Abstract
Age-related changes in the basic functional unit of the neuromuscular system, the motor unit, and its neural inputs have a profound effect on motor function, especially among the expanding number of old (older than ∼60 yr) and very old (older than ∼80 yr) adults. This review presents evidence that age-related changes in motor unit morphology and properties lead to impaired motor performance that includes 1) reduced maximal strength and power, slower contractile velocity, and increased fatigability; and 2) increased variability during and between motor tasks, including decreased force steadiness and increased variability of contraction velocity and torque over repeat contractions. The age-related increase in variability of motor performance with aging appears to involve reduced and more variable synaptic inputs that drive motor neuron activation, fewer and larger motor units, less stable neuromuscular junctions, lower and more variable motor unit action potential discharge rates, and smaller and slower skeletal muscle fibers that coexpress different myosin heavy chain isoforms in the muscle of older adults. Physical activity may modify motor unit properties and function in old men and women, although the effects on variability of motor performance are largely unknown. Many studies are of cross-sectional design, so there is a tremendous opportunity to perform high-impact and longitudinal studies along the continuum of aging that determine 1) the influence and cause of the increased variability with aging on functional performance tasks, and 2) whether lifestyle factors such as physical exercise can minimize this age-related variability in motor performance in the rapidly expanding numbers of very old adults.
Keywords: aging, motor unit, power, strength, voluntary activation, contractile velocity, muscle fatigue, steadiness
the motor unit is the basic functional unit in the neuromuscular system that allows production of force and movement (37). The motor unit consists of the alpha motor neuron and the muscle fibers it innervates. Force produced during maximal and submaximal contractions performed by skeletal muscles is controlled by varying the number of motor units recruited and the discharge rate of the action potentials that innervate each active motor unit (21, 38). Thus, age-related changes in motor unit size, properties, and morphology, as well as altered inputs from the nervous system, can profoundly change motor function and performance in old adults (age, older than ∼60 yr).
Age-related changes to the neuromuscular system and the resulting motor performance, however, do not appear to be uniform among old adults. Some old adults have much greater impairments in motor function compared with others of the same age and sex, so between-subject variability is typically greater than young adults (27, 126, 150). Aging is also accompanied by greater inconsistency of motor performance by an old adult when repeating a task so that the within-subject variability is larger than it is in young adults (27, 126, 150). This between-subject and among-subject variability that increases with aging may appear even greater in men and women aged >80 yr [e.g., see Degens and Korhonen (27), Rantanan et al. (126), and Vanden Noven et al. (150)]. Thus, in addition to the reduction in motor performance (e.g., reduced strength and power, increased fatigability) in old and very old adults compared with young adults, we argue that impaired motor performance with advanced age also includes increased variability between trials and within trials for a given motor task (within-subject variability) [e.g., see Christou (20)]. This variability leads to less predictable and less accurate performance that may compound motor function that is already compromised with aging. We present evidence that a large source of the within-subject variability that increases with aging arises from neural mechanisms.
The large age-related variability among and within older adults, however, can be masked when the average or best performances of the age groups or individuals are compared, so that subtle age-related changes in physiology and function are not always detected. Contributing factors that interact with biological aging to increase variability between and within old adults includes physical activity, genetics, nutritional status, hormonal status, and inflammatory status among other mediators that will modify the motor unit and motor performance between people as they age and into very old age (27). Some of this large variability may also be exacerbated (or dampened) by the sampling bias associated with cross-sectional design studies that also average the motor performance data of subjects over a large age range (60–90 yr).
In this review, we focus on the age-related changes in the motor unit and the inputs it receives from spinal and supraspinal sources, and then highlight several areas of motor performance that are directly influenced by these changes, including maximal strength and power, velocity of contraction, fatigability, and steadiness during submaximal contractions.
AGING AND THE MOTOR UNIT
The motor neuron is the final common pathway through which all synaptic inputs are translated to motor function by the musculoskeletal system. Aging is accompanied by a net loss of motor units (16, 145), changes to the morphology and properties of existing motor units (55), and altered inputs from peripheral, spinal, and supraspinal centers [e.g., see Seidler et al. (132) and Tracy et al. (147)]. Ultimately, motor performance is impaired and its variability is greater with advanced age.
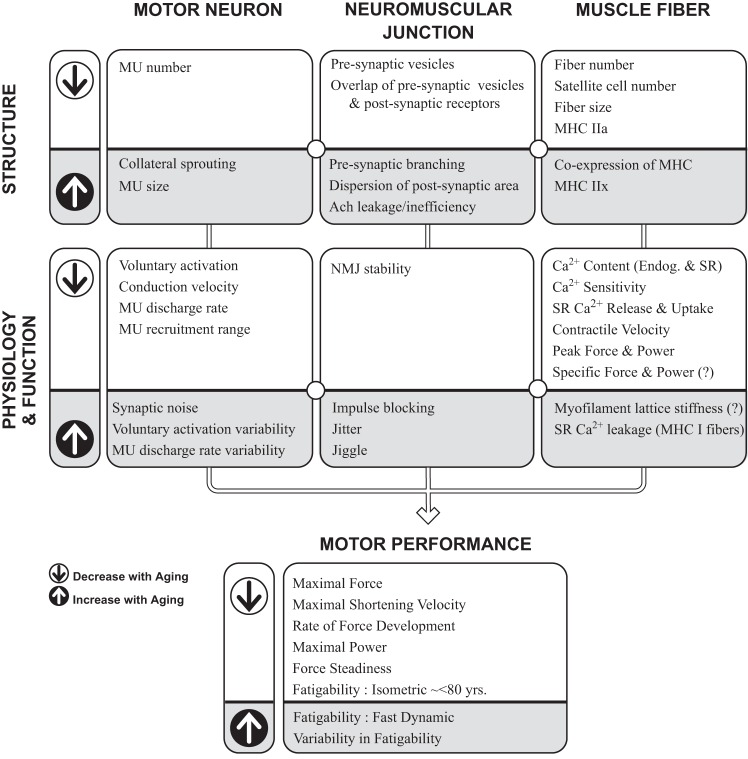
Sarcopenia is the age-related loss of muscle mass and associated reductions in maximal muscle strength, power, and physical function (10, 42, 54). It is predictive of disability, loss of independence, and mortality (108) and has a strong neurogenic component (86). One of the primary causes of sarcopenia is the loss of muscle fiber innervation by alpha motor neurons caused by spinal motor neuron apoptosis and distal axon retraction. Spinal motor neuron apoptosis can begin gradually, early in life, and accelerate after the age of 60 yr and beyond (16, 145) with more rapid declines in human muscle mass and function from approximately 75 to 80 yr of age [e.g., see Andersen (2), Justice et al. (70), and McNeil et al. (104)]. In addition, age-related morphological changes in the alpha motor neuron occur during normal aging is in part due to oxidative stress and inflammation (115), and the neuromuscular junction becomes less stable (31, 55). These events alter the normal cycle of denervation and reinnervation of muscle fibers that occurs over the life span in skeletal muscle. The damaged motor axon withdraws from the muscle fibers it innervates, and many but not all of these fibers are then reinnervated by adjacent axons through collateral sprouting (55). This process of denervation and reinnervation accelerates with advanced age and leads to fewer but larger surviving motor units (16, 43), ultimately resulting in lasting functional consequences for men and women starting as early as age 50–60 yr that can be magnified in very old adults (<80 yr) [e.g., see Reid and Fielding (127)]. Regular physical activity, however, may modify age-related changes in motor unit structure and function across the life span, with examples provided throughout this review. Figure 1 summarizes some of these age-related changes in structure at the different levels of the motor unit and the accompanying physiological and functional changes that ultimately contribute to declines in motor performance in old and very old adults. It is important to note that although the role of physical activity is highlighted in this review, other processes such as genetics, nutrition (decreased total caloric and protein intake), altered hormonal status, inflammatory mediators, and factors resulting in altered protein synthesis and sarcopenia (35) may also be powerful modifiers to the aging neuromuscular system, possibly contributing to the large variability in motor performance between old adults and very old adults (27). The contribution of biological aging, physical activity, and these other modulators on the motor unit and the subsequent neuromuscular system is not well understood because many of the studies in humans are of cross-sectional design and not able to control for many of these potential modifiers. In this context, the following section will broadly highlight some of the known age-related changes in motor unit at the level of the 1) motor neuron, 2) neuromuscular junction, and 3) muscle fiber, and are summarized in the model presented in Figure 1.
Fig. 1.
Structural and physiological changes to the aging motor unit (MU) and motor performance outcomes. Shown is a working model of some of the known age-related changes in structures (top) and physiology and function (middle) that are thought to occur at each level of the MU identified as the motor neuron, neuromuscular junction, and muscle fibers (top row). Arrows (left) indicate the direction of change that occurs with advanced age. The supporting evidence varies for those variables identified, so a question mark (?) denotes when there is conflicting or small amounts of evidence. Furthermore, other factors (not shown) such as physical activity, nutrition, genetics, and inflammation among other possible modifiers, can interact with biological aging to alter motor performance in old and very old adults. Supraspinal and spinal inputs are not highlighted here but are influential. Motor performance outcomes (bottom) are each influenced to varying degrees by the variables in the model. The panels are interconnected so that the changes in physiology and function in one column (e.g., the motor neuron) are profoundly affected by age-related changes in the structures and physiology from the other levels (columns) of the motor unit (e.g., the neuromuscular junction and the muscle fibers). Ach, acetylcholine; Ca2+, calcium; Endog, endogenous; MHC, myosin heavy chain; NMJ, neuromuscular junction; SR, sarcoplasmic reticulum.
Motor Neuron
Skeletal muscle contraction is the end result of a series of events that is initiated with the transmission of synaptic inputs to motor neurons. Thus, age-associated changes in the synaptic input can change the output (i.e., force and movement) and the age effect determined by the gain of the system and its sensitivity to different inputs. “Gain” is the ratio of the output to the input, and motor neurons in the spinal cord are able to alter the gain (e.g., excitability) of the system, thereby providing a change in the output for a given input. For example, monoaminergic projections from the brainstem have a powerful role in influencing the gain of the spinal motor neurons (e.g., by either increasing or decreasing excitability) and thus motor function (58, 69). The synaptic input onto motor neurons that originate from multiple sites within the neuromuscular system change with age, although the current review will highlight those age-related changes from supraspinal and peripheral reflex pathways. In addition, the importance of age-related changes in both independent and shared common synaptic inputs to motor neurons within and across muscles that govern motor control are highlighted. These common synaptic inputs play an integral role in determining the input-output relationships (i.e., gain) of the motor neuron pool, and these inputs coordinate motor unit activity.
Cortical inputs to motor neurons.
Significant age-associated changes in the structure and function of cortical neurons contribute to the dysfunction of the aging neuromuscular system. Neuronal atrophy is ubiquitous with advancing age throughout the central nervous system (156), with ∼40% reduction in the volume/size of motor cortical cell bodies by 80 yr of age (51), and general reductions in both gray matter (103, 130) and white matter (98) volume throughout the cortex. In contrast to spinal motor neurons, there is limited evidence that the number of neurons change substantially in the cortex with advancing age (130). Nonetheless, the functional importance of the reduced gray matter thickness is shown by its association with reduced motor performance in old adults during motor tasks (76, 136).
In addition to age-related structural changes, the function of cortical neurons is marked by a significant decrease in inhibition and increased activity in many cortical areas in old adults. The decreased inhibition with advancing age has been assessed predominantly with transcranial magnetic stimulation (TMS) and quantified as 1) decreased interhemispheric inhibition between cortices (100, 156); 2) shorter silent period in electromyographic (EMG) recordings in response to TMS before and after fatiguing exercise (63, 113, 131); and 3) reduced short-interval intracortical inhibition (53, 99). In addition, there is greater activation during motor tasks throughout the aging cortex in the contralateral and ipsilateral hemispheres (100, 156). An inability to inhibit appropriate cortical areas during focused and targeted motor tasks can lead to inappropriate activation of motor units (132). Accordingly, greater muscle activity is frequently observed when older adults perform fine motor tasks (74, 135). It is unknown whether these age-related changes are the result of dysfunction or are compensatory [i.e., increased cortical activity to compensate for decreased cortical matter (132) or increased activity to stiffen the joint for postural support (74)], but ultimately, they influence corticospinal inputs leading to altered synaptic inputs at the motor neuron.
Reflex responses.
Afferent feedback from sensory receptors to spinal motor neurons evokes rapid responses in muscle that are reduced with advancing age. One hallmark of aging is a slowing of responses due to a reduction in peripheral efferent and afferent axon action potential conduction velocity (36, 129) due at least in part, to declines in the density of unmyelinated and myelinated neurons (64). Furthermore, the Hoffman (H) reflex, which predominantly assesses the efficacy of Ia afferents to activate spinal motor neurons, is often reduced in old adults compared with young adults, although these effects are highly variable and dependent on the task being performed (6, 8). Moreover, the function of peripheral reflexes changes with advanced age and can be accompanied by corresponding compensatory responses in the cortex; for example, the lower efficacy of Ia afferents to discharge spinal motor neurons is accompanied by greater corticospinal excitability in old adults compared with young adults (9).
Independent and common inputs to motor neurons.
Spinal motor neurons receive tens of thousands of synaptic inputs from a multitude of different sources, with many synaptic inputs shared across motor neurons within and across populations of motor neurons (52). In addition to age-associated changes in the timing and magnitude of synaptic inputs onto motor neurons, changes in the common synaptic inputs across many motor neurons also play an important role in coordinating muscle activation in old adults. One frequently used method to assess coordination of motor neuron activity is to quantify the common modulation of synaptic inputs from central and peripheral sources onto spinal motor neurons, which influences motor function (29, 149). This common synaptic input is received by a population of motor neurons and drives the discharge rates of these motor neurons at a common low frequency that affects the force exerted by the muscle (112). Common modulation of the motor unit activity is typically quantified by performing a coherence analysis between two physiological signals, including motor unit action potential discharge times, and EMG and electroencephalographic (EEG) recordings. Coherence is a measure of the correlation between two signals in the frequency domain and is quantified between values of zero to 1. Coherent motor unit activity may be critical during the performance of fine motor skills, including that by older adults. However, the association between oscillatory activity (identified by coherence analysis) and motor function is unclear. Although it has been suggested that the oscillatory activity may improve the recruitment of motor units and facilitate coordination of motor unit activity across multiple muscles, the coherent activity is more commonly viewed to be maladaptive; for example, in the generation of essential tremor and Parkinsonian tremor (48, 124). Fine motor skills may require the separate and independent activation of multiple muscles, which would require decoupling from this coherent activity that occurs throughout the neuromuscular system to produce precise forces and movements (4).
Consistent with this view, a number of studies have reported increased coherence between different physiological signals in old compared with young adults. First, old adults demonstrate greater coherence than young adults between the discharge times of pairs of concurrently active motor units in the first dorsal interosseus from 2 to 20 Hz during the performance of an index finger abduction task (133). Second, EEG-EMG and magnetoencephalographic (MEG)-EMG coherence was increased in old adults across a wide frequency range (8–32 Hz) during the performance of hand tasks (68, 73). Third, interhemispheric EEG-EEG coherence between 1 to 30 Hz was increased in old adults compared with young adults during attention-demanding tasks (102, 142). These studies taken together point to an inability of old adults to appropriately regulate and coordinate the common synaptic inputs onto spinal motor neurons. Because the common oscillatory synaptic input likely represents the effective neural drive to muscle (112), greater fluctuations in this common oscillatory input in old adults likely explain in large part the increased variability in motor unit action potential discharge rate (47, 67, 147) and impaired motor performance in old compared with young adults (133).
Neuromuscular Junction
The neuromuscular junction is the synapse between an alpha motor neuron and a skeletal muscle fiber (55). Aging is associated with remodeling of the neuromuscular junction and impaired neuromuscular transmission (31, 55) that may increase the variability in motor unit activation among old adults. Typically, the amount of the neurotransmitter released in the postsynaptic membrane of the neuromuscular junction is in excess of what is necessary, so there is a safety factor (157) to ensure efficient transmission of the potential even in high-demand events such as a fatiguing contraction. The neuromuscular junction typically allows a 1:1 transmission of the action potential from the motor neuron to the muscle fiber, but this reliability declines with aging (157), and it probably reflects age-related changes and remodeling of the neuromuscular junction both morphologically and physiologically (55).
Evidence for age-related changes in the neuromuscular junction exists mostly in reports from animal studies with indirect evidence in humans. Morphological changes observed in aged animals include increased presynaptic branching, larger and more dispersed postsynaptic endplate areas, and a reduction in the coupling of the presynaptic vesicles and postsynaptic receptors [e.g., see reviews by Deschenes (31) and Hepple and Rice (55)]. The presynaptic nerve terminal branches become greater in number and length and are accompanied by a larger area of vesicles (31). However, as neuromuscular branching increases, vesicle density decreases along with the acetylcholine stores and release (31). Primary factors underlying these changes and instability of the neuromuscular junction probably involve greater mitochondrial dysfunction, oxidative stress, inflammation, and neurodegeneration, all of which increase with aging (144). The functional consequences of these age-related changes in the neuromuscular junction include impaired neuromuscular transmission, which may result in complete blocking of the action potential (impulse blocking) and inefficient and more variable activation of the muscle. Ultimately, such changes may lead to sarcopenia. For example, in mice, a reduction in fiber size was shown to be preceded by neuromuscular junction disruption (32); however, whether sarcopenia is secondary to this disruption at the neuromuscular junction in old adults or vice versa is not understood (144).
In humans, insight into age-related changes of the neuromuscular junction is observed with increased “jitter” (12) and “jiggle,” which are both detected with EMG recordings of single motor unit potentials (56, 137). Jitter is the variability in the time interval of two muscle fiber action potentials from different fibers of the same motor unit and is larger with greater collateral sprouting. Jiggle is the variability in the shape of a single motor unit across consecutive discharges (56) and is related to decreased transmission stability due to increased jitter and impulse blocking (55). Cross-sectional studies show that jiggle is greater in very old adults (age, ∼80 yr) compared with young adults (age, <30 yr) (56), and in very old elite masters athletes (age, ∼80 yr) jiggle is reduced relative to age-matched nonathletes (119). In contrast, endurance training can alter the morphology of the neuromuscular junction and neuromuscular transmission in several muscles of young mice, but this effect is dampened or does not occur with training in old mice (31, 33). Thus, physical activity and training may not always preserve the neuromuscular junctions in old and very old muscle, and other factors may preserve their function in very-old-age lifetime exercisers (119). Calorie restriction, for example, can reduce oxidative stress and preserve function of the neuromuscular junction with aging in old mice (115). Longitudinal studies, however, would provide greater insight into the role that exercise and other factors such as nutrition and genetics play in the change in neuromuscular junction stability that occurs with advanced age.
Muscle Fiber
Advanced age is accompanied by a net loss of innervated muscle fibers and a reduction in the fiber size of the surviving motor units (93). Fiber loss results from motor unit remodeling (a greater rate of fiber denervation relative to reinnervation) (55) and also may include increased oxidative stress and apoptosis, which reduces satellite cell regeneration of muscle fibers (11, 111). Old adults typically have a larger innervation ratio than that of young adults so that there are more muscle fibers within each motor unit [e.g., see Fling et al. (43)], and the fibers of a single motor unit are more likely to be clustered within a specific region of the muscle (fiber-type grouping) (91).
Fibers from the existing motor units are generally smaller in old and very old adults compared with young adults, especially in lower limb muscles [e.g., see Hunter et al. (62), Lexell et al. (93), and Venturelli et al. (152)]. The age-related reduction in fiber cross-sectional area usually occurs across all fibers, although there is some evidence that this atrophy occurs to a greater extent in fibers expressing myosin heavy chain (MHC) II isoforms in old adults younger than ∼80 yr (62, 93, 123). In very old adults (older than ∼80 yr) atrophy can be quite marked in all fibers (123). These age-related reductions in fiber size, however, are not reported by all studies and may differ between muscles and the sexes. For example, compared with young adults, there were no differences in the size of vastus lateralis muscle fibers in old men (age, ∼69 yr) (109) and very old men and women (age, 80–88 yr) (122, 148, 152), nor in the fibers of the biceps brachii of very old women (age, ∼88 yr) (152). Aside from the possible influence of lifestyle modifiers such as physical activity or nutrition, the disparities of findings may be influenced by sampling bias of cross-sectional designed studies, the variability associated with muscle biopsies, and small numbers of subjects in some studies.
Smaller fibers in old adults compared with young adults are associated with lower protein synthesis and less satellite cells in type II fibers, which are needed for skeletal muscle growth and repair (71, 153, 154). Some fibers of older adults are also quite small and angular in shape (92), possibly representing denervation (123). Furthermore, with aging, muscle fibers of animals and humans are more likely to coexpress multiple MHC isoforms in single fibers, suggesting age-related changes in denervation-innervation of muscle fibers (3, 109, 122). Myosin ATPase histochemical staining techniques, however, are not able to easily identify coexpression of the different MHCs (123), so caution is required in categorizing fibers in older adults on the basis of type I and type II categories alone without considering the MHC isoform composition.
In general, the smaller muscle of old adults (age, approximately 70–73 yr) has been shown to have a smaller proportional area that expresses MHC IIa isoforms compared with that of young adults [e.g., D'Antona et al. (26) and Lamboley et al. (87)]. This is consistent with studies that show increased atrophy of type II fibers (identified with myosin ATPase histochemical staining) compared with type I fibers in old adults (age, up to ∼83 yr) (62, 93). Furthermore, in old and very old adults there is evidence of a shift to a proportional increase in the expression of the MHC IIx isoform (26, 109, 122, 152), with less coexpression in very old master athletes compared with age-matched nonathletic controls (122).
Although the size of muscle fibers decreases with advanced age, their specific tension (force per unit area of muscle) may be preserved with aging especially when young and old adults are matched for physical activity levels (148, 152). This finding, however, is not universal because other studies report lower specific tension across different fibers of muscle in old individuals (mean age, approximately 70–73 yr) (26, 87, 90, 122) and in very old master athletes (age, ∼80 yr) (122). Lower intracellular calcium (endogenous and sarcoplasmic reticulum) in the skeletal muscle fibers of old adults (∼70 yr) compared with that in young adults, and a lower calcium sensitivity to activation could in part be responsible for an age-related reduction in the specific tension (87). In fact, there are several age-related mechanisms informed by animal studies that can reduce effective excitation-coupling and be responsible for lower specific tension in old skeletal muscle [see review by Delbono (28)]. In contrast, specific tension of MHC I and IIa single fibers in old women (age, ∼68 yr) was greater than that in activity-matched young women and men, possibly due to increased myofilament lattice stiffness (109). Specific tension of all fiber types in old men and women (age, ∼74 yr) were elevated in a 3-yr longitudinal follow-up study (128). Taken together, the age differences in the specific tension of single fibers may not be uniform across the population of motor units and people. Physical activity may in part modulate age differences in specific tension observed in old adults <75 yr, but does not seem to be able to prevent the reduction of specific tension even in very old athletes (122).
Muscle fibers of old adults also typically exhibit reduced contractile speed compared with those of young adults. For example in single fibers, lower rates of force development in active and inactive older adults (age, ∼80 yr) (122) and lower maximal shortening velocity (26, 85, 90, 122) are reported, although not all studies show this to be the case in active, older adults (age, ∼78 yr) (148). The reduced velocity of the muscle fibers is associated with slower cross-bridge kinetics (109, 122). Shortening velocity can be 3× faster and peak power output up to 6× greater in MHC IIa fibers compared with MHC I fibers (148), so that any shift toward a reduction in MHC IIa isoforms in old muscle would reduce its contractile speed (90).
Consistent with the slowing of contractile speed, the rate of muscle relaxation of whole muscle slows with aging [e.g., see Callahan and Kent-Braun (14), Hunter et al. (62, 63), and Molenaar et al. (110)], probably due to slower cross-bridge mechanics and slower rates of Ca2+ uptake into the sarcoplasmic reticulum. For example, in old women (age, ∼71 yr), the rate of relaxation of the quadriceps muscles was associated with a lower proportional area of type IIa fibers (% area of sample), and lower sarcoplasmic reticulum maximal rate of Ca2+ uptake and Ca2+ ATPase activity analyzed from biopsy samples of the vastus lateralis muscle (62). In the same study, 12 wk of strength training of the quadriceps muscles decreased the age difference in sarcoplasmic reticulum Ca2+ uptake and Ca2+ ATPase activity in the old women, but the Ca2+ uptake and Ca2+ ATPase activity in the young women did not change (62). Thus, the initial age difference in calcium kinetics was due in part to age differences in physical activity (62). Thus, the slower and small muscle fibers of old adults can be influenced by sedentary behavior, which can also increase the variability of fiber characteristics among old adults.
AGE-RELATED CHANGES IN MOTOR PERFORMANCE
This section will highlight the consequences of altered motor unit morphology and properties on motor function, with reference to distinct areas of motor performance including strength, power, contraction velocity, fatigability, and force steadiness.
Strength and Power
Strength.
The age-related reduction in maximal isometric strength largely parallels the loss of muscle mass (44, 107). Lower specific force (force per unit cross-sectional area) of limb muscles in old adults is explained in part by greater infiltration of fat and connective tissue that may be ameliorated with physical activity (46). Based on cross-sectional studies of lower limb muscles, strength is usually reduced ∼10% per decade with reductions starting at approximately 40–50 yr of age, with evidence of accelerated declines in very old age so that the average strength of an 80-yr-old can be ∼40% that of a same-sex 20- to 30-yr-old (61, 94). The findings from cross-sectional studies, however, may underestimate the actual age-related reductions of strength in old adults [e.g., see Frontera et al. (44) and Metter et al. (106)]. The limited number of longitudinal studies indicate the variability between individuals and the rate of strength reduction is greater with increasing age [e.g., see Charlier et al. (18), Frontera et al. (44), Hughes et al. (57), Metter et al. (106, 107), and Rantanen et al. (126)] possibly more so in men (106) and with chronic disease in old adults (126). Furthermore, there can also be variability in strength between muscles with advanced age because the age-related reductions in isometric strength are typically greater in muscles of the lower limb such as the knee extensors than for the upper limb such as the finger flexor and elbow flexor muscles (44, 61, 125, 152). This muscle group difference may be explained in part by the variability in the rate of sarcopenia in response to differing use of muscles groups between the limbs (27, 61, 66, 151).
Maximal torque during isokinetic dynamic contractions is also reduced with advanced age in both men and women [e.g., see Lindle et al. (94)]. At fast speeds of shortening (concentric) contractions, the age-related reduction in maximal torque for some muscles such as the knee extensor muscles is larger than for slower speeds (89), and this difference can vary across muscle groups (125) (see Power). The age difference in maximal torque, however, differs with contraction mode with less of an age reduction in torque for lengthening (eccentric) than shortening (concentric) contractions (81, 94, 120, 121, 125). For example, for lengthening contractions performed between 15 to 360 deg/s with the ankle dorsiflexor muscles, old men (age, ∼76 yr) had a larger eccentric:isometric torque ratio than young adults (121). The greater preservation of torque during lengthening contractions may be larger in old women than old men (81, 94). The mechanisms for the age and sex-related differences in lengthening contraction torque is not due to cocontraction (121) but may involve elastic, structural, and cross-bridge properties of the muscle. Specifically, the contributing mechanisms likely involve slower cross-bridge kinetics, an increased proportion of weakly bound compared with strongly bound cross-bridges, and a stiffer musculotendinous complex in the muscle of old adults compared with that of young adults (81, 90, 120, 121).
Power.
Age-related reductions in maximal power are greater in magnitude than for maximal isometric strength (105, 125, 127, 134, 143). Power is also more strongly associated with functional performance tasks such as stair climbing, ambulation, and rising time from a chair, than age-associated reductions in isometric strength (127). Importantly, the measure of leg power during shortening contractions is predictive of functional tasks and disability (127).
The age difference in power widens as the velocity demands increase, particularly for the knee extensor muscles, with some older adults unable to reach velocities of >270 deg/s (14, 89, 127). The greater age reductions in power of the lower limb at fast velocities are associated with the reductions in maximal shortening velocity of single fibers, especially MHC IIa fibers (85, 90), reduced muscle mass, and altered muscle architecture (127, 143). Furthermore, the role of inadequate activation of the motor units during fast contractions performed by older adults is discussed below (80, 127), and its effect on age-related reduction in power probably underestimated. Increasing physical activity, in particular with resistance and power training of the lower limbs, can have large effects on increasing power and decreasing risk of disability in old and very old adults who are close to a critical threshold of functional independence (127).
Contraction velocity and rate of force development.
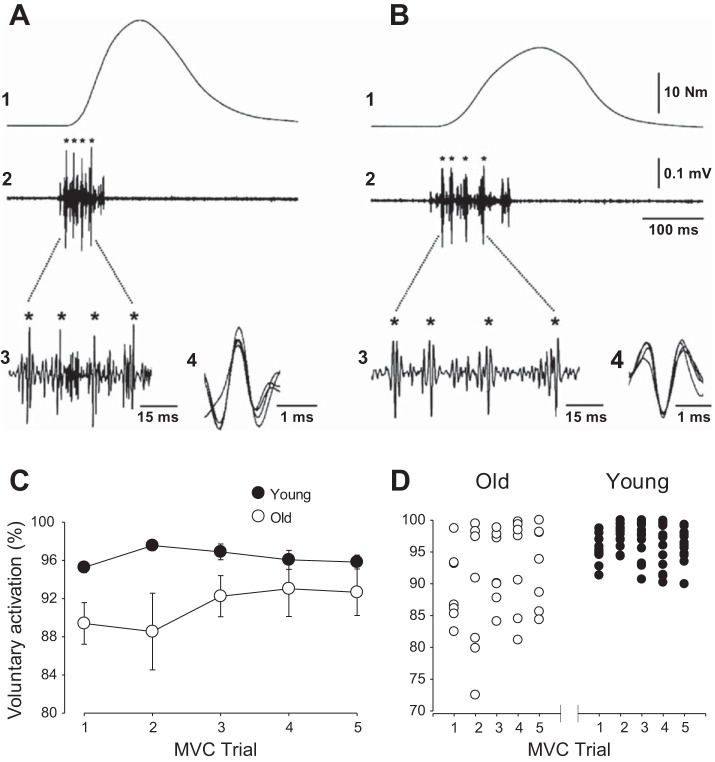
The slowing of whole muscle with aging contributes to the reduced power in old and very old adults [e.g., see McNeil and Rice (105)]. The whole muscles of older adults exhibit lower rates of force development and slower relaxation rates than young during voluntary contractions [e.g., see Klass et al. (80)] and evoked contractions (independent of voluntary activation) in both upper and lower limbs [e.g., see Callahan and Kent-Braun (14), Hunter et al. (62), Klass et al. (80), Molenaar et al. (110), and Yoon et al. (159)]. Age-related slowing of whole muscle is accompanied by a reduced proportional area of MHC II fibers and lower maximal shortening velocity of single muscle fibers even in MHC I fibers of very old active and inactive old adults [see Krivickas et al. (85), Larsson et al. (90), and Power et al. (122); cf. Trappe et al. (148)]. There is also evidence of lower rates of force development of the lower limb muscles in old adults (age, ∼76 yr) compared with young adults (80). Because in old adults the rapid force development of isometric contractions with ankle dorsiflexor muscles is greater during voluntary than electrically evoked contractions (by ∼10%), inadequate activation of the motor units likely contribute to the slower force development in old adults (80) (Fig. 2A).
Fig. 2.
Age-related change in neural activation during voluntary maximal effort contractions. Torque (1) and behavior of single motor units (2) from the tibialis anterior of a young (A) and an old (B) adult during a brief isometric contraction performed during rapid force development. Intramuscular electromyographic (EMG) recordings are shown over the same time interval as the torque (2) and also with a greater time resolution inset of the EMG data (3). *Discharge of the same motor unit (2 and 3) and their traces are superimposed (4). Both the recruitment threshold [2–3% maximum voluntary contraction (MVC)] and the peak torque (∼50% of MVC) were similar for the two motor units identified with intramuscular EMG recordings. The maximal rate of torque development was lower in the old subject (480% MVC/s) compared with the young subject (784% MVC/s). The instantaneous discharge rate during the first three interspike intervals were 78, 71, and 61 Hz, respectively, for the young adult; and 63, 40, and 34 Hz, respectively, for the old adult. [A and B are borrowed from Fig. 6 in Klass et al. (80)]. C and D: voluntary activation (estimated from interpolating cortical stimulation during MVC) for each individual during five brief MVCs with the elbow flexor muscles in young (n = 17; age, 25.5 ± 3.6 yr) and old (n = 7; age, 73.0 ± 3.3 yr) adults. C: old adults had lower voluntary activation compared with young adults during trials 1–3, but similar values were obtained between groups during trials 4 and 5 (age × trial interaction, P < 0.01). D: larger variability in voluntary activation between the old adults compared with young adults for each of the trials. [C and D are borrowed from Fig. 2 in Hunter et al. (63)].
Voluntary activation.
During a maximal-effort isometric contraction and with adequate practice, old adults can achieve high levels of activation, although this may vary with the muscle group involved, velocity of contraction, physical activity level, and age of the old adult (82). Inadequate activation of the surviving motor units in old adults may contribute to age-related losses of strength and power. Evidence for inadequate activation comes from experiments showing that discharge rates of motor units are typically lower with aging during maximal and submaximal isometric contractions in upper and lower limb muscles [e.g., see Barry et al. (5), Connelly et al. (22), Dalton et al. (23), Kamen et al. (72), and Piasecki et al. (118)], presumably representing age-related deficits in voluntary activation (83). Slower contractile properties of old muscle such as longer contraction times and relaxation times were thought to compensate for the lower maximal discharge rates (22, 72), but these associations are not found in all muscles (23) and are probably not causally related (45, 55).
Voluntary activation of motor units can also be assessed by stimulating the muscle or motor cortex during maximal efforts and is most easily performed during isometric contractions (141). Age differences in voluntary activation can depend on contraction velocity, the muscle groups involved, whether practice of maximal efforts is provided, and physical activity levels (82). Some studies show that old adults consistently have lower or more variable activation levels during maximal isometric contractions than young adults [e.g., De Serres and Enoka (30), Hunter et al. (63), Stevens et al. (139), and Yoon et al. (158)], whereas others indicate that old adults can activate to similar levels as young people after practice or familiarization when the best attempt at activating the muscle is compared [e.g., Hunter et al. (63) Jakobi and Rice (65), Molenaar et al. (110), and Yoon et al. (158); see Fig. 2C]. Young adults do not need as much practice to achieve consistent levels of activation, but practice can improve activation levels in old and very old adults in one session or even within a session (63, 65). Furthermore, less regular physical activity among old adults may exacerbate age differences in voluntary activation (49). Accordingly, old men and women are more variable in their activation levels across trials and among themselves (63, 158) (Fig. 2D). Implications from these findings are that the activation may be inadequate during tasks that require/allow only one attempt as routinely observed in real-world situations (e.g., recovering from a disturbance in balance to prevent a fall). Although maximal voluntary contractions that are practiced in the laboratory allow comparison of the best attempts of young and old adults, the larger variability in voluntary activation within older adults potentially exacerbates the age-related declines in strength and power required during daily tasks.
During slow-to-moderate velocity-dynamic contractions, young and old adults appear to have similar levels of voluntary activation for upper limb muscles (V. Rozand, J. Senefeld, and S. K. Hunter, unpublished findings) and lower limb muscles (81) when assessed with the interpolated stimulus technique. This technique, however, is difficult to accomplish during fast contractions at which age differences in power become large (89). Insight has been gained from single motor unit recordings showing lower rates of motor unit discharge and torque development in old adults immediately before contractions requiring rapid force development (80) (Fig. 2, A and B). Such differences in neural activation of the muscle may also occur with aging during fast-velocity contractions (155), but further exploration is needed in older women and across many muscle groups in both sexes.
Fatigability
Performance fatigability is the reduction in force or power of a muscle in response to exercise (40). The consequences of greater fatigability with aging are significant because fatigability further exacerbates the age-related loss of strength and power in old adults observed before exercise. In addition, fatigue-induced variability of force or power, which is greater with aging (78, 134), may further impair performance of daily activities.
Age-related changes in the inputs to the motor neuron and motor unit morphology and physiology as described earlier, can alter the rates at which the motor unit is stressed in young, old, and very old adults during a fatiguing task, resulting in an age difference in fatigability. Furthermore, when the demands of the task are altered, such as the intensity, velocity and mode of contraction (dynamic vs. isometric tasks), the muscle group involved, and age of the older adult, the magnitude of age difference in fatigability also changes [e.g., Christie et al. (19), Justice et al. (70), McNeil and Rice (105), Senefeld et al. (134), and Yoon et al. (161)].
For maximal and submaximal isometric fatiguing contractions, healthy old adults (age, 60–75 yr) are typically less fatigable than young adults (77) even when matched for strength (60) for both men and women (59). The greater fatigue resistance of old adults is associated with slower contractile properties, a shift to a lower proportional area of fibers expressing MHC IIa isoforms, and a lower reliance on glycolytic metabolism in old adults compared with young adults (15, 62, 63, 77). Consequently, during isometric fatiguing contractions, the skeletal muscles of old adults have a lower accumulation of inorganic phosphate and hydrogen ions that potentially interfere with force production (88). The fatigue resistance of sustained isometric contractions may be relevant only in old adults aged <75 yr; adults aged >75 yr exhibited greater fatigability in the lower limb rather than the fatigue resistance observed in the those <75 yr (70). More studies are needed to determine the mechanisms for the shift in fatigability from the old to very old age groups across many muscle groups and determine the effect on physical function and disability.
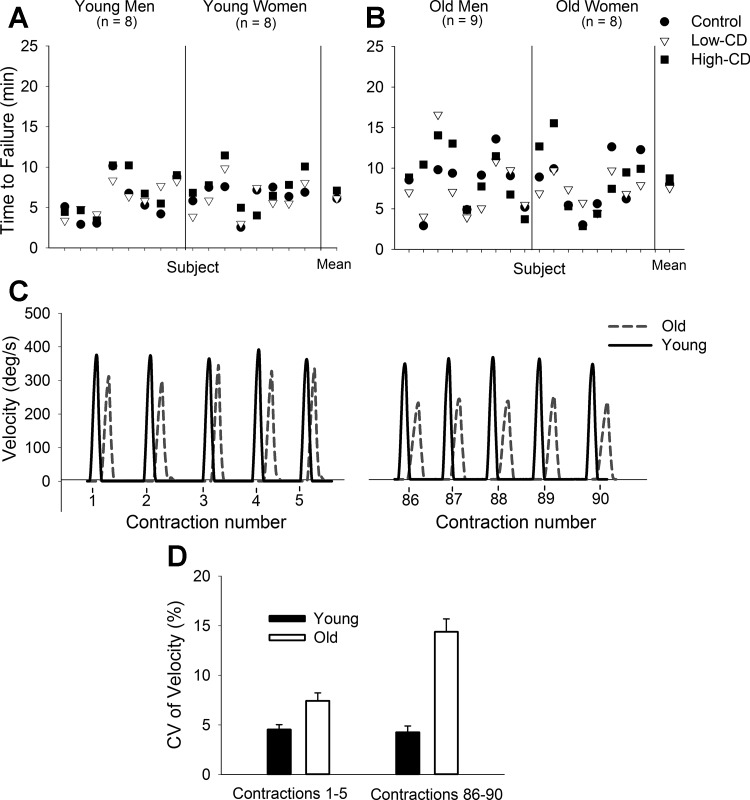
Cortical inputs, however, are often increased during daily fatiguing tasks (e.g., increased attention), and these inputs can influence performance of fatiguing contractions differently for young and old adults. This was observed by imposing a difficult cognitive challenge (mental math) while a participant sustained a submaximal isometric contraction with the elbow flexors muscles (75, 116, 160) and ankle dorsiflexor muscles (150). Young women, for example, exhibited greater fatigability (decreased time to task failure) only when a high cognitive challenge was imposed during elbow flexion contraction compared with the control task (75, 160), whereas the time to task failure for old women was reduced when either a low- or high-level cognitive challenge was imposed (116). The mechanisms for the greater fatigability with superimposition of a cognitive challenge are not understood. However, these studies are important because imposition of a cognitive challenge more closely resembles real-life activities, which commonly require dual tasks. Furthermore, the variability between the task durations (coefficient of variation of the time to failure across the tasks with and without a cognitive challenge) can be much larger for old adults than for young adults (150). This was observed in the ankle dorsiflexor muscles despite no differences in the mean time to failure with and without the cognitive challenge imposed (Fig. 3, A and B) (150). These data suggest that a lack of age difference when group averages of best performances are compared may not effectively detect the poor motor performance among old adults.
Fig. 3.
Age-related variability during fatiguing contractions. Top: time to task failure during fatiguing contractions with the ankle dorsiflexor muscles for young (A) and old (B) adults during control (circle), low-cognitive demand (subtracting by 1) (Low-CD, triangles), and high-cognitive demand (subtracting by 13) (High-CD, squares) sessions. Data for each individual are displayed for each session (men and women are separated by the middle vertical line). The mean for each session is on the ri ght. The range and variability of time to task failure among the old adults and women were greater than for the young adults and men, respectively, for each session. [A and B are Fig. 4 from Vanden Noven et al. (150), CC BY 3.0, http://journal.frontiersin.org/article/10.3389/fnagi.2014.00097/full.] C: representative data of maximal velocity of knee extensor muscles during the first (1–5) and last (86–90) contractions performed during a fatiguing contraction with a load equivalent to 20% of MVC for a young man (black line) and old man (gray dashed line). Contractions were performed once every 3 s. Overall, the old adults (n = 32, 71.3 ± 6.3 yr; 14 women) had greater reductions in velocity at the last five contractions compared with young adults (n = 35; age, 21.0 ± 2.6 yr; 19 women). On average, there was ∼35% age-related difference in the reduction of velocity. D: coefficient of variation (CV) of velocity during the same contractions depicted in C. Old adults had greater CV of power compared with young adults during the first five contractions. At the last five contractions old adults had a greater increase in CV of power than young adults. [C and D are from data extracted from Senefeld et al. (134)].
The age difference in fatigability also depends on the contraction velocity. For example, the age difference in fatigability observed for isometric contractions was diminished for a dynamic task with slow-to-moderate velocity contractions (<180 deg/s) for both the elbow flexor and knee extensor muscles (13, 24, 161). During high-velocity concentric contractions, old and very old adults exhibit greater fatigability than young adults when contractions are performed with the lower limb muscles (14, 25, 105, 155); for example, for contractions >180 deg/s with the knee extensor muscles, old adults were more fatigable (greater reductions in power) than young adults (14, 24). The mechanism for the velocity-dependent age difference in fatigability appears to involve processes that are associated with contractile speed, a slower shortening velocity that occurs with age (7, 14, 161), and also a reduced ability to rapidly activate the agonist muscle during plantar flexion for old men aged ∼78 yr (155). The extent that inadequate activation during repeated fast contractions contributes to the age differences in fatigability in women, is not known. It also appears that the fatigability of fast contractions with advanced age is even larger in very old men (age, >80 yr) than men aged 60–70 yr (105). There are gaps in the literature in understanding the mechanisms for the greater fatigability with aging, particularly in women, and in general the effect of fatigability in predicting functional performance, frailty and disability.
An important but understudied aspect of performance fatigability that is altered with age is the variability of force or power during the fatiguing task. For example, variability of the maximal torque obtained during repeated dynamic contractions of the knee extensor muscles (120 deg/s) was greater in old women compared with young women and old women who were not mobility impaired (78). Similarly, maximal velocity during a dynamic fatiguing task with the knee extensor muscles was more variable [a larger coefficient of variation (CV)] for old adults compared with young adults (134) (Fig. 3D). However, there were no age differences in variability for the same task conducted with the elbow flexor muscles (134). Although the mechanism was not identified, these data are consistent with increased variability in voluntary activation and motor performance with aging and suggests that neural mechanisms may contribute to increased variability of motor output in older adults across a fatiguing task.
Force Steadiness
Age-related changes in motor unit behavior affect the control of force among old adults. Typically, old adults are less steady than young adults (39), especially during light-load tasks that are often required during activities of daily living (79). One metric of force steadiness is the amplitude of force fluctuations around a target and is quantified as the standard deviation (SD) of the force (39). In young and old adults, the SD of isometric force is associated with the mean target force so that the SD is larger in high-intensity than low-intensity contractions, commonly referred to as signal-dependent noise (50, 138). To allow comparisons between contractions and among groups of individuals who have large strength differences (e.g., young vs. old), the SD is normalized to the mean force and expressed as the CV (39). Motor unit behavior and the inputs to the motor unit are largely responsible for the magnitude of force fluctuations (41). Single motor unit recordings and decomposition of surface EMG from multiple-array electrodes demonstrate that the variability of the motor unit discharge rate at low frequencies (1–2 Hz) can explain up to ∼70% of the force steadiness in young adults (41). Supraspinal centers are also involved in the fluctuations of force in young adults and include both cortical and subcortical areas in the motor area and frontal lobes such as the putamen, insula, and contralateral superior frontal gyrus (162). Although previous research has found increased coherence in higher frequency bands (>2 Hz) in old compared with young adults (68, 73, 102, 133, 142), whether age-related differences exist in coherence at low frequencies (≤2 Hz) is not known. Changes within these cortical areas with advancing age might be expected to similarly influence low-frequency inputs onto the motor neuron pool.
The larger CV of force during isometric contractions (decreased steadiness) with advanced age occurs in both the upper- and lower-extremity muscles, primarily at lower contraction intensities [2–10% maximum voluntary contraction (MVC)] (117, 150). At these low forces, greater variability in the discharge rates of motor units in old adults are primarily responsible for the larger CV of force that occurs with aging (47, 147) and not the larger size of the motor units as was once thought (39, 140). The source of the age-related variability in discharge rates could arise from many different sources, including the synaptic input, the intrinsic properties of the motor neuron, and a more variable after-hyperpolarization, which is known to impair the time course of a subsequent action potential (101). All these alterations in the aging motor neuron potentially can induce greater oscillations in the neural signal and synaptic noise (101) that ultimately will increase the variability of discharge rate of motor units in older adults. Simulation studies show that synaptic noise primarily accounts for the force fluctuations at very low contraction intensities (34), and therefore may explain the age differences.
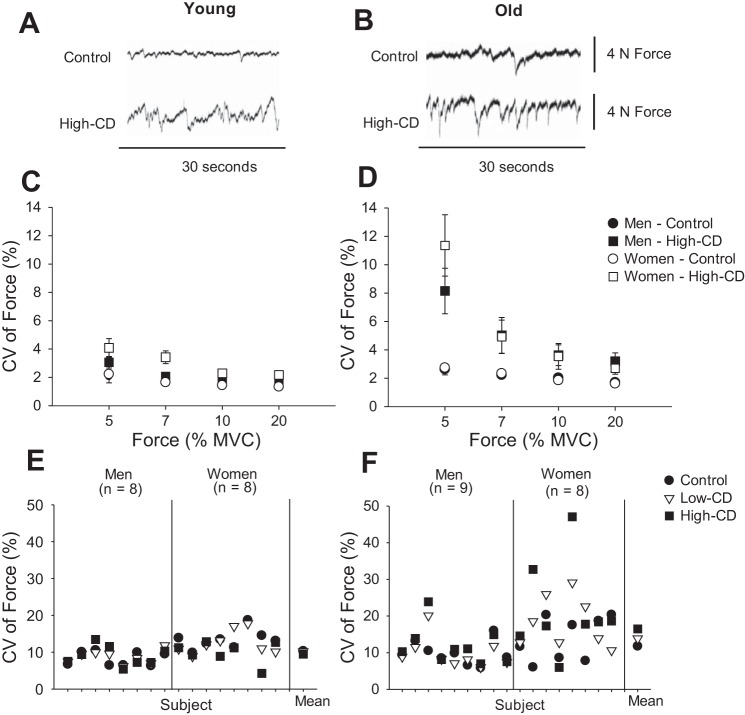
Age-related differences in steadiness are further exacerbated when old adults are required to perform a dual task that involves simultaneously maintaining a force steadiness task and cognitive challenge (116, 117, 150). For example, force steadiness of the elbow flexor muscles was reduced (greater CV of force) when a cognitive challenge (mental math) was performed during isometric contractions in young and old adults (117) (Fig. 4, A and B). The greatest differences occurred during a very-low-intensity contraction (e.g., 5% MVC) for old adults and women (117) compared with young adults and men (Fig. 4, C and D). When the cognitive challenge was imposed, the increased CV of force during contractions at 5% MVC with the ankle dorsiflexor muscles was variable between old adults and women (Fig. 4, E and F) (150), although the mechanisms of this are not known.
Fig. 4.
Force steadiness with and without imposition of a cognitive challenge in young and (A, C, and E) old (B, D, and F) adults. A and B: representative force signals of the elbow flexor muscles in a young and an old woman performed at 5% of MVC for a control and high-cognitive demand trial (subtracting by 13). C and D: CV of force for the elbow flexor muscles during contractions at 5, 7, 10, and 20% of MVC. Values are means ± SE for men (filled symbols) and women (open symbols) during the control session (circles) and high-cognitive demand session (subtracting by 13) (High-CD, squares). [Data for A and B are from Pereira et al. (116), and for the 7–20% MVC from Pereira HM and Hunter SK, unpublished.] E and F: CV of force for the ankle dorsiflexor muscles during contractions at 5% MVC performed by men and women for a control (filled circles), low-cognitive demand (Low-CD, subtracting by 1; open triangles) and high-cognitive demand (High-CD, subtracting by 13; filled squares) trials. The mean is shown for each session for young (E) and old (F) adults. E and F show that old adults, especially women, exhibited greater between trial variability in CV of force with imposition of the cognitive demand. [E and F were created using data presented in Vanden Noven et al. (150).]
The motor output variability in young and old adults is associated with functional performance tasks. For example, larger variability during sinusoidal tracking with the ankle dorsiflexor muscles was associated with poor reactive driving performance in old adults (95), and low force steadiness of the upper limb muscles was associated with pegboard tests of dexterity (1, 97). Furthermore, force steadiness was worse in old adults who had a history of falling compared with those who did not have a fall history (age, >70 yr) (17). Interventions to improve steadiness (reduced CV of force) among old adults have usually involved practice and strength training with the aim to reduce discharge rate variability [e.g., Griffin et al. (47) and Kornatz et al. (84)]. Large improvements in force steadiness were obtained after short-term (<4 wk), light-load strength training and practice, highlighting the improvements that arise from neural sources (84, 96, 146). Modulation of motor unit spikes between 13 and 30 Hz indicate that cortical centers may be responsible for the reduction in the variability of discharge rate with practice in old adults (114). There is limited information on how such improvements in steadiness and discharge rate variability in response to physical training ultimately affect functional performance tasks.
CONCLUSIONS
Motor performance declines with advanced age and is accelerated in very old age (>80 yr), involving muscles that are weaker, slower, less powerful, less steady, and more fatigable during high-velocity dynamic tasks. The reduced maximal power of lower-extremity muscles is predictive of functional performance and disability, with less understanding of the predictive strength of other aspects of motor performance, including steadiness and fatigability, thus providing opportunities for high-impact studies.
An important but often overlooked aspect of age-related declines in motor performance is the large within- and between-subject variability of many aspects of motor performance (e.g., force, velocity, and fatigability) that increase with advanced aging, and lead to low predictability of an already compromised neuromuscular system in old and very old adults. Imposing a cognitive challenge, which often accompanies real-life motor tasks, further exacerbates variability in motor performance among older adults. The mechanisms for the greater variability between trials with advanced age are not well understood, but they may have a neural origin as evidenced by increased variability in voluntary activation and slower and more variable motor unit discharge rates in old adults. Practice and enhanced physical activity appear to ameliorate some of differences in variability of performance and function of the motor unit, which are often attributed to aging. Many studies describing the change in motor performance and variability across the continuum of ages are cross-sectional in design. Thus, the future challenge is to perform adequately powered longitudinal studies to determine the involved mechanisms of impaired motor performance and the increased variability, and the effect on functional performance and disability in the old and very old men and women.
GRANTS
This work was in part supported by National Institute of Aging Grant R21-AG-045766 to S. K. Hunter.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.K.H. conception and design of research; S.K.H., H.M.P., and K.G.K. interpreted results of experiments; S.K.H., H.M.P., and K.G.K. prepared figures; S.K.H., H.M.P., and K.G.K. drafted manuscript; S.K.H., H.M.P., and K.G.K. edited and revised manuscript; S.K.H., H.M.P., and K.G.K. approved final version of manuscript.
REFERENCES
- 1.Almuklass AM, Price RC, Gould JR, Enoka RM. Force steadiness as a predictor of time to complete a pegboard test of dexterity in young men and women. J Appl Physiol 120: 1410–1417, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports 13: 40–47, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Andersen JL, Terzis G, Kryger A. Increase in the degree of coexpression of yosin heavy chain isoforms in skeletal muscle fibers of the very old. Muscle Nerve 22: 449–454, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Baker SN, Kilner JM, Pinches EM, Lemon RN. The role of synchrony and oscillations in the motor output. Exp Brain Res 128: 109–117, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Barry BK, Pascoe MA, Jesunathadas M, Enoka RM. Rate coding is compressed but variability is unaltered for motor units in a hand muscle of old adults. J Neurophysiol 97: 3206–3218, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Baudry S, Enoka RM. Influence of load type on presynaptic modulation of Ia afferent input onto two synergist muscles. Exp Brain Res 199: 83–88, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Baudry S, Klass M, Pasquet B, Duchateau J. Age-related fatigability of the ankle dorsiflexor muscles during concentric and eccentric contractions. Eur J Appl Physiol 100: 515–525, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Baudry S, Maerz AH, Enoka RM. Presynaptic modulation of Ia afferents in young and old adults when performing force and position control. J Neurophysiol 103: 623–631, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baudry S, Penzer F, Duchateau J. Input-output characteristics of soleus homonymous Ia afferents and corticospinal pathways during upright standing differ between young and elderly adults. Acta Physiol (Oxf) 210: 667–677, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev 107: 123–136, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Blau HM, Cosgrove BD, Ho AT. The central role of muscle stem cells in regenerative failure with aging. Nat Med 21: 854–862, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bromberg MB, Scott DM. Single fiber EMG reference values: reformatted in tabular form. Ad Hoc Committee of the AAEM Single Fiber Special Interest Group. Muscle Nerve 17: 820–821, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Callahan DM, Foulis SA, Kent-Braun JA. Age-related fatigue resistance in the knee extensor muscles is specific to contraction mode. Muscle Nerve 39: 692–702, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callahan DM, Kent-Braun JA. Effect of old age on human skeletal muscle force-velocity and fatigue properties. J Appl Physiol 111: 1345–1352, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callahan DM, Umberger BR, Kent JA. Mechanisms of in vivo muscle fatigue in humans: investigating age-related fatigue resistance with a computational model. J Physiol 594: 3407–3421, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell MJ, McComas AJ, Petito F. Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry 36: 174–182, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carville SF, Perry MC, Rutherford OM, Smith IC, Newham DJ. Steadiness of quadriceps contractions in young and older adults with and without a history of falling. Eur J Appl Physiol 100: 527–533, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Charlier R, Knaeps S, Mertens E, Van Roie E, Delecluse C, Lefevre J, Thomis M. Age-related decline in muscle mass and muscle function in Flemish Caucasians: a 10-year follow-up. Age (Dordr) 38: 36, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christie A, Snook EM, Kent-Braun JA. Systematic review and meta-analysis of skeletal muscle fatigue in old age. Med Sci Sports Exerc 43: 568–577, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christou EA. Aging and variability of voluntary contractions. Exerc Sport Sci Rev 39: 77–84, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clamann HP. Statistical analysis of motor unit firing patterns in a human skeletal muscle. Biophys J 9: 1233–1251, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol 87: 843–852, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Dalton BH, Jakobi JM, Allman BL, Rice CL. Differential age-related changes in motor unit properties between elbow flexors and extensors. Acta Physiol (Oxf) 200: 45–55, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Dalton BH, Power GA, Vandervoort AA, Rice CL. The age-related slowing of voluntary shortening velocity exacerbates power loss during repeated fast knee extensions. Exp Gerontol 47: 85–92, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Dalton BH, Power GA, Vandervoort AA, Rice CL. Power loss is greater in old men than young men during fast plantar flexion contractions. J Appl Physiol 109: 1441–1447, 2010. [DOI] [PubMed] [Google Scholar]
- 26.D'Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol 552: 499–511, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Degens H, Korhonen MT. Factors contributing to the variability in muscle ageing. Maturitas 73: 197–201, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Delbono O. Expression and regulation of excitation-contraction coupling proteins in aging skeletal muscle. Curr Aging Sci 4: 248–259, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Control scheme governing concurrently active human motor units during voluntary contractions. J Physiol 329: 129–142, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Serres S, Enoka RM. Older adults can maximally activate the biceps brachii muscle by voluntary command. J Appl Physiol 84: 284–291, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Deschenes MR. Motor unit and neuromuscular junction remodeling with aging. Curr Aging Sci 4: 209–220, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol 45: 389–393, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deschenes MR, Roby MA, Glass EK. Aging influences adaptations of the neuromuscular junction to endurance training. Neuroscience 190: 56–66, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dideriksen JL, Negro F, Enoka RM, Farina D. Motor unit recruitment strategies and muscle properties determine the influence of synaptic noise on force steadiness. J Neurophysiol 107: 3357–3369, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol 95: 1717–1727, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Dorfman LJ, Bosley TM. Age-related changes in peripheral and central nerve conduction in man. Neurology 29: 38–44, 1979. [DOI] [PubMed] [Google Scholar]
- 37.Duchateau J, Enoka RM. Human motor unit recordings: origins and insight into the integrated motor system. Brain Res 1409: 42–61, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Enoka RM. Morphological features and activation patterns of motor units. J Clin Neurophysiol 12: 538–559, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Enoka RM, Christou EA, Hunter SK, Kornatz KW, Semmler JG, Taylor AM, Tracy BL. Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kinesiol 13: 1–12, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol 586: 11–23, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farina D, Negro F. Common synaptic input to motor neurons, motor unit synchronization, and force control. Exerc Sport Sci Rev 43: 23–33, 2015. [DOI] [PubMed] [Google Scholar]
- 42.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences International working group on sarcopenia. J Am Med Dir Assoc 12: 249–256, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fling BW, Knight CA, Kamen G. Relationships between motor unit size and recruitment threshold in older adults: implications for size principle. Exp Brain Res 197: 125–133, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol 88: 1321–1326, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Fuglevand AJ, Keen DA. Re-evaluation of muscle wisdom in the human adductor pollicis using physiological rates of stimulation. J Physiol 549: 865–875, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goodpaster BH, Chomentowski P, Ward BK, Rossi A, Glynn NW, Delmonico MJ, Kritchevsky SB, Pahor M, Newman AB. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol 105: 1498–1503, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griffin L, Painter PE, Wadhwa A, Spirduso WW. Motor unit firing variability and synchronization during short-term light-load training in older adults. Exp Brain Res 197: 337–345, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Halliday DM, Conway BA, Farmer SF, Shahani U, Russell AJ, Rosenberg JR. Coherence between low-frequency activation of the motor cortex and tremor in patients with essential tremor. Lancet 355: 1149–1153, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Harridge SD, Kryger A, Stensgaard A. Knee extensor strength, activation, and size in very elderly people following strength training. Muscle Nerve 22: 831–839, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature 394: 780–784, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Haug H, Eggers R. Morphometry of the human cortex cerebri and corpus striatum during aging. Neurobiol Aging 12: 336–338; discussion 352–355, 1991. [DOI] [PubMed] [Google Scholar]
- 52.Heckman CJ, Enoka RM. Physiology of the motor neuron and the motor unit. In: Clinical neurophysiology of motor neuron diseases, edited by Eisen A. Boston: Elsevier, 2004, p. 119–147. [Google Scholar]
- 53.Heise KF, Zimerman M, Hoppe J, Gerloff C, Wegscheider K, Hummel FC. The aging motor system as a model for plastic changes of GABA-mediated intracortical inhibition and their behavioral relevance. J Neurosci 33: 9039–9049, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hepple RT. Muscle atrophy is not always sarcopenia. J Appl Physiol 113: 677–679, 2012. [DOI] [PubMed] [Google Scholar]
- 55.Hepple RT, Rice CL. Innervation and neuromuscular control in ageing skeletal muscle. J Physiol 594: 1965–1978, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hourigan ML, McKinnon NB, Johnson M, Rice CL, Stashuk DW, Doherty TJ. Increased motor unit potential shape variability across consecutive motor unit discharges in the tibialis anterior and vastus medialis muscles of healthy older subjects. Clin Neurophysiol 126: 2381–2389, 2015. [DOI] [PubMed] [Google Scholar]
- 57.Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Fiatarone Singh MA. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci 56: B209–B217, 2001. [DOI] [PubMed] [Google Scholar]
- 58.Hultborn H, Brownstone RB, Toth TI, Gossard JP. Key mechanisms for setting the input-output gain across the motoneuron pool. Prog Brain Res 143: 77–95, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Hunter SK, Critchlow A, Enoka RM. Influence of aging on sex differences in muscle fatigability. J Appl Physiol 97: 1723–1732, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Hunter SK, Critchlow A, Enoka RM. Muscle endurance is greater for old men compared with strength-matched young men. J Appl Physiol 99: 890–897, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Hunter SK, Thompson MW, Adams RD. Relationships among age-associated strength changes and physical activity level, limb dominance, and muscle group in women. J Gerontol A Biol Sci Med Sci 55: B264–B273, 2000. [DOI] [PubMed] [Google Scholar]
- 62.Hunter SK, Thompson MW, Ruell PA, Harmer AR, Thom JM, Gwinn TH, Adams RD. Human skeletal sarcoplasmic reticulum Ca2+ uptake and muscle function with aging and strength training. J Appl Physiol 86: 1858–1865, 1999. [DOI] [PubMed] [Google Scholar]
- 63.Hunter SK, Todd G, Butler JE, Gandevia SC, Taylor JL. Recovery from supraspinal fatigue is slowed in old adults after fatiguing maximal isometric contractions. J Appl Physiol 105: 1199–1209, 2008. [DOI] [PubMed] [Google Scholar]
- 64.Jacobs JM, Love S. Qualitative and quantitative morphology of human sural nerve at different ages. Brain 108: 897–924, 1985. [DOI] [PubMed] [Google Scholar]
- 65.Jakobi JM, Rice CL. Voluntary muscle activation varies with age and muscle group. J Appl Physiol 93: 457–462, 2002. [DOI] [PubMed] [Google Scholar]
- 66.Janssen I, He ymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol 89: 81–88, 2000. [DOI] [PubMed] [Google Scholar]
- 67.Jesunathadas M, Marmon AR, Gibb JM, Enoka RM. Recruitment and derecruitment characteristics of motor units in a hand muscle of young and old adults. J Appl Physiol 108: 1659–1667, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson AN, Shinohara M. Corticomuscular coherence with and without additional task in the elderly. J Appl Physiol 112: 970–981, 2012. [DOI] [PubMed] [Google Scholar]
- 69.Johnson MD, Heckman CJ. Gain control mechanisms in spinal motoneurons. Front Neural Circuits 8: 81, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Justice JN, Mani D, Pierpoint LA, Enoka RM. Fatigability of the dorsiflexors and associations among multiple domains of motor function in young and old adults. Exp Gerontol 55: 92–101, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kadi F, Ponsot E. The biology of satellite cells and telomeres in human skeletal muscle: effects of aging and physical activity. Scand J Med Sci Sports 20: 39–48, 2010. [DOI] [PubMed] [Google Scholar]
- 72.Kamen G, Sison SV, Du CC, Patten C. Motor unit discharge behavior in older adults during maximal-effort contractions. J Appl Physiol 79: 1908–1913, 1995. [DOI] [PubMed] [Google Scholar]
- 73.Kamp D, Krause V, Butz M, Schnitzler A, Pollok B. Changes of cortico-muscular coherence: an early marker of healthy aging? Age (Dordr) 35: 49–58, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Keenan KG, Massey WV. Control of fingertip forces in young and older adults pressing against fixed low- and high-friction surfaces. PLoS One 7: e48193, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keller-Ross ML, Pereira HM, Pruse J, Yoon T, Schlinder-Delap B, Nielson KA, Hunter SK. Stressor-induced increase in muscle fatigability of young men and women is predicted by strength but not voluntary activation. J Appl Physiol 116: 767–778, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kennedy KM, Raz N. Age, sex and regional brain volumes predict perceptual-motor skill acquisition. Cortex 41: 560–569, 2005. [DOI] [PubMed] [Google Scholar]
- 77.Kent-Braun JA. Skeletal muscle fatigue in old age: whose advantage? Exerc Sport Sci Rev 37: 3–9, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kent-Braun JA, Callahan DM, Fay JL, Foulis SA, Buonaccorsi JP. Muscle weakness, fatigue, and torque variability: effects of age and mobility status. Muscle Nerve 49: 209–217, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kern DS, Semmler JG, Enoka RM. Long-term activity in upper- and lower-limb muscles of humans. J Appl Physiol 91: 2224–2232, 2001. [DOI] [PubMed] [Google Scholar]
- 80.Klass M, Baudry S, Duchateau J. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J Appl Physiol 104: 739–746, 2008. [DOI] [PubMed] [Google Scholar]
- 81.Klass M, Baudry S, Duchateau J. Aging does not affect voluntary activation of the ankle dorsiflexors during isometric, concentric, and eccentric contractions. J Appl Physiol 99: 31–38, 2005. [DOI] [PubMed] [Google Scholar]
- 82.Klass M, Baudry S, Duchateau J. Voluntary activation during maximal contraction with advancing age: a brief review. Eur J Appl Physiol 100: 543–551, 2007. [DOI] [PubMed] [Google Scholar]
- 83.Knight CA, Kamen G. Relationships between voluntary activation and motor unit firing rate during maximal voluntary contractions in young and older adults. Eur J Appl Physiol 103: 625–630, 2008. [DOI] [PubMed] [Google Scholar]
- 84.Kornatz KW, Christou EA, Enoka RM. Practice reduces motor unit discharge variability in a hand muscle and improves manual dexterity in old adults. J Appl Physiol 98: 2072–2080, 2005. [DOI] [PubMed] [Google Scholar]
- 85.Krivickas LS, Suh D, Wilkins J, Hughes VA, Roubenoff R, Frontera WR. Age- and gender-related differences in maximum shortening velocity of skeletal muscle fibers. Am J Phys Med Rehabil 80: 447–457, 2001. [DOI] [PubMed] [Google Scholar]
- 86.Kwan P. Sarcopenia, a neurogenic syndrome? J Aging Res 2013: 791679, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lamboley CR, Wyckelsma VL, Dutka TL, McKenna MJ, Murphy RM, Lamb GD. Contractile properties and sarcoplasmic reticulum calcium content in type I and type II skeletal muscle fibres in active aged humans. J Physiol 593: 2499–2514, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lanza IR, Larsen RG, Kent-Braun JA. Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow. J Physiol 583: 1093–1105, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lanza IR, Towse TF, Caldwell GE, Wigmore DM, Kent-Braun JA. Effects of age on human muscle torque, velocity, and power in two muscle groups. J Appl Physiol 95: 2361–2369, 2003. [DOI] [PubMed] [Google Scholar]
- 90.Larsson L, Li X, Frontera WR. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol Cell Physiol 272: C638–C649, 1997. [DOI] [PubMed] [Google Scholar]
- 91.Lexell J, Downham DY. The occurrence of fibre-type grouping in healthy human muscle: a quantitative study of cross-sections of whole vastus lateralis from men between 15 and 83 years. Acta Neuropathol 81: 377–381, 1991. [DOI] [PubMed] [Google Scholar]
- 92.Lexell J, Taylor CC. Variability in muscle fibre areas in whole human quadriceps muscle: effects of increasing age. J Anat 174: 239–249, 1991. [PMC free article] [PubMed] [Google Scholar]
- 93.Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294, 1988. [DOI] [PubMed] [Google Scholar]
- 94.Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol 83: 1581–1587, 1997. [DOI] [PubMed] [Google Scholar]
- 95.Lodha N, Moon H, Kim C, Onushko T, Christou EA. Motor output variability impairs driving ability in older adults. J Gerontol A Biol Sci Med Sci. First published March 2, 2016. [DOI] [PubMed] [Google Scholar]
- 96.Marmon AR, Gould JR, Enoka RM. Practicing a functional task improves steadiness with hand muscles in older adults. Med Sci Sports Exerc 43: 1531–1537, 2011. [DOI] [PubMed] [Google Scholar]
- 97.Marmon AR, Pascoe MA, Schwartz RS, Enoka RM. Associations among strength, steadiness, and hand function across the adult life span. Med Sci Sports Exerc 43: 560–567, 2011. [DOI] [PubMed] [Google Scholar]
- 98.Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol 462: 144–152, 2003. [DOI] [PubMed] [Google Scholar]
- 99.Marneweck M, Loftus A, Hammond G. Short-interval intracortical inhibition and manual dexterity in healthy aging. Neurosci Res 70: 408–414, 2011. [DOI] [PubMed] [Google Scholar]
- 100.Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in human motor function. Neurology 58: 630–635, 2002. [DOI] [PubMed] [Google Scholar]
- 101.Matthews PB. Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise. J Physiol 492: 597–628, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maurits NM, Scheeringa R, van der Hoeven JH, de Jong R. EEG coherence obtained from an auditory oddball task increases with age. J Clin Neurophysiol 23: 395–403, 2006. [DOI] [PubMed] [Google Scholar]
- 103.McGinnis SM, Brickhouse M, Pascual B, Dickerson BC. Age-related changes in the thickness of cortical zones in humans. Brain Topogr 24: 279–291, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve 31: 461–467, 2005. [DOI] [PubMed] [Google Scholar]
- 105.McNeil CJ, Rice CL. Fatigability is increased with age during velocity-dependent contractions of the dorsiflexors. J Gerontol A Biol Sci Med Sci 62A: 624–629, 2007. [DOI] [PubMed] [Google Scholar]
- 106.Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci 52: B267–B276, 1997. [DOI] [PubMed] [Google Scholar]
- 107.Metter EJ, Lynch N, Conwit R, Lindle R, Tobin J, Hurley B. Muscle quality and age: cross-sectional and longitudinal comparisons. J Gerontol A Biol Sci Med Sci 54: B207–B218, 1999. [DOI] [PubMed] [Google Scholar]
- 108.Metter EJ, Talbot LA, Schrager M, Conwit RA. Arm-cranking muscle power and arm isometric muscle strength are independent predictors of all-cause mortality in men. J Appl Physiol 96: 814–821, 2004. [DOI] [PubMed] [Google Scholar]
- 109.Miller MS, Bedrin NG, Callahan DM, Previs MJ, Jennings ME 2nd, Ades PA, Maughan DW, Palmer BM, Toth MJ. Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J Appl Physiol 115: 1004–1014, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Molenaar JP, McNeil CJ, Bredius MS, Gandevia SC. Effects of aging and sex on voluntary activation and peak relaxation rate of human elbow flexors studied with motor cortical stimulation. Age (Dordr) 35: 1327–1337, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Narasimhan M, Hong J, Atieno N, Muthusamy VR, Davidson CJ, Abu-Rmaileh N, Richardson RS, Gomes AV, Hoidal JR, Rajasekaran NS. Nrf2 deficiency promotes apoptosis and impairs PAX7/MyoD expression in aging skeletal muscle cells. Free Radic Biol Med 71: 402–414, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Negro F, Farina D. Linear transmission of cortical oscillations to the neural drive to muscles is mediated by common projections to populations of motoneurons in humans. J Physiol 589: 629–637, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, Ranieri F, Di Lazzaro V. Effects of aging on motor cortex excitability. Neurosci Res 55: 74–77, 2006. [DOI] [PubMed] [Google Scholar]
- 114.Onushko T, Baweja HS, Christou EA. Practice improves motor control in older adults by increasing the motor unit modulation from 13 to 30 Hz. J Neurophysiol 110: 2393–2401, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Opalach K, Rangaraju S, Madorsky I, Leeuwenburgh C, Notterpek L. Lifelong calorie restriction alleviates age-related oxidative damage in peripheral nerves. Rejuvenation Res 13: 65–74, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pereira HM, Spears VC, Schlinder-Delap B, Yoon T, Harkins A, Nielson KA, Hoeger Bement M, Hunter SK. Sex differences in arm muscle fatigability with cognitive demand in older adults. Clin Orthop Relat Res 473: 2568–2577, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pereira HM, Spears VC, Schlinder-Delap B, Yoon T, Nielson KA, Hunter SK. Age and sex differences in steadiness of elbow flexor muscles with imposed cognitive demand. Eur J Appl Physiol 115: 1367–1379, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Piasecki M, Ireland A, Stashuk D, Hamilton-Wright A, Jones DA, McPhee JS. Age-related neuromuscular changes affecting human vastus lateralis. J Physiol 594: 4525–4536, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Power GA, Allen MD, Gilmore KJ, Stashuk DW, Doherty TJ, Hepple RT, Taivassalo T, Rice CL. Motor unit number and transmission stability in octogenarian world class athletes: can age-related deficits be outrun? J Appl Physiol. First published March 24, 2016; doi: 10.1152/japplphysiol.00149.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Power GA, Flaaten N, Dalton BH, Herzog W. Age-related maintenance of eccentric strength: a study of temperature dependence. Age (Dordr) 38: 43, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Power GA, Makrakos DP, Stevens DE, Rice CL, Vandervoort AA. Velocity dependence of eccentric strength in young and old men: the need for speed! Appl Physiol Nutr Metab 40: 703–710, 2015. [DOI] [PubMed] [Google Scholar]
- 122.Power GA, Minozzo FC, Spendiff S, Filion ME, Konokhova Y, Purves-Smith MF, Pion C, Aubertin-Leheudre M, Morais JA, Herzog W, Hepple RT, Taivassalo T, Rassier DE. Reduction in single muscle fiber rate of force development with aging is not attenuated in world class older masters athletes. Am J Physiol Cell Physiol 310: C318–C327, 2016. [DOI] [PubMed] [Google Scholar]
- 123.Purves-Smith FM, Sgarioto N, Hepple RT. Fiber typing in aging muscle. Exerc Sport Sci Rev 42: 45–52, 2014. [DOI] [PubMed] [Google Scholar]
- 124.Raethjen J, Govindan RB, Kopper F, Muthuraman M, Deuschl G. Cortical involvement in the generation of essential tremor. J Neurophysiol 97: 3219–3228, 2007. [DOI] [PubMed] [Google Scholar]
- 125.Raj IS, Bird SR, Shield AJ. Aging and the force-velocity relationship of muscles. Exp Gerontol 45: 81–90, 2010. [DOI] [PubMed] [Google Scholar]
- 126.Rantanen T, Masaki K, Foley D, Izmirlian G, White L, Guralnik JM. Grip strength changes over 27 yr in Japanese-American men. J Appl Physiol 85: 2047–2053, 1998. [DOI] [PubMed] [Google Scholar]
- 127.Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev 40: 4–12, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Reid KF, Pasha E, Doros G, Clark DJ, Patten C, Phillips EM, Frontera WR, Fielding RA. Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur J Appl Physiol 114: 29–39, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rivner MH, Swift TR, Malik K. Influence of age and height on nerve conduction. Muscle Nerve 24: 1134–1141, 2001. [DOI] [PubMed] [Google Scholar]
- 130.Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex 14: 721–730, 2004. [DOI] [PubMed] [Google Scholar]
- 131.Sale MV, Semmler JG. Age-related differences in corticospinal control during functional isometric contractions in left and right hands. J Appl Physiol 99: 1483–1493, 2005. [DOI] [PubMed] [Google Scholar]
- 132.Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34: 721–733, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Semmler JG, Kornatz KW, Enoka RM. Motor-unit coherence during isometric contractions is greater in a hand muscle of older adults. J Neurophysiol 90: 1346–1349, 2003. [DOI] [PubMed] [Google Scholar]
- 134.Senefeld J, Yoon T, Hunter SK. Age differences in fatigability of arm and leg muscles of men and women: associations with physical function. Exp Geront, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shinohara M, Keenan KG, Enoka RM. Contralateral activity in a homologous hand muscle during voluntary contractions is greater in old adults. J Appl Physiol 94: 966–974, 2003. [DOI] [PubMed] [Google Scholar]
- 136.Sridharan A, Willette AA, Bendlin BB, Alexander AL, Coe CL, Voytko ML, Colman RJ, Kemnitz JW, Weindruch RH, Johnson SC. Brain volumetric and microstructural correlates of executive and motor performance in aged rhesus monkeys. Front Aging Neurosci 4: 31, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stålberg EV, Sonoo M. Assessment of variability in the shape of the motor unit action potential, the “jiggle,” at consecutive discharges. Muscle Nerve 17: 1135–1144, 1994. [DOI] [PubMed] [Google Scholar]
- 138.Stein RB, Gossen ER, Jones KE. Neuronal variability: noise or part of the signal? Nat Rev Neurosci 6: 389–397, 2005. [DOI] [PubMed] [Google Scholar]
- 139.Stevens JE, Stackhouse SK, Binder-Macleod SA, Snyder-Mackler L. Are voluntary muscle activation deficits in older adults meaningful? Muscle Nerve 27: 99–101, 2003. [DOI] [PubMed] [Google Scholar]
- 140.Taylor AM, Christou EA, Enoka RM. Multiple features of motor-unit activity influence force fluctuations during isometric contractions. J Neurophysiol 90: 1350–1361, 2003. [DOI] [PubMed] [Google Scholar]
- 141.Taylor JL. Point: the interpolated twitch does/does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol 107: 354–355, 2009. [DOI] [PubMed] [Google Scholar]
- 142.ten Caat M, Maurits NM, Roerdink JB. Data-driven visualization and group analysis of multichannel EEG coherence with functional units. IEEE Trans Vis Comput Graph 14: 756–771, 2008. [DOI] [PubMed] [Google Scholar]
- 143.Thom JM, Morse CI, Birch KM, Narici MV. Influence of muscle architecture on the torque and power-velocity characteristics of young and elderly men. Eur J Appl Physiol 100: 613–619, 2007. [DOI] [PubMed] [Google Scholar]
- 144.Tintignac LA, Brenner HR, Ruegg MA. Mechanisms regulating neuromuscular junction development and function and causes of muscle wasting. Physiol Rev 95: 809–852, 2015. [DOI] [PubMed] [Google Scholar]
- 145.Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci 34: 213–219, 1977. [DOI] [PubMed] [Google Scholar]
- 146.Tracy BL, Enoka RM. Steadiness training with light loads in the knee extensors of elderly adults. Med Sci Sports Exerc 38: 735–745, 2006. [DOI] [PubMed] [Google Scholar]
- 147.Tracy BL, Maluf KS, Stephenson JL, Hunter SK, Enoka RM. Variability of motor unit discharge and force fluctuations across a range of muscle forces in older adults. Muscle Nerve 32: 533–540, 2005. [DOI] [PubMed] [Google Scholar]
- 148.Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol 552: 47–58, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vallbo AB, Wessberg J. Organization of motor output in slow finger movements in man. J Physiol 469: 673–691, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vanden Noven ML, Pereira HM, Yoon T, Stevens AA, Nielson KA, Hunter SK. Motor variability during sustained contractions increases with cognitive demand in older adults. Front Aging Neuro sci 6: 97, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Venturelli M, Morgan GR, Donato AJ, Reese V, Bottura R, Tarperi C, Milanese C, Schena F, Reggiani C, Naro F, Cawthon RM, Richardson RS. Cellular aging of skeletal muscle: telomeric and free radical evidence that physical inactivity is responsible and not age. Clin Sci (Lond) 127: 415–421, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Venturelli M, Saggin P, Muti E, Naro F, Cancellara L, Toniolo L, Tarperi C, Calabria E, Richardson RS, Reggiani C, Schena F. In vivo and in vitro evidence that intrinsic upper- and lower-limb skeletal muscle function is unaffected by ageing and disuse in oldest-old humans. Acta Physiol 215: 58–71, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 292: E151–E157, 2007. [DOI] [PubMed] [Google Scholar]
- 154.Wall BT, Gorissen SH, Pennings B, Koopman R, Groen BB, Verdijk LB, van Loon LJ. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS One 10: e0140903, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wallace JW, Power GA, Rice CL, Dalton BH. Time-dependent neuromuscular parameters in the plantar flexors support greater fatigability of old compared with younger males. Exp Gerontol 74: 13–20, 2016. [DOI] [PubMed] [Google Scholar]
- 156.Ward NS. Compensatory mechanisms in the aging motor system. Ageing Res Rev 5: 239–254, 2006. [DOI] [PubMed] [Google Scholar]
- 157.Wood SJ, Slater CR. Safety factor at the neuromuscular junction. Prog Neurobiol 64: 393–429, 2001. [DOI] [PubMed] [Google Scholar]
- 158.Yoon T, De-Lap BS, Griffith EE, Hunter SK. Age-related muscle fatigue after a low-force fatiguing contraction is explained by central fatigue. Muscle Nerve 37: 457–466, 2008. [DOI] [PubMed] [Google Scholar]
- 159.Yoon T, Doyel R, Widule C, Hunter SK. Sex differences with aging in the fatigability of dynamic contractions. Exp Gerontol 70: 1–10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yoon T, Keller ML, De-Lap BS, Harkins A, Lepers R, Hunter SK. Sex differences in response to cognitive stress during a fatiguing contraction. J Appl Physiol 107: 1486–1496, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yoon T, Schlinder-Delap B, Hunter SK. Fatigability and recovery of arm muscles with advanced age for dynamic and isometric contractions. Exp Gerontol 48: 259–268, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yoon T, Vanden Noven ML, Nielson KA, Hunter SK. Brain areas associated with force steadiness and intensity during isometric ankle dorsiflexion in men and women. Exp Brain Res 232: 3133–3145, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]