We developed FloWave.US, publicly distributed software for automated ultrasound blood flow analysis. The software provides efficient and reliable measures of blood velocity and vessel diameter that are valid with a skeletal muscle flow phantom and independent of the ultrasound scanner. Open-source access to this software reduces barriers to use and enables community improvements and customization of analysis features to advance vascular physiology research.
Keywords: edge detection, wave tracking, flow-mediated dilation, Doppler, regional blood flow
Abstract
Automated software improves the accuracy and reliability of blood velocity, vessel diameter, blood flow, and shear rate ultrasound measurements, but existing software offers limited flexibility to customize and validate analyses. We developed FloWave.US—open-source software to automate ultrasound blood flow analysis—and demonstrated the validity of its blood velocity (aggregate relative error, 4.32%) and vessel diameter (0.31%) measures with a skeletal muscle ultrasound flow phantom. Compared with a commercial, manual analysis software program, FloWave.US produced equivalent in vivo cardiac cycle time-averaged mean (TAMean) velocities at rest and following a 10-s muscle contraction (mean bias <1 pixel for both conditions). Automated analysis of ultrasound blood flow data was 9.8 times faster than the manual method. Finally, a case study of a lower extremity muscle contraction experiment highlighted the ability of FloWave.US to measure small fluctuations in TAMean velocity, vessel diameter, and mean blood flow at specific time points in the cardiac cycle. In summary, the collective features of our newly designed software—accuracy, reliability, reduced processing time, cost-effectiveness, and flexibility—offer advantages over existing proprietary options. Further, public distribution of FloWave.US allows researchers to easily access and customize code to adapt ultrasound blood flow analysis to a variety of vascular physiology applications.
NEW & NOTEWORTHY
We developed FloWave.US, publicly distributed software for automated ultrasound blood flow analysis. The software provides efficient and reliable measures of blood velocity and vessel diameter that are valid with a skeletal muscle flow phantom and independent of the ultrasound scanner. Open-source access to this software reduces barriers to use and enables community improvements and customization of analysis features to advance vascular physiology research.
doppler ultrasonography can be used to measure blood flow in the peripheral vasculature with protocols designed to investigate specific aspects of vascular physiology including endothelial and vascular smooth muscle cell function (16, 36, 38). However, analyzing ultrasound blood flow data can be time-consuming, dependent on manufacturer settings, and susceptible to bias (6, 22). To address these limitations, many research laboratories now use either commercial products, such as Medical Imaging Applications' Vascular Research Tools (17, 23, 27) and Quipu's FMD-Studio (10-12), or custom software (2, 15, 20, 28, 29, 32–34, 37, 41) to analyze ultrasound blood flow data. Although the potential benefits of these programs include improved accuracy and reliability as well as significant reduction in processing time due to automated measurement of blood velocity and vessel diameter, existing software is expensive, requires specialized equipment, and offers limited options for customization.
Further, it is unclear if existing automated software has been validated with clinical standards (13). Comparisons of automated software output to in vivo data (23, 29, 32) or to other automated techniques (10, 28, 33) lack true reference values. Other studies have validated automated software results against criteria such as computer-generated ultrasound images (12, 20, 22, 34) or water bath phantoms (15, 41), but these experimental preparations fail to recreate heterogeneous image properties and noise observed in vivo. Automated ultrasound blood flow analyses, however, should be validated with phantoms that simulate the acoustic and mechanical properties of human soft tissue to permit accurate application of software across a range of vessel images (e.g., small, large, superficial, deep) and experimental conditions (19, 38). Once validated, blood velocity and vessel diameter can be measured and used to calculate numerous vascular health metrics such as blood flow, shear rate, arterial stiffness, and flow-mediated dilation (11, 14–16, 21, 25, 38, 39).
Here, we describe our newly developed and validated open-source software for ultrasound blood flow analysis, FloWave.US (part 1). We initiated the current study to validate our software with an ultrasound flow phantom (part 2); to compare a manual, commercial software program with FloWave.US (part 3); and to demonstrate an in vivo case study highlighting the advantages of FloWave.US for ultrasound blood flow data collection and analysis (part 4).
METHODS
Ultrasound Image Acquisition and Analysis
We acquired duplex ultrasound images with two ultrasound scanners and the following probe, pulse wave mode (PWM), and hardware configurations: 1) 8-MHz linear array vascular probe model 8L-RS, 4.4-MHz PWM frequency, General Electric (GE) LOGIQ Book e (Milwaukee, WI); and 2) 9-MHz linear array vascular probe model 9L4, 4.0-MHz PWM frequency, Siemens Acuson SC2000 (Malvern, PA). Sampling parameters were selected to optimize B-mode and continuous Doppler velocity (insonation angle ≤ 60°) data in a 2-cm length of the anterior tibial artery (ATA), ∼2–3 cm distal to the fibular head, or over the center of the artery mimics for in vivo and phantom studies, respectively. Once a satisfactory image was obtained, transducer position and scanner settings were held constant for all trials. Because of logistical constraints, we used only the GE LOGIQ Book e for the manual vs. automated software comparison study (part 3) and the in vivo case study (part 4).
Digital video (640 × 480-pixel screen resolution) was recorded from the ultrasound scanner via the composite video out port to a DVD at 30 Hz (VRD-MC6 multifunctional DVD recorder; Sony, Tokyo, Japan) and converted into an audio video interleaved (AVI) file format (WinX HD Converter Deluxe; Digiarty Software). The same investigator collected and edited all video recordings. FloWave.US (version 0.2.0) was executed in MATLAB (release 2015a) on a Dell Precision T7600 computer (Dell, Round Rock, TX) with an Intel Xeon 3.30-GHz processor (Intel, Santa Clara, CA) and 32.0-GB random access memory.
Part 1: FloWave.US Overview
FloWave.US is hosted in a version control GitHub repository (http://github.com/ccoolbaugh/FloWave.US) and can be executed in MATLAB (The Mathworks, Natick, MA) release 2011b or newer. The code repository contains FloWave.US and its associated subroutines, an ultrasound setup program (UsSetup.m), and a separate vessel diameter measurement program (BMode.m). Investigators can access the public GitHub repository to download this code, read instruction manuals and image quality guidelines, test demonstration data, and report “issues” (i.e., bugs and suggestions) to improve the code.
In the following sections, we present an overview of the main features of FloWave.US including 1) custom video settings, 2) blood velocity measurement, 3) vessel diameter measurement, 4) beat-to-beat analysis, and 5) data summary.
Custom video settings.
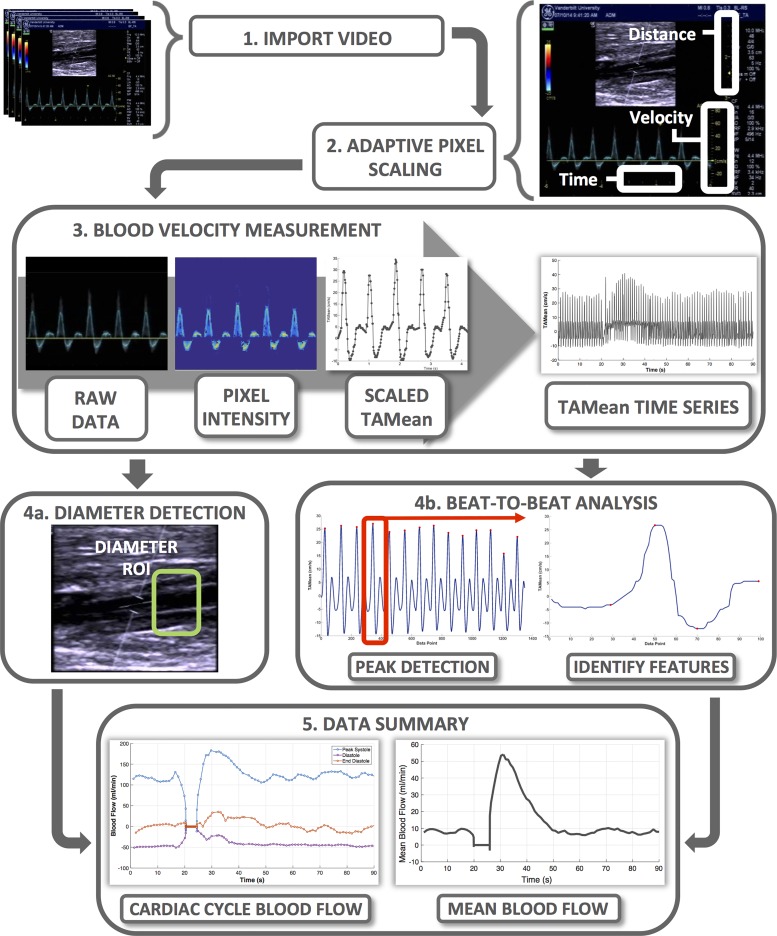
FloWave.US can analyze digital video recordings of duplex or brightness mode (B-mode) ultrasound screen captures (Fig. 1, process 1, “Import Video”). Imported video must include on-screen display of calibrated ultrasound scales and be formatted to a MATLAB-compatible video file type. Operators can create custom screen setting files (UsSetup.m) and select scaling factors (e.g., the number of pixels in a cm, cm/s, or s) to adapt FloWave.US to a variety of ultrasound scanners and experimental conditions (Fig. 1, process 2, “Adaptive Pixel Scaling”).
Fig. 1.
Overview of FloWave.US processing sequence, features, and outcomes.
Blood velocity measurement.
We developed a technique to estimate time-averaged mean (TAMean) velocity [an intensity-weighted mean of Doppler shifts in the blood velocity data (12)] from the Doppler pulse wave spectrum (Fig. 1, process 3, “Blood Velocity Measurement”). The code includes options to recreate TAMean velocities from a real-time colored overlay (TAMean is shown as a teal line in the “raw data” panel of Fig. 1, process 3) or from the Doppler pulse wave spectrum envelope. First, video images are cropped to a region of interest (ROI) around the Doppler pulse wave spectrum. Within this ROI, a pixel's color intensity is calculated if its color is within a threshold of either the TAMean color overlay or the Doppler pulse wave spectrum. Next, an iterative search identifies the location of the maximum pixel intensity (TAMean color overlay) or the boundaries of the Doppler envelope. The pixel position data are then converted to a positive (antegrade) or negative (retrograde) velocity (cm/s) according to the pixel scaling factor and its position relative to the zero-velocity baseline. Finally, an interactive cursor allows the operator to select time points in the TAMean velocity data associated with specific events (e.g., baseline, muscle contraction, recovery). These time event markers are logged in the program for subsequent use in the “Vessel Diameter Measurement” and “Beat-to-Beat Analysis” code.
Vessel diameter measurement.
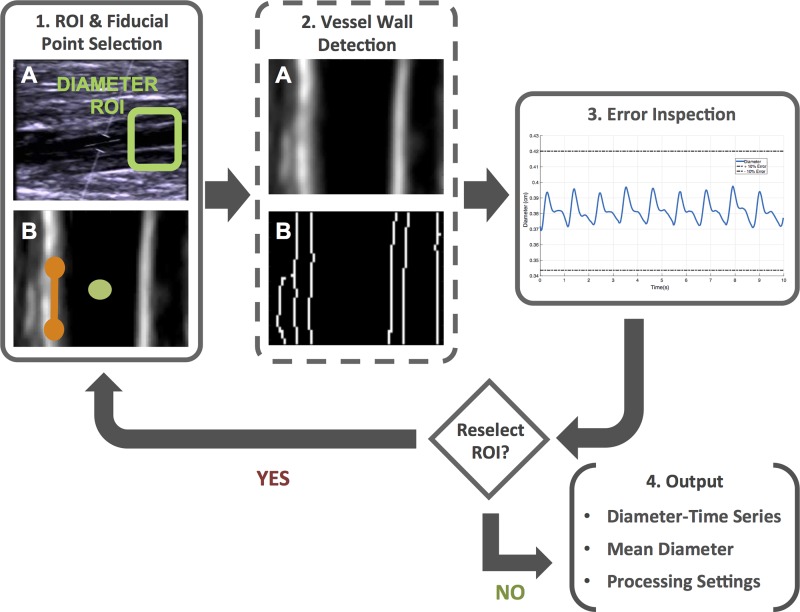
Vessel diameter is measured via a semiautomated edge detection code within FloWave.US (Fig. 1, process 4a, “Diameter Detection,” and Fig. 2). In the first video frame, the operator selects a ROI where the vessel walls are most visible in the B-mode image and identifies the vessel center and vessel wall angle. These image fiducial markers are combined with the MATLAB edge function with the “Canny” option specified to detect the position of the vessel walls. In brief, the Canny method locates edges by determining the local maxima of a gradient based on the derivative of a Gaussian filtered image (5). For each video frame, the perpendicular distance between the near and far vessel wall for each pixel in the ROI is found, converted to centimeters, and the mean diameter for all pixels in the image is calculated. The operator can choose to select a new vessel ROI at each time event marker or insert zero values to skip analysis (e.g., a motion artifact). The diameter time series is combined for all images in the video, smoothed with a Savitsky-Golay least squares filter (3rd order, 11-point segment length) (30), and presented to the operator for visual inspection. If errors are present, the vessel diameter measurement code can be repeated with different time event markers and vessel ROI selections. Alternatively, a separate program (BMode.m) can be used to acquire a single diameter value from a high-resolution B-mode video as a supplement to duplex ultrasound data with poor vessel wall visibility.
Fig. 2.
Workflow for the diameter detection subroutine (BMode.m). Solid and dashed boxes indicate user and algorithm actions, respectively. In step 1a, the user selects the diameter region of interest (ROI) to begin the processing sequence. In step 1b, the ROI is cropped and transposed to allow the user to select the vessel center (green circle) and wall angle (orange line). In step 2a, a Gaussian filter is applied to the image to improve detection of the vessel walls via the (step 2b) Canny edge detection method. After repeating these steps for each image frame in the digital video, an aggregate diameter-time series is displayed (blue line) with ±10% error limits (dashed lines) (step 3). If the diameter values appear erroneous, the operator can choose to reselect the ROI or (step 4) record the subroutine output.
Beat-to-beat analysis.
FloWave.US includes an automated method to analyze beat-to-beat alterations in blood velocity and vessel diameter with respect to phases in the cardiac cycle (Fig. 1, process 4b, “Beat-to-Beat Analysis”). First, TAMean velocity time series data are partitioned into distinct periods using the time event markers. For each time period, the peak TAMean velocity of each cardiac cycle is detected according to operator-defined thresholds. A windowing function, based on the cardiac cycle frequency in the specific time period, isolates data associated with a single cardiac cycle. Within this cycle, features of the TAMean velocity waveform are used to identify and log the TAMean velocity and time point associated with the start of systole, peak systole, nadir of diastole, and end diastole. In addition, the durations of systole (SysTime) and diastole (DiasTime) are calculated for each cardiac cycle.
Data summary.
FloWave.US exports the measured data (blood velocity, vessel diameter, beat-to-beat data), calculated values (blood flow, shear rate, blood flow at points of interest in the cardiac cycle), and operator processing settings (pixel scaling factors, time event markers, peak find threshold) to a set of comma-separated variable (CSV) files (Fig. 1, process 5, “Data Summary”). To account for inherent differences in sampling rate, a linear interpolation resamples TAMean velocity (cm/s) and vessel diameter (cm) data to 0.01-s intervals, which permits calculation of blood flow (ml/min) (Eq. 1) and shear rate (s−1) (Eq. 2):
| (1) |
| (2) |
In addition, systole and diastole time points identified in the “Beat-to-Beat Analysis” are used to calculate mean (Eq. 3) and weighted mean (Eq. 4) blood flow for each cardiac cycle [T1, time (s) at the start of systole; T2, time (s) at the end of diastole]:
| (3) |
| (4) |
Note that mean blood flow (Eq. 3) and weighted mean blood flow (Eq. 4) represent the net area under the curve or the sum of positive (antegrade) and negative (retrograde) TAMean velocity components across the cardiac cycle.
Part 2: Flow Phantom Study
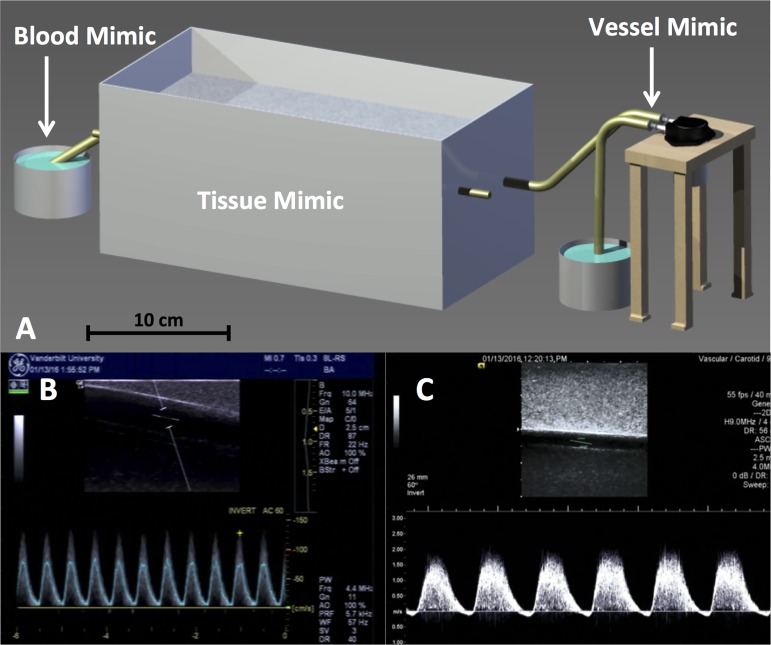
We constructed an ultrasound flow phantom (Fig. 3A) to assess the validity of the “Blood Velocity Measurement” and “Vessel Diameter Measurement” features in the FloWave.US software.
Fig. 3.
Schematic of the vascular ultrasound flow phantom (A) and example screen captures displaying the General Electric (brachial artery mimic, fast pump speed, time-averaged mean velocity color overlay) (B) and Siemens (anterior tibial artery mimic, slow pump speed, Doppler pulse wave spectrum) (C) ultrasound trials. Tissue, blood, and vessel mimic locations are indicated. A peristaltic vacuum pump connected to the vessel mimic generated flow of the blood mimic through the phantom at slow (70 pulses/min) and fast (110 pulses/min) speeds. Time-averaged mean (TAMean) velocities were also extracted from the General Electric system for the Doppler pulse wave spectrum without the color overlay condition (not shown).
Flow phantom configuration.
Two latex tubes, with known inner diameters of 0.410 and 0.314 cm (confirmed with a digital caliper), were suspended in a tissue mimic formulated with agar, graphite, and n-propanol to match the rigidity, attenuation coefficient, and speed of sound of skeletal muscle tissue (18, 19). The tubes were placed at depths of 1.0 and 2.5 cm to reproduce anatomical characteristics of the brachial artery (BA) and ATA, respectively. A peristaltic vacuum pump (Pump in Style Advanced; Medela, McHenry, IL) circulated a 1% concentration cornstarch-saline solution at slow (70 pulses/min) and fast (110 pulses/min) suction rates through the tubes to generate acoustic backscatter similar to physiological Doppler velocity signals. Flow through the artery mimic was collected in a 150-ml reservoir placed on a scale [AWS-600 (0.1-g resolution); American Weigh Scales, Norcross, GA] as a direct measure of volume for each trial.
Blood velocity measurement validation.
Doppler velocity signals were continuously recorded in the flow phantom using the 1) GE TAMean color overlay (GE TAMean), 2) GE Doppler pulse wave spectrum (GE Pulse), and 3) Siemens Doppler pulse wave spectrum (Siemens Pulse) settings. Prior to data collection, ultrasound gate width was adjusted to encompass the full diameter of the vessel mimic, and gain and dynamic range were optimized to avoid saturation and aliasing of the Doppler signal. Three trials of blood velocity data were collected for ∼15 s at both the slow and fast peristaltic pump settings in each vessel mimic (12 trials per ultrasound velocity setting; 36 total trials). Vessel mimic diameter was assumed constant for each trial; therefore differences in volume (the integral of the flow-time curve) would reflect the error of the TAMean velocities estimated by the “Blood Velocity Measurement” feature in FloWave.US. An expert processed each trial with FloWave.US and applied the known vessel mimic diameter to calculate the flow-time curve and the automated volume.
Vessel diameter measurement validation.
A 15-s video of the longitudinal B-mode view of each vessel mimic was recorded with the GE and Siemens scanners. During data collection, the peristaltic pump circulated the blood mimic solution at the slow pump speed to enable visualization of the vessel mimic walls. An expert processed each video five times with the BMode.m program to calculate a mean automated vessel mimic diameter. The vessel ROI was selected to include the borders of the near and far vessel walls, and on-screen feedback (x and y position of the interactive ROI selection cursor) was used to maintain the size and position of the ROI between trials.
Statistical analysis.
Differences between measured (phantom reservoir) and automated (FloWave.US) volume were averaged for the three trials for each experimental condition. Similarly, the mean automated diameter values were compared with the known vessel mimic diameters to assess absolute error in the “Vessel Diameter Measurement” feature. Values are means ± SE.
Part 3: Manual vs. Automated Software Comparison Study
We recorded blood velocity and vessel diameter during a muscle contraction experiment to 1) validate cardiac cycle TAMean velocities measured by the “Beat-to-Beat Analysis” feature and 2) compare operator reliability and 3) compare processing time between a manual, commercial software program (GE LOGIQ Book e) and FloWave.US. The Vanderbilt University Institutional Review Board approved the study, and the subject, a healthy man (aged 19 yr), gave informed, written consent. The subject lay supine on a patient bed with his nondominant foot secured at a 90° angle in a custom-built device for performing isometric ankle dorsiflexions, and five resting video trials were collected. Next, the subject completed five 10-s maximal isometric ankle dorsiflexion contraction trials at 90-s intervals. A 10-s contraction was chosen to alter cardiac cycle blood velocity for a sustained duration to create ample replicates for analysis (42). Ultrasound data were stored in 60–90-s cinematic loops on the ultrasound scanner and replayed for subsequent digital video recording as described previously.
Two trained raters processed the 10 trials with both the manual and automated software. To reduce the overall processing time, only the first 40 s of the resting data and the first 30 postcontraction cardiac cycles were analyzed. Using the GE-supplied software, raters identified each cardiac cycle and recorded the cycle number, cycle start time, peak systolic velocity, nadir diastolic velocity, end diastolic velocity, TAMean velocity, and volume flow. Manual processing time was defined as the time taken to record these variables in a notebook and transfer them to a Microsoft Excel spreadsheet. Raters also analyzed each edited digital video file with FloWave.US. Automated processing time was defined as the time taken to import the video in MATLAB and save the results to CSV files. Each rater analyzed the trials on two occasions. Trials were randomized by analysis method, and raters were blinded to the automated video processing order.
Statistical analysis.
The 95% limits of agreement were calculated to determine the equivalency of cardiac cycle TAMean velocities measured with each analysis method (3). In addition, the standard error of estimate (SEE) for the resting and postcontraction data was calculated to measure the prediction accuracy of the “Beat-to-Beat Analysis” feature. Intrarater and interrater operator reliabilities were found using the class two intraclass correlation coefficient (ICC) of absolute agreement for the single observation [ICC (2,1)] and average values [ICC (2,k)], respectively, for each trial condition (resting or postcontraction) and each analysis method (31). Comparisons between the manual and automated processing times were made by paired t-test. The level of significance was set at P < 0.05. Statistical analyses were performed with R (R Foundation for Statistical Computing).
Part 4: In Vivo Case Study of a Lower Extremity Muscle Contraction Experiment
Our test case for FloWave.US demonstrates the flexibility and utility of the software for measuring lower extremity blood flow during a brief muscle contraction experiment. Blood velocity and vessel diameter ultrasound data were collected from a healthy, active male subject (aged 21 yr) as part of a separate research study. The Vanderbilt University Institutional Review Board had also approved this study, and the subject gave informed, written consent. The muscle contraction protocol was carried out as described in part 3; however, the subject performed only a 1-s maximal isometric ankle dorsiflexion. Digital video was recorded for 20 s prior and 75 s following the muscle contraction. An expert used FloWave.US to measure TAMean velocity and vessel diameter and calculate weighted mean blood flow during the trial. A Savitsky-Golay smoothing filter (3rd order, 11-point segment length) was applied to the data to remove noise associated with the subject's respiratory rate.
RESULTS
Part 2: Flow Phantom Study
Blood velocity measurement validation.
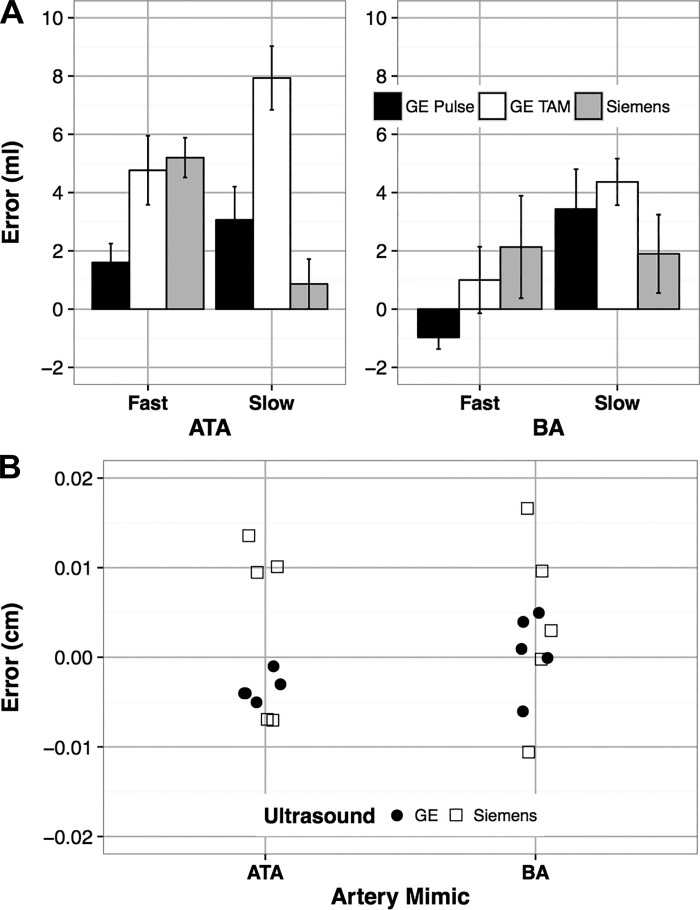
Typical screen captures acquired from the GE and Siemens ultrasound scanners are presented in Fig. 3, B and C, respectively. Different scaling factors were used for each ultrasound velocity setting, and measured volumes varied across trials (Table 1). Automatically detected volumes (FloWave.US) were similar in absolute terms but underestimated measured (phantom reservoir) volumes for most of the conditions (Fig. 4A). Mean volume errors were low across all conditions and both ultrasound scanners (2.94 ± 0.45 ml or a relative error of 4.32% given an average measured volume of 68.1 ± 1.33 ml). Mean volume errors of <1 ml were observed for the GE Pulse (fast pump speed, −0.9 ± 0.4 ml) and Siemens Pulse (slow pump speed, 0.9 ± 0.8 ml) conditions in the BA and ATA vessel mimics, respectively. The GE TAMean condition demonstrated the largest errors for both the BA (slow pump speed, 4.4 ± 0.8 ml) and ATA mimics (slow pump speed, 7.9 ± 1.1 ml) due to a possible limitation of the phantom.
Table 1.
Summary of the flow phantom volume validation study parameters
| Fast, 110 ppm |
Slow, 70 ppm |
|||||
|---|---|---|---|---|---|---|
| GE (TAMean) | GE (Pulse) | Siemens (Pulse) | GE (TAMean) | GE (Pulse) | Siemens (Pulse) | |
| Brachial artery mimic, diameter = 0.410 cm | ||||||
| Known volume, ml | 63.9 ± 2.1 | 71.0 ± 0.9 | 78.4 ± 3.7 | 64.5 ± 1.2 | 66.0 ± 1.6 | 80.9 ± 7.7 |
| Auto volume, ml | 62.9 ± 0.8 | 71.9 ± 1.3 | 76.3 ± 5.9 | 60.1 ± 2.5 | 62.6 ± 2.4 | 79.0 ± 6.6 |
| Scaling, cm·s−1·pixel−1 | 1.06 ± 1.15 × 10−4 | 1.07 ± 1.99 × 10−3 | 1.29 ± 2.89 × 10−3 | 1.07 ± 6.01 × 10−3 | 1.07 ± 3.23 × 10−3 | 1.64 ± 2.72 × 10−16 |
| Anterior tibial artery mimic, diameter = 0.314 cm | ||||||
| Known volume, ml | 64.4 ± 1.3 | 65.8 ± 1.7 | 68.1 ± 5.5 | 67.8 ± 7.6 | 73.3 ± 7.2 | 53.0 ± 2.4 |
| Auto volume, ml | 59.9 ± 8.5 | 64.2 ± 1.5 | 62.8 ± 4.7 | 59.9 ± 8.5 | 70.3 ± 5.6 | 52.1 ± 3.4 |
| Scaling, cm·s−1·pixel−1 | 1.95 ± 4.56 × 10−3 | 1.96 ± 2.14 × 10−3 | 1.80 ± 3.45 × 10−3 | 2.24 ± 8.65 × 10−3 | 2.24 ± 6.16 × 10−3 | 2.00 ± 2.08 × 10−3 |
Values are means ± SD. ppm, pulses per minute; GE, General Electric LOGIQ Book e ultrasound; Siemens, Siemens Acuson SC2000 ultrasound; TAMean, time-averaged mean velocity extraction method; Pulse, Doppler pulse wave velocity extraction method.
Fig. 4.
Summary of volume (A) and vessel diameter (B) absolute errors (known − automated) for the flow phantom validation study. Trials evaluated automated volumes (ml) extracted from the General Electric (GE) time-averaged mean color overlay (GE TAM), GE Doppler pulse wave spectrum (GE Pulse), and Siemens Acuson Doppler pulse wave spectrum (Siemens) in response to slow and fast pump speeds in the anterior tibial artery (ATA) and brachial artery (BA) vessel mimics of the phantom. Values are means ± SE. Automated diameter errors (cm) represent differences with known ATA and BA vessel mimics obtained from B-mode images acquired with the GE and Siemens ultrasound scanners.
Vessel diameter measurement validation.
Automated vessel diameters measured for the BA (GE, 0.409 ± 0.002 cm; Siemens, 0.406 ± 0.005 cm) and ATA (GE, 0.317 ± 0.001 cm; Siemens, 0.310 ± 0.004 cm) mimics demonstrated low absolute errors compared with the known diameters (BA, 0.410 cm; ATA, 0.314 cm) (Fig. 4B). Processing trials for the GE scanner used a lower distance pixel scaling factor (BA, 0.0074 cm/pixel; ATA, 0.0067 cm/pixel) than the Siemens scanner (BA, 0.0101 cm/pixel; ATA, 0.0107 cm/pixel).
Part 3: Manual vs. Automated Software Comparison Study
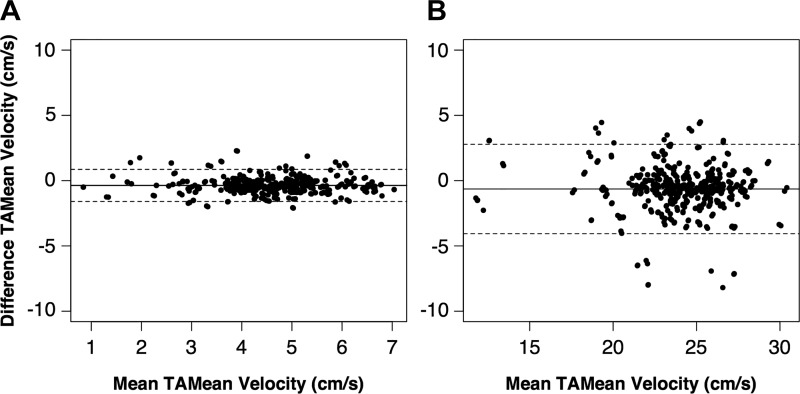
The “Beat-to-Beat Analysis” feature underestimated cardiac cycle TAMean velocities for both resting [−0.37 cm/s; 95% confidence interval (CI), −0.42 to −0.31 cm/s] and postcontraction (−0.64 cm/s; 95% CI, −0.80 to −0.48 cm/s) conditions compared with the native method implemented on the GE LOGIQ Book e. Limits of agreement (Fig. 5) for resting (−1.59 to 0.86 cm/s) and postcontraction (−4.05 to 2.78 cm/s) data were small. SEE values (Table 2) were similar in magnitude to the pixel scaling factors (resting, 0.64 cm·s−1·pixel−1; postcontraction, 1.07 cm·s−1·pixel−1). ICC values were in the excellent range (ICC = 0.85–1.00) for both the intrarater and interrater analyses (Table 2), and use of FloWave.US resulted in a slight improvement of agreement between raters for both the resting (manual ICC, 0.85; vs. auto ICC, 0.99) and postcontraction (manual ICC, 0.90; vs. auto ICC, 0.99) conditions. Automated analysis also reduced processing time by an average of 9.8-fold (total manual time/total automated time; Table 3).
Fig. 5.
Automated and manually assessed time-averaged mean (TAMean) velocity values (cm/s) were equivalent for both resting (A) and postcontraction (B) cardiac cycles. The center solid line represents the mean difference (auto − manual), and the outer dashed lines show the 95% limits of agreement on each Bland-Altman plot. Note the increased x-axis range for the postcontraction data.
Table 2.
Reliability and prediction accuracy of the manual vs. automated analysis of cardiac cycle TAMean velocities
| Resting |
Postcontraction |
|||||
|---|---|---|---|---|---|---|
| Manual ICC | Auto ICC | SEE, cm/s | Manual ICC | Auto ICC | SEE, cm/s | |
| Interrater | 0.85 (0.78–0.9) | 0.99 (0.99–0.99) | 0.60 (12.6%) | 0.90 (0.86–0.93) | 0.99 (0.98–0.99) | 1.63 (6.7%) |
| Intrarater, rater 1 | 1.00 (1.00–1.00) | 0.99 (0.99–1.00) | 0.38 (7.83%) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.38 (5.72%) |
| Intrarater, rater 2 | 0.94 (0.92–0.96) | 0.99 (0.99–0.99) | 0.74 (15.6%) | 1.00 (0.99–1.00) | 1.00 (1.00–1.00) | 1.81 (7.39%) |
ICC values are given as ICC (95% confidence interval). Interrater ICC method (2,k) calculated as the average of analysis trials 1 and 2 for each rater. Intrarater ICC method (2,1) calculated as the agreement between analysis trials 1 and 2 for each rater. SEE values are given as SEE (SEE %). SEE % represents the SEE divided by the average TAMean velocity for the manual method multiplied by 100. ICC, intraclass correlation coefficient; SEE, standard error estimate.
Table 3.
Data processing times using the manual and automated analysis methods
| Rater 1 |
Rater 2 |
|||
|---|---|---|---|---|
| Method | Manual | Auto | Manual | Auto |
| Total time, min | 1,157.4 | 131.7 | 953.4 | 87.8 |
| Trial time, min | 57.8 ± 13.9 | 6.58* ± 2.46 | 47.7 ± 13.0 | 4.39* ± 1.1 |
| Efficiency | 8.79 | 10.86 | ||
Values are means ± SD. Efficiency = total manual time/total automated time.
Significantly different from manual analysis (P < 0.05).
Part 4: In Vivo Case Study of a Lower Extremity Muscle Contraction Experiment
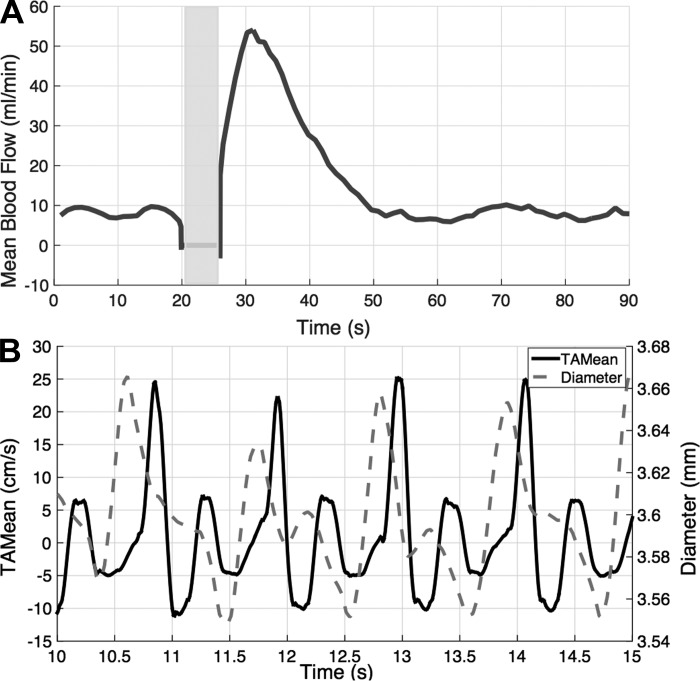
Fig. 6A shows the weighted mean time course of ATA blood flow in response to a 1-s maximal ankle dorsiflexion in a trained male subject. Peak flow increased 6.4-fold above rest and occurred 4.3 s following the contraction. Figure 6B demonstrates the ability of FloWave.US to provide synchronous measures of TAMean velocity and vessel diameter during the protocol. Noticeably, acute changes in vessel diameter on the order of 0.02 mm were detected throughout the cardiac cycle.
Fig. 6.
Representative time course of anterior tibial artery mean blood flow before and after a 1-s maximum voluntary contraction (indicated by the gray box) (A). Small changes in vessel diameter (dashed line) and TAMean velocity (solid line) can be distinguished throughout the cardiac cycle (B).
DISCUSSION
We developed FloWave.US—open-source software for automated vascular ultrasound analysis—and demonstrated the validity of its blood velocity and vessel diameter measures with a skeletal muscle ultrasound flow phantom. Compared with the GE LOGIQ Book e manual analysis software, FloWave.US produced equivalent cardiac cycle TAMean velocities and reduced operator bias and data processing time. Finally, our case study of a lower extremity muscle contraction experiment highlighted software features that can be adapted to a variety of vascular physiology research applications.
Part 1: FloWave.US Overview
FloWave.US offers a cost-effective solution to create custom, adaptable, and high-quality methods to analyze ultrasound blood flow data. Here, we have focused on providing valid measures of blood velocity, vessel diameter, and beat-to-beat analysis, but these variables permit calculation of a myriad of other vascular health parameters including blood flow, flow-mediated dilation, arterial stiffness, antegrade and retrograde shear patterns, compliance during the cardiac cycle, and low flow-mediated vasoconstriction (11, 14–16, 21, 25, 38, 39). With FloWave.US, researchers have the freedom to access, customize, and develop new code to perform these advanced analyses. As the code evolves, maintained archives allow software upgrades to occur as needed, and use of digital video and custom screen settings enables FloWave.US to adapt to a variety of commercial ultrasound scanners and experimental conditions. Creating new, valid, and robust analyses, however, depends on community support and participation (1). Therefore we encourage researchers to become active members of the FloWave.US development network, submit feedback, and report issues to help the software continue to improve and be applied to new areas of vascular physiology research.
Part 2: Flow Phantom Study
Blood velocity measurement validation.
The “Blood Velocity Measurement” feature provided a robust and accurate analysis of TAMean velocity from the Doppler pulse wave spectrum. We are aware of only one other validation of automated ultrasound blood flow analysis software with a flow phantom (15), and compared with these data, aggregate volume errors for our study represent a substantial improvement in accuracy (4.32 vs. 13.8%). It is important to note, however, that constraints of the flow phantom may have increased the magnitude and variability of the errors for the different ultrasound velocity settings. For some trials, the abrupt stop of the pulsatile vacuum pump caused artifacts in the simulated Doppler velocity signal that were difficult to measure with the software. Although a construct of the flow phantom, similar artifacts may occur during in vivo data collection (e.g., probe movement) and adversely impact the accuracy of the estimated TAMean velocity data.
Vessel diameter measurement validation.
Automatically detected vessel diameters were accurate compared with the known BA and ATA mimic diameters in the flow phantom. Absolute errors were within clinical standards (vertical distance error ≤ 1.5 mm) for B-mode ultrasound imaging (13) and consistent with errors reported for existing ultrasound edge detection methods validated with simulated images (12, 20) and plastic phantoms (35, 41). In contrast to these previous studies, we designed the ultrasound flow phantom to assess the effects of soft tissue and vessel depth on the accuracy of the vessel diameter measurement. For the ATA mimic, increased sound attenuation and echogenic backscatter reduced the clarity of the vessel wall edges and increased error for both ultrasound scanners (19). Lower errors for the GE scanner compared with the Siemens scanner, however, reflected the effect of pixel scaling. Recall that the pixel scaling factor determines the amount of data—distance (cm), velocity (cm/s), or time (s)—contained in a single pixel. Greater magnification of the vessel walls using the zoom tool in the GE-acquired B-mode images, therefore, increased the amount of distance information in each pixel. Together, these findings highlight the importance of image quality and careful selection of the vessel ROI and pixel scaling factors to the performance of the “Vessel Diameter Measurement” feature.
Part 3: Manual vs. Automated Software Comparison Study
Comparison of cardiac cycle TAMean velocities measured with a manual, commercial software program and the “Beat-to-Beat Analysis” feature in FloWave.US suggests the analysis methods can be used interchangeably. Mean bias between methods equated to <1 pixel for both the resting and postcontraction data. The limits of agreement, however, indicate errors could be as large as ∼4 (resting) and 6 (postcontraction) pixels or 2 and 3% error (full screen resolution, 180 pixels). Further, the similarity of SEE values to the pixel scaling factor indicates that the automated analysis method is unable to detect subpixel velocities because of the inherent limitation of recreating a screen capture. Investigators can improve the pixel scaling factor and subsequent detection of cardiac cycle TAMean velocities by optimizing the ultrasound pulse repetition frequency to record the full dynamic range of expected Doppler velocity signals for a given data collection session.
Consistent with existing automated ultrasound analysis software (10, 12, 15, 22, 29, 32, 34, 41), FloWave.US improved reproducibility and lowered processing time compared with the commercial, manual analysis technique. A priori training of raters resulted in no change between trials, but a slight improvement in interrater reliability suggests that automated analysis reduced measurement error of the cardiac cycle TAMean velocities. FloWave.US also minimized subjective assessments, such as placing cardiac cycle time markers and detecting vessel wall edges. Automation of these steps simplified training for new users, removed operator selection bias, and cut processing time from almost an hour to <10 min per trial.
Part 4: In Vivo Case Study of a Lower Extremity Muscle Contraction Experiment
Blood velocity, vessel diameter, and blood flow data obtained during an in vivo muscle contraction experiment highlighted the capabilities of FloWave.US to analyze images with heterogeneous quality. In vivo ultrasound recordings require substantial technical skill particularly for dynamic experimental conditions such as muscle contraction or exercise where movement of the underlying tissue makes acquisition of high-quality images a challenge. In our case study, blood flow analyses were possible throughout baseline, ∼1 s following muscle contraction, and recovery despite transducer movements associated with the protocol. Notably, deflections in vessel diameter were detected in synchrony with TAMean velocity suggesting FloWave.US has the spatial and temporal resolution needed to detect small vessel diameter changes expected during the cardiac cycle (9). Moreover, the ability to extend the duration of the ultrasound data recording (∼95 s in this example) beyond the 30–60-s segments typical of cinematic loop formats permitted analysis of time course data that may provide additional insight into the kinetics of blood flow in response to brief muscle contractions (8, 40), dynamic exercise (15, 26, 33), or cuff occlusion (16, 36, 38).
Future Directions
FloWave.US will benefit from continued testing and coding developments. We demonstrated the functionality of the software with two commercial ultrasound scanners, but it is necessary to evaluate the compatibility of the software with an array of commercial ultrasound scanners. We anticipate similar performance if the ultrasound scanner allows 1) capture of the video output signal and 2) on-screen display of calibrated scales. However, it is unclear how additional on-screen markings or low screen resolution will affect interoperability. Further, FloWave.US should be compared with commercially available software, such as Medical Imaging Applications' Vascular Research Tools and Quipu's FMD-Studio, with a flow phantom experiment to allow comparisons of blood velocity and vessel diameter data obtained in previous studies. In addition, pixel color threshold settings for the TAMean velocity overlay condition (blood velocity measurement validation in methods) require additional optimization. For pixels located at extreme blood velocity values, overlap of the autocalculation color overlay and the background pulse wave data may blur the pixel color and restrict its inclusion in the automated TAMean velocity data. Although these errors are minimal, improvement of threshold settings may mitigate underestimation of TAMean velocities when applying ultrasound scanner autocalculation features. Finally, upgrade to the “Beat-to-Beat Analysis” feature would enable estimation of cardiac cycle time points to extract antegrade and retrograde shear rate measures for clinical studies (4, 7, 24).
In summary, we developed, validated, and demonstrated the features of FloWave.US: accurate, accessible, flexible, and cost-effective software for ultrasound blood flow analysis. Current software applications include automated blood velocity measurement, vessel diameter edge detection, and calculation of blood flow in the peripheral vasculature. Continued community contributions have the potential to improve the quality and extend analytical capabilities of the software for a variety of applied vascular physiology research areas.
GRANTS
Funding support for this research was provided by National Institutes of Health Grants 1R0I-AR-050101, T32-EB-001628, and UL1-TR-000445.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.L.C., E.C.B., C.F.C., and T.F.T. conception and design of research; C.L.C. and E.C.B. performed experiments; C.L.C. analyzed data; C.L.C., C.F.C., B.M.D., and T.F.T. interpreted results of experiments; C.L.C. and E.C.B. prepared figures; C.L.C. drafted manuscript; C.L.C., E.C.B., C.F.C., B.M.D., and T.F.T. edited and revised manuscript; C.L.C., E.C.B., C.F.C., B.M.D., and T.F.T. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Shea Sabin, Patrick Givens, Efrain Salazar, and Samuel Klockenkemper for their assistance in pilot testing the software and collecting data for the manual and automated analysis comparison study. We also thank Dr. Brett Byram, Dr. Adrienne Dula, and Dr. Lori Arlinghaus for loaning equipment for the phantom study. Finally, we are grateful to the subjects for their participation.
REFERENCES
- 1.Ball P. Openness makes software better sooner. Nat News (June 25, 2003). doi: 10.1038/news030623-6. [DOI] [Google Scholar]
- 2.Black MA, Cable NT, Thijssen DHJ, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 51: 203–210, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat 17: 571–582, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bombardini T, Gemignani V, Bianchini E, Venneri L, Petersen C, Pasanisi E, Pratali L, Alonso-Rodriguez D, Pianelli M, Faita F, Giannoni M, Arpesella G, Picano E. Diastolic time-frequency relation in the stress echo lab: filling timing and flow at different heart rates. Cardiovasc Ultrasound 6: 15, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canny J. A computational approach to edge detection. IEEE Trans Pattern Anal Mach Intell 8: 679–698, 1986. [PubMed] [Google Scholar]
- 6.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Chung CS, Karamanoglu M, Kovács SJ. Duration of diastole and its phases as a function of heart rate during supine bicycle exercise. Am J Physiol Heart Circ Physiol 287: H2003–H2008, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Credeur DP, Holwerda SW, Restaino RM, King PM, Crutcher KL, Laughlin MH, Padilla J, Fadel PJ. Characterizing rapid onset vasodilation to single muscle contractions in the human leg. J Appl Physiol (1985) 118: 455–464, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eriksen M. Noninvasive measurement of arterial diameters in humans using ultrasound echoes with prefiltered waveforms. Med Biol Eng Comput 25: 189–194, 1987. [DOI] [PubMed] [Google Scholar]
- 10.Faita F, Masi S, Loukogeorgakis S, Gemignani V, Okorie M, Bianchini E, Charakida M, Demi M, Ghiadoni L, Deanfield JE. Comparison of two automatic methods for the assessment of brachial artery flow-mediated dilation. J Hypertens 29: 85–90, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Gemignani V, Bianchini E, Faita F, Giannarelli C, Plantinga Y, Ghiadoni L, Demi M. Ultrasound measurement of the brachial artery flow-mediated dilation without ECG gating. Ultrasound Med Biol 34: 385–391, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Gemignani V, Faita F, Ghiadoni L, Poggianti E, Demi M. A system for real-time measurement of the brachial artery diameter in B-mode ultrasound images. IEEE Trans Med Imaging 26: 393–404, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Goodsitt MM, Carson PL, Witt S, Hykes DL, Kofler JM. Real-time B-mode ultrasound quality control test procedures: report of AAPM Ultrasound Task Group No. 1. Med Phys 25: 1385–1406, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Gori T, Dragoni S, Lisi M, Di Stolfo G, Sonnati S, Fineschi M, Parker JD. Conduit artery constriction mediated by low flow: a novel noninvasive method for the assessment of vascular function. J Am Coll Cardiol 51: 1953–1958, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Green D, Cheetham C, Reed C, Dembo L, O'Driscoll G. Assessment of brachial artery blood flow across the cardiac cycle: retrograde flows during cycle ergometry. J Appl Physiol 93: 361–368, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris RA, Padilla J, Hanlon KP, Rink LD, Wallace JP. The flow-mediated dilation response to acute exercise in overweight active and inactive men. Obesity 16: 578–584, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Hoskins PR. Physical properties of tissues relevant to arterial ultrasound imaging and blood velocity measurement. Ultrasound Med Biol 33: 1527–1539, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Hoskins PR. Simulation and validation of arterial ultrasound imaging and blood flow. Ultrasound Med Biol 34: 693–717, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Hunt BE, Flavin DC, Bauschatz E, Whitney HM. Accuracy and robustness of a simple algorithm to measure vessel diameter from B-mode ultrasound images. J Appl Physiol (1985) 120: 1374–1379, 2016. [DOI] [PubMed] [Google Scholar]
- 21.Kizhakekuttu TJ, Gutterman DD, Phillips SA, Jurva JW, Arthur EI, Das E, Widlansky ME. Measuring FMD in the brachial artery: how important is QRS gating? J Appl Physiol 109: 959–965, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang W, Browning RL, Lauer RM, Sonka M. Automated analysis of brachial ultrasound time series. Proc SPIE 3337, 108–118, 1998. [Google Scholar]
- 23.Mancini GB, Yeoh E, Abbott D, Chan S. Validation of an automated method for assessing brachial artery endothelial dysfunction. Can J Cardiol 18: 259–262, 2002. [PubMed] [Google Scholar]
- 24.Merkus D, Kajiya F, Vink H, Vergroesen I, Dankelman J, Goto M, Spaan JA. Prolonged diastolic time fraction protects myocardial perfusion when coronary blood flow is reduced. Circulation 100: 75–81, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Myers KA, Clough A. Making Sense of Vascular Ultrasound: A Hands-On Guide. New York: Arnold, 2004. [Google Scholar]
- 26.Padilla J, Harris RA, Fly AD, Rink LD, Wallace JP. A comparison between active- and reactive-hyperaemia-induced brachial artery vasodilation. Clin Sci (Lond) 110: 387–392, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Padilla J, Johnson BD, Newcomer SC, Wilhite DP, Mickleborough TD, Fly AD, Mather KJ, Wallace JP. Normalization of flow-mediated dilation to shear stress area under the curve eliminates the impact of variable hyperemic stimulus. Cardiovasc Ultrasound 6: 44, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol 298: H1128–H1135, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Preik M, Lauer T, Heiss C, Tabery S, Strauer BE, Kelm M. Automated ultrasonic measurement of human arteries for the determination of endothelial function. Ultraschall Med 21: 195–198, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Savitzky A, Golay MJ. Smoothing and differentiation of data by simplified least squares procedures. Anal Chem 36: 1627–1639, 1964. [Google Scholar]
- 31.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86: 420–428, 1979. [DOI] [PubMed] [Google Scholar]
- 32.Sidhu JS, Newey VR, Nassiri DK, Kaski JC. A rapid and reproducible on line automated technique to determine endothelial function. Heart 88: 289–292, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simmons GH, Padilla J, Young CN, Wong BJ, Lang JA, Davis MJ, Laughlin MH, Fadel PJ. Increased brachial artery retrograde shear rate at exercise onset is abolished during prolonged cycling: role of thermoregulatory vasodilation. J Appl Physiol 110: 389–397, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonka M, Liang W, Lauer RM. Automated analysis of brachial ultrasound image sequences: early detection of cardiovascular disease via surrogates of endothelial function. IEEE Trans Med Imaging 21: 1271–1279, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Stadler RW, Karl WC, Lees RS. New methods for arterial diameter measurement from B-mode images. Ultrasound Med Biol 22: 25–34, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Stoner L, Sabatier MJ. Use of ultrasound for non-invasive assessment of flow-mediated dilation. J Atheroscler Thromb 19: 407–421, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Stoner L, Sabatier MJ, Mahoney ET, Dudley GA, McCully KK. Electrical stimulation-evoked resistance exercise therapy improves arterial health after chronic spinal cord injury. Spinal Cord 45: 49–56, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Thijssen DHJ, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 66: 698–722, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Towse TF, Slade JM, Ambrose JA, DeLano MC, Meyer RA. Quantitative analysis of the postcontractile blood-oxygenation-level-dependent (BOLD) effect in skeletal muscle. J Appl Physiol 111: 27–39, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol 91: 929–937, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Zou GY. Sample size formulas for estimating intraclass correlation coefficients with precision and assurance. Stat Med 31: 3972–3981, 2012. [DOI] [PubMed] [Google Scholar]