Despite important clinical and physiological significance there is currently limited information on how irradiation interacts with skeletal muscle unloading. Our study examined the independent and combined effects of acute irradiation exposure and short-term unloading on muscle Akt signaling. We report that an acute dose of irradiation is sufficient to alter unloading-induced regulation of Akt signaling.
Keywords: atrophy, microgravity, MuRF-1, mTORC1, unloading, protein turnover
Abstract
Muscle irradiation (IRR) exposure can accompany unloading during spaceflight or cancer treatment, and this has been shown to be sufficient by itself to induce skeletal muscle signaling associated with a remodeling response. Although protein kinase B/Akt has an established role in the regulation of muscle growth and metabolism, there is a limited understanding of how Akt signaling in unloaded skeletal muscle is affected by IRR. Therefore, we examined the combined effects of acute IRR and short-term unloading on muscle Akt signaling. Female C57BL/6 mice were subjected to load bearing or hindlimb suspension (HS) for 5 days (n = 6/group). A single, unilateral hindlimb IRR dose (0.5 Gy X-ray) was administered on day 3. Gastrocnemius muscle protein expression was examined. HS resulted in decreased AktT308 phosphorylation, whereas HS+IRR resulted in increased AktT308 phosphorylation above baseline. HS resulted in reduced AktS473 phosphorylation, which was rescued by HS+IRR. Interestingly, IRR alone resulted in increased phosphorylation of AktS473, but not that of AktT308. HS resulted in decreased mTORC1 signaling, and this suppression was not altered by IRR. Both IRR and HS resulted in increased MuRF-1 expression, whereas atrogin-1 expression was not affected by either condition. These results demonstrate that either IRR alone or when combined with HS can differentially affect Akt phosphorylation, but IRR did not disrupt suppressed mTORC1 signaling by HS. Collectively, these findings highlight that a single IRR dose is sufficient to disrupt the regulation of Akt signaling in atrophying skeletal muscle.
NEW & NOTEWORTHY
Despite important clinical and physiological significance there is currently limited information on how irradiation interacts with skeletal muscle unloading. Our study examined the independent and combined effects of acute irradiation exposure and short-term unloading on muscle Akt signaling. We report that an acute dose of irradiation is sufficient to alter unloading-induced regulation of Akt signaling.
skeletal muscle comprises ∼40% of total body mass, and the maintenance of this mass is critical for metabolic health and physical function (52). Muscle mass and function is dramatically influenced by mechanical loading, and disuse atrophy is accompanied by disrupted muscle protein turnover that involves suppressed protein synthesis and activated protein degradation (27). Ionizing radiation exposure can accompany spaceflight-induced unloading or cancer treatment-induced disuse (1, 4, 50), and has the potential to disrupt the normal muscle responses to these stimuli (20, 25). Furthering our understanding of the interaction of ionizing radiation with skeletal muscle plasticity has clear clinical significance for the health of patients with cancer and for space travel.
The serine/threonine kinase Akt, also known as protein kinase B, plays a critical role in muscle in the successful integration of anabolic and catabolic signaling, which are initiated by growth factors, nutrients, and muscle use (44). Akt activation is primarily controlled by phosphorylation at two primary residues. The serine/threonine kinase phosphoinositide-dependent kinase 1 (PDK1) is responsible for T308 phosphorylation (3), whereas the rapamycin-insensitive mTOR complex (mTORC2) can phosphorylate S473 (18). Phosphorylation of S473 is responsible for the stabilization of Akt in its active conformation state (55). Enhanced Akt signaling leads to downstream activation of protein synthesis through the mammalian target of rapamycin complex 1 (mTORC1) and glycogen synthase kinase-3β (GSK3β) (44). Akt directly phosphorylates and inhibits GSK3β at S9, leading to the activation of eukaryotic translation initiation factor 2B (eIF2B) and protein synthesis (15, 42, 44). Additionally, Akt can indirectly activate mTORC1 through the phosphorylation of tuberous sclerosis 2 (TSC2), which relieves the inhibitory effects of the TSC1/2 complex on mTORC1 and the subsequent downstream activation of P70S6K and ribosomal protein S6 (RPS6) (30, 56). In addition to its effects on protein synthesis, Akt can also inhibit catabolic processes through the phosphorylation and inhibition of the forkhead box O (FOXO) transcription factor, which regulates the expression of muscle-specific E3 ligases atrogin-1 and MuRF-1 (36, 43, 44).
Akt is sensitive to skeletal muscle loading. Akt/mTORC1 signaling is increased during overload-induced skeletal muscle hypertrophy (9, 49) and is decreased with muscle unloading (2, 11, 26). Akt phosphorylation is reduced at both regulatory sites during hindlimb suspension (HS)-induced atrophy, and is accompanied by protein synthesis suppression and protein breakdown activation (9, 17, 34). Irradiation exposure in nonmuscle cells induces Akt phosphorylation, which is thought to be related to cell survival and apoptosis regulation (29, 45–47). Ionizing radiation can promote oxidative stress to organelles, DNA damage, and subsequent cell death (5, 10, 25). Classically, radiation was used to assess muscle satellite cell function during overload-induced hypertrophy and muscle regeneration (7, 10, 16, 28, 33). Studies have demonstrated that radiation exposure can disrupt muscle morphology, production of reactive oxygen species, and angiogenesis (19, 21, 32). Interestingly, cyclic stretch has been shown to maintain proliferative capacity in irradiated muscle cells, which may indicate that loading could play a protective role in skeletal muscle and satellite cells (13). Thus, lack of mechanical loading may increase a muscle's sensitivity to irradiation, however, this has not been investigated. Furthermore, the effect of irradiation on muscle Akt signaling in either basal or atrophic conditions is not well understood.
We have previously reported that skeletal muscle remodeling and oxidative stress are induced by varying doses of X-ray irradiation (25). However, the interaction of ionizing radiation and muscle signaling during disuse atrophy has not been clearly defined. Therefore, the purpose of this study was to determine the effect of acute irradiation on unloading-induced regulation of muscle Akt signaling. We hypothesized that unloading-induced suppression of muscle Akt phosphorylation and associated downstream signaling would be disrupted by acute irradiation. To test this hypothesis, female C57BL/6 mice were subjected to load bearing (LB) or HS for 5 days. On day 3, mice received a single, unilateral hindlimb irradiation dose (0.5 Gy X-ray). Akt and downstream signaling were examined in the gastrocnemius muscle, which underwent disuse atrophy and has been widely studied in the disuse field (8, 9, 12). Our results demonstrate that Akt phosphorylation at T308 and S473 in atrophying muscle is differentially affected by irradiation.
METHODS
Animals.
Twelve female C57BL/6 mice 12 wk of age were purchased from Charles River Laboratory (Charleston, SC) and housed at the animal resource facility at Clemson University. Mice were single housed, given access to food and water ad libitum, and kept on a 12:12-h light-dark cycle. The study was approved by the Institutional Animal Care and Use Committee at Clemson University.
Experimental design.
At 12 wk of age mice were randomly assigned and subjected to LB or HS conditions for 5 days (n = 6/group). Mice in the LB condition group were allowed normal cage ambulation. In contrast, mice in the HS condition group were unloaded during the treatment period. All mice in the study (n = 12) received a single radiation dose (0.5 Gy X-ray) to the right hindlimb on day 3 of treatment. The left hindlimb of each animal served as an intra-animal, contralateral control (Fig. 1). Mice were killed on day 5 of unloading treatment, 2 days following acute radiation exposure.
Fig. 1.
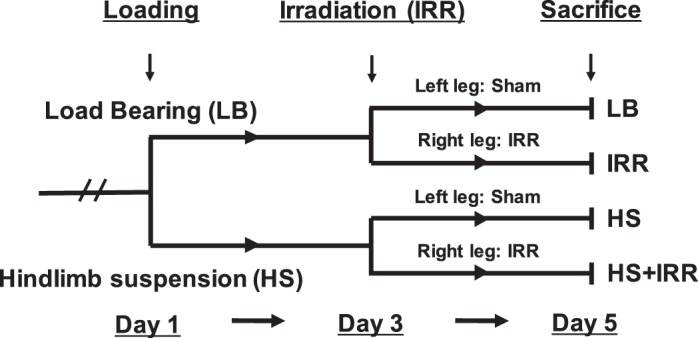
Experimental design. Twelve female C57BL/6 mice were assigned to load bearing (LB) or hindlimb suspension (HS) for 5 days (n = 6/group). All mice received irradiation (IRR) (0.5 Gy X-ray) to the right hindlimb on day 3 of the study. The left hindlimb served as the nonirradiated intra-animal control. All mice were killed on day 5 of the study.
Hindlimb suspension.
Muscle unloading was achieved by using HS as previously described (48). Briefly, mice were anesthetized with 2.5% isoflurane, and tails were cleaned with rubbing alcohol and air dried, covered with a light coat of benzoin tincture, and dried with a hair dryer until the surface was tacky. Strips of elastoplast (Biersdorf, Norwalk, CT) adhesive bandage were applied to the proximal two-thirds of all sides of the tail and looped through a swivel attachment mounted above the cage designed to allow the animal to move rotationally 360° with only the forelimbs able to come into contact with the cage floor. The animals were provided food and water ad libitum and were monitored daily for signs of lethargy or illness.
Hindlimb irradiation.
All mice received hindlimb irradiation on day 3 of the study as previously described with slight modifications (25, 51). While the mice were under anesthesia (1.5% isoflurane) they were irradiated using a 150-kV industrial portable X-ray unit (Phillips Medical Systems, Bothell WA) (51). The collimator was adjusted so the radiation field included the proximal femur to the distal toe of the right hindlimb. Mice received a single dose of 0.5 Gy X-ray to the right hindlimb at a nominal dose rate of 1.37 Gy/min with an exposure time of 0.36 min. The rest of each animal's body was shielded from irradiation during the entire procedure. Mice were returned to their respective cages following the procedure upon recovery from anesthesia, but no loading was achieved in the HS group.
Tissue collection.
On day 5 of the study mice were killed via cervical dislocation. Hindlimb skeletal muscles were excised, snap-frozen in liquid nitrogen, and stored at −80°C until analysis. The tibia and femur were removed, cleaned of all soft tissue, and stored for additional analysis.
Western blot analysis.
Western blot analysis was performed as previously described (24, 41). Briefly, frozen gastrocnemius muscle was homogenized in Mueller buffer and protein concentration was determined by the Bradford method. Crude muscle homogenates (10–40 μg) were fractionated on 8–15% polyacrylamide gels and transferred to polyvinylidene difluoride membranes overnight at 4°C. Equal protein loading of the gels was assessed by Ponceau staining. Membranes were blocked in 5% milk-TBST for 1 to 2 h at room temperature. Primary antibodies for p-Akt (T308); p-Akt (S473); Akt, p-RPS6 (S235/236); p-RPS6 (S240/244); RPS6, p-P70S6K (T389); P70S6K, p-TSC2 (T1462); TSC2, p-PRAS40 (T246); PRAS40, p-FOXO1 (S256); FOXO1, p-FOXO3 (S253); FOXO3, p-GSK3β (S9); GSK3β; atrogin-1; MuRF-1 (ECM Biosciences); and GAPDH were incubated at 1:2,000 to 1:10,000 dilutions in 5% milk-TBST for 2 h at room temperature or overnight at 4°C. Secondary anti-rabbit or anti-mouse IgG-conjugated antibodies were incubated with membranes at a 1:2,000 dilution in 5% milk-TBST for 1 h at room temperature. All antibodies were purchased from Cell Signaling Technology (Danvers, MA) unless otherwise stated. Enhanced chemiluminescence was used to visualize the antibody-antigen interaction and was developed using autoradiography (Kodak, Biomax) or digital imaging (G-Box, Syngene). Immunoblots were analyzed by measuring the integrated optical density of each band using ImageJ software (National Institutes of Health, Bethesda, MD). Data were normalized to LB controls.
Statistical analysis.
Results are reported as means ± SE. A repeated-measures two-way (loading × irradiation) ANOVA was used to determine differences between treatment groups. Post hoc analyses were performed with Tukey's multiple comparison test when appropriate. Significance was set at P < 0.05. Statistical analysis was performed using SigmaStat version 3.5 (Systat Software, Richmond, CA).
RESULTS
Effects of HS and irradiation on body weight and muscle mass.
Mice that underwent HS had higher body weight at death compared with mice in LB group (Table 1). Despite being heavier, mice subjected to HS had lower muscle mass irrespective of irradiation (Table 1). Similar to absolute, relative muscle mass was decreased by HS irrespective of irradiation (Table 1). Neither absolute or relative muscle mass was altered by irradiation irrespective of the loading condition. Collectively, these results demonstrate that 5 days of unloading induced skeletal muscle atrophy, and that irradiation had no effect on muscle mass alone or when combined with unloading.
Table 1.
Effect of hindlimb suspension and irradiation on body weight and muscle mass
| LB | HS | IRR | HS+IRR | |
|---|---|---|---|---|
| Body weight, g | 17.1 ± 0.4 | 21.2 ± 0.6* | 17.1 ± 0.4 | 21.2 ± 0.6* |
| Gastrocnemius mass | ||||
| Absolute, mg | 74 ± 2.4 | 62 ± 2.7* | 76 ± 1.5 | 62 ± 2.4* |
| Relative, mg/g | 4.3 ± 0.1 | 2.9 ± 0.2* | 4.4 ± 0.1 | 2.9 ± 0.1* |
Values are means ± SE. Body weight and muscle mass were measured when animals died. Irradiation was performed on the right leg; the left leg served as an intra-animal control. Body weights are the same for treatment groups within the same animal. Relative muscle mass is calculated as mg of muscle/g body weight.
LB, load bearing; HS, hindlimb suspension; IRR, irradiation.
Signifies main effect of HS.
Effects of HS and irradiation on differential Akt phosphorylation.
Gastrocnemius muscle Akt phosphorylation was examined at the two sites (T308 and S473) known to regulate protein turnover. HS resulted in decreased muscle AktT308 phosphorylation (Fig. 2A), whereas the combination of HS and irradiation blocked the reduction in muscle AktT308 phosphorylation. Irradiation alone had no effect on muscle AktT308 phosphorylation. Similar to AktT308, HS resulted in reduced muscle AktS473 phosphorylation (Fig. 2B), and when combined with irradiation, AktS473 phosphorylation was returned to baseline values. Interestingly, irradiation induced AktS473 phosphorylation independent of HS. These results demonstrate that irradiation affected unloading-induced regulation of Akt at both regulatory sites. However, in the LB condition, only AktS473 was sensitive to irradiation.
Fig. 2.
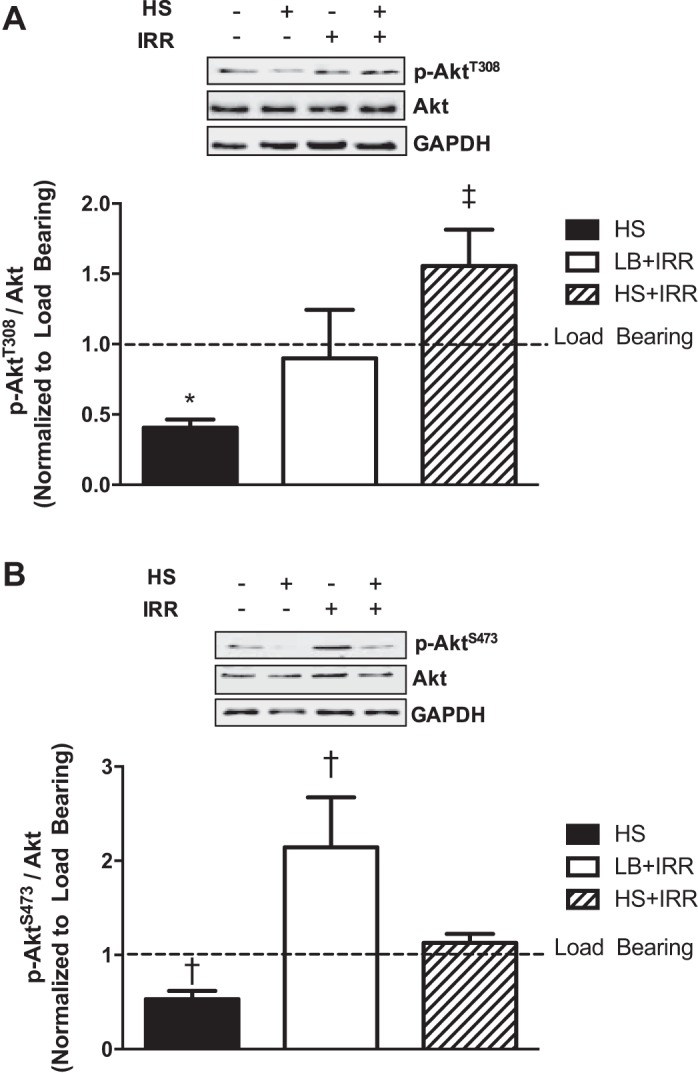
Akt phosphorylation in response to unloading and irradiation. A: representative immunoblots of AktT308, total Akt, and GAPDH in gastrocnemius muscle (top); quantification of phosphorylated to total Akt protein expression (bottom). B: representative immunoblots AktS473, total Akt, and GAPDH in gastrocnemius muscle (top); quantification of phosphorylated to total Akt protein expression (bottom). All samples were run on the same gel. Values are means ± SE. Statistical significance was set at P < 0.05. Data are normalized to LB controls. *Significantly different from LB; †significantly different from all groups; ‡significantly different from HS.
Effects of hindlimb unloading and irradiation on direct downstream Akt targets.
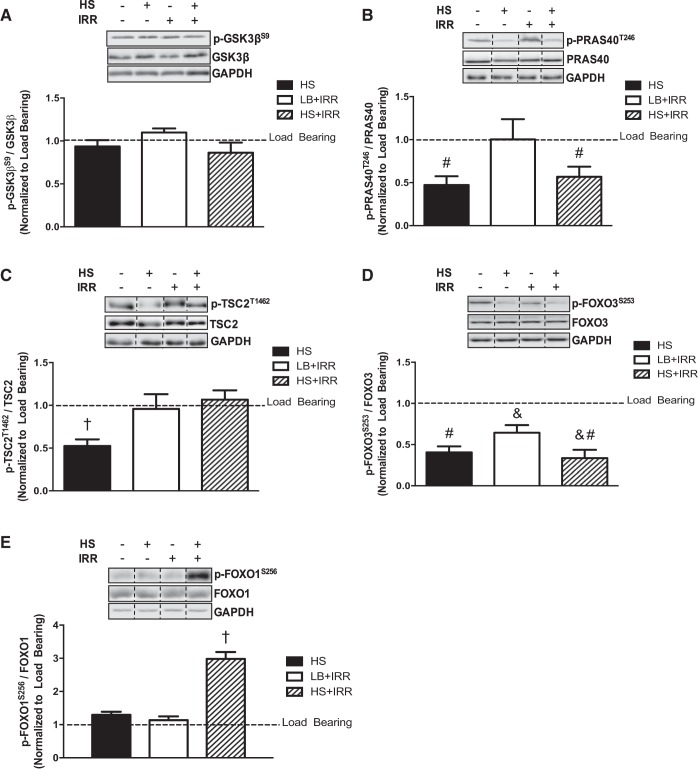
We next examined the effect of irradiation on the disuse regulation of direct downstream Akt targets in atrophying muscle. GSK3βS9 phosphorylation was not altered by HS or irradiation (Fig. 3A). Irradiation did not alter the HS suppression of PRAS40T246 phosphorylation, and there was no effect of irradiation alone (Fig. 3B). Irradiation resulted in a reversal of the HS suppression of TSC2T1462 phosphorylation, whereas in LB muscle, irradiation did not affect TSC2T1462 phosphorylation (Fig. 3C). Irradiation had no effect on the HS-induced suppression of FOXO3S253 phosphorylation (Fig. 3D), but also reduced FOXO3S253 phosphorylation independent of HS (Fig. 3D). Interestingly, irradiation induced FOXO1S256 phosphorylation in muscle that underwent HS (Fig. 3E). These results demonstrate that irradiation altered the HS regulation of TSC2T1462 and FOXO1S256 phosphorylation. Additionally, muscle FOXO3S253 phosphorylation was sensitive to irradiation independent of muscle atrophy.
Fig. 3.
Direct downstream targets of Akt signaling in response to unloading and irradiation. A: representative immunoblots of GSK3βS9, total GSK3β, and GAPDH in gastrocnemius muscle (top); quantification of phosphorylated to total GSK3β protein expression (bottom). B: representative immunoblots of PRAS40T246, total PRAS40, and GAPDH in gastrocnemius muscle (top); quantification of phosphorylated to total PRAS40 protein expression (bottom). C: representative immunoblots of TSC2T1462, total TSC2, and GAPDH in gastrocnemius muscle (top); quantification of phosphorylated to total TSC2 protein expression (bottom). D: representative immunoblots of FOXO3S253, total FOXO3, and GAPDH in gastrocnemius muscle (top); quantification of phosphorylated to total FOXO3 protein expression (bottom). E: representative immunoblots of FOXO1S256, total FOXO1, and GAPDH in gastrocnemius muscle (top); quantification of phosphorylated to total FOXO1 protein expression (bottom). All samples were run on the same gel. Values are means ± SE. Statistical significance was set at P < 0.05. Data are normalized to LB controls. Dashed lines indicate adjacent lanes were cropped for representative immunoblots. #Main effect of HS; &main effect of irradiation; †significantly different from all groups.
Effects of hindlimb unloading and irradiation on mTORC1 signaling.
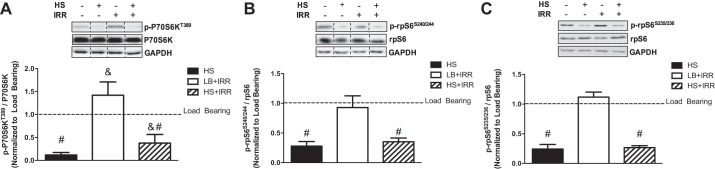
We next examined whether irradiation could alter the HS suppression of mTORC1 signaling. We first examined the direct mTORC1 target, P70S6K. Although irradiation did not alter the HS suppression of P70S6KT389 phosphorylation, irradiation induced P70S6KT389 phosphorylation irrespective of HS (Fig. 4A). Next, we examined RPS6 phosphorylation, the direct P70S6K target involved in translation initiation and ribosomal biogenesis. Irradiation had no effect on the HS suppression of RPS6S235/326 and RPS6S240/244 phosphorylation (Fig. 4, B and C). These results demonstrate that irradiation was not sufficient to alter HS suppression of mTORC1 signaling. However, muscle P70S6K phosphorylation was sensitive to irradiation independent of atrophy.
Fig. 4.
Mammalian target of rapamycin complex 1 (mTORC1) signaling in response to unloading and irradiation. A: representative immunoblots of P70S6KT389, total P70S6K, and GAPDH in gastrocnemius muscle (top); quantification of phosphorylated to total P70S6K protein expression (bottom). B: representative immunoblots of rpS6S240/244, total rpS6, and GAPDH in gastrocnemius muscle (top); quantification of phosphorylated to total rpS6 protein expression (bottom). C: representative immunoblots rpS6S235/236, total rpS6, and GAPDH in gastrocnemius muscle (top); quantification of phosphorylated to total rpS6 protein expression (bottom). All samples were run on the same gel. Values are means ± SE. Statistical significance was set at P < 0.05. Data are normalized to LB controls. Dashed lines indicate adjacent lanes were cropped for representative immunoblots. #Main effect of HS; &main effect of irradiation.
Effects of hindlimb unloading and irradiation on muscle-specific E3 ligase protein expression.
Akt can regulate protein breakdown through inhibition of FOXO transcriptional activation of atrogenes. Therefore, we examined the protein expression of the muscle-specific E3 ligases atrogin-1 and MuRF-1. Although irradiation did not affect the HS induction of MuRF-1 protein expression, irradiation was sufficient to increase MuRF-1 expression in LB muscle (Fig. 5A). Muscle atrogin-1 protein expression did not change with any loading or irradiation condition (Fig. 5B). These results demonstrate that skeletal muscle MuRF-1 expression, but not atrogin-1, was sensitive to irradiation exposure in unloaded and LB conditions.
Fig. 5.
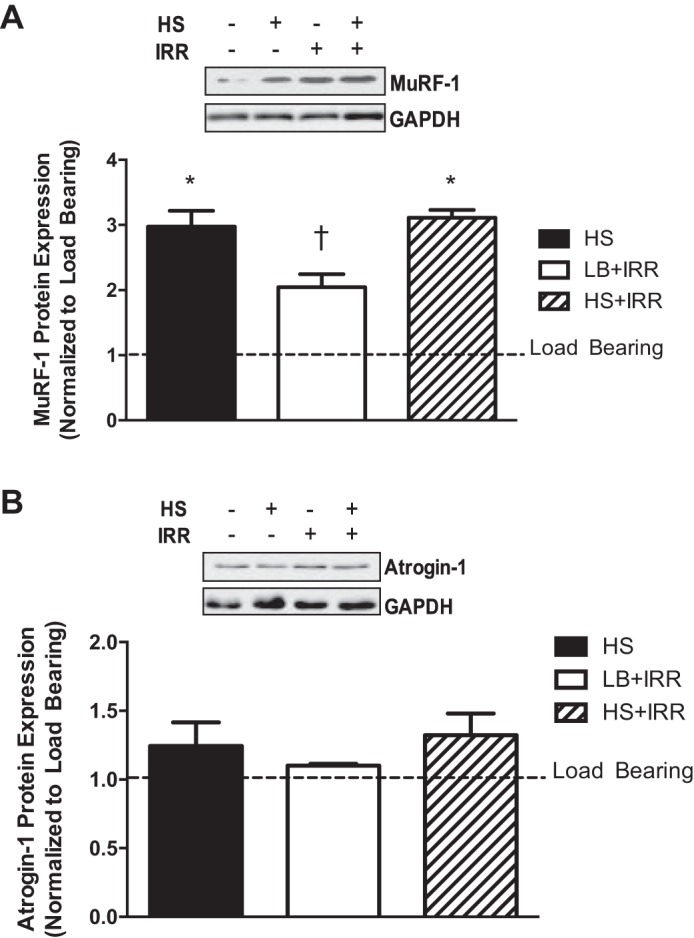
Muscle E3 ligase protein expression in response to unloading and irradiation. A: representative immunoblots of MuRF-1 and GAPDH in gastrocnemius muscle (top); quantification of total MuRF-1 protein expression (bottom). B: representative immunoblots of atrogin-1 and GAPDH in gastrocnemius muscle (top); quantification of total atrogin-1 protein expression (bottom). All samples were run on the same gel. Values are means ± SE. Statistical significance was set at P < 0.05. Data are normalized to LB controls. *Significantly different from LB; †significantly different from all groups.
DISCUSSION
Significant progress has been made in our understanding of the cellular signaling that regulates muscle protein turnover during unloading-induced atrophy. However, there is little direct information on how irradiation could affect this atrophy signaling in muscle. Individuals can be exposed to a combination of muscle unloading and irradiation during spaceflight or with disuse associated with cancer treatment. Thus, an improved understanding of the radiation-sensitive cellular responses in unloaded muscle could provide insight into preventive or therapeutic treatments that could avoid deleterious outcomes. Our study reports the novel finding that irradiation can alter muscle cellular regulation induced by unloading. Specifically, we found that irradiation altered the unloading suppression of Akt phosphorylation at two different regulatory sites. Downstream of Akt we also found that irradiation affected the unloading suppression of TSC2 phosphorylation and the induction of FOXO1 phosphorylation. Furthermore, we also found that irradiation was sufficient to induce Akt signaling pathways in LB muscle. Irradiation-sensitive targets in LB muscle included Akt, P70S6K, and MuRF-1. Collectively, these results demonstrate that acute irradiation can alter unloading-induced muscle Akt signaling, and further work is justified to determine the functional and metabolic ramifications of these changes.
Akt has emerged as a critical regulator of muscle mass through the activation of protein synthesis and inhibition of protein degradation (9, 38, 44). Whereas increased mechanical loading is associated with Akt activation, reductions in both phosphorylation sites (S473 or T308) have been observed during unloading-induced muscle atrophy (9, 22, 34). Although we also report that during the initiation of disuse atrophy muscle Akt phosphorylation was suppressed at both sites, the interactive effects between irradiation and unloading also involve both Akt T308 and S473 phosphorylation sites. Because this interaction occurred at the onset of atrophy and without any additive effect on muscle mass loss, further research is needed to determine whether there is any additional effect on either the degree of atrophy with long-term disuse or the ability to recover muscle mass upon reloading. Interestingly, Akt S473 phosphorylation was also induced by irradiation alone, making this phosphorylation site independently sensitive to either unloading or irradiation. Phosphorylation of Akt S473 is a downstream target of mTORC2 (18) and is responsible for the stabilization of Akt in its active conformation state (55). mTORC2-dependent signaling plays critical roles in cellular processes related to cell survival and metabolism (31), which may account for why irradiation alone did not involve atrophic changes. This signaling response may be involved in radiation-induced oxidative stress and remodeling, which has been previously found in irradiated muscle (25). Further research is needed to determine the physiological and functional ramifications of disrupted Akt phosphorylation during the onset of unloading-induced atrophy.
mTORC1 has emerged as a critical regulator of muscle protein synthesis, and this signaling pathway can be regulated by Akt activity (44). Akt/mTORC1 signaling is suppressed during the initial stages of disuse atrophy (17, 26). In line with these observations, unloading resulted in reduced phosphorylation of direct Akt targets PRAS40 and TSC2, which corresponded with reduced mTORC1 signaling through P70S6K and RPS6 phosphorylation. Interestingly, there were differential effects of irradiation on the regulation of this load-sensitive signaling. Irradiation relieved suppressed TSC2 phosphorylation in atrophying muscle, but did not alter the suppression of PRAS40 or mTORC1 downstream signaling. The metabolic and growth significance related to the differential sensitivity of Akt targets to irradiation requires further study. Furthermore, irradiation in weight-bearing muscle resulted in increased Akt S473 phosphorylation, with no corresponding change in direct Akt targets. Irradiation has been shown to induce Akt phosphorylation in nonmuscle cells (29, 45–47), and this activation may be related to radioprotective signaling pathways that regulate cell survival and apoptosis. Nonetheless, although we found that irradiation could increase P70S6K phosphorylation independent of atrophy, there was no effect on its downstream target, RPS6. These findings corroborate previously published results reporting that acute irradiation is not sufficient to reduce LB muscle mass in mice (25). It is intriguing to speculate that loading may be protective to the effects of irradiation in muscle; cyclic stretch has been shown to reduce the negative consequences of irradiation to cultured muscle cells (10, 13). These findings provide a rationale for further examining the potential beneficial effects of mechanical signaling in muscle subjected to radiation exposure.
Akt can also regulate muscle mass through phosphorylation and inhibition of the FOXO family of transcription factors. Indeed, disuse atrophy is associated with FOXO activation and increased atrogene expression (17, 35, 37, 54). During the initiation of disuse atrophy, FOXO3 phosphorylation, but not that of FOXO1, was suppressed, and this was associated with increased MuRF-1 protein expression. Irradiation did not alter the unloading response of FOXO3. However, FOXO3 was a target of irradiation in LB muscle. The activation of FOXO3 appeared to be independent of classical Akt regulation (9), which may further support that concept that Akt S473 activation reflects radiation-sensitive pathways that are uncoupled from the regulation of skeletal muscle mass. Oxidative stress has been reported to enhance FOXO transcriptional activity in skeletal muscle (14, 40), and we have previously demonstrated that irradiation can induce oxidative stress independent of alterations in LB muscle mass (25). Further research is needed to mechanistically determine whether radiation promotes oxidative stress during the initiation of disuse atrophy.
It is well established that exposure to irradiation can alter rodent skeletal muscle response to overload-induced hypertrophy or injury (16, 28, 33, 39), and can induce remodeling in LB mouse muscle independent of reductions in muscle mass (6, 25, 45). It has also been recently demonstrated in the nonlocomotive laryngeal muscle that radiation-induced myofiber atrophy, which coincided with myosin heavy chain loss and increased MuRF-1 protein expression (23). As briefly discussed, irradiation and unloading both independently resulted in induced MuRF-1 protein expression. It has been reported that FOXO transcriptional activity may not be required for MuRF-1 expression during the initiation of disuse atrophy (53); whether this regulation could occur in response to irradiation is not certain. Nonetheless, our results demonstrate that FOXO and MuRF-1 activation are targets of irradiation stimuli. These results suggest that irradiation does not exacerbate the unloading induced changes in muscle protein turnover signaling.
Conclusion.
In summary, we demonstrate that unloading can alter a muscle's sensitivity to irradiation. We report the novel finding that acute irradiation can disrupt unloading-induced regulation of muscle Akt and downstream signaling. This was evident by the irradiation induction of Akt phosphorylation in unloaded skeletal muscle. We also report that the direct Akt targets TSC2 and FOXO1 were sensitive to the combination of unloading and irradiation. Additionally, irradiation suppressed FOXO3 activation independent of Akt and unloading. Interestingly, although MuRF-1 was the primary E3 ligase that was activated during the initiation of disuse atrophy, it was also sensitive to irradiation in LB muscle. Our results have important implications for understanding the effect of irradiation exposure on muscle growth and metabolic signaling, and also provide a rationale for the need to further define the interaction of mechanical signaling and irradiation sensitivity.
GRANTS
This work was supported by National Institutes of Health Grants R01-CA-121249 to J. A. Carson and 5P30 GM-103336 to the Center for Colon Cancer Research at the University of South Carolina, and in part by funds from the SPARC Graduate Research Grant from the Office of the Vice President for Research at the University of South Carolina to J. P. Hardee.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.K.F. performed experiments; D.K.F. and J.P.H. analyzed data; D.K.F., J.P.H., and J.A.C. interpreted results of experiments; D.K.F. prepared figures; D.K.F. and J.P.H. drafted manuscript; D.K.F., J.P.H., T.A.B., and J.A.C. edited and revised manuscript; D.K.F., J.P.H., T.A.B., and J.A.C. approved final version of manuscript; T.A.B. and J.A.C. conception and design of research.
REFERENCES
- 1.Adams GR. Human unilateral lower limb suspension as a model for spaceflight effects on skeletal muscle. J Appl Physiol 93: 1563–1566, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Adams GR, Haddad F, Bodell PW, Tran PD, Baldwin KM. Combined isometric, concentric, and eccentric resistance exercise prevents unloading-induced muscle atrophy in rats. J Appl Physiol 103: 1644–1654, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol 7: 261–269, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Allen DL, Yasui W, Tanaka T, Ohira Y, Nagaoka S, Sekiguchi C, Hinds WE, Roy RR, Edgerton VR. Myonuclear number and myosin heavy chain expression in rat soleus single muscle fibers after spaceflight. J Appl Physiol 81: 145–151, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Azimzadeh O, Scherthan H, Sarioglu H, Barjaktarovic Z, Conrad M, Vogt A, Calzada-Wack J, Neff F, Aubele M, Buske C, Atkinson MJ, Tapio S. Rapid proteomic remodeling of cardiac tissue caused by total body ionizing radiation. Proteomics 11: 3299–3311, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Bandstra ER, Thompson RW, Nelson GA, Willey JS, Judex S, Cairns MA, Benton ER, Vazquez ME, Carson JA, Bateman TA. Musculoskeletal changes in mice from 20–50 cGy of simulated galactic cosmic rays. Radiat Res 172: 21–29, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Bergstrom RM, Salmi A. Radiation-induced damage in the ultrastructure of striated muscle. Exp Cell Res 26: 226–228, 1962. [DOI] [PubMed] [Google Scholar]
- 8.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Caiozzo VJ, Giedzinski E, Baker M, Suarez T, Izadi A, Lan M, Cho-Lim J, Tseng BP, Limoli CL. The radiosensitivity of satellite cells: cell cycle regulation, apoptosis and oxidative stress. Radiat Res 174: 582–589, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannavino J, Brocca L, Sandri M, Bottinelli R, Pellegrino MA. PGC1-alpha over-expression prevents metabolic alterations and soleus muscle atrophy in hindlimb unloaded mice. J Physiol 592: 4575–4589, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cannavino J, Brocca L, Sandri M, Grassi B, Bottinelli R, Pellegrino MA. The role of alterations in mitochondrial dynamics and PGC-1alpha over-expression in fast muscle atrophy following hindlimb unloading. J Physiol 593: 1981–1995, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho-Lim JJ, Caiozzo VJ, Tseng BP, Giedzinski E, Baker MJ, Limoli CL. Satellite cells say NO to radiation. Radiat Res 175: 561–568, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clavel S, Siffroi-Fernandez S, Coldefy AS, Boulukos K, Pisani DF, Dérijard B. Regulation of the intracellular localization of Foxo3a by stress-activated protein kinase signaling pathways in skeletal muscle cells. Mol Cell Biol 30: 470–480, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol 2: 769–776, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Darden EB., Jr Changes in membrane potentials, K content, and fiber structure in irradiated frog sartorius muscle. Am J Physiol 198: 709–714, 1960. [DOI] [PubMed] [Google Scholar]
- 17.Dupont E, Cieniewski-Bernard C, Bastide B, Stevens L. Electrostimulation during hindlimb unloading modulates PI3K-AKT downstream targets without preventing soleus atrophy and restores slow phenotype through ERK. Am J Physiol Regul Integr Comp Physiol 300: R408–R417, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Fayard E, Tintignac LA, Baudry A, Hemmings BA. Protein kinase B/Akt at a glance. J Cell Sci 118: 5675–5678, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Fedorova M, Kuleva N, Hoffmann R. Reversible and irreversible modifications of skeletal muscle proteins in a rat model of acute oxidative stress. Biochim Biophys Acta 1792: 1185–1193, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Gillette EL, Mahler PA, Powers BE, Gillette SM, Vujaskovic Z. Late radiation injury to muscle and peripheral nerves. Int J Radiat Oncol Biol Phys 31: 1309–1318, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Grabham P, Sharma P. The effects of radiation on angiogenesis. Vasc Cell 5: 19, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haddad F, Baldwin KM, Tesch PA. Pretranslational markers of contractile protein expression in human skeletal muscle: effect of limb unloading plus resistance exercise. J Appl Physiol 98: 46–52, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Han X, Pires L, Browne JD, Sullivan CA, Zhao W, Feng X. Increased expression of MuRF1 is associated with radiation-induced laryngeal muscle atrophy. Anticancer Res 35: 6049–6056, 2015. [PubMed] [Google Scholar]
- 24.Hardee JP, Mangum JE, Gao S, Sato S, Hetzler KL, Puppa MJ, Fix DK, Carson JA. Eccentric contraction-induced myofiber growth in tumor-bearing mice. J Appl Physiol 120: 29–37, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardee JP, Puppa MJ, Fix DK, Gao S, Hetzler KL, Bateman TA, Carson JA. The effect of radiation dose on mouse skeletal muscle remodeling. Radiol Oncol 48: 247–256, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hornberger TA, Hunter RB, Kandarian SC, Esser KA. Regulation of translation factors during hindlimb unloading and denervation of skeletal muscle in rats. Am J Physiol Cell Physiol 281: C179–C187, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Kelleher AR, Kimball SR, Dennis MD, Schilder RJ, Jefferson LS. The mTORC1 signaling repressors REDD1/2 are rapidly induced and activation of p70S6K1 by leucine is defective in skeletal muscle of an immobilized rat hindlimb. Am J Physiol Endocrinol Metab 304: E229–E236, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan MY. Radiation-induced changes in skeletal muscle. An electron microscopic study. J Neuropathol Exp Neurol 33: 42–57, 1974. [DOI] [PubMed] [Google Scholar]
- 29.Kim MJ, Byun JY, Yun CH, Park IC, Lee KH, Lee SJ. c-Src-p38 mitogen-activated protein kinase signaling is required for Akt activation in response to ionizing radiation. Mol Cancer Res 6: 1872–1880, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Kimball SR, Farrell PA, Jefferson LS. Invited Review: Role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. J Appl Physiol 93: 1168–1180, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci 122: 3589–3594, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leach JK, Van Tuyle G, Lin PS, Schmidt-Ullrich R, Mikkelsen RB. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res 61: 3894–3901, 2001. [PubMed] [Google Scholar]
- 33.Lewis RB. Changes in striated muscle following single intense doses of x-rays. Lab Invest 3: 48–55, 1954. [PubMed] [Google Scholar]
- 34.Liu H, Blough ER, Arvapalli R, Wang Y, Reiser PJ, Paturi S, Katta A, Harris R, Nepal N, Wu M. Regulation of contractile proteins and protein translational signaling in disused muscle. Cell Physiol Biochem 30: 1202–1214, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Lomonosova YN, Shenkman BS, Nemirovskaya TL. Attenuation of unloading-induced rat soleus atrophy with the heat-shock protein inducer 17-(allylamino)-17-demethoxygeldanamycin. FASEB J 26: 4295–4301, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Milan G, Romanello V, Pescatore F, Armani A, Paik JH, Frasson L, Seydel A, Zhao J, Abraham R, Goldberg AL, Blaauw B, DePinho RA, Sandri M. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat Commun 6: 6670, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagatomo F, Fujino H, Kondo H, Suzuki H, Kouzaki M, Takeda I, Ishihara A. PGC-1alpha and FOXO1 mRNA levels and fiber characteristics of the soleus and plantaris muscles in rats after hindlimb unloading. Histol Histopathol 26: 1545–1553, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Akt (protein kinase B) isoform phosphorylation and signaling downstream of mTOR (mammalian target of rapamycin) in denervated atrophic and hypertrophic mouse skeletal muscle. J Mol Signal 7: 7, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phelan JN, Gonyea WJ, Norrby M, Evertsson K, Fjallström AK, Svensson A, Tågerud S. Effect of radiation on satellite cell activity and protein expression in overloaded mammalian skeletal muscle. Anat Rec 247: 179–188, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Powers SK, Wiggs MP, Duarte JA, Zergeroglu AM, Demirel HA. Mitochondrial signaling contributes to disuse muscle atrophy. Am J Physiol Endocrinol Metab 303: E31–E39, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puppa MJ, Gao S, Narsale AA, Carson JA. Skeletal muscle glycoprotein 130's role in Lewis lung carcinoma-induced cachexia. FASEB J 28: 998–1009, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3: 1009–1013, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle 1: 4, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shtifman A, Pezone MJ, Sasi SP, Agarwal A, Gee H, Song J, Perepletchikov A, Yan X, Kishore R, Goukassian DA. Divergent modification of low-dose 56Fe-particle and proton radiation on skeletal muscle. Radiat Res 180: 455–464, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toulany M, Rodemann HP. Phosphatidylinositol 3-kinase/Akt signaling as a key mediator of tumor cell responsiveness to radiation. Semin Cancer Biol 35: 180–190, 2015. [DOI] [PubMed] [Google Scholar]
- 47.Viniegra JG, Martinez N, Modirassari P, Hernández Losa J, Parada Cobo C, Sánchez-Arévalo Lobo VJ, Aceves Luquero CI, Alvarez-Vallina L, Ramón y Cajal S, Rojas JM, Sánchez-Prieto R. Full activation of PKB/Akt in response to insulin or ionizing radiation is mediated through ATM. J Biol Chem 280: 4029–4036, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Washington TA, White JP, Davis JM, Wilson LB, Lowe LL, Sato S, Carson JA. Skeletal muscle mass recovery from atrophy in IL-6 knockout mice. Acta Physiol (Oxf) 202: 657–669, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White JP, Wrann CD, Rao RR, Nair SK, Jedrychowski MP, You JS, Martínez-Redondo V, Gygi SP, Ruas JL, Hornberger TA, Wu Z, Glass DJ, Piao X, Spiegelman BM. G protein-coupled receptor 56 regulates mechanical overload-induced muscle hypertrophy. Proc Natl Acad Sci USA 111: 15756–15761, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Widrick JJ, Trappe SW, Romatowski JG, Riley DA, Costill DL, Fitts RH. Unilateral lower limb suspension does not mimic bed rest or spaceflight effects on human muscle fiber function. J Appl Physiol 93: 354–360, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Willey JS, Lloyd SA, Robbins ME, Bourland JD, Smith-Sielicki H, Bowman LC, Norrdin RW, Bateman TA. Early increase in osteoclast number in mice after whole-body irradiation with 2 Gy X rays. Radiat Res 170: 388–392, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 84: 475–482, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Wu CL, Cornwell EW, Jackman RW, Kandarian SC. NF-κB but not FoxO sites in the MuRF1 promoter are required for transcriptional activation in disuse muscle atrophy. Am J Physiol Cell Physiol 306: C762–C767, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu CL, Kandarian SC, Jackman RW. Identification of genes that elicit disuse muscle atrophy via the transcription factors p50 and Bcl-3. PLoS One 6: e16171, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang J, Cron P, Good VM, Thompson V, Hemmings BA, Barford D. Crystal structure of an activated Akt/protein kinase B ternary complex with GSK3-peptide and AMP-PNP. Nat Struct Biol 9: 940–944, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12: 21–35, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]