This study applied an engineering tool, computational fluid dynamics, to noninvasively estimate the pharyngeal air pressure field based on dynamic MR images acquired during relaxed wakeful tidal breathing. The slope of cross-sectional area vs. airway pressure was defined as the “effective compliance” and was often negative, which is attributed to strong activation of airway dilators during inspiration. Effective compliance in the nasopharynx was more negative in girls with obstructive sleep apnea syndrome and correlated with Pcrit.
Keywords: human, adolescent, airway compliance, dynamic MRI, rhinomanometry
Abstract
Obstructive sleep apnea syndrome (OSAS) is associated with anatomical abnormalities restricting upper airway size and functional factors decreasing pharyngeal dilator activity in sleep. In this study we hypothesized that OSAS is also associated with altered pharyngeal mechanical compliance during wakefulness. Five OSAS and six control obese girls between 14 and 18 years of age were studied. All underwent polysomnography, critical closing pressure (Pcrit) studies, and dynamic MRI of the upper airway during awake tidal breathing. Effective airway compliance was defined as the slope of cross-sectional area vs. average pressure between maximum inspiration and maximum expiration along the pharyngeal airway. Pharyngeal pressure fields were calculated by using image-based computational fluid dynamics and nasal resistance. Spearman correlations were calculated to test associations between apnea-hypopnea index (AHI), Pcrit, and airway compliance. Effective compliances in the nasopharynx (CNP) and velopharynx (CVP) were lower and negative in OSAS compared with controls: −4.4 vs. 1.9 (mm2/cmH2O, P = 0.012) and −2.1 vs. 3.9 (mm2/cmH2O, P = 0.021), respectively, suggesting a strong phasic pharyngeal dilator activity during inspiration in OSAS compared with controls. For all subjects, CNP and AHI correlated negatively (rS = −0.69, P = 0.02), and passive Pcrit correlated with CNP (rS = −0.76, P = 0.006) and with AHI (rS = 0.86, P = 0.0006). Pharyngeal mechanics obtained during wakefulness could be used to characterize subjects with OSAS. Moreover, negative effective compliance during wakefulness and its correlation to AHI and Pcrit suggest that phasic dilator activity of the upper pharynx compensates for negative pressure loads in these subjects.
NEW & NOTEWORTHY
This study applied an engineering tool, computational fluid dynamics, to noninvasively estimate the pharyngeal air pressure field based on dynamic MR images acquired during relaxed wakeful tidal breathing. The slope of cross-sectional area vs. airway pressure was defined as the “effective compliance” and was often negative, which is attributed to strong activation of airway dilators during inspiration. Effective compliance in the nasopharynx was more negative in girls with obstructive sleep apnea syndrome and correlated with Pcrit.
obese children are at a particular risk for developing obstructive sleep apnea syndrome (OSAS), with an estimated prevalence of 50% (1) compared with 2–4% in the general pediatric population (12). Moreover, compared with normal weight children, obese children respond poorly to treatment directed to affect the anatomy of the upper airway such as adenotonsillectomy (AT), with residual OSAS exceeding 50% (4). Such discrepancy in prevalence and response to treatment is not well understood and may be related to functional mechanisms promoting OSAS in these children.
Some insight into such functional attributes of the upper airway in obese children is provided by a recent study by Huang et al. (9) of obese children with OSAS [apnea-hypopnea index (AHI) > 5 events/h] and obese or lean controls without OSAS (AHI < 1.5 events/h). The investigators examined the critical airway collapse pressure in sleep measured by the critical closing pressure (Pcrit) passive protocol (airway stability measured under brief transient drops in nasal pressure, which maintains the airway in a relatively passive state). Passive Pcrit was higher in both obese groups. But the activated protocol Pcrit (airway stability under gradually decreased nasal pressure, allowing an active response to low pressure) was higher only in the obese group with OSAS. These results suggest that the airway is weaker in many obese children, but that some obese children are protected from OSAS by a more robust neuromuscular response during sleep. However, such functional data related to airway collapsibility do not explain the temporal or specific anatomical location(s) leading to obstruction and therefore have limited therapeutic value.
Computational fluid dynamics (CFD) modeling based on MRI or CT (19, 22, 24–26) has been validated by several groups and shown to be a robust tool to understand the biomechanical properties of the airway in children with OSAS. Our team recently introduced CFD based on static wake MRI images to characterize the upper airway biomechanical properties of obese children with and without OSAS (24). This study demonstrated that obese children with OSAS have larger pressure drop in the pharynx where the soft palate, tonsils, and adenoids maximally restrict the airway, compared with obese controls, and the pressure drop correlates with OSAS severity. These results suggest that increased pressure loading due to anatomical restriction of the airway may play a role in destabilizing the airway in sleep. A second study used MR image-based CFD to compare pressure drop before and after AT surgery (13); reduced pressure drop correlated strongly with improvements in apnea severity, reinforcing the first study's results and suggesting that CFD may be useful in planning adenotonsillectomy surgery. Both studies had some outliers, which may be due to structural differences reflected in airway mechanical properties, but the static MRI protocols used in these studies cannot resolve airway tissue mechanical properties.
Dynamic MR imaging combined with CFD modeling could potentially advance the field by demonstrating both structural and functional data about the pharynx that relates to OSAS. Wagshul et al. (23) developed a novel retrospective respiratory-gated MRI protocol that acquires data from a series of normal tidal breaths to assemble a series of dynamic images of the airway over the respiratory cycle. In several relaxed, awake subjects, the entire pharynx appeared to expand during inspiration and contract during expiration as one coordinated structure in subjects without OSAS. But in one subject with severe OSAS the airway above the maximum restriction expanded during inspiration, similar to controls, but the airway below the maximum restriction was out of phase and expanded during expiration, suggesting structural or functional differences that may be tied to apnea. Persak et al. (20) have used CFD to estimate the pressure field in the deforming upper airway at different phases of inspiration, to quantify the airway cross-sectional area vs. internal pressure. The “effective compliance,” the slope of the area-pressure curve, can be calculated at any cross section based on the CFD models, and was higher in young children with OSAS during sedative-induced sleep.
In this study, CFD modeling derived from dynamic MRI of the upper airway was used to quantify differences in local airway area-pressure relationships in awake subjects. We hypothesize that biomechanical properties of the upper airway in wakefulness are associated with functional data regarding airway collapsibility in sleep and severity of OSAS. Thus the main goal of this study is to establish a relationship between upper airway compliance during wake tidal breathing derived from CFD and dynamic MRI, upper airway collapsibility (Pcrit) measured during sleep, and clinical severity of OSAS measured by AHI.
METHODS
Subjects.
The study was approved by the Albert Einstein College of Medicine Internal Review Board (Protocol #: 2010-207). Subjects or the parents of minors gave written, informed consent at enrollment; all minor subjects gave assent to the study. Subjects were girls, age 14 to 18, recruited from endocrine and obesity clinics at the Children's Hospital at Montefiore, and were part of a larger study of polycystic ovarian syndrome (PCOS), obesity, and OSAS. The current study includes girls both with and without PCOS.
Overnight polysomnography was performed (Xltek, Oakville, Canada) in the Sleep Center at Children's Hospital At Montefiore. Subjects slept between 6 and 8 h under natural conditions. Sleep was staged by standard criteria (10), and respiratory events were scored by pediatric standards (2, 10). Hypopnea was defined as 30% decrease in amplitude of nasal pressure transducer associated with a fall in 3% or more of basal SpO2 or with an arousal. The major metrics of OSAS were apnea index (AI), AHI, and SpO2 nadir. Abnormal values included AI of >1 episodes/h and/or AHI >5 episodes/h, and or SpO2 nadir <90%.
Pcrit.
Critical airway closing pressures were measured by both passive and activated Pcrit protocols as described by Marcus et al. (9, 14) with a Pcrit device (modified Respironics BiPAP Harmony S/T device, Respironics, Monroeville, PA) during a second overnight sleep study.
Upper airway MRI.
Respiratory-gated dynamic MRI of the upper airway was performed as described previously (23), producing 3D volume images 18 × 24 × 4 cm (anterior-posterior × rostral-caudal × lateral-medial, isotropic 1.1 mm voxels) of the airway and surrounding tissues at 10 equal phase increments of the respiratory cycle.
Effective compliance calculations.
To compute local biomechanics of the airway, CFD was used to compute the air pressure field as described below. At several representative locations along the airway length (see Fig. 2), airway cross-sectional area was plotted against average air pressure at each phase of respiration. The slope of the area-pressure curve from peak inspiratory flow to peak expiratory flow was defined as the effective compliance. For a relatively passive airway, the compliance is positive; increasing internal pressure passively causes the airway to expand. For a very active airway, the airway may even expand when pressure is decreasing, and the effective compliance will be negative. Therefore the sign of the effective compliance indicates the strength of dilator activity of discrete regions of the pharynx. The effective compliance at each monitored cross section, Ci, was calculated as the slope of the pressure-area curve between the point of maximum inspiratory and expiratory flows:
| (1) |
where i is the cross-sectional location, and pi and Ai, are the area-averaged pressure and cross-sectional area at location i during the corresponding phase increment. Ci was calculated at the nasopharynx, velopharynx, oropharynx behind the tongue, and hypopharynx (see Fig. 2).
Fig. 2.
Sample CFD model results for a subject with severe OSAS. Pharynx anatomy with pressure contours at peak inspiration phase (phase = 3, top left) and peak expiration phase (phase = 8, top right); note larger cross-sectional area during inspiration in nasopharynx (NP), velopharynx (VP), and hypopharynx (HP). Only retrolingual oropharynx (OP) cross section is smaller during inspiration. Bottom left: cross-sectional areas and flow rate vs. phase increment. Bottom right: area-pressure curves for each subject. Effective compliance is defined as the pressure-area slope of the section, between maximum inspiratory and maximum expiratory flow (lines). Sections NP, VP, and HP have negative effective compliance; the airway expands the airway during inspiration, while OP has positive slope, indicating net passive response to airway pressure loads.
Patient-specific CFD.
CFD methods were based on a previously described noninvasive image-based method for estimating pressure and local compliance in the pharyngeal airway (20), but with the novel imaging protocol which provided increased MRI-based axial resolution, and with patient-specific nasal resistances. Patient-specific modeling boundary conditions were determined by first measuring resting nasal tidal breathing waveforms with a pneumotachometer (RSS 100HR Research, Hans Rudolph). Then nasal flow-resistance curves were recorded (3 times for each nasal passage) with an anterior rhinomanometer (NR-6, GM Instruments, Kilwinning, United Kingdom) with 150 Pa threshold as previously described (21). Eight Rohrer coefficients were extracted to characterize linear (viscous) and second-order (acceleration) pressure-flow characteristics of each nasal passage during inspiration and expiration. Dynamic 3D MR images of the deforming pharyngeal airway at 10 phase increments of the respiratory cycle were acquired by a retrospective respiratory-gating method (23). Subjects were supine, in a relaxed wakeful state during physiological and imaging studies, and data were acquired only during normal tidal breaths as determined by the period and amplitudes of the flow waveform collected from a nasal cannula.
The modeling scheme is outlined in Fig. 1. Velocity, pressure, and turbulence statistics (kinetic energy k and specific dissipation rate ω) were calculated in the pharyngeal airway at up to 10 respiratory phase increments by using CFD. For each phase modeled, the pharyngeal airway 3D volume was segmented from MR images acquired during that phase with commercial image-based modeling software (Materialize Mimics). The airway volume was edited and meshed with commercial modeling software (Materialize 3matics and ANSYS Gambit). For inspiratory phases, a 10+ diameter extension from the tracheal end of the model domain was added to avoid boundary artifacts due to backflow into the outlet and to accelerate model convergence; similarly, for expiratory phases, 10+ diameter nasal extensions to the choanae were created. The flow and turbulence fields were computed in commercial finite volume CFD software (ANSYS Fluent) by using an unsteady low-Reynolds number k-ω model, as previously described (13, 20, 24, 25). Inspiratory velocity inlet boundary conditions at the choanae and expiratory velocity boundary conditions at the tracheal extension were set to uniform normal velocity at each inlet based on the phase average flow rate, with 10% turbulent kinetic energy and a 1-cm dissipation length scale. For inspiratory phases, an outflow boundary was applied at the tracheal extension, while for expiratory phases, velocity inlets with uniform outgoing velocity were set with the right/left flow split predicted by the nasal resistances. For both inspiratory and expiratory phases, average pressure was set at the choanae by using the flow rate, cross-sectional areas, and the parallel nasal resistances of the nasal passages. The unsteady flow field was solved to convergence as judged by normalized residuals <10−6 for a period of 100 ms.
Fig. 1.
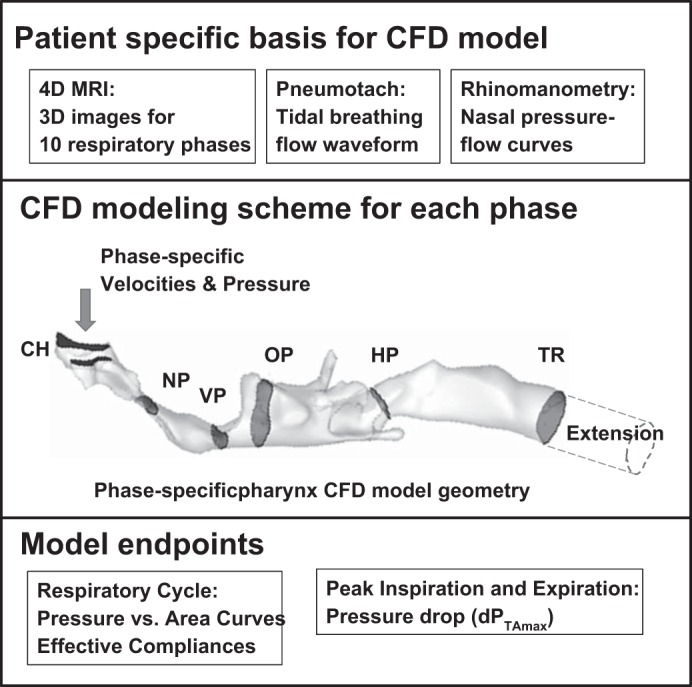
CFD modeling scheme. The CFD model is based on dynamic MR image sets representing airway anatomy at 10 equal phase (time) increments during tidal breaths. Average flow waveforms and nasal passage pressure-flow curves are obtained to set flow and pressure boundary conditions at the choanae. A separate CFD model is created for each phase increment modeled and used to compute airway pressure, velocity, and turbulence fields. Model endpoints are extracted from the CFD model pressure data.
Area-averaged pressure was computed at representative cross sections in the nasopharynx (at the level of the hard palate), velopharynx (maximum restriction posterior to the soft palate and between the tonsils), retrolingual oropharynx (behind the tongue and below the level of the soft palate), and hypopharynx (below the level of the epiglottis), as well as the entry to the trachea. Area-pressure curves during tidal breathing were created by plotting cross-sectional area vs. pressure for all phases modeled.
CFD endpoints were generated by models of the phases corresponding to maximum inspiratory flow and maximum expiratory flow. The inspiratory pressure drop from choanae to maximum overlap cross section was defined as dPTAmax, and used to characterize the anatomical flow resistance in the pharynx (24). The minimum airway wall pressure in the overlap region was defined as Pmin (24) and was used to characterize the pressure loading due to airway anatomy restriction. Effective compliance was calculated with Eq. 1.
Statistical analysis.
Subjects were classified by polysomnography results into OSAS (AHI > 5 or AI > 1 and oxygen saturation nadir < 90%) and control groups. Mean values of clinical and CFD model endpoints were compared by using Student's t-test for normally distributed statistics (Microsoft Excel), or median values were compared by using Wilcoxon rank sum test for apparently nonnormal statistics (Matlab). Spearman rank correlations were computed between CFD model outcomes, AHI, and Pcrit for all subjects (Matlab).
RESULTS
We have studied 11 female subjects, 5 with OSAS (4 with PCOS) and 6 nonOSAS controls (2 with PCOS). Demographics, polysomnography, and Pcrit results are summarized in Table 1. All subjects were obese, with body mass index (BMI) ranging from 29.6 to 55 kg/m2, and BMI was similar in OSAS and controls (and PCOS vs. non-PCOS). In the OSAS group, four subjects had severe OSAS, with AHI ranging from 18 to 38 events/h, and one subject had mild-moderate OSAS (AHI = 4.6, SpO2 nadir 83%). Respiratory events in all subjects were primarily hypopneas, with AI <1 in all but one subject. There was a trend toward higher passive Pcrit in OSAS that did not reach statistical significance, and activated Pcrit was not significantly different. There was also a trend toward higher AHI for subjects with PCOS that did not reach statistical significance (P = 0.052); other differences between PCOS and non-PCOS groups were less significant (data not shown).
Table 1.
Demographics, PSG, and Pcrit
| OSAS | Control | P | |
|---|---|---|---|
| N | 5 | 6 | |
| Age, yr | 15.6 ± 1.1 | 17.0 ± 1.5 | NS |
| BMI, kg/m2 | 42.1 ± 9.4 | 42.8 ± 5.9 | NS |
| AHI, events/h | 20.9 ± 12.2 | 4.3 ± 1.4 | 0.009 |
| AI, events/h | 2.4 ± 4.2 | 0.2 ± 0.4 | NS |
| HI, events/h | 18.1 ± 9.1 | 3.8 ± 1.0 | 0.004 |
| Central apnea index, events/h | 0.4 ± 0.4 | 0.3 ± 0.3 | NS |
| SpO2 nadir, % | 84 ± 6 | 93 ± 3 | 0.02 |
| Pcrit (passive), cm H2O | −2 ± 3 | −10 ± 8 | 0.052 |
| Pcrit (activated), cm H2O | −10 ± 6 | −13 ± 12 | NS |
Values are means ± SD. NS, not significant.
CFD modeling and outcomes.
We first describe our results in a sample subject, and then give statistical results. CFD model results from one subject with severe OSAS are shown in Fig. 2. The pharyngeal airway cross section was narrowed in the nasopharynx (NP) and reached a minimum near the velopharynx (VP) because of the overlap of adenoids, tonsils, and soft palate/uvula in this region. The retrolingual oropharynx (OP) typically had the largest cross-sectional area. During inspiration, the pressure drops in the nasopharynx and reaches a local minimum near the velopharynx, before recovering slightly behind the tongue. Pressure falls again in the narrower hypopharynx (HP). The pressure drop in the nasal passages was larger than in the pharynx. During expiration, the tongue, posterior soft palate, and lateral walls were pushed apart in the retrolingual oropharynx. Pressure dropped rapidly to a local minimum around the velopharynx, recovering slightly in the nasopharynx. The area waveforms of the NP, VP, and HP sections were different from the OP section: the OP was larger during expiration and smaller during inspiration, and apparently passively followed the pressure waveform. The other cross sections were larger during inspiration, actively opposing the pressure loads in the airway. This qualitative difference is also evident in cross-sectional area vs. average local pressure plots for each cross section. For section OP the slope is positive, giving a compliance of COP = 4.9 mm2/cmH2O, while the pressure-area slopes for the other cross sections were negative, with slopes from −5 to −9 mm2/cmH2O.
For the group comparisons, effective compliances of the nasopharynx and velopharynx tended to be negative in subjects with OSAS, and were significantly lower in OSAS compared with controls (Table 2). Compliances in the lower pharynx (hypopharynx and oropharynx behind the tongue) were not significantly different. Inspiratory pressure drop through the maximum airway restriction (dPTAmax) trended higher in OSAS but did not reach statistical significance. Expiratory dPTAmax and minimum airway pressures were not significantly different.
Table 2.
CFD outcomes: Pmin, dPTAmax, and effective compliances
| OSAS | Control | P | |
|---|---|---|---|
| N | 6 | 5 | |
| Pmin (inspiration), Pa | −173 ± 85 | −130 ± 86 | NS |
| Pmin (expiration), Pa | 181.2 ± 116.5 | 182.6 ± 164.1 | NS |
| dPTAmax (inspiration), Pa | −74.4 ± 80.7 | −13.3 ± 16.1 | 0.052 |
| dPTAmax (expiration), Pa | 38.2 ± 91.9 | 1.0 ± 3.2 | NS |
| CNP, mm2/cmH2O | −4.4 ± 3.6 | 1.9 ± 3.1 | 0.012 |
| CVP, mm2/cmH2O | −2.1 ± 2.4 | 3.9 ± 4.2 | 0.021 |
| COP, mm2/cmH2O | −1.1 ± 6.9 | 5.2 ± 5.6 | NS |
| CHP, mm2/cmH2O | −2.7 ± 4.2 | 0.1 ± 4.7 | NS |
Values are means ± SD.
Nasopharynx effective compliance (CNP) was significantly negatively correlated to AHI, as well as hypopnea index (Table 3). Other airway compliances were not significantly correlated to AHI, although velopharynx compliance correlated significantly to CNP (Table 3). Passive Pcrit was strongly correlated to AHI (Fig. 3), and CNP and passive Pcrit had a significant negative correlation (Fig. 4).
Table 3.
Correlates of effective compliances, Pcrit, and AHI
| R | P | |
|---|---|---|
| Pcrit passive vs. AHI | 0.86 | 0.0006 |
| CNP vs. AHI | −0.69 | 0.02 |
| CNP vs. Pcrit passive | −0.76 | 0.006 |
| CVP vs. AHI | −0.49 | 0.13 |
| COP vs. AHI | −0.32 | 0.34 |
| CHP vs. AHI | −0.45 | 0.17 |
| CVP vs. CNP | 0.92 | 0.0001 |
Spearman correlation coefficients and (unadjusted) P values.
Fig. 3.
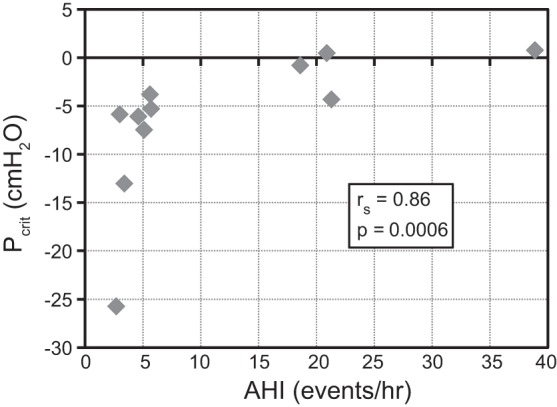
Passive Pcrit vs. AHI. AHI increases with increasing Pcrit.
Fig. 4.
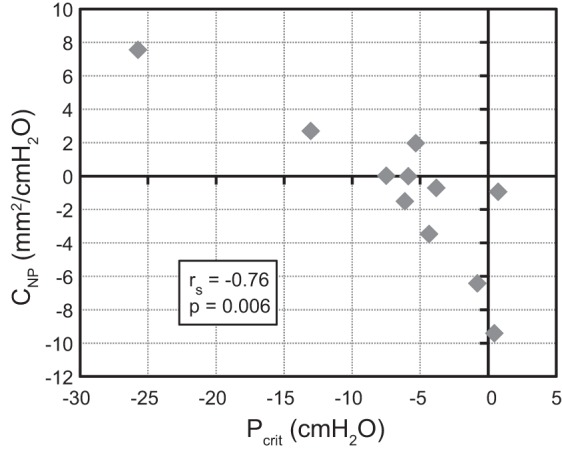
Passive Pcrit vs. CNP. Low critical pressure in the passive airway during sleep (less collapsible airway) correlated with positive and higher nasopharynx compliance (more passive mechanical compliance) when awake.
DISCUSSION
The primary mechanisms causing OSAS are not well understood in part because the pharynx has a complex shape and size controlled by several muscle groups, and muscle activity varies with sleep state and within each breath and phase of respiration. Passive Pcrit measures passive airway biomechanics in sleep, and activated Pcrit measures airway responsiveness during sleep, but both protocols have a disadvantage in that the locations of flow limitation and airway expansion are not known. Dynamic MRI can quantify airway shape and motion, but lacks biomechanical context because airway loads are unknown. Image-based CFD is a modeling tool that can generate a wealth of data about the airway flow field that may provide important insight regarding the pathophysiology of OSAS. In this study, CFD was used to noninvasively estimate local air pressure, which was used to calculate the effective compliance, at several locations along the pharynx. We found that effective compliance in the nasopharynx correlated with polysomnography data and passive Pcrit.
In many of the subjects studied, effective compliances at several locations were negative, i.e., the airway was larger during inspiration, in spite of negative pressure loads that tend to reduce the airway size. Negative effective compliance may be evidence of strong phasic activation of pharyngeal dilators, overcompensating for negative pressure loads during inspiration when subjects are awake. A reflex response to negative pharyngeal pressure has been demonstrated by genioglossal electromyogram (EMGGG) measurements, and this reflex is inhibited by topical upper airway anesthesia (5, 7, 8). EMGGG during awake tidal breathing is higher in children (11) and adults (18) with OSAS compared with controls. Acoustic pharyngometry measurements before and after topical upper airway anesthesia suggest that airway dilator activation due to negative pressure reflex is much stronger in children with OSAS than in controls (6). These studies are consistent with our observations of more negative effective compliance, i.e., a more active airway, in awake subjects with OSAS.
Passive Pcrit and AHI were positively correlated, which is consistent with previous studies measuring higher Pcrit in children with OSAS (15, 16). The activated Pcrit did not correlate significantly with AHI, in contrast to a recent larger study (9), but this may be due to the small sample size in the current study or differences between girls with PCOS and the general obese pediatric population. Passive Pcrit is conceptually distinct from airway compliance; Pcrit estimates the collapse pressure of the upper airway during non-rapid eye movement sleep, by using brief negative pressure challenges while otherwise maintaining the airway in a relatively passive state by using CPAP. A high or positive Pcrit indicates a more collapsible airway, and a more negative Pcrit indicates an airway that can withstand lower air pressures without collapsing. In contrast, CNP (awake) estimates sensitivity of nasopharynx airway cross section to internal pressure changes, measured during awake tidal breathing. Given the distinct differences between these endpoints, a strong correlation between CNP and Pcrit was not entirely expected, and suggests that in many subjects a more active airway was required to maintain the patency of a weaker, more collapsible airway. Strong phasic activation may overcompensate for the pressure loads since the airway expands on inspiration.
The nasopharynx was the only airway segment with significant correlation between compliance and AHI in this study, although effective compliance of the velopharynx (CVP) was correlated to CNP and was also significantly lower in OSAS. Our results suggest that the nasopharynx is more critical because it narrows the airway leading into the velopharynx, setting up the low local airway pressure and high pressure drop upstream of the apparently more passive oropharynx. An alternative explanation is that in children with OSAS, pharyngeal dilators are less effective in controlling the airway cross section at the velopharynx, compared with the nasopharynx, leading to more expansion at the nasopharynx.
The correlations between CNP, Pcrit, and AHI suggest the possibility of using effective compliance to improve treatment selection for obese patients. In a previous study of the effects of adenotonsillectomy surgery, postop changes in CFD pharyngeal pressure drop based on static MRI anatomy correlated well to improvement in AHI in patients that improved after surgery, but did not identify the patients who did not benefit from surgery (13). In the current study, effective compliances did not correlate strongly to pharyngeal pressure drop, which raises the possibility that effective compliance is independent from pressure drop and might help to identify patients who would not benefit from adenotonsillectomy; we are planning a longitudinal study to evaluate this hypothesis. CNP may also be a noninvasive surrogate for passive Pcrit. CNP in its current form is limited to basic research because it requires dynamic imaging and CFD modeling. But in the near future effective compliance might be measured with a much less expensive and faster optical coherence tomography system (17). CNP has several advantages over current diagnostic parameters. It can be performed either awake or simultaneously with sleep recordings, and in its current form is noninvasive. It is also performed without manipulating nasal pressure, so it simulates normal tidal breathing more than the response to a negative pressure challenge used in a Pcrit study. CNP might be related to clinical measurements of airway collapsibility by using negative expiratory pressure; the relative increase in expiratory flow with 5 cmH2O negative expiratory pressure during quiet wakeful breathing is inversely correlated to the pressure-flow slope in Pcrit tests of adolescent subjects (3).
Our finding of negative effective compliance in OSAS contrasts with our earlier pilot study (20) that was based on images of sedated children. In that study, effective compliance was positive and a higher magnitude in OSAS, consistent with sedation causing a relatively passive airway that did not maintain the airway cross section during tidal breathing from atmospheric pressure. Future studies should also estimate effective compliance in sleeping subjects. Changes in effective compliance with sleep may prove useful for identifying different phenotypes of OSAS, for example, helping to quantify and localize functional abnormalities in subjects with relatively unrestricted upper airways.
There are several limitations to the current study. The sample size was small, so some endpoints with lesser significance may not have been identified. We did not measure airway muscle activation with EMG, and we did not obtain images of the airway during sleep. The CFD model assumes the airway tissues are stationary during each phase increment and does not model airway collapse. Dynamic MRI are obtained over multiple tidal breaths. Abnormal breaths are discarded during imaging, and subjects with image sets that contained artifacts due to change in posture were excluded, but the method averages the airway shape during each phase and might introduce subtle imaging artifacts. We used a two-equation turbulence model for the CFD calculations. This method is less accurate than much more expensive direct numerical simulations of turbulence, but it has been experimentally verified for modeling the wall pressure fields in the upper airway by us and others (19, 24–26), with sufficient accuracy for the purposes of the present study (19). Finally, the image-based CFD model domain is limited to the pharynx with very short sections of the nasal passage and trachea, and with uniform velocity inlets rather than the more rounded developing velocity profiles that could be computed with anatomical nasal passages and thoracic airways. However, we have compared our method with those using more developed entering velocity profiles in a sample subject, and observed minimal effects on pressure drops (<2%) and effective compliances (<0.4%), and we believe that we would reach the same results with a larger model domain.
In conclusion, we report the first application of dynamic image-based CFD and rhinomanometry to estimate effective compliance of four segments of the pharyngeal airway in awake obese adolescent children with and without OSAS. Effective compliance in the nasopharynx during wakefulness was significantly associated with AHI and significantly lower in subjects with OSAS. Passive critical closing pressure was also significantly correlated to AHI as well as nasopharynx effective compliance. These results suggest that stronger activation of nasopharyngeal dilators in awake subjects with OSAS overcompensates for weaker passive airway properties that are observed during sleep. The significant correlations between CNP, Pcrit, and AHI support further study of effective compliance in awake subjects as a diagnostic tool in OSAS.
GRANTS
Support for this study was provided by National Heart, Lung, and Blood Institute Grant 5 R01 HL-105212, National Science Foundation Grant 959915, and the C.V. Starr Research Foundation at The Cooper Union.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.M.W., J.M.M., M.E.W., C.R.I., and R.A. conception and design of research; D.M.W., S.S., H.L., A.Y., and M.E.W. performed experiments; D.M.W., S.S., H.L., A.Y., and M.E.W. analyzed data; D.M.W., S.S., H.L., A.Y., J.M.M., M.E.W., C.R.I., and R.A. interpreted results of experiments; D.M.W., H.L., and A.Y. prepared figures; D.M.W. drafted manuscript; D.M.W., S.S., H.L., J.M.M., M.E.W., C.R.I., and R.A. edited and revised manuscript; D.M.W., S.S., H.L., A.Y., J.M.M., M.E.W., C.R.I., and R.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Alan Schwartz, MD, Jason Kirkness, PhD, and Philips Respironics.
REFERENCES
- 1.Arens R, Muzumdar H. Childhood obesity and obstructive sleep apnea syndrome. J Appl Physiol 108: 436–444, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 8: 597–619, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrera HL, Marcus CL, McDonough JM, Morera JC, Huang J, Farre R, Montserrat JM. Negative expiratory pressure technique: an awake test to measure upper airway collapsibility in adolescents. Sleep 38: 1783–1791, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa DJ, Mitchell R. Adenotonsillectomy for obstructive sleep apnea in obese children: a meta-analysis. Otolaryngol Head and Neck Surg 140: 455–460, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Fogel RB, Malhotra A, Shea SA, Edwards JK, White DP. Reduced genioglossal activity with upper airway anesthesia in awake patients with OSA. J Appl Physiol 88: 1346–1354, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Gozal D, Burnside MM. Increased upper airway collapsibility in children with obstructive sleep apnea during wakefulness. Am J Respir Crit Care Med 169: 163–167, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Horner RL, Innes JA, Holden HB, Guz A. Afferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesia. J Physiol 436: 31–44, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol 436: 15–29, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Pinto SJ, Yuan H, Katz ES, Karamessinis LR, Bradford RM, Gallagher PR, Hannigan JT, Nixon T, Ward MB, Lee YN, Marcus CL. Upper airway collapsibility and genioglossus activity in adolescents during sleep. Sleep 35: 1345–1352, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine, 2007. [Google Scholar]
- 11.Katz ES, White DP. Genioglossus activity in children with obstructive sleep apnea during wakefulness and sleep onset. Am J Respir Crit Care Med 168: 664–670, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 5: 242–252, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo H, Sin S, McDonough JM, Isasi CR, Arens R, Wootton DM. Computational fluid dynamics endpoints for assessment of adenotonsillectomy outcome in obese children with obstructive sleep apnea syndrome. J Biomech 47: 2498–2503, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcus CL, Fernandes Do Prado LB, Lutz J, Katz ES, Black CA, Galster P Carson KA. Developmental changes in upper airway dynamics. J Appl Physiol 97: 98–108, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Marcus CL, Katz ES, Lutz J, Black CA, Galster P, Carson KA. Upper airway dynamic responses in children with the obstructive sleep apnea syndrome. Pediatr Res 57: 99–107, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Marcus CL, McColley SA, Carroll JL, Loughlin GM, Smith PL, Schwartz AR. Upper airway collapsibility in children with obstructive sleep apnea syndrome. J Appl Physiol 77: 918–924, 1994. [DOI] [PubMed] [Google Scholar]
- 17.McLaughlin RA, Noble PB, Sampson DD. Optical coherence tomography in respiratory science and medicine: from airways to alveoli. Physiology 29: 369–380, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J Clin Invest 89: 1571–1579, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mylavarapu G, Murugappan S, Mihaescu M, Kalra M, Khosla S, Gutmark E. Validation of computational fluid dynamics methodology used for human upper airway flow simulations. J Biomech 42: 1553–1559, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Persak SC, Sin S, McDonough JM, Arens R, Wootton DM. Noninvasive estimation of pharyngeal airway resistance and compliance in children based on volume-gated dynamic MRI and computational fluid dynamics. J Appl Physiol 111: 1819–1827, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sin S, Wootton DM, McDonough JM, Nandalike K, Arens R. Anterior nasal resistance in obese children with obstructive sleep apnea syndrome. Laryngoscope 124: 2640–2644, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vos W, De Backer J, Devolder A, Vanderveken O, Verhulst S, Salgado R, Germonpre P, Partoens B, Wuyts F, Parizel P, De Backer W. Correlation between severity of sleep apnea and upper airway morphology based on advanced anatomical and functional imaging. J Biomech 40: 2207–2213, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Wagshul ME, Sin S, Lipton ML, Shifteh K, Arens R. Novel retrospective, respiratory-gating method enables 3D, high resolution, dynamic imaging of the upper airway during tidal breathing. Magn Reson Med 70: 1580–1590, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wootton DM, Luo H, Persak SC, Sin S, McDonough JM, Isasi CR, Arens R. Computational fluid dynamics endpoints to characterize obstructive sleep apnea syndrome in children. J Appl Physiol 116: 104–112, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu C, Sin S, McDonough JM, Udupa JK, Guez A, Arens R, Wootton DM. Computational fluid dynamics modeling of the upper airway of children with obstructive sleep apnea syndrome in steady flow. J Biomech 39: 2043–2054, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Zhao M, Barber T, Cistulli P, Sutherland K, Rosengarten G. Computational fluid dynamics for the assessment of upper airway response to oral appliance treatment in obstructive sleep apnea. J Biomech 46: 142–150, 2013. [DOI] [PubMed] [Google Scholar]



