA nephropathy. J Clin Lab Anal. 2003;17:73–76. doi: 10.1002/jcla.10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novak J., Julian B.A., Tomana M., Mesteck J. Progress in molecular and genetic studies of IgA nephropathy. J Clin Immunol. 2001;21:310–327. doi: 10.1023/a:1012284402054. [DOI] [PubMed] [Google Scholar]
- 9.Yanagawa H., Suzuki H., Suzuki Y., Kiryluk K., Gharavi A.G., Matsuoka K., Makita Y., Julian B.A., Novak J., Tomino Y. A panel of serum biomarkers differentiates IgA nephropathy from other renal diseases. PLoS One. 2014;9:e98081. doi: 10.1371/journal.pone.0098081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasutake J., Suzuki Y., Suzuki H., Hiura N., Yanagawa H., Makita Y., Kaneko E., Tomino Y. Novel lectin-independent approach to detect galactose-deficient IgA1 in IgA nephropathy. Nephrol Dial Transplant. 2015;30:1315–1321. doi: 10.1093/ndt/gfv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan L.Y., Leung J.C., Tsang A.W., Tang S.C., Lai K.N. Activation of tubular epithelial cells by mesangial-derived TNF-alpha: glomerulotubular communication in IgA nephropathy. Kidney Int. 2005;67:602–612. doi: 10.1111/j.1523-1755.2005.67116.x. [DOI] [PubMed] [Google Scholar]
- 12.Sonoda Y., Gohda T., Suzuki Y., Omote K., Ishizaka M., Matsuoka J., Tomino Y. Circulating TNF receptors 1 and 2 are associated with the severity of renal interstitial fibrosis in IgA nephropathy. PLoS One. 2015;10:e0122212. doi: 10.1371/journal.pone.0122212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asao R., Asanuma K., Kodama F., Akiba-Takagi M., Nagai-Hosoe Y., Seki T., Takeda Y., Ohsawa I., Mano S., Matsuoka K., Kurosawa H., Ogasawara S., Hirayama Y., Sekine S., Horikoshi S., Hara M., Tomino Y. Relationship between levels of urinary podocalyxin, number of podocytes, and histological injury in adult patients with IgA nephropathy. Clin J Am Soc Nephrol. 2012;7:1385–1393. doi: 10.2215/CJN.08110811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onda K., Ohsawa I., Ohi H., Tamano M., Mano S., Wakabayashi M., Toki A., Horikoshi S., Fujita T., Tomino Y. Excretion of complement proteins and its activation marker C5b-9 in IgA nephropathy in relation to renal function. BMC Nephrol. 2011;12:64. doi: 10.1186/1471-2369-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hotta O., Miyazaki M., Furuta T., Tomioka D., Chiba S., Horigome I., Abe K., Taguma Y. Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. Am J Kidney Dis. 2001;38:736–743. doi: 10.1053/ajkd.2001.27690. [DOI] [PubMed] [Google Scholar]
- 16.Xie Y., Nishi S., Ueno M., Imai N., Sakatsume M., Narita I., Suzuki Y., Akazawa K., Shimada H., Arakawa M., Gejyo F. The efficacy of tonsillectomy on long-term renal survival in patients with IgA nephropathy. Kidney Int. 2003;63:1861–1867. doi: 10.1046/j.1523-1755.2003.00935.x. [DOI] [PubMed] [Google Scholar]
- 17.Matsuzaki K., Suzuki Y., Nakata J., Sakamoto N., Horikoshi S., Kawamura T., Matsuo S., Tomino Y. Nationwide survey on current treatments for IgA nephropathy in Japan. Clin Exp Nephrol. 2013;17:827–833. doi: 10.1007/s10157-013-0779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki Y., Tomino Y. Potential immunopathogenic role of the mucosa-bone marrow axis in IgA nephropathy: insights from animal models. Semin Nephrol. 2008;28:66–77. doi: 10.1016/j.semnephrol.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Nakata J., Suzuki Y., Suzuki H., Sato D., Kano T., Yanagawa H., Matsuzaki K., Horikoshi S., Novak J., Tomino Y. Changes in nephritogenic serum galactose-deficient IgA1 in IgA nephropathy following tonsillectomy and steroid therapy. PLoS One. 2014;9:e98081. doi: 10.1371/journal.pone.0089707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawamura T., Yoshimura M., Miyazaki Y., Okamoto H., Kimura K., Hirano K., Matsushima M., Utsunomiya Y., Ogura M., Yokoo T., Okonogi H., Ishii T., Hamaguchi A., Ueda H., Furusu A., Horikoshi S., Suzuki Y., Shibata T., Yasuda T., Shirai S., Imasawa T., Kanozawa K., Wada A., Yamaji I., Miura N., Imai H., Kasai K., Soma J., Fujimoto S., Matsuo S., Tomino Y., The Special IgA Nephropathy Study Group A multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy in patients with immunoglobulin A nephropathy. Nephrol Dial Transplant. 2014;29:1548–1553. doi: 10.1093/ndt/gfu020. [DOI] [PMC free article] [PubMed] [Google Scholar]