Supplemental digital content is available in the text.
Abstract
Background
The poor prognosis classically associated with Banff grade 2 acute cell-mediated rejection (CMR) may be due to unrecognized antibody-mediated damage. We thus performed a systematic review of the literature to determine the rate of response to treatment in kidney transplant recipients with pure CMR, stratified by Banff class.
Methods
In addition to a manual search, databases interrogated included Excerpta Medica Database (EMBASE), Medical Literature Analysis and Retrieval System Online (MEDLINE), Evidence-Based Medicine (EBM) databases, Central, PubMed and CINAHL. Studies providing functional and/or histological response rates to the treatment of CMR rejection by Banff class (1997 or more recent) were included.
Results
Among the 746 articles identified, 5 articles were included in the final review. Two studies excluded some, and 2 excluded all features of antibody-mediated rejection, while providing data on functional recovery. The absence of functional recovery was reported in 4% of borderline, 15% for Banff grade 1A and IB pooled, 0% to 25% of Banff grade 1B alone, 11% to 20% of Banff grade 2A, and 38% of Banff grade 2B rejections.
Conclusions
The rate of functional recovery of pure Banff IIA CMR overlapped with that of Banff grade 1 CMR, whereas Banff grade 2B showed worse prognosis. There was important heterogeneity in the definition of response to treatment and paucity of data describing the histological response to treatment stratified by Banff class. There is a pressing need to standardize outcome metrics for the reversibility of rejection in kidney transplant recipients in order to design high-quality trials for novel therapeutic alternatives.
Given the improvements in immunosuppressive protocols over the past decades, the rate of acute rejection has decreased to approximately 15% to 20% in kidney transplant recipients.1 Yet, rejection remains an important cause of death-censored graft loss.2 There is wide heterogeneity in the severity of rejection episodes and their response to treatment. The functional response to therapy, or the restoration of kidney function to prerejection levels, is a strong determinant of graft survival.3-5 A lower rate of histological response to therapy on control biopsies has been linked to recurrent rejections.6 Hence, although their correlation seems to be poor,6 both functional and histological responses to therapy are important outcomes to assess in transplant recipients with acute rejection.
Cell-mediated rejections (CMR) involving the graft vasculature (Banff grades 2 and 3) have classically been associated with poorer response to steroid treatment7 and inferior graft survival.8 The functional reversibility of CMRs upon treatment is also reportedly lower in the presence of vascular involvement.9 However, recent data suggest that the adverse impact of vascular involvement on graft survival is most important in the presence of antibody-mediated damage.10 Features of antibody-mediated rejection (AMR) often coexist with Banff grade 2 and 3 CMRs.11 In eras where C4d staining and donor-specific antibodies (DSA) were not systematically assessed, episodes of mixed AMR and CMR may have been misclassified as pure CMR, leading to inappropriate therapy and adverse outcomes.10 Furthermore, rejection outcomes may be different in the era of more potent, modern maintenance immunosuppressive regimen. There is thus uncertainty as to the rate of response that should be expected in patients with CMR episodes in the absence of features of antibody-mediated damage.
We performed a systematic review of the literature to determine the rate of functional and/or histological response to treatment in clinical, pure CMR based on the most recent versions of the Banff classification system (1997 and above) which provide elements to distinguish cell-mediated and antibody-mediated features.
MATERIALS AND METHODS
Study Eligibility
We performed a systematic review of the literature to identify original studies which describe therapy and outcomes of pure acute CMR in kidney transplant patients. Studies providing the response rate to the treatment of clinical rejection episodes in terms of function or histology by Banff class in kidney transplant recipients were included. We excluded studies that were not published in French or English, those reporting on ABO-incompatible transplantations or on other solid organ transplants, studies whose sole focus was AMR, case reports, studies reporting on pediatric populations, and those using a Banff version earlier than 1997, as the distinction between CMR and AMR was not expected to be made explicitly at that time.
Data Sources and Search Strategy
A search strategy (Table S1, SDC, http://links.lww.com/TXD/A30) was developed by a medical librarian and run up to October 28, 2015. Databases interrogated included EMBASE, MEDLINE, EBM, Central, PubMed and CINAHL (EBSCO). To complement the electronic search, the reference list of selected reviews on acute kidney graft rejection (from the Cochrane library and others) was also checked manually. All citations were downloaded on the EndNote reference manager system.
Study Selection and Data Collection
After the exclusion of duplicates, a first screening was performed by 2 independent investigators (C.L., J.M.C.) by reading the titles and/or the abstracts of selected articles. After this first step, the 2 independent investigators read the full text of selected articles and extracted the data on case report forms. Articles were then excluded if they filled 1 of the exclusion criteria above, or if there was no sufficient information provided to extract the data to answer the study question. Articles were also excluded if on careful examination, they were found to report outcomes for the same patients as another publication. In that case, the most recent report was chosen. Final data extraction was performed by 2 other investigators (H.C., L.S.) based on the case report forms filled by the first 2 investigators and the articles. The corresponding authors of selected papers were contacted through emails to gather missing information.
Data Items
The following characteristics were collected from each study: authors, journal and year of publication, study design, study period, study sample size, treatment protocols, version of Banff classification system used, explicit exclusion of AMR, definition of treatment response that was used, and response to treatment by rejection class in each study. When possible, we extracted data for patients who specifically had no features suggestive of antibody-mediated damage.
RESULTS
We identified 746 unique citations through our search strategy. After the exclusion of irrelevant citations by title and abstract review, 342 articles were selected. After the full text review, 5 articles provided data for this review (Figure 1).
FIGURE 1.
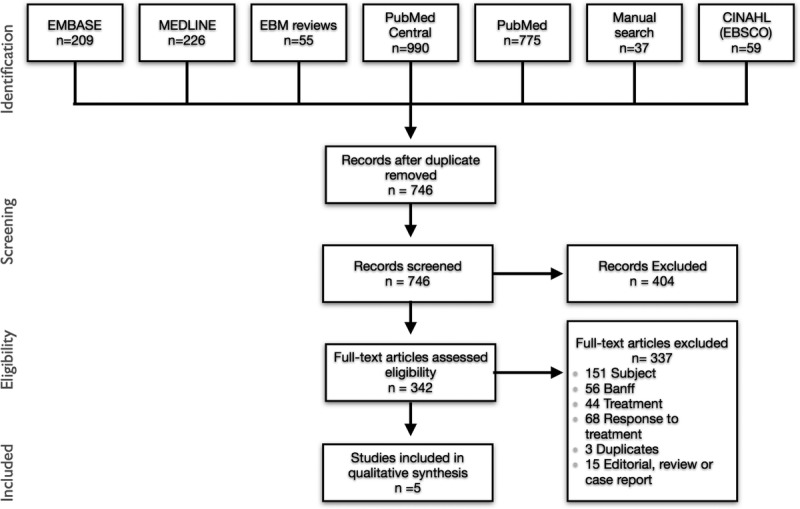
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) diagram.
Clinical and Methodological Heterogeneity Among Selected Studies
Study Populations
All the studies selected were retrospective, including patients transplanted between 1985 and 2012 (Table 1).11,13-16 Only 2 studies included patients transplanted after 2010.13,14 One report was restricted to patients with steroid resistant rejection.13 Biopsies were performed for clinical motives in all studies, with indications including delayed graft function,15,16 and increase in serum creatinine levels.11,13-16 One study included a small percentage of rejections diagnosed on surveillance biopsies (3%).14 The 1997 version of the Banff scoring system was used to classify rejection episodes in 3 studies (60%),12,13,16 whereas the 2007 and 2009 versions were used in 1 report each.14,15 Antibody-mediated rejections were explicitly excluded in 2 (40%) studies,15,16 and 2 other studies (40%) excluded C4d-positive rejections but not patients with positive DSA.13,14
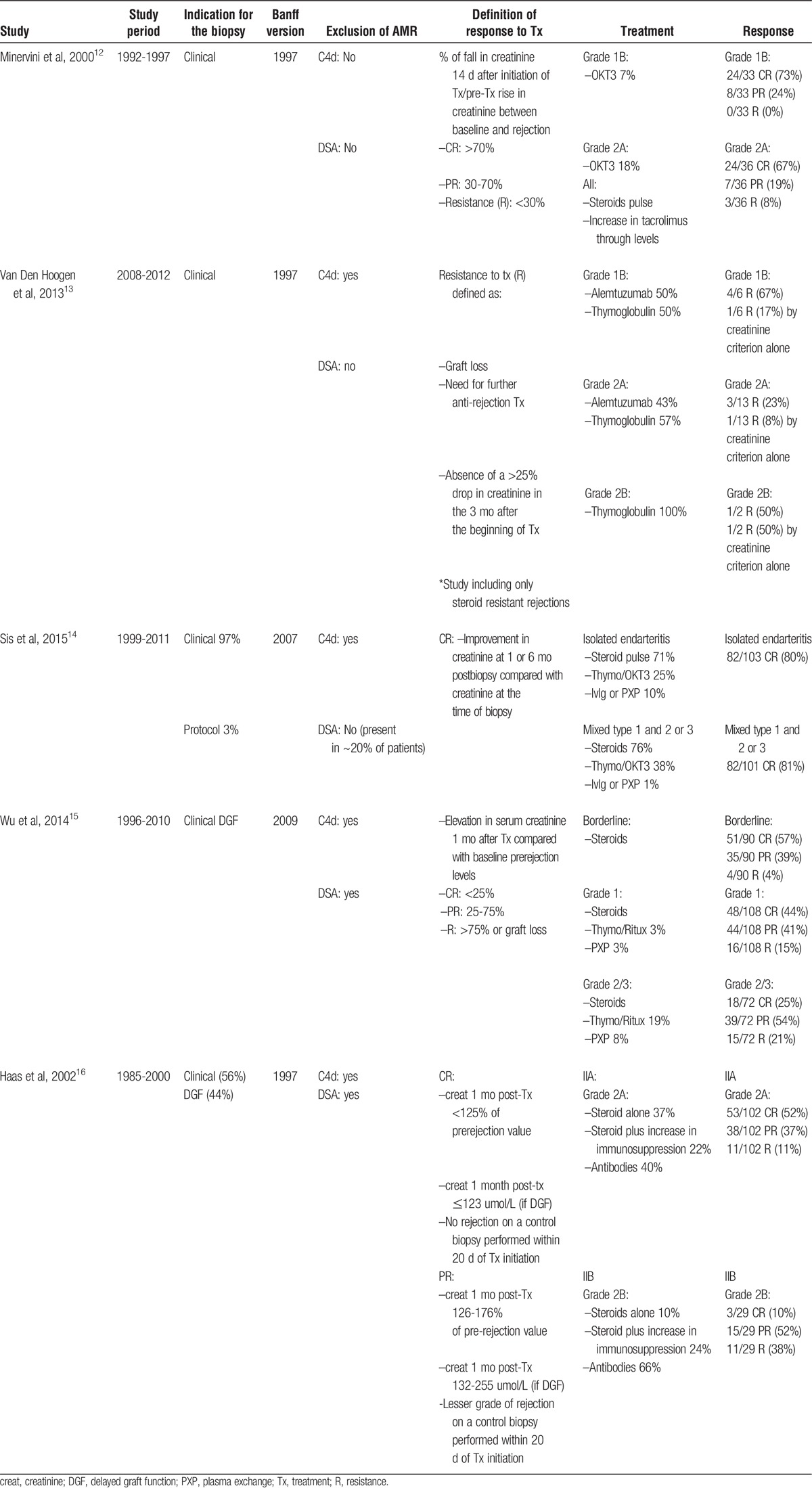
TABLE 1.
Functional response to treatment in selected studies
Treatment Protocols
Except for 1 report in which the use of first-line therapy with corticosteroids was 74%,14 all patients with acute CMRs of Banff grade 1 and above received corticosteroids as first-line treatment. Treatment protocols with regards to corticosteroids were defined in only 1 study,13 with a regimen varying between methylprednisolone 0.5 to 1 g once a day for 3 to 6 consecutive days. In 2 studies, some or all patients received increased doses of maintenance immunosuppressive agents without conversion.12,16 The most commonly used depleting agents consisted of thymoglobulin,13-16 and OKT3,12,14,16 and were used either as first line therapy for Banff grade 2 and above rejections16 or more commonly, as a second-line for steroid-resistant patients.12,13,15 Atgam,16 alemtuzumab,13 rituximab,15 and intravenous immunoglobulin14 were each reported in 1 study. Although patients with DSA or C4d positive staining were excluded, use of plasmapheresis was reported in 3% of patients with Banff grade 1 and 8% of those with Banff grade 2/3 rejection in 1 report.15
Definition of Treatment Response
The definition of a functional response to treatment varied markedly across studies. All studies included at least 1 biochemical/functional criterion to define the reversibility of rejection.12-16 In 2 studies, complete responses (CR) and partial responses (PR) were defined as a serum creatinine 1 month posttreatment that had returned within 125% (CR) or between 125% and 175% (PR) of the prerejection value.15,16 Other functional criteria included a greater than 25% decrease in peak serum creatinine 3 months after treatment,13 lower levels of serum creatinine 1 and 6 months postbiopsy compared with serum creatinine at the time of biopsy,14 and the ratio of the difference between serum creatinine on day 14 posttreatment and peak level at the time rejection to the difference between the latter and baseline creatinine. A CR was called when this ratio had a value greater than 70%, and a PR when its value was between 30% and 70%.12 In 2 reports, biochemical criteria were supplemented by other elements to define response to treatment. Haas and colleagues16 added clearance of rejection on a control biopsy as a histological criterion for patients with delayed graft function after transplant because prerejection creatinine is not a reliable reference in those patients. Van Der Hoogen et al13 added graft loss and need for further antirejection treatment as criteria for nonresponse in addition to the biochemical definition used.
We could not identify any reports providing data on the histological response to treatment of clinical rejection episodes through our search strategy.
Rate of Response to Treatment
In the studies reporting a functional response to the treatment of rejection based on improvements in serum creatinine after treatment compared with before treatment,11-13,15,16 the CR rate varied from 57% in borderline,15 73% to 75% in Banff grade 1B,12,13 52% to 80% in Banff grade 2A,12,14,16 and 10% in Banff grade 2B rejections.16 No specific data were provided for Banff grades 1A and 3 separately, but some studies reported complete functional responses of 44% for Banff grade 1 rejections (IA and IB pooled), and between 25% and 81% for Banff grades 2 and 3 pooled as a group.14,15
A PR was observed in 39% of borderline,15 24% of Banff grade 1B,12 19% to 37% in Banff grade 2A,12,16 and 52% in Banff grade 2B rejections.16 Again, specific data was not provided for Banff grades 1A and 3 separately, but some studies reported partial functional responses of 41% for Banff grade 1 rejections (1A and 1B pooled),15 and 54% for Banff grades 2 and 3 pooled as a group.15 Overall, resistant rejection rates by these functional criteria were 4% in borderline,15 0% to 25% in Banff grade 1B,12,13 11% to 20% in Banff grade 2A,12,14,16 and 38% in Banff grade 2B rejections.16 Functional resistance rates of 15% for Banff grade 1 rejections (1A and 1B pooled),15 and between 19% and 21% for Banff grades 2 and 3 pooled as a group.15
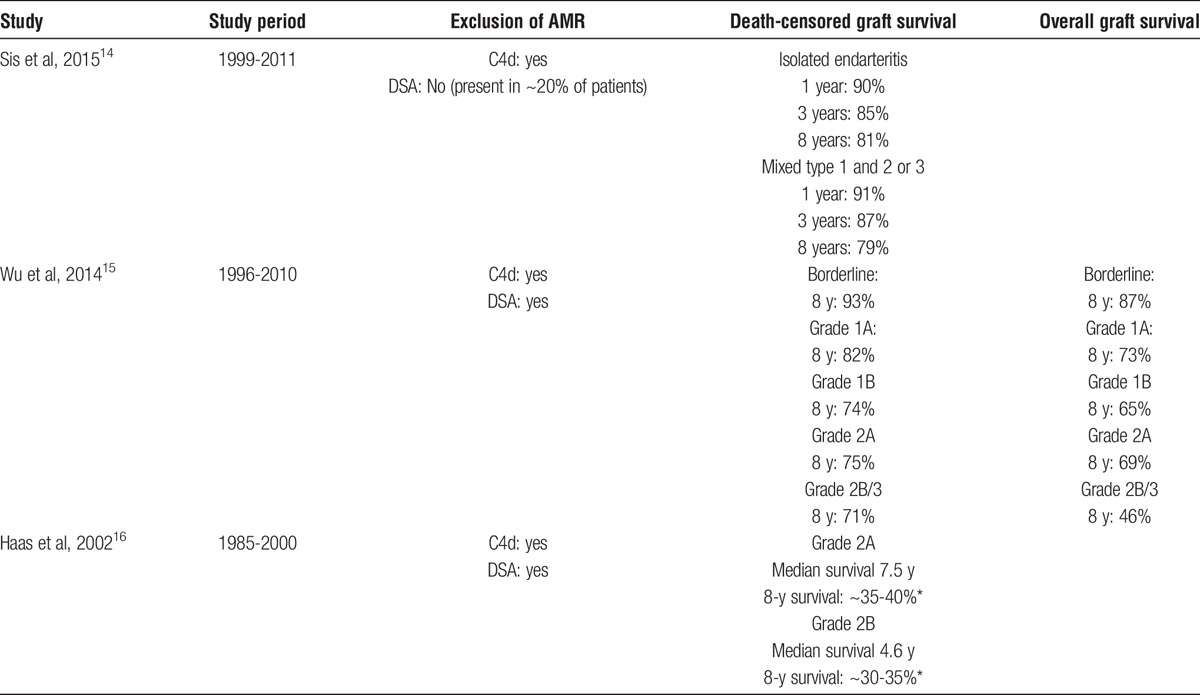
The rates of death-censored graft survival (Table 2) were 90% to 91% for pooled grades 2 and 3 rejections at 1 year,14 and 85% to 87% at 3 years.14 Reported death-censored graft survival at 8 years is 93% for borderline,15 82% for Banff grade 1A,15 and 73% for Banff grade 1B rejections.15 For Banff grade 2A and 2B rejections, 2 recent studies report rates of graft survival of 71% to 79% at 8 years,14,15 whereas in an older publication, lower of figures of 30% to 40% were reported for this group of patients.16
TABLE 2.
Long-term graft survival by rejection class in selected studies
DISCUSSION
Functional recovery after the treatment of rejection is an important prognostic factor for long-term graft survival,3-5 and a key element considered in clinical practice to make decisions to repeat graft biopsy or treatment. Furthermore, the efficacy of potential novel therapies to reverse CMR should be compared with that of current practice. Given the increased strength in maintenance immunosuppressive regimen used in the past decades, and because previously unrecognized antibody-mediated processes may have led to underestimate the reversibility of CMR, we performed a systematic review of the literature to define the functional or histological reversibility of CMR episodes categorized by the Banff (version 1997 and above) classification.
The first finding from this systematic review is the paucity of data regarding the reversibility of pure CMR episodes by Banff class. Of the initial 746 articles identified by our search strategy, only 5 provided data on the functional reversibility of rejection, and none provided data on histological reversibility. In only 2 of these studies were AMRs completely excluded.15,16 Two other selected reports excluded C4d positive cases, but not patients with DSA,13,14 which may have led to misclassification of C4d negative AMRs as pure CMRs. Some Banff categories were also regrouped in some reports,15 making reversibility figures impossible to extract by specific Banff class.
We observed heterogeneity in treatment protocols, but overall, almost all patients received intravenous corticosteroids.12-16 Lymphocyte-depleting agents were more commonly used as second-line agents when intravenous corticosteroids had failed, although 1 study used them as first line therapy for Banff grade 2 or higher rejections.16
The heterogeneity in the criteria used to define reversibility made a meta-analysis of data impossible. However, the complete functional response rates of Banff grade 1 pooled and grade 1B separately (44-73%)12,15 overlapped with those of Banff grade 2A (52-80%),12,14,16 whereas lower figures were reported for Banff grade 2B rejections (10%).16 The same is true for functional resistance, with estimated rates of 0% to 16% for Banff grade 1 pooled and for grade 1B separately,12,15 8% to 20% for grade 2A,12,14,16 and 38% for grade 2B.16 We excluded figures from the study with steroid-resistant cases only, because this is a different subpopulation of patients. For Banff grade 1B and 2A rejections, the study that did not exclude any AMRs12 did not report higher functional resistance rates compared to those that did.14-16 For Banff grades 1B and 2A, functional resistance rates were again at least as low in the studies performed in earlier eras12,16 compared with more recent ones.14,15 Although an era effect for the functional reversibility of rejection was hence not apparent, death-censored graft survival rates at 8 years were higher in 2 recent reports14,15 compared with an older one16 for grade 2A rejections.
Through our search strategy, we could not identify studies that described the histological clearance of CMR episodes after treatment. Two studies were initially identified but not retained as their main focus was the evolution of subclinical rejection. One study included only cases of subclinical borderline rejections, and found an increased Banff chronicity score 1 year after the rejection episode in patients who had not received corticosteroid treatment compared to patients who had been treated.17 The other provided incomplete response data in patients with subclinical rejections only.18 Although previous work suggests that functional and histological reversibility of rejection are not well correlated,6 both aspects seem to be clinically relevant, because the lack of histological response is predictive of recurrent clinical rejection.6
Our work highlights the lack of consensus among investigators for defining therapeutic protocols and metrics for measuring their efficacy, leading to a wide variability of response rates reported in the treatment of CMR. In the late 1990s, investigators from multiple centers and countries proposed a consensus definition for the functional response to therapy.19 Successful treatment was defined as a serum creatinine level of 110% or less of the serum creatinine on the day of the rejection diagnosis by 5 days of therapy, with maintenance of this response for a minimum of 30 days.19 None of the studies we identified used this criterion to define reversibility. However, the need for consensual definition of metrics used to define successful treatment remains an important endeavor to design rigorous studies to test novel therapeutic alternatives.
In conclusion, this systematic review of the literature underscores the paucity and heterogeneity of existing literature on the topic of rejection reversibility. Taking these limitations into account, functional reversibility rates of Banff grade 2A rejections overlapped with those of Banff grade 1 rejections, and lower reversibility figures were reported for Banff grade 2B rejections. We could not see an apparent effect of era or specific exclusion of antibody-mediated processes on functional reversibility rates reported, although the small number of studies precludes a definite conclusion on this topic. Long-term graft survival did appear to be higher for vascular rejections in more recent studies.
There is a pressing need to improve uniformity among centers in terms of therapeutic protocols and definition of outcome metrics in the treatment of CMR. The rate of functional and histological responses to the treatment of CMR, classified according to the new Banff 2013 classification, also needs to be defined in future studies. This will be key to provide patients with prognostic information, and to develop high-quality trials for new therapeutic options for CMR, which could positively affect graft outcomes.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge their 2 medical librarians, Mme Marie-Marthe Gagnon and Mme Daniela Ziegler who have developed and updated the search strategy.
Footnotes
Published online 15 November, 2016.
C.L., L.S., and H.C. are investigators, Canadian National Transplant Research Program (CNTRP).
The authors declare no conflicts of interest.
This work is supported by the Canadian National Transplant Research Program (CNTRP).
C.L., J.M.C., L.S., and H.C. performed article selection and data extraction. C.L. and H.C. drafted the article. J.M.C. and L.S. reviewed and edited the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2011 Annual Data Report: kidney. Am J Transplant. 2013;13(Suppl 1):11–46. [DOI] [PubMed] [Google Scholar]
- 2.El-Zoghby ZM, Stegall MD, Lager DJ, et al. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9:527–535. [DOI] [PubMed] [Google Scholar]
- 3.Vereerstraeten P, Abramowicz D, de Pauw L, et al. Absence of deleterious effect on long-term kidney graft survival of rejection episodes with complete functional recovery. Transplantation. 1997;63:1739–1743. [DOI] [PubMed] [Google Scholar]
- 4.Madden RL, Mulhern JG, Benedetto BJ, et al. Completely reversed acute rejection is not a significant risk factor for the development of chronic rejection in renal allograft recipients. Transpl Int. 2000;13:344–350. [DOI] [PubMed] [Google Scholar]
- 5.Meier-Kriesche HU, Ojo AO, Hanson JA, et al. Increased impact of acute rejection on chronic allograft failure in recent era. Transplantation. 2000;70:1098–1100. [DOI] [PubMed] [Google Scholar]
- 6.Gaber LW, Moore LW, Gaber AO, et al. Correlation of histology to clinical rejection reversal: a thymoglobulin multicenter trial report. Kidney Int. 1999;55:2415–2422. [DOI] [PubMed] [Google Scholar]
- 7.Palomar R, Ruiz JC, Zubimendi JA, et al. Is there any correlation between pathologic changes for acute rejection in kidney transplantation (Banff 97) and graft function? Transplant Proc. 2002;34:349. [DOI] [PubMed] [Google Scholar]
- 8.Mueller A, Schnuelle P, Waldherr R, et al. Impact of the Banff '97 classification for histological diagnosis of rejection on clinical outcome and renal function parameters after kidney transplantation. Transplantation. 2000;69:1123–1127. [DOI] [PubMed] [Google Scholar]
- 9.Gaber LW, Moore LW, Alloway RR, et al. Correlation between Banff classification, acute renal rejection scores and reversal of rejection. Kidney Int. 1996;49:481–487. [DOI] [PubMed] [Google Scholar]
- 10.Lefaucheur C, Loupy A, Vernerey D, et al. Antibody-mediated vascular rejection of kidney allografts: a population-based study. Lancet. 2013;381:313–319. [DOI] [PubMed] [Google Scholar]
- 11.Sis B, Einecke G, Chang J, et al. Cluster analysis of lesions in nonselected kidney transplant biopsies: microcirculation changes, tubulointerstitial inflammation and scarring. Am J Transplant. 2010;10:421–430. [DOI] [PubMed] [Google Scholar]
- 12.Minervini MI, Torbenson M, Scantlebury V, et al. Acute renal allograft rejection with severe tubulitis (Banff 1997 grade IB). Am J Surg Pathol. 2000;24:553–558. [DOI] [PubMed] [Google Scholar]
- 13.van den Hoogen MW, Hesselink DA, van Son WJ, et al. Treatment of steroid-resistant acute renal allograft rejection with alemtuzumab. Am J Transplant. 2013;13:192–196. [DOI] [PubMed] [Google Scholar]
- 14.Sis B, Bagnasco SM, Cornell LD, et al. Isolated endarteritis and kidney transplant survival: a multicenter collaborative study. J Am Soc Nephrol. 2015;26:1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu K, Budde K, Lu H, et al. The severity of acute cellular rejection defined by Banff classification is associated with kidney allograft outcomes. Transplantation. 2014;97:1146–1154. [DOI] [PubMed] [Google Scholar]
- 16.Haas M, Kraus ES, Samaniego-Picota M, et al. Acute renal allograft rejection with intimal arteritis: histologic predictors of response to therapy and graft survival. Kidney Int. 2002;61:1516–1526. [DOI] [PubMed] [Google Scholar]
- 17.Min SI, Park YS, Ahn S, et al. Chronic allograft injury by subclinical borderline change: evidence from serial protocol biopsies in kidney transplantation. J Korean Surg Soc. 2012;83:343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kee TY, Chapman JR, O'Connell PJ, et al. Treatment of subclinical rejection diagnosed by protocol biopsy of kidney transplants. Transplantation. 2006;82:36–42. [DOI] [PubMed] [Google Scholar]
- 19.Guttmann RD, Soulillou JP, Moore LW, et al. Proposed consensus for definitions and endpoints for clinical trials of acute kidney transplant rejection. Am J Kidney Dis. 1998;31(6 Suppl 1):S40–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


