The age-related increase in muscle and tendon tissue stiffness and reduction in active force capacity of the muscle compromise elastic energy utilization and positive work production, which may require the recruitment of additional muscle volume potentially contributing to the increased cost of locomotion observed in older individuals.
Keywords: aging, elastic energy, cost of locomotion, fibrosis, ankle joint work
Abstract
Efficient muscle-tendon performance during cyclical tasks is dependent on both active and passive mechanical tissue properties. Here we examine whether age-related changes in the properties of muscle-tendon units (MTUs) compromise their ability to do work and utilize elastic energy storage. We empirically quantified passive and active properties of the medial gastrocnemius muscle and material properties of the Achilles tendon in young (∼6 mo) and old (∼32 mo) rats. We then used these properties in computer simulations of a Hill-type muscle model operating in series with a Hookean spring. The modeled MTU was driven through sinusoidal length changes and activated at a phase that optimized muscle-tendon tuning to assess the relative contributions of active and passive elements to the force and work in each cycle. In physiologically realistic simulations where young and old MTUs started at similar passive forces and developed similar active forces, the capacity of old MTUs to store elastic energy and produce positive work was compromised. These results suggest that the observed increase in the metabolic cost of locomotion with aging may be in part due to the recruitment of additional muscles to compensate for the reduced work at the primary MTU. Furthermore, the age-related increases in passive stiffness coupled with a reduced active force capacity in the muscle can lead to shifts in the force-length and force-velocity operating range that may significantly impact mechanical and metabolic performance. Our study emphasizes the importance of the interplay between muscle and tendon mechanical properties in shaping MTU performance during cyclical contractions.
NEW & NOTEWORTHY
The age-related increase in muscle and tendon tissue stiffness and reduction in active force capacity of the muscle compromise elastic energy utilization and positive work production, which may require the recruitment of additional muscle volume potentially contributing to the increased cost of locomotion observed in older individuals.
locomotion is more energetically costly in older individuals. A comparison of healthy young men (∼27 yr old) and healthy older men (∼74 yr old) walking at varying speeds found that the metabolic cost of walking increased by an average of 31% in older men (39). Similarly, during running, older individuals have decreased overall efficiency, requiring muscle to consume significantly more metabolic energy per unit mechanical work with each step (15). While this may be partially due to a decreased efficiency of muscle contraction (16), a reduction in effective energy storage could also contribute to an increase in the metabolic cost of locomotion.
The dynamic function of tendon is integral to reducing the metabolic cost of locomotion. These energetic savings may occur by changing where muscles operate on their force-length and force-velocity curves (35), by reducing muscle work (6), or by reducing active muscle volume (24). Regardless of the mechanism by which tendons reduce the metabolic cost of muscle contraction, changes in the active and passive mechanical properties of muscles and tendons could reduce the ability of a muscle-tendon unit to effectively use these cost-saving mechanisms.
The mechanical properties of tendon are critical in determining tendon dynamics and function (8). However, there is little consensus on how aging affects tendon properties. Changes in modulus and cross-sectional area can alter the functional stiffness of tendon thereby changing tendon dynamics during cyclical tasks such as walking and running. Additionally, changes in the tendon stiffness will lead to changes in the natural frequency of muscle-tendon units and limbs, potentially compromising the effective use of resonance during such tasks (56). Increases in tendon cross-sectional area (37, 54) and collagen cross-linking due to an accumulation of advanced glycation end products (32) have been demonstrated with age. These changes are likely to increase tendon stiffness (48). The majority of experimental data comparing young and old connective tissues in various animal systems support this and suggest an increase in elastic modulus with age (1, 13, 14, 18, 21, 54). Some studies, however, especially in vivo studies on humans, have shown a decrease in the Young's modulus of tendons (9, 29, 30, 33, 43, 44, 59). The lack of consensus in the literature suggests that the effect of aging on the material properties of tendon may vary on the basis of preparation, the tendon being studied, the study species, and the age cohorts being compared. This lack of consensus has made it difficult to relate changes in tendon properties to the metabolic cost of movement.
Age-related reduction in the force capacity of older muscles is well established. A reduction in maximum isometric force can be as high as 2% per year (55) and is often associated with dimensional changes in the muscles and an overall loss of muscle mass due to atrophy (60). However, a reduction in muscle mass or cross-sectional area (CSA) does not fully explain the decline in force output in the elderly. Maximum isometric stress (force/CSA) also decreases significantly with age. This has been attributed to age-related increases in noncontractile tissues such as fat and collagen (21, 31, 47), changes in muscle architecture (42, 57), and increased stiffness of intramuscular connective tissues (31). The age-related reduction in muscle force can decrease the ability of a muscle to stretch its external tendon and compromise the storage and return of elastic energy.
The purpose of this study was to examine the age-related changes in muscle-tendon unit function. We use a well-established animal model of aging, the F344xBN rat (5, 25). The use of an animal model provides a more controlled system than the cross-sectional studies of humans, which are often fraught with significant interindividual variation, and a more practical system than longitudinal studies of human subjects that can extend over several decades. In addition, the use of an animal model allows us to study both isolated components of the musculoskeletal system as well as the integrated and intact system in order to better understand the specific structures responsible for functional decrements. Finally, the rat strain used in the study (developed by the National Institute on Aging) is well suited for studies of healthy aging without the confounding pathologies common in other model systems. In this study we first measured the contractile, morphological, and mechanical properties of the medial gastrocnemius muscle and tendon. We then used these experimental variables as inputs to a muscle-tendon model (Hill-type model operating with an in-series spring) to simulate cyclic contractions (i.e., work loops) and test 1) whether old muscle-tendon units (MTUs) can perform as much mechanical work as young ones and 2) whether elastic energy storage and return is compromised in old MTUs as a result of nonoptimal interaction between active and passive MTU properties.
MATERIALS AND METHODS
Experimentally measured muscle-tendon properties.
Active and passive muscle-tendon unit properties (Table 1) were measured in situ in young [n = 8, age 5-9 mo, body mass 362 ± 32 (SD) g] and old (n = 8, age 33–34 mo, body mass 489 ± 19 g) male Brown Norway x F344 F1 hybrid rats, Rattus norwegicus, from the National Institute on Aging (F344BN; Bethesda, MD). The animal ages were chosen to ensure that the effect we documented was one of senescence and not developmental maturation (41). The medial gastrocnemius (MG) was identified as a good muscle model because of its size and accessibility and its significant contribution to ankle power. All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of California, Irvine.
Table 1.
Empirically derived model parameters for young and old rat medial gastrocnemius muscle-tendon unit
| Young | Old | |
|---|---|---|
| Active parameters | ||
| Fmax,a N | 14.7 ± 2.77 (n = 8) | 7.1 ± 1.41 (n = 8) |
| Vmax,b m/s | 0.122 (n = 8) | 0.107 (n = 8) |
| tact,c s | 0.062 | 0.062 |
| tdeact,c s | 0.071 | 0.081 |
| Passive parameters | ||
| L0 muscle, m | 0.039 ± 0.022 (n = 11) | 0.033 ± 0.0086 (n = 3) |
| Lr (L0 muscle)d | 1.1 | 1.0 |
| kmuscle, N/m | 420 ± 87.3 (n = 7) | 970 ± 160.9 (n = 5) |
| Emuscle, kPa | 0.79 ± 0.40 (n = 5) | 1.74 ± 0.87 (n = 7) |
| Lslack tendon, m | 0.0153 ± 0.018 (n = 5) | 0.0115 ± 0.013 (n = 5) |
| ktendon, N/m | 1,102 ± 313 (n = 5) | 1,505 ± 333 (n = 5) |
| Etendon,e GPa | 6.1 ± 1.78 (n = 5) | 10.2 ± 1.11 (n = 5) |
Values are means ± SD.
Emuscle and Etendon, muscle and tendon elastic modulus respectively.
Mean peak isometric stress multiplied by mean triceps surae cross-sectional area and corrected for the medial gastrocnemius proportion of complex (see materials and methods).
Value was estimated by fitting a single curve to the compiled data for each age group.
Selected to match the force rise (tact) and force decay (tdeact) of a representative in situ tetanic contraction at L0.
Length at which muscle develops passive force.
Calculated for 5% strain and 5-Hz cycling.
The passive and active force-length properties of the intact muscle were first determined in situ as previously described by Holt et al. (23). The rats were anesthetized using 2% isoflurane, maintained on a closed-system anesthesia machine (Parkland Scientific, Coral Springs, FL), and placed, prone, on a heat mat. The sciatic nerve was exposed via a small incision running from the caudal midline of the hind limb toward the base of the tail. A nerve cuff containing a stimulus and a ground electrode was placed around the nerve, and the nerve was severed proximally. The area around the electrode was filled with warmed mineral oil, and the suture was closed. The Achilles tendon was then exposed, and all tendons, except that of the gastrocnemius, were severed. The calcaneus was cut leaving a small amount of bone attached to the MG tendon. This bone and tendon were secured in a custom-made clamp, as close to the end of the muscle fibers as possible. An incision was made on the lateral aspect of the thigh, and the femur was clamped into a stereotaxic frame. The distal clamp was connected to the lever arm of an ergometer (310 B-LR; Aurora Scientific, Aurora, ON, Canada) using steel cable (10 cm). Care was taken to minimize the compliance of the setup. The muscle was wrapped in saline-moistened gauze and Saran wrap, and muscle temperature was maintained at 37°C using a heat lamp.
Isometric, fixed-end, twitch contractions were elicited, by applying a single stimulus pulse to the sciatic nerve, at a range of lengths to produce a twitch force-length curve (Grass S48 stimulator; Grass Medical Instruments, Quincy, MA). This allowed us to quantify the optimal length of the muscle. Force and ergometer position data were collected at 1,000 Hz using a National Instruments AD board (NI USB-6212; Austin, TX) and recorded using Igor Pro 6.31 software (Wavemetrics, Lake Oswego, OR). A single tetanic contraction was elicited at the optimal muscle length (L0 muscle, Table 1) by applying a train of 0.2-ms square wave pulses at 100 Hz for 400 ms (Fmax, Table 1). Because the lateral gastrocnemius was still attached to the MG and was also stimulated by the sciatic nerve, force values (Fmax) were corrected on the basis of the relative size of the two muscles.
After-loaded isotonic tetanic contractions were used to determine the force-velocity relationship of the muscle with the same stimulation protocol used for isometric tetanic contractions (23). Force was allowed to rise to a defined level (10, 30, 50, 70, or 90% of Fmax), and the muscle was allowed to shorten to maintain a constant force. The order of force levels was randomized, and a 5-min rest period was allowed for recovery between contractions. Another isometric contraction was performed after the series of isometric contractions to monitor muscle fatigue and health; muscle force never fell below 90% of its original value. Maximum shortening velocity (Vmax) was obtained by fitting the Hill equation to the pooled data for each age group. Activation (tact) and deactivation (tdeact) time constants were defined on the basis of activation equations from Zajac (61) and were chosen to match the force profile of a representative tetanus for each age group (Fig. 1).
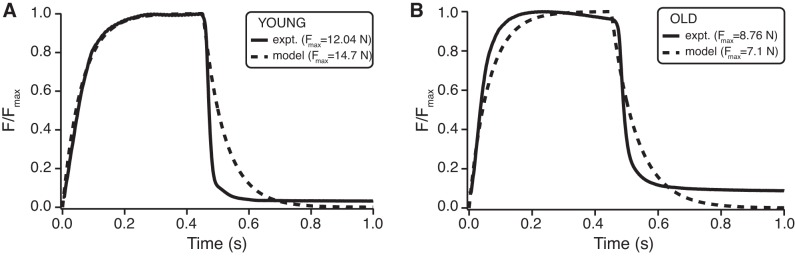
Fig. 1.
Simulated and experimental tetanic contractions. Experimental data (solid lines) consist of a single tetanic contraction of the intact medial gastrocnemius, at L0, with a 450-ms stimulation. Computational simulations (dashed lines) match experimental data reasonably well in both the young (A) and old (B) cases, with the most obvious difference being a slower rate of force decay in the modeled data. Fmax is the maximum isometric force.
Passive stiffness of the muscle (kmuscle, Table 1) was measured during passive stretches and calculated from the slope of a linear fit to passive force vs. muscle strain (relative to L0 muscle) in Igor Pro 6.31 (Wavemetrics). The length of the contralateral MG tendon (L0 tendon) was measured in situ using calipers with the knee and ankle at 90°. The tendon was harvested immediately following euthanasia and frozen in physiological saline for up to 1 yr before testing. Tendon stiffness (ktendon) was measured as follows. One end of the tendon was attached by Kevlar thread to an ergometer (model 360C; Aurora Scientific), and the other was held in place with a stationary clamp. Each tendon was repeatedly stretched to 2 and 5% of its resting length for five cycles at two frequencies, 2.5 Hz (2 s) and 5 Hz (1 s). The strain profiles selected encompassed the predicted tendon strains during walking and running (25), reached linear stress-strain profile, and avoided plastic deformation. Tendon stiffness (N/mm) was calculated as the slope of the linear region of the third stretching cycle.
Computational muscle-tendon model.
We used Simulink (MathWorks, Natick, MA) to develop a computational model (50) and ran simulations using empirical data from either young or old muscles. Briefly, the models consisted of a Hill-type muscle model with nonlinear force-length and force-velocity properties in series with a linearly elastic tendon. Muscle activation was modeled after Zajac (61). The young and old models were validated by comparing the force profiles of tetanic contractions measured in situ in young and old muscles with the simulated output force profile of the models (Fig. 1).
Once validated, young and old muscle-tendon unit models were oscillated through 3-Hz stretch-shorten cycles with an amplitude that corresponded to 25% of their resting length (muscle L0 plus tendon slack length, Lslack tendon; 13.5 mm for young) starting at 1.1L0. In length-matched simulations, old muscle-tendon unit models were also oscillated through 3-Hz stretch-shorten cycles with an amplitude that corresponded to 25% of their resting length (muscle L0 plus tendon slack length, Lslack tendon; 11.2 mm). However, the differences in the empirically derived passive stiffness of the young and old muscles (Table 1) meant that while these simulations provided a length-matched comparison between young and old muscle, they did not provide a reasonable force-matched comparison. To achieve a force-matched simulation, we also cycled old muscle through a 3-Hz stretch-shorten cycle with a starting length of 1.0L0 and an amplitude corresponding to 12% MTU strain. This resulted in more comparable passive forces both at the beginning of the cycle and throughout the stretch-shorten cycle.
In all simulations the muscle was stimulated for 10% of the cycle. The stimulus was applied at 12.5% intervals to find the timing of stimulation that minimized net muscle work. In all simulations the optimal phase, resulting in the least net work, was 37.5%, where 0% is the beginning of lengthening (Fig. 2).
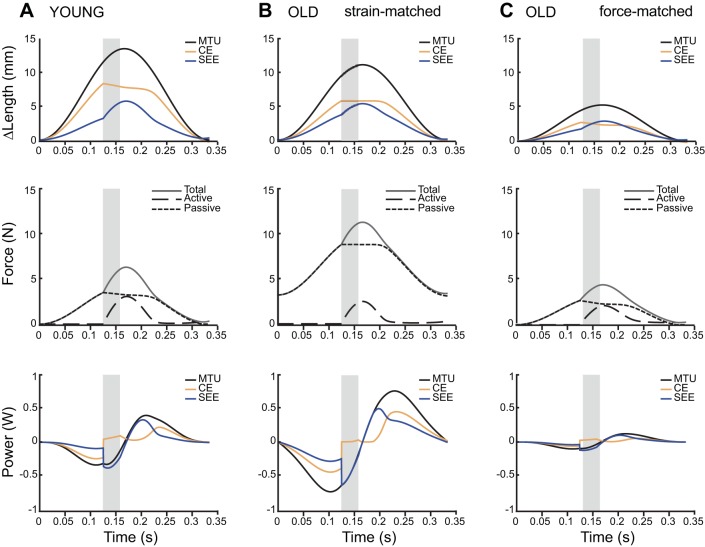
Fig. 2.
Time series of length change, force development, and power during simulations (3 Hz with 10% duty stimulation starting at 37.5%) for the three conditions tested. The period of stimulation is indicated by the shaded grey region. Total muscle-tendon unit (MTU) length change is different for young and old MTUs (A and B) because resting MTU length differed in the two age groups (54 mm for young vs. 44.6 mm for old MTU when measured with the ankle and knee at 90°). During strain-matched simulations when the starting length and strain amplitudes are the same (A and B), the old MTU develops high passive forces prior to activation. During force-matched simulations when initial passive force and total maximum force are similar (A and C), old MTU develops significantly less power and stores less energy in series elastic elements. CE is the contractile element, and SEE is the series elastic element.
RESULTS
Our empirical measurements are largely consistent with previous studies describing age-related changes to muscle and tendon. We found that aging results in a decrease in both maximum isometric force (Fmax) and maximum shortening velocity (Vmax) (Table 1). The passive stiffness of the muscle was higher, and passive tension developed at shorter lengths (1.0L0) in old muscles, compared with young muscles (1.1L0) (Table 1). We also found that the stiffness of the series elastic tendon was higher in old compared with young muscles (Table 1). Activation time constant (tact) was not different between young and old muscles, but old muscles took longer to deactivate (tdeact; Table 1) as has been observed elsewhere (10).
To validate the outputs of the muscle-tendon model, we compared an empirical tetanic contraction from an in situ preparation with the predicted force profile from a model simulation (Fig. 1). To simulate the removal of the tendon from the in situ preparation, we set tendon stiffness to an unrealistically high value prior to our validation trials. The model predicted the path of force rise and decay reasonably well, with the most obvious difference lying in the rate of force decay. In the empirical tetanic contractions, force dropped at a faster rate than in the computational model (Fig. 1).
First we compared young and old MTUs under strain-matched conditions where the length trajectories imposed on the virtual MTUs were identical. Both young and old muscles start the work loop cycle at 1.0L0 and undergo sinusoidal length changes with 25% MTU strain (Fig. 2, A and B). The young contractile element (CE; Fig. 2A) initially shortens internally against the tendon while the old CE produces force nearly isometrically. Additionally, in the young MTU the CE makes up nearly twice as much of the total MTU strain as the series elastic element (SEE), but a larger proportion of MTU strain is due to the stretch of the tendon (SEE) in the old MTU (Fig. 2B). On the other hand, as the MTU is being passively stretched prior to stimulation, the increased stiffness of the old muscle and tendon result in higher initial and maximum passive forces in the old MTU (Fig. 2B). Under these strain-matched conditions, old MTUs seemed better tuned for elastic energy utilization than young MTUs. The old CE performed less active work, and the SEE cycled nearly 100% of the elastic energy stored in it (Fig. 3, A and B).
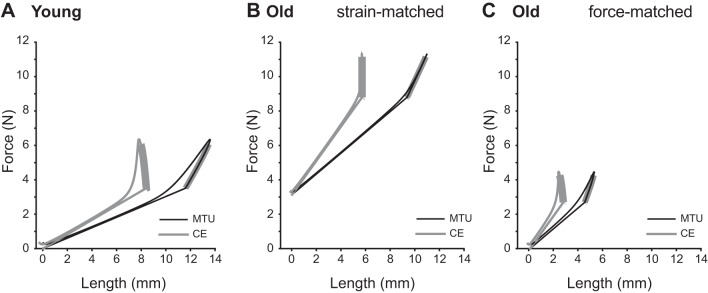
Fig. 3.
Muscle-tendon unit (MTU) and contractile element (CE) work loops from simulations of MTU at 3 Hz with 10% duty stimulation starting at 37.5% of the cycle (with respect to the beginning of shortening). When young and old MTUs are cycled under the same conditions (A and B), old MTUs start at a higher passive force and, when stimulated, contract nearly isometrically performing less positive work than young muscles (CE). However, if the initial passive force and the maximum total force are matched between young and old MTUs (A and C), old MTUs are stretched by only 12% of their resting length, and the muscle starts contracting at 1.0L0 compared with 1.1L0 of the young CE. Bolded regions of the work loops represent periods of active force production.
Second, we compared young and old MTUs under force-matched conditions, where the length trajectories imposed on the virtual MTU were adjusted to produce similar passive forces prior to activation. This resulted in a passive force of 3.5 N for the young MTU and 2.7 N in the old MTU under force-matched conditions, compared with a passive force prior to stimulation of 8.8 N in the old MTU under strain-matched conditions. To match passive force profiles, old work loops were performed with shorter initial muscle length (1.0 vs. 1.1 L0 muscle) and at nearly half the amplitude (12 vs. 25% MTU strain) compared with young (Fig. 2C). Under these conditions, muscle and tendon took up equal amounts of the imposed MTU strain prior to stimulation. At stimulation the muscle shortened against the tendon but produced less active force than the young muscle (Fig. 2C). Under these force-matched conditions, positive MTU work was reduced to nearly a third of young MTU work (Fig. 3C).
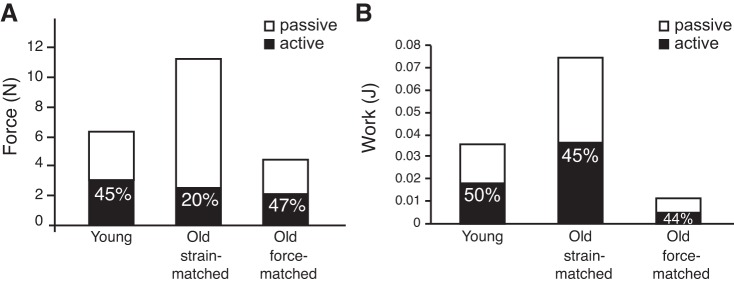
We also compared the absolute and relative contributions of active and passive forces to total force and work (Fig. 4). Young MTUs produced a maximum force of 6 N while strain-matched old MTUs produced nearly twice as much force (11.1 N). However, 80% of the total force came from passive tissues in the old MTU, compared with 55% in the young MTU (Fig. 4A). Under force-matched conditions, the proportion of passive to total force in old MTUs dropped to 53%, similar to young MTUs. We also calculated the amount of positive work done by the muscle (active) and series elastic elements (passive) and the proportion of total positive work (CE + SEE) done by each element (Fig. 4B). Although the proportion of elastic potential energy, calculated as the product of total force and SEE length change, was similar in all three simulation conditions (44–50%), the absolute magnitudes of these differed greatly, with old MTUs under strain-matched conditions producing the most work but old MTUs in the force-matched model producing the least.
Fig. 4.
Contributions of passive (SEE) and active (CE) elements to total force and work production, under each of the three simulation conditions. A: when old MTUs were cycled with the same conditions as young MTUs, the peak total force doubled because of a large increase in passive force and a reduction in active muscle force. When old MTUs were allowed to begin cycling at shorter lengths and with smaller strain excursions, both the absolute and relative passive and active peak force patterns resembled that of young MTUs. B: in young MTUs, CE and SEE both contribute 50% of the work in a work loop. The relative values are similar in old MTUs cycled under the same conditions, but the absolute value of work is more than doubled. However, when the peak muscle force is kept at realistic values for old MTUs (initial muscle length at 1L0 and 12% MTU strain), the whole MTU performs less than a third of the work that a young MTU does under physiological conditions.
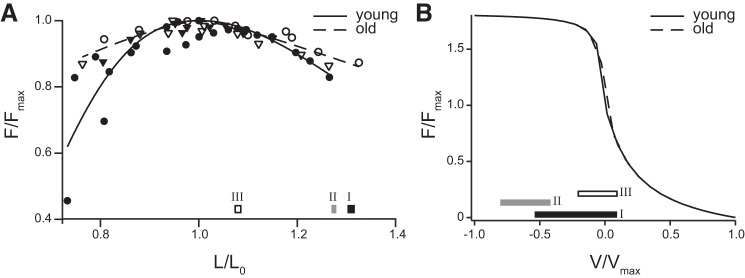
To examine whether age-related changes affected where muscles operated on the force-length and force-velocity curves, we plotted the active muscle lengths and shortening velocities on empirically derived force-length and force-velocity curves (Fig. 5). The operating length of the muscle was largely determined by the initial length of the MTU and prescribed strain patterns. Therefore, during strain-matched simulations, young and old muscles operated over similar regions of the force-length curve (simulations I and II, Fig. 5A). However, during force-matched simulations, old MTUs operated on the plateau and underwent less length change (simulation III, Fig. 5A). Young MTUs underwent significant periods of active shortening (positive V/Vmax) and also operated eccentrically near Fmax for part of the cycle (simulation I, Fig. 5B). In the strain-matched condition, old MTUs never shortened and operated closer to Fmax (simulation II, Fig. 5B). In force-matched conditions, however, they behave more like the young MTU, undergoing both concentric and eccentric contractions albeit at lower average lengthening velocities (simulation III, Fig. 5B).
Fig. 5.
Operating range of young and old muscles under three simulation conditions, mapped on experimentally derived force-length and force-velocity curves: simulation I, young; simulation II, old, strain-matched conditions; simulation III, old, force-matched conditions. Data are shown only for the active portion of the cycle. A: young and old muscle acting under the same strain stretch-shorten conditions (muscle Linitial = 1.1L0, 25% MTU strain) operate on the descending limb of the force-length curve. However, old muscle acting under conditions that match the MTU passive force produced during stretching operate on the plateau, nearer to L0. B: young muscle operates over a broader range of velocities than old muscle under either strain-matched or force-matched condition.
DISCUSSION
We used empirically informed simulations to assess how age-related changes in the mechanical properties of an MTU affect how mechanical work is distributed between the muscle and the series elastic elements during cyclical contractions and how such changes affect muscle operating lengths and velocities.
Changes in MTU mechanical properties with age.
We found that in the rat gastrocnemius both the muscle and tendon increased in stiffness with advanced aging, similar to what has been reported in other studies (1, 13, 14, 18, 21, 54) (Table 1). Muscle fibrosis and increased deposition of collagen in the extracellular matrix is common among neuromuscular pathologies, atrophy, and aging (36). During aging, muscle stem cells disproportionately shift from a myogenic (muscle-forming cell) fate to a fibrogenic fate and cause a relative increase in collagen content, leading to a “fibrotic” muscle phenotype (1, 21, 32). Fibrotic muscles have increased passive stiffness and develop passive tension at shorter relative lengths (Table 1; 36).
Despite numerous studies that have measured the tensile properties of tendons, there has been no consensus on whether advanced aging increases (13, 54), decreases (9, 30, 59), or has no effect (29, 40) on tendon stiffness or modulus. This is surprising given the observed decrease in the crimp angle of collagen and increased fiber cross-linking with aging (18), which suggest that structural changes should lead to an increase in Young's modulus (18, 58). However, none of these studies concerned the Achilles tendon of rats. Tendons have been shown to have varying structural and material properties depending on their in vivo function (8, 54). Therefore it is worth conducting future studies to explore how age-related effects might differ among muscle-tendon groups with varying functions.
We also documented a reduction in maximum isometric muscle force with aging (Table 1) similar to numerous other studies. However, our results suggest that at longer MTU lengths, passive forces may compensate for the loss of active force capacity in old muscles (Fig. 2). This result may help explain previous findings that have shown that the age-related loss of force is more pronounced during concentric or isometric tasks compared with eccentric tasks where passive forces are more likely to contribute (27).
MTU strain and work.
In the old MTU, we matched either total MTU strain (strain-matched simulation) or total MTU passive force (force-matched simulation) to the values seen in young MTUs. The reduction in active force capacity coupled with the increased stiffness of the muscle and series elastic elements meant that when young and old MTUs undergo the same strain cycle, passive forces contributed much more to the total MTU force and positive work in old MTUs. However, the passive stretch of the old muscle requires more force production, either by the contraction of antagonists or by the generation of increased inertial loads, both of which are likely to bear an energetic burden. The strain-matched conditions are highly unlikely under physiologically realistic conditions. First, antagonist muscles are likely to show a similar decline in active force capacity and therefore may not be able to generate sufficient forces to stretch muscles to such high passive tensions. Similarly, given that body mass is unlikely to increase in old animals and vertical velocity during walking is actually reduced (25, 45), the inertial/gravitational forces loading the muscle-tendons would not be high enough to stretch old muscle to lengths that correspond to significant passive force. The reduction in MTU amplitude from 25 to 12% in the force-matched simulations is likely still an overestimate of the strain antagonist muscles would be able to impose given the high passive stiffness of gastrocnemius MTU and the 50% reduction in force-producing capacity in old rats (Table 1).
The force-matched simulations are therefore more likely to represent realistic conditions under which to compare the young and old MTU. It has been previously shown that variation in the passive stiffness of a muscle shifts the operating length such that muscles are recruited at similar passive forces rather than similar lengths (4). The results of the force-matched model potentially explain some of the distinct gait changes that occur with aging in a diversity of animal groups including humans (25, 53). Under such conditions an old MTU performs about one-third of the work per stride compared with young MTUs (Fig. 4A). This result is consistent with the reduction in preferred walking speed with age in humans (38) and the lower stride length observed in older humans and animals (25, 53). Therefore a scenario of muscles operating at shorter lengths and undergoing lower muscle excursions may be more representative of in vivo muscle conditions in older animals and humans.
The reduction in operating lengths and strain amplitude suggested by our simulations (Fig. 5A) may also protect old muscle from damage. Operating on the descending limb of the force-length relationship and undergoing larger lengthening strains are associated with an increase in the likelihood of muscle damage (22). Avoiding such longer lengths may be particularly beneficial as fibers from old muscles are more prone to being damaged (12) and less efficient at recovering from injury (11).
Elastic energy storage was not always compromised in old MTUs. From the strain-matched models we could see that if there was enough force available to stretch the MTU to similar strains in the aged animals, elastic energy storage would increase because of the increased stiffness of the muscle and tendon (Fig. 4). Although the absolute amount of elastic energy stored is much reduced in the force-matched model, the proportion of total work done by the passive elements is actually slightly increased. Hence, while the total work of the system may decrease with age, the contribution of the tendon does not necessarily change.
Energetic implications of changes in MTU properties due to aging.
The physiologically more relevant force-matched simulations of the old MTU may have some potential implications for the energetic cost of locomotion. The reduced work production by the gastrocnemius in this condition will reduce the range of motion at the ankle, the primary joint where work is done during push off (19). This will require more work to be done by the more proximal muscles around the knee and hip joints (26). This shift in joint work results in a shift in the architecture of the muscles used from short-fibered pennate muscles with long tendons in the distal limb to long-fibered parallel muscles with little external tendon in the proximal limb. This shift in the architecture of muscles used may increase the cost of generating mechanical work (34, 49). Smaller muscle moment arms during crouched postures (7, 25) further contribute to a reduction in ankle work. Similar kinematic and energetic changes were observed when ankle excursion and work were limited by a rigid ankle orthotic prosthesis in humans (28).
Additionally, there is a well-documented increase in both synergist and antagonist muscle cocontraction with aging (20, 39, 46). It has been hypothesized that cocontraction of antagonists may provide additional joint stability to support the body weight (39) given the reduced capacity of each muscle to produce force. Furthermore, the results of our force-matched simulations show that cocontraction of synergistic muscles is a means of compensating for reduced work due to the changes in active and passive mechanical properties of the MTU. If the age-related changes we observe in rats are similar in humans, then the recruitment of additional muscles may contribute to the additional metabolic cost of locomotion in the elderly; cocontraction of knee extensors and flexors alone can account for 28–52% of the increased metabolic cost of walking (39).
Changes in the stiffness of an MTU also alter its natural frequency. Resonance occurs when a system is driven at its natural frequency and has been shown to significantly affect both MTU dynamics (51) and the metabolic efficiency of locomotion (17). However, in our analyses we used a single driving frequency for two different mechanical systems. This could explain why we observed no shift in the stimulation onset that minimized active muscle work, similar to what has been observed in the onset of gastrocnemius activity in a speed-matched study of young and old men walking (39). In an unconstrained work loop experiment it was shown that when MTUs of different mechanical properties were cycled at their natural frequency, stimulation onset shifted relative to length changes to tune MTUs for maximum force and elastic energy storage (51). By constraining the driving frequency of the MTU or during speed-matched in vivo studies we are likely forcing energy-inefficient dynamics onto systems whose stiffness differs by nearly twofold.
Potential limitations.
There were a number of simplifications we made to the structural morphology of our MTU model that are worth addressing. First, our model is a one-dimensional muscle model that can account for the functional effects of certain morphological changes along the line of action of the free tendon (e.g., reduction in the cross-sectional area of muscles and tendon) but cannot address off-axis shape changes that occur during in vivo contractions. For example, our simulations do not account for three-dimensional effects such as muscle gearing (2) that have been shown to be significantly affected by age (23). In addition, our simulations used a series elastic element with a linear stress-strain relationship and a constant stiffness. The stress-strain curve of tendons has a nonlinear stiffness at lower strains (toe region), which may have some small impact on MTU dynamics at the lowest force levels. In addition, aponeuroses have been shown to deform biaxially during stretch-shorten cycles and to function as variable stiffness springs (3). Thus it is possible that the effects of aging on the dynamic changes in aponeurosis stiffness may also alter the length trajectory of an MTU.
Aspects of our work loop protocol may also deviate from capturing all of the features of in vivo MTU dynamics. Work loops that strictly enforce MTU length change patterns and muscle stimulation phase provide a highly controlled experimental framework that is useful for gaining initial insights (52). However, while our imposed length trajectories were symmetrical sine waves, the actual length trajectories of MTUs like the gastrocnemius may be more accurately characterized as asymmetrical (51). In addition, we note that cyclic contractions in freely moving animals are more likely the result of the dynamic interaction between MTU force output, the load of the body, and the dynamics of the environment (51). Finally, any extrapolation from our results to the mechanics and energetics of human locomotion is based on the assumption that mechanical changes that occur with age are shared between our rodent model and humans. Despite these limitations, our study is the first to use empirically informed simulations to compare the dynamics of old and young MTUs and provides fundamental insight into the mechanical interactions of muscles and tendons.
Conclusions.
In this study we used an empirically driven simulation of muscle-tendon unit dynamics to explore the effect of age-related disruptions to active and passive properties on the exchanges in energy during cyclical contractions. Our results suggest that the ability to store and return energy in tendons is not always compromised with age as passive forces compensate for a decline in active force production. However, age-related increases in passive stiffness are likely to reduce MTU strain and therefore mechanical work. This reduced work capacity is likely to significantly impact energetic performance as more muscle mass may be required to retain comparable joint dynamics.
GRANTS
This study was funded by National Institutes of Health Grant AR-055295 and National Science Foundation Grant 1436476.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.D. and E.A. conception and design of research; N.D. performed experiments; N.D. analyzed data; N.D., N.C.H., G.S.S., and E.A. interpreted results of experiments; N.D. prepared figures; N.D. drafted manuscript; N.D., N.C.H., G.S.S., and E.A. edited and revised manuscript; N.D., N.C.H., G.S.S., and E.A. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of N. Danos: Biology Department, University of San Diego, 5998 Alcalá Park, San Diego CA 92110.
We thank Emily Abbott for help during experiments and for useful discussions at the early stages of this study.
REFERENCES
- 1.Alnaqeeb MA, Zaid Al NS, Goldspink G. Connective tissue changes and physical properties of developing and ageing skeletal muscle. J Anat 139, Part 4: 677–689, 1984. [PMC free article] [PubMed] [Google Scholar]
- 2.Azizi E, Brainerd EL, Roberts TJ. Variable gearing in pennate muscles. Proc Natl Acad Sci U S A 105: 1745–1750, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azizi E, Roberts TJ. Biaxial strain and variable stiffness in aponeuroses. J Physiol 587: 4309–4318, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azizi E. Locomotor function shapes the passive mechanical properties and operating lengths of muscle. Proc Biol Sci 281: 20132914, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballak SB, Degens H, de Haan A, Jaspers RT. Aging related changes in determinants of muscle force generating capacity: a comparison of muscle aging in men and male rodents. Ageing Res Rev 14: 43–55, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Biewener AA, Roberts TJ. Muscle and tendon contributions to force, work, and elastic energy savings: a comparative perspective. Exerc Sport Sci Rev 28: 99–107, 2000. [PubMed] [Google Scholar]
- 7.Biewener AA. Scaling body support in mammals: limb posture and muscle mechanics. Science 245: 45–48, 1989. [DOI] [PubMed] [Google Scholar]
- 8.Birch HL. Tendon matrix composition and turnover in relation to functional requirements. Int J Exp Pathol 88: 241–248, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blevins FT, Hecker AT, Bigler GT, Boland AL, Hayes WC. The effects of donor age and strain-rate on the biomechanical properties of bone-patellar tendon-bone allografts. Am J Sports Med 22: 328–333, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 404: 71–82, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks SV, Faulkner JA. Contraction-induced injury: recovery of skeletal-muscles in young and old mice. Am J Physiol Cell Physiol 258: C436–C442, 1990. [DOI] [PubMed] [Google Scholar]
- 12.Brooks SV, Faulkner JA. The magnitude of the initial injury induced by stretches of maximally activated muscle fibres of mice and rats increases in old age. J Physiol 497: 573–580, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canon F, Gamet D, Perot C. Passive stiffness of rat soleus muscle from weaning to senescence. Comput Methods Biomech Biomed Eng 11: 49–50, 2008. [Google Scholar]
- 14.Carroll CC, Dickinson JM, Haus JM, Lee GA, Hollon CJ, Aagaard P, Magnusson SP, Trappe TA. Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol 105: 1907–1915, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavagna GA, Legramandi MA, Peyre-Tartaruga LA. Old men running: mechanical work and elastic bounce. Proc Biol Sci 275: 411–418, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conley KE. Mitochondria to motion: optimizing oxidative phosphorylation to improve exercise performance. J Exp Biol 219: 243–249, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dean JC, Kuo AD. Energetic costs of producing muscle work and force in a cyclical human bouncing task. J Appl Physiol 110: 873–880, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diamant J, Keller A, Baer E, Litt M, Arridge R. Collagen: ultrastructure and its relation to mechanical properties as a function of ageing. Proc R Soc Lond B Biol Sci 180: 293–315, 1972. [DOI] [PubMed] [Google Scholar]
- 19.Farris DJ, Sawicki GS. The mechanics and energetics of human walking and running: a joint level perspective. J R Soc Interface 9: 110–118, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franz JR, Kram R. How does age affect leg muscle activity/coactivity during uphill and downhill walking? Gait Posture 37: 378–384, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Y, Kostrominova TY, Faulkner JA, Wineman AS. Age-related changes in the mechanical properties of the epimysium in skeletal muscles of rats. J Biomech 41: 465–469, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gosselin LE, Burton H. Impact of initial muscle length on force deficit following lengthening contractions in mammalian skeletal muscle. Muscle Nerve 25: 822–827, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Holt NC, Danos N, Roberts TJ, Azizi E. Stuck in gear: age-related loss of variable gearing in skeletal muscle. J Exp Biol 219: 998–1003, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holt NC, Wakeling JM, Biewener AA. The effect of fast and slow motor unit activation on whole-muscle mechanical performance: the size principle may not pose a mechanical paradox. Proc Biol Sci 281: 20140002, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horner AM, Russ DW, Biknevicius AR. Effects of early-stage aging on locomotor dynamics and hindlimb muscle force production in the rat. J Exp Biol 214: 3588–3595, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Hortobágyi T, Finch A, Solnik S, Rider P, DeVita P. Association between muscle activation and metabolic cost of walking in young and old adults. J Gerontol A Biol Sci Med Sci 66: 541–547, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hortobágyi T, Zheng D, Weidner M, Lambert NJ, Westbrook S, Houmard JA. The influence of aging on muscle strength and muscle fiber characteristics with special reference to eccentric strength. J Gerontol A Biol Sci Med Sci 50: B399–B406, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Huang TWP, Shorter KA, Adamczyk PG, Kuo AD. Mechanical and energetic consequences of reduced ankle plantar-flexion in human walking. J Exp Biol 218: 3541–3550, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson GA, Tramaglini DM, Levine RE, Ohno K, Choi NY, Woo SL. Tensile and viscoelastic properties of human patellar tendon. J Orthop Res 12: 796–803, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Karamanidis K, Arampatzis A. Mechanical and morphological properties of different muscle-tendon units in the lower extremity and running mechanics: effect of aging and physical activity. J Exp Biol 208: 3907–3923, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Kent-Braun JA, Ng AV, Young K. Skeletal muscle contractile and noncontractile components in young and older women and men. J Appl Physiol 88: 662–668, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Kragstrup TW, Kjaer M, Mackey AL. Structural, biochemical, cellular, and functional changes in skeletal muscle extracellular matrix with aging. Scand J Med Sci Sports 21: 749–757, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Kubo K, Kanehisa H, Miyatani M, Tachi M, Fukunaga T. Effect of low-load resistance training on the tendon properties in middle-aged and elderly women. Acta Physiol Scand 178: 25–32, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Lichtwark GA, Wilson AM. Effects of series elasticity and activation conditions on muscle power output and efficiency. J Exp Biol 208: 2845–2853, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Lichtwark GA, Wilson AM. In vivo mechanical properties of the human Achilles tendon during one-legged hopping. J Exp Biol 208: 4715–4725, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Lieber RL, Ward SR. Cellular mechanisms of tissue fibrosis: 4. structural and functional consequences of skeletal muscle fibrosis. Am J Physiol Cell Physiol 305: C241–C252, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magnusson SP, Hansen P, Aagaard P, Brond J, Dyhre-Poulsen P, Bojsen-Moller J, Kjaer M. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol Scand 177: 185–195, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Malatesta D, Simar D, Dauvilliers Y, Candau R, Ben Saad H, Prefaut C, Caillaud C. Aerobic determinants of the decline in preferred walking speed in healthy, active 65- and 80-year-olds. Pflugers Arch 447: 915–921, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Mian OS, Thom JM, Ardigò LP, Narici MV, Minetti AE. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiol (Oxf) 186: 127–139, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Nakagawa Y, Hayashi K, Yamamoto N, Nagashima K. Age-related changes in biomechanical properties of the Achilles tendon in rabbits. Eur J Appl Physiol Occup Physiol 73: 7–10, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull 95: 139–159, 2010. [DOI] [PubMed] [Google Scholar]
- 42.Narici MV, Maganaris CN, Reeves ND, Capodaglio P. Effect of aging on human muscle architecture. J Appl Physiol 95: 2229–2234, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Noyes FR, Grood ES. The strength of the anterior cruciate ligament in humans and Rhesus monkeys. J Bone Joint Surg Am 58: 1074–1082, 1976. [PubMed] [Google Scholar]
- 44.Onambele GL, Narici MV, Maganaris CN. Calf muscle-tendon properties and postural balance in old age. J Appl Physiol 100: 2048–2056, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Ortega JD, Farley CT. Minimizing center of mass vertical movement increases metabolic cost in walking. J Appl Physiol 99: 2099–2107, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Ortega JD, Farley CT. Effects of aging on mechanical efficiency and muscle activation during level and uphill walking. J Electromyogr Kinesiol 25: 193–198, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramaswamy KS, Palmer ML, van der Meulen JH, Renoux A, Kostrominova TY, Michele DE, Faulkner JA. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J Physiol 589: 1195–1208, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reddy GK, Stehno-Bittel L, Enwemeka CS. Glycation-induced matrix stability in the rabbit Achilles tendon. Arch Biochem Biophys 399: 174–180, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Roberts TJ. The integrated function of muscles and tendons during locomotion. Comp Biochem Physiol A Mol Integr Physiol 133: 1087–1099, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Robertson BD, Sawicki GS. Exploiting elasticity: modeling the influence of neural control on mechanics and energetics of ankle muscle-tendons during human hopping. J Theor Biol 353: 121–132, 2014. [DOI] [PubMed] [Google Scholar]
- 51.Robertson BD, Sawicki GS. Unconstrained muscle-tendon workloops indicate resonance tuning as a mechanism for elastic limb behavior during terrestrial locomotion. Proc Natl Acad Sci U S A 112: E5891–E5898, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sawicki GS, Robertson BD, Azizi E, Roberts TJ. Timing matters: tuning the mechanics of a muscle-tendon unit by adjusting stimulation phase during cyclic contractions. J Exp Biol 218, Part 19: 3150–3159, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schultz AB. Mobility impairment in the elderly: challenges for biomechanics research. J Biomech 25: 519–528, 1992. [DOI] [PubMed] [Google Scholar]
- 54.Shadwick RE. Elastic energy storage in tendons: mechanical differences related to function and age. J Appl Physiol (1985) 68: 1033–1040, 1990. [DOI] [PubMed] [Google Scholar]
- 55.Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65–89 years. Age Ageing 23: 371–377, 1994. [DOI] [PubMed] [Google Scholar]
- 56.Takeshita D, Shibayama A, Muraoka T, Muramatsu T, Nagano A, Fukunaga T, Fukashiro S. Resonance in the human medial gastrocnemius muscle during cyclic ankle bending exercise. J Appl Physiol 101: 111–118, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Thom JM, Morse CI, Birch KM, Narici MV. Influence of muscle architecture on the torque and power-velocity characteristics of young and elderly men. Eur J Appl Physiol 100: 613–619, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Thompson JI, Czernuszka JT. The effect of two types of cross-linking on some mechanical properties of collagen. Biomed Mater Eng 5: 37–48, 1995. [PubMed] [Google Scholar]
- 59.Vogel HG. Influence of maturation and aging on mechanical and biochemical properties of connective tissue in rats. Mech Ageing Dev 14: 283–292, 1980. [DOI] [PubMed] [Google Scholar]
- 60.Young A, Stokes M, Crowe M. The size and strength of the quadriceps muscles of old and young men. Clin Physiol 5: 145–154, 1985. [DOI] [PubMed] [Google Scholar]
- 61.Zajac FE. Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng 17: 359–411, 1989. [PubMed] [Google Scholar]