Supplemental digital content is available in the text.
Abstract
Background
Epitope matching, which evaluates mismatched amino acids within antigen-antibody interaction sites (eplets), may better predict acute rejection than broad antigen matching alone. We aimed to determine the association between eplet mismatches and acute rejection in kidney transplant recipients.
Methods
The association between eplet mismatches, broad antigen mismatches and acute rejection was assessed using adjusted Cox proportional hazard regression. Model discrimination for acute rejection was evaluated using the area under receiver operating characteristic curves.
Results
Of the 3,499 kidney transplant recipients from 2006 to 2011, the average (SD) number of broad antigen and eplet mismatches were 3.4 (1.7) and 22.8 (12.2), respectively. Compared with 0 to 2 eplet mismatches, the adjusted hazard ratio (HR) for acute rejection among those with 20 or greater eplet mismatches was 2.16 (95% confidence interval [CI], 1.33-3.52; P = 0.001). The adjusted area under the curve for broad antigen mismatches was 0.58 (95% CI, 0.56-0.61), similar to that for eplet mismatches (HR, 0.59; 95% CI, 0.56-0.61; P = 0.365). In recipients who were considered as low immunological risk (0-2 broad antigen HLA-ABDR mismatch), those with 20 or greater eplet mismatches experienced an increased risk of rejection compared to those with less than 20 mismatches (adjusted HR, 1.85; 95% CI, 1.11-3.08; P = 0.019).
Conclusions
Increasing number of eplet mismatches is associated with acute rejection in kidney transplant recipients. Consideration of eplet HLA mismatches may improve risk stratification for acute rejection in a selected group of kidney transplant candidates.
HLA compatibility between donor and recipient is an important factor that influences graft outcomes after kidney transplantation whereby an increasing number of class I (HLA-AB) and II (HLA-DR) mismatches are associated with an increased risk of acute rejection and graft loss.1,2 Even in the era of modern immunosuppression, HLA matching remains one of the major criterion in deceased donor kidney allocation in Australia and worldwide.3-5 Serological typing at the HLA-ABDR loci is used universally to determine the number broad antigen HLA mismatches and is the standard triage test for the assessment of immunological compatibility between potential donors and recipients. However, molecular HLA typing may allow accurate evaluation of the immunogenic potential of broad antigen HLA mismatches.
The immunogenicity of HLA antigens is determined by continuous and discontinuous short sequences of amino acids that form the antibody-accessible regions within each HLA allele known as epitopes. HLAMatchmaker, a computer algorithm that calculates the number of epitope mismatches between donors and recipients by considering each HLA allele as a combination of distinct epitopes known as triplets (continuous amino acid sequences) or eplets (closely located contiguous amino acid sequences). Mismatches of these HLA epitopes have been linked to the development of de novo anti-HLA donor-specific alloantibodies after transplantation,6,7 which is associated with up to a 20-fold increased risk of acute rejection and graft loss after kidney transplantation.8 Although a direct and positive association between HLA epitope mismatches and transplant glomerulopathy has been reported, it remains unclear whether epitope HLA mismatches may improve the discrimination HLA mismatches in predicting acute rejection compared to broad antigen HLA mismatches. We aimed to determine the association between broad antigen HLA mismatches, eplet HLA mismatches and acute rejection in a cohort of kidney transplant recipients.
MATERIALS AND METHODS
Study Population
Using data from the Australia and New Zealand Dialysis and Transplant (ANZDATA) registry and National Organ Matching System (NOMS), all primary living and deceased donor kidney transplant recipients in Australia between 2006 and 2011 were included in this study. All transplant recipients had negative complement-dependent cytotoxicity T-cell crossmatch before transplantation. Flow cytometry and B cell complement-dependent cytotoxicity crossmatches are not routinely performed before deceased donor transplantation in the Australian population. Multiple-organ transplant recipients and recipient-donor pairs without recorded HLA-A, B, or -DR typing were excluded (n = 395). Serological HLA-A, -B, and -DR typing for donors and recipients were performed by local tissue typing laboratories, which were extracted from NOMS and linked to a dataset with matching recipients' details from ANZDATA. Our local institutional ethics committee (Sir Charles Gairdner Hospital, Perth, Australia) granted a waiver of consent for this study.
Data Collection
The baseline data included recipient characteristics, such as age, sex, race, causes of end-stage renal disease, preemptive transplantation, peak percentage panel-reactive antibody (PRA), waiting time on dialysis (in months), diabetes, coronary artery disease and smoking history (categorized as current smokers, former smokers or nonsmokers); donor characteristics including age, sex and type (living or deceased); and transplant-related characteristics including the use of induction antibody therapy (interleukin-2 receptor antibody or T cell–depleting antibody), total ischaemic time (in hours) and transplant year. Information regarding the class and intensity of pretransplant DSA were not available.
Calculating the Number of Eplet HLA Mismatches Using HLAMatchmaker
The number of eplet HLA mismatches for each recipient and donor pair at HLA class I (HLA-AB) and class II (HLA-DR) loci was calculated using HLAMatchmaker (Version 2.1) (available from: www.hlamatchmaker.net). The HLAMatchmaker program converts the 2-digit HLA serologic typing to 4-digit alleleic typing according to the most common alleles derived from various populations worldwide.9 Typing of HLA-Cw, -DP, and -DQ were not routinely performed in Australia within the study period and as such were not included in analyses. Analyses were conducted for HLA-A, -B, -AB (class I), and -DR (class II) eplet mismatches in isolation and/or in combination within the individual loci.
Graft Outcomes
The primary clinical outcome of this study included any acute rejection episode occurring during the follow-up period. Data on acute rejection is reported to the registry biannually and, although not a requirement, is reported in accordance with Banff criteria where available by each transplanting centre. We also performed a subgroup analysis restricting to those who had experienced antibody-mediated rejections (AMR).
Statistical Analysis
Statistical analyses were performed using SPSS (version 20; SPSS Inc. Chicago, IL) and SAS (SAS Institute Inc. Cary, NC). Data were expressed as mean and SD, median and interquartile range, or frequencies and proportion (%). Patients were grouped according to whether they experienced at least 1 episode of acute rejection or not for comparison. Groups were then compared using independent-samples t tests for continuous variables and chi-square analysis for categorical variables.
First we explored the association between broad antigen and eplet HLA mismatches using Pearson correlations. A Pearson’s correlation coefficient of 0.1 to 0.3 was considered weak, 0.4 to 0.6 moderate and 0.7 to 0.9 strong correlations. Correlations were presented with 95% confidence intervals (95% CI) and P values.
Second, we tested linearity between broad antigen, eplet HLA mismatches and acute rejection using restricted cubic splines. We used univariate and multivariable Cox proportional hazard regression models to explore the association between the exposure and the binary outcomes. Recipients who did not experience any acute rejection episodes, lost to follow-up, or died were censored at the end of the follow-up period. Covariates examined in the Cox regression models included recipient characteristics (age, sex, height, weight, waiting time on dialysis, and peak PRA), donor characteristics (type, age, and sex), transplant characteristics (ischemia time and use of induction therapy). Covariates with a P value less than 0.20 in the unadjusted model were included in the multivariable-adjusted analyses. However, PRA, recipient and donor age, donor type and ischemia time were included in all models irrespective of the association in the univariate models. Results were expressed as hazards ratios (HR) with 95% CI.
Third, to assess the test performance of broad antigen and eplet HLA mismatches in predicting acute rejection risk, we considered the discrimination of the 2 different models using the area under the curve (AUC) of the receiver operating characteristic (ROC) curves. Discrimination refers to the ability of the model to distinguish individuals with and without the outcomes of interest whereby an AUC of 1 implies perfect discrimination and an AUC of 0.5 represents random discrimination. The sidak option provides adjusted P values comparing the ROC areas between the 2 models, assuming a “gold standard” being broad antigen HLA mismatches.
RESULTS
Baseline Characteristics of Patient Cohort
The characteristics of recipients and their donors are shown in Table 1 and Figure 1. Of the 3844 primary kidney transplant recipients, complete HLA-ABDR typing was available for donors and recipients in 3449 transplants (90%) with median (interquartile range) follow-up of 3.4 (2.1-5.1) years resulting in 11 775 patient-years. The mean (SD) age of the recipients was 4710 years and 2113 (61%) received deceased donor kidney transplants. Six hundred fifty-seven (19%) recipients experienced at least 1 episode of acute rejection during the follow-up period. Over 97% (640 of 657) of acute rejection episodes were biopsy-proven. Compared with recipients who did not experience acute rejection, recipients that experienced acute rejection were more likely to have had diabetes mellitus, received a deceased donor kidney transplant or had a greater number of broad antigen (3.7 vs 3.3) and eplet (25.7 vs 23.0) HLA-ABDR mismatches. Recipient sensitisation status, as measured by PRA, was not significantly different between rejection status. The majority of primary kidney transplant recipients during the study period were maintained on a combination of prednisolone (99%), cyclosporine (30%) or tacrolimus (70%), and mycophenolate (97%).11 Of the 647 acute rejection episodes, 157 (24%) and 37 (5%) were AMR and vascular rejection respectively (Figure 1).
TABLE 1.
Demographics and characteristics of the cohort of primary graft recipients
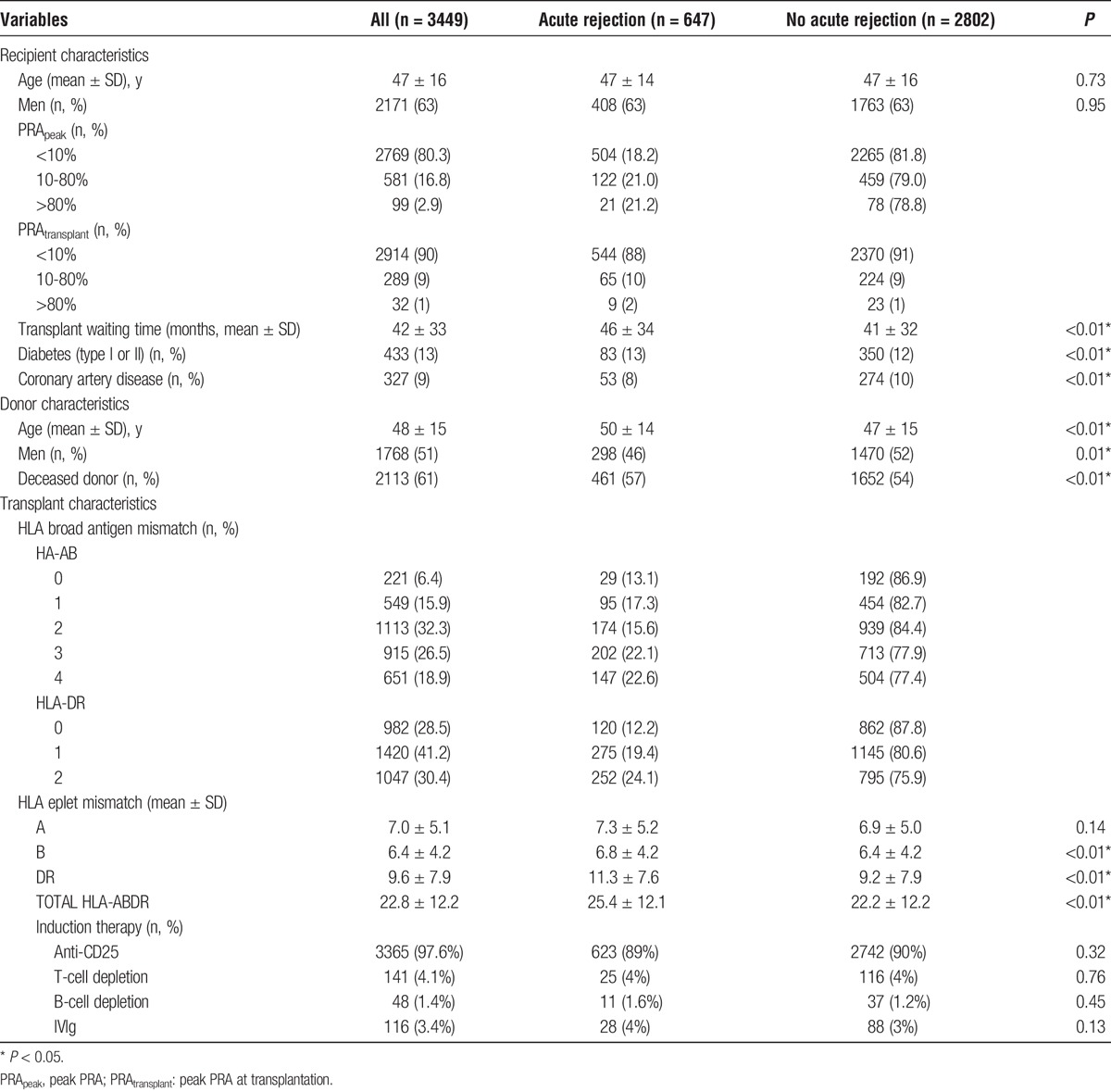
FIGURE 1.
Distribution of rejection episodes by kidney transplantation type.
Associations Between Broad Antigen and Eplet HLA Mismatches
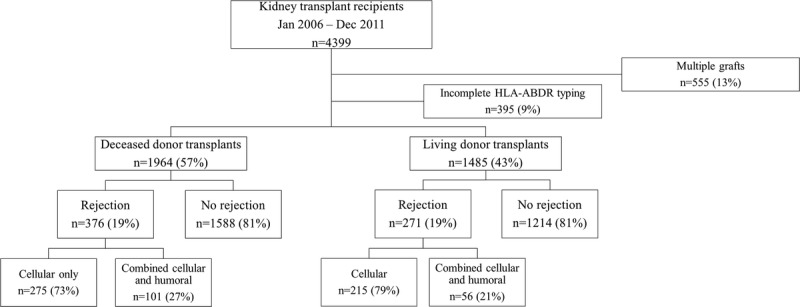
There was a positive and linear correlation between class I, II, and I + II broad antigen and eplet HLA mismatches with Pearson’s r-values (95% CI) of 0.80 (0.78-0.82), 0.84 (0.83-0.86) and 0.85 (0.84-0.87) respectively (Figure 2).
FIGURE 2.
Scatter plot of correlation between broad antigen and eplet HLA mismatches by HLA class I (HLA-A and HLA-B), class II (HLA-DR) and combined class I & II (HLA-ABDR) loci and associated correlation matrix showing Pearson r values.
Associations Between Broad Antigen or Eplet HLA Mismatches and Acute Rejection
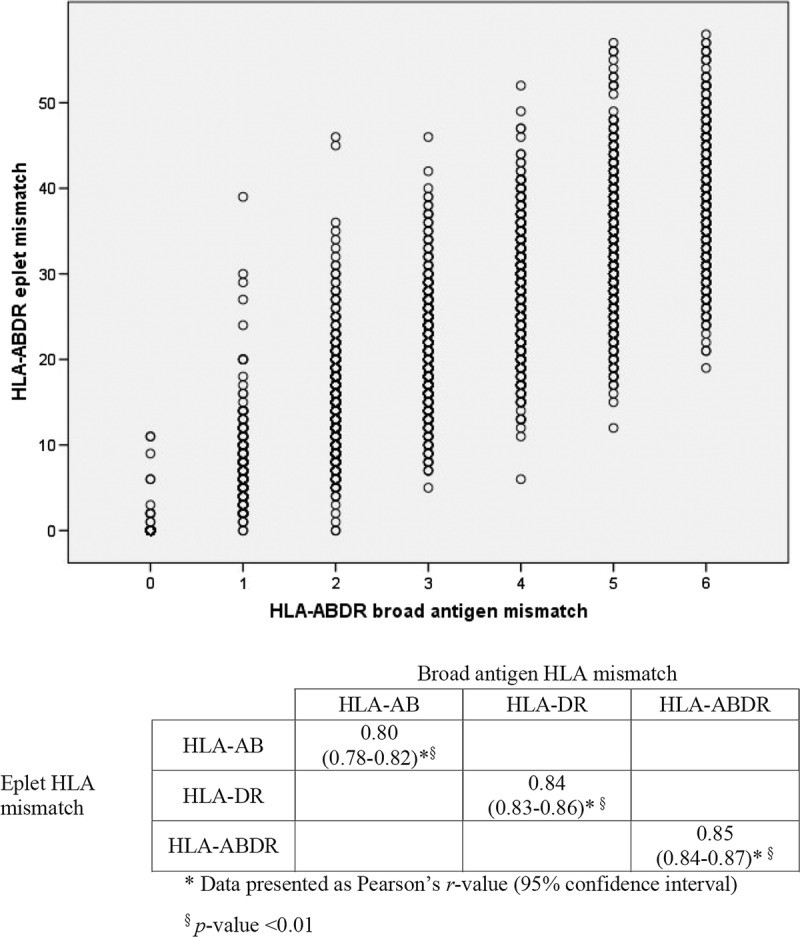
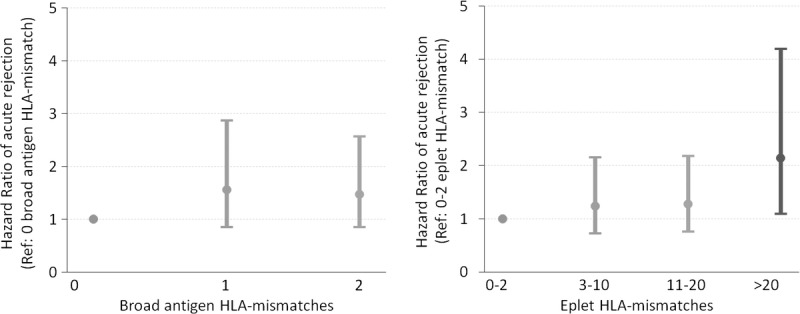
The restricted cubic spline showed a linear association between broad antigen HLA mismatch and acute rejection (P value for deviation from linearity = 0.803, Fig. S1a, SDC, http://links.lww.com/TXD/A31). For each HLA mismatch, there was at least a 10% increased risk of acute rejection (adjusted HR, 1.17; 95% CI, 1.11-1.24; P < 0.01). In contrast, the association between eplet HLA mismatches and acute rejection was nonlinear (P value for deviation from linearity < 0.001, Fig. S1b, SDC, http://links.lww.com/TXD/A31). After logarithmic transformation, the total number of eplet HLA-ABDR mismatches exhibited a linear relationship with acute rejection, which best fit into 4 categories of 0 to 2, 3 to 10, 11 to 20, and >20 eplet HLA-ABDR mismatches (Fig. S2, SDC, http://links.lww.com/TXD/A31). Compared to recipients with 0 to 2 eplet mismatches, the adjusted hazards ratio (95% CI) for experiencing any acute rejection in recipients with 3 to 10, 11 to 20, or more than 20 eplet mismatches were 1.21 (0.70-2.07), 1.51 (0.92-2.49), and 2.16 (1.33-3.52), respectively (Figure 3). Significant covariates included in the multivariable regression model included recipient male sex (adjusted HR, 1.26; P = 0.004) and increasing donor age (adjusted HR, 1.01; P < 0.001). Analyses restricting to recipients with 1, 2, 3, or 4 HLA-ABDR broad antigen mismatches showed similar results.
FIGURE 3.
Hazard ratio plot of adjusted rejection risk according to (A) HLA-ABDR broad antigen mismatch and (B) HLA-ABDR eplet mismatch. Logistic regression model adjusted for recipient age, sex, height, weight, waiting time on dialysis, peak PRA, donor source, donor age, gender, and use of induction therapy.
In an analysis restricting to recipients with 0 to 2 HLA-ABDR broad antigen mismatches (n = 1221, 35%), acute rejection was observed in 175 (14%) of recipients. Of these, 49 (28%) were AMR. The incidences of acute rejection stratified by broad antigen HLA mismatches and by categories of eplet mismatches are shown in Table 2. Figure 4 shows the adjusted hazard ratios for acute rejection according to categories of eplet HLA mismatches in recipients with 0 to 2 broad antigen HLA-ABDR mismatches. Compared with recipients with less than 20 eplet HLA mismatches, those with 20 or greater eplet mismatches were more likely to experience any acute rejection with adjusted HR (95% CI) of 1.85 (1.11-3.08, P = 0.019). Among recipients with 0 to 2 broad antigen HLA mismatches, approximately 10% (n = 126) had greater than 20 eplet HLA mismatches. Adding eplet HLA mismatches to broad antigen HLA mismatches did not improve the prediction for any acute rejection compared with broad antigen HLA mismatches alone (adjusted AUC [95% CI] of 0.68 [0.64-0.73] and 0.69 [0.65-0.74), respectively).
TABLE 2.
Proportion of acute rejection in recipients with 0 to 2 HLA-ABDR broad antigen mismatches
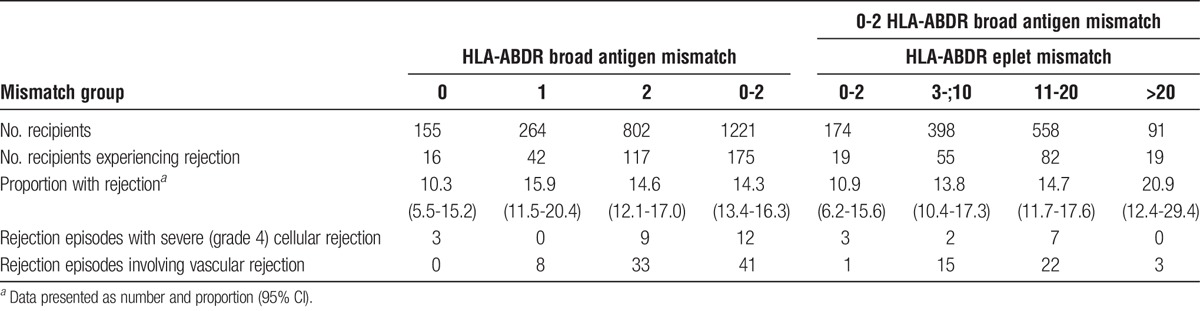
FIGURE 4.
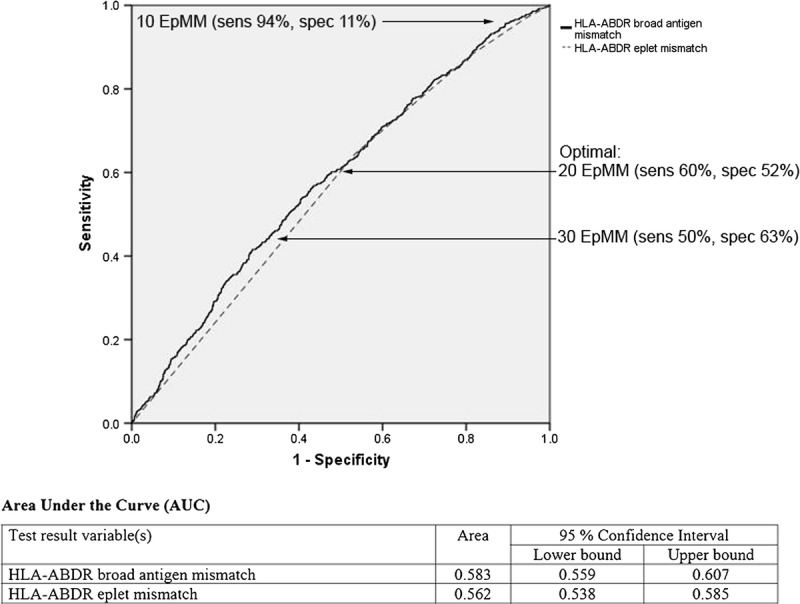
Receiver operating characteristic curves of HLA-ABDR broad antigen and eplet mismatch for acute rejection episode. Models adjusted for recipient age, sex, height, weight, waiting time on dialysis, peak PRA, donor source, donor age, sex, and use of induction therapy.
Model Discrimination for Acute Rejection
The adjusted AUC (95% CI) using broad antigen HLA mismatches in predicting acute rejection was 0.58 (0.56-0.61), which was similar to combined broad antigen and eplet HLA mismatches (0.59 [0.56-0.61], P = 0.365) (Figure 5). The threshold for predicting acute rejection balancing test sensitivity and specificity was 20 eplet mismatches, with test sensitivity and specificity 60% and 52%, respectively. This is represented by the point on the ROC curve closest to the (0,1) on the x and y axes as demonstrated in Figure 5.
FIGURE 5.
Hazard ratio plot of adjusted rejection risk restricted to 0 to 2 broad antigen HLA-ABDR mismatches according to broad antigen and eplet HLA mismatches. Cox's proportional hazards regression model adjusted for recipient age, sex, height, weight, waiting time on dialysis, peak PRA, donor source, donor age, sex, and use of induction therapy.
Subgroup Analysis: Antibody-Mediated Rejection
One hundred fifty-seven (of 2449) recipients experienced AMR with an average time to rejection of 23 days. Of these, 12 (7.6%) had peak PRA >80%. Compared to recipients with 0 to 2 HLA-ABDR eplet mismatches, the adjusted HR (95% CI) for AMR in recipients experiencing AMR with 3 to 10, 11 to 20 and greater than 20 eplet mismatches were 1.14 (0.36-3.60), 1.93 (0.68-5.50) and 2.24 (0.80-6.32), respectively. Using the adjusted models for predicting AMR, the adjusted AUC (95% CI) of eplet HLA mismatches were 0.62 (0.59-0.65), comparable to the AUC (95% CI) for broad antigen HLA mismatches alone of 0.63 (0.60-0.65).
DISCUSSION
Our study findings suggested that there is a strong and positive correlation between eplet and broad antigen HLA-ABDR mismatches. The association between broad antigen HLA mismatches and acute rejection appears to be linear whereas the association between eplet HLA mismatches and acute rejection is exponential. Although adding eplet to broad antigen HLA mismatches did not improve the accuracy in the prediction of clinical events such as acute rejection, understanding the extent of eplet mismatches may improve the assessment of immunological risk in kidney transplant recipients. Recipients who were considered as low-immunological risk (0-2 broad antigen HLA-ABDR mismatched kidneys) but with high donor-recipient eplet HLA mismatches (≥20) experienced a twofold increased risk of acute rejection in comparison to recipients with low eplet HLA mismatches.
HLA compatibility between donors and recipients is a major determinant of acute rejection and graft loss after kidney transplantation.1,2 The evolution of HLA typing methods from serological to molecular typing permits accurate assessment of donor-recipient compatibility at the epitope level. Epitopes comprised of triplets or eplets, which are the amino acid sequences within each HLA antigen capable of eliciting an immune response. Although epitope mismatches have been shown to predict the development of donor-specific anti-HLA antibody (DSA), it is unclear whether epitope mismatches are superior to broad antigen HLA mismatches in predicting patient relevant outcomes such as rejection and/or graft loss.6,7,12 Our study findings suggested that we could identify a high-risk subgroup using eplet matching in those where were otherwise considered as low risk using broad antigen HLA mismatches (ie, 0-2 mismatches). For kidney transplant recipients who received 0 to 2 broad antigen HLA-ABDR mismatched kidneys, there was up to a twofold increased risk of acute rejection in recipients with ≥20 eplet mismatches compared to recipients with <20 eplet mismatches. A lack of observed benefit with eplet matching in our subgroup analyses of sensitised recipients is unexpected and may be attributed to the small sample size and limited power to detect any statistically significant effects.
In an analysis of 31 879 kidney transplant recipients from the United Network for Organ Sharing and Eurotransplant registries,13 increasing number of triplet HLA mismatches was associated with reduced graft and patient survivals. In recipients who had received kidneys with <5 triplet mismatches, the risk of 5-year graft loss was significantly lower than those with 5 or greater triplet HLA mismatches. On the contrary, an observational analysis of 16 997 kidney transplant recipients using data from the Collaborative Transplant Study did not show a statistically significant association between epitope mismatches (categorized as 0-6, 7-8, 10-12, and ≥13 triplet HLA mismatches) and graft survival.14 In a small single-center study of 101 kidney transplant recipients with follow-up period of less than 2 years, transplant recipients with greater than 10 class I triplet mismatches experienced an increased risk of grafts loss in excess by 5 times compared to those with 10 or lesser class I triplet mismatches.15 However, a similar association was not observed between class I eplet HLA mismatches and overall graft loss.16 These studies were limited by short follow-up period and analysis restricting to predominantly class I mismatches.
Recent studies investigating the utility of epitope matching in kidney transplantation have focused on the development of de novo DSA (dnDSA) and transplant glomerulopathy. In a study of 286 kidney transplant patients between 1999 and 2008, Wiebe et al10 showed a positive association between class II HLA epitope mismatches and dnDSA such that greater than 10 HLA-DR eplet mismatches or greater than 17 HLA-DQ eplet mismatches was associated with a greater occurrence of dnDSA against HLA-DR or HLA-DR, respectively. There was no association between class II epitope mismatches and acute rejection. Interestingly, the occurrence of clinical rejection episodes was identified as an independent predictor of dnDSA development (odds ratio, 2.6 per rejection episode, P < 0.001). In a separate study of 1753 kidney transplant recipients followed for 3.97 ± 2.71 years, Sapir-Pichhadze et al17 showed an association between class II HLA eplet mismatches and the development of transplant glomerulopathy.17 Compared to less than 27 class II (HLA-DR + DQ) eplet mismatches, 27 to 43 eplet mismatches were associated with a greater than twofold risk of transplant glomerulopathy (odds ratio, 2.84; P = 0.043).
Unlike broad antigen HLA mismatches, we have shown that the association between eplet HLA mismatches and risk of acute rejection is nonlinear, and may explain the lack of a defined threshold and inconsistent findings from studies that have evaluated the association between epitope or eplet HLA mismatches and development of de novo DSA and other clinical outcomes.16-19 Although increasing number of eplet HLA mismatches may increase the chance of developing dnDSA and acute rejection, the immunogenic potential of eplet mismatches may be dependent on the nature and binding capacity of each eplet HLA mismatch.20,21
Despite the positive association between eplet and broad antigen HLA mismatches with acute rejection, the addition of eplet HLA mismatches did not improve the accuracy in predicting acute rejection over broad antigen HLA mismatches. It is not clear why the HLAMatchmaker program predicted a higher rate of acute rejection since there is no known relationship between B and T cell epitopes.22
Our study has several limitations. First, complete HLA typing was not available for 10% of the study population but had similar baseline characteristics to our study cohort. Given molecular 4-digit typing is essential in determining the number of eplet HLA mismatches, the use of the HLAMatchmaker program to convert donor and recipient from serological to molecular typing may introduce inaccuracies because it is dependent on the estimations of the most frequently defined alleles for each 2-digit serologically defined HLA antigens from various populations worldwide.23 The lack of HLA typings at the -DQ, -DP and -Cw loci would likely have underestimated the total number of broad antigen and eplet HLA mismatches. Even though HLA-DQ DSA is the most common de novo DSA occurring after transplantation, we may have underestimated the clinical benefit of adding eplet HLA matching to broad antigen HLA matching, but this is likely to be minimal as de novo DSA are more important in predicting late rejection or chronic AMR.24-27 However, the link with clinical outcomes remains unclear, as the studies investigating the association of HLA-DQ dnDSA with acute rejection, chronic rejection or graft loss have yielded conflicting results.24,26,27 Given high linkage disequilibrium, a potential means of circumventing this issue may be to infer HLA-DQ alleles based on HLA-DR. However, the lack of acuity for all HLA-DR-DQ haplotypes introduces uncertainties.28 Our study has focussed only on the risk of early acute rejection within 12 months. Longer-term clinical outcomes data including transplant glomerulopathy and chronic AMR, histological data and data pertaining to development of de novo DSA were unavailable within out study cohort. Although we have performed analyses restricted to AMR, the low event rate may result in reduced statistical power to draw meaningful conclusions. Also, our registry does not collect granular data including dnDSA and histological details of kidney biopsies, but our study represents the largest study evaluating the association between epitope mismatches and acute rejection.
IMPLICATIONS FOR FUTURE RESEARCH
Our study raises a number of important and clinically relevant issues, which may be a focus of future research. Although associated with increased morbidity, early acute rejection does not always translate to a reduction in graft and/or patient survival thus raising the need to evaluate the association between eplet HLA mismatches and these longer-term outcomes such as de novo DSA development, chronic AMR, transplant glomerulopathy and graft failure. Also, external validity of the current study should be examined through cross validation with an external cohort. Furthermore, uncertainties surrounding the accuracy of converting 2-digit to 4-digit HLA typing using HLAMatchmaker suggested the need to explore the correlation between HLAMatchmaker conversion and molecular typing performed in the laboratory.
CONCLUSIONS
An increasing number of eplet HLA mismatches is associated with an increased risk of acute rejection. Eplet HLA mismatches may provide better risk stratification for acute rejection among those who are otherwise classified as low immunological risk at the broad antigen level. Future studies incorporating mismatches at other HLA loci and considering longer-term clinical outcomes are required to determine the utility of epitope matching in kidney transplantation.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to gratefully acknowledge the substantial contributions of the entire Australian and New Zealand nephrology community (physicians, surgeons, database managers, nurses, renal operators and patients) that provide information to, and maintain, the ANZDATA database. The data reported here have been supplied by ANZDATA and NOMS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of ANZDATA.
Footnotes
Germaine Wong and Wai H Lim are Joint senior authors.
H.D.N. was supported by the Jacquot Research Entry Scholarship (administered by the Royal Australian College of Physicians) and the National Health and Medical Research Council Postgraduate Medical Scholarship for stipend.
The authors have no conflicts of interest to disclose.
H.D.N. participated in research design, performance of the research, data analysis and writing of the article. G.W. participated in research design, supervising the research, data analysis and writing of the article. J.R.C. participated in research design and writing of the article. S.P.M. participated in research design and writing of the article. P.T.C. participated in research design and writing of the article. N.W. participated in research design and writing of the article. G.R.R. participated in research design and writing of the article. L.D.O. participated in research design and writing of the article. W.H.L. participated in research design, supervising the research, data analysis and writing of the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Lim WH, Chadban SJ, Clayton P, et al. Human leukocyte antigen mismatches associated with increased risk of rejection, graft failure, and death independent of initial immunosuppression in renal transplant recipients. Clin Transplant. 2012;26:E428–E437. [DOI] [PubMed] [Google Scholar]
- 2.Opelz G, Döhler B. Effect of human leukocyte antigen compatibility on kidney graft survival: comparative analysis of two decades. Transplantation. 2007;84:137–143. [DOI] [PubMed] [Google Scholar]
- 3.The Transplantation Society of Australia and New Zealand. Organ Transplantation from Deceased Donors: Consensus Statement on Eligibility Criteria and Allocation Protocols. 2011.
- 4.United Network for Organ Sharing. Kidney Allocation Policies. 2011.
- 5. Eurotransplant. Eurotransplant Manual. March, 2014.
- 6.Dankers MK, Witvliet MD, Roelen DL, et al. The number of amino acid triplet differences between patient and donor is predictive for the antibody reactivity against mismatched human leukocyte antigens. Transplantation. 2004;77:1236–1239. [DOI] [PubMed] [Google Scholar]
- 7.Kosmoliaptsis V, Bradley JA, Sharples LD, et al. Predicting the immunogenicity of human leukocyte antigen class I alloantigens using structural epitope analysis determined by HLAMatchmaker. Transplantation. 2008;85:1817–1825. [DOI] [PubMed] [Google Scholar]
- 8.Lefaucheur C, Loupy A, Hill GS, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21:1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Galarza FF, Christmas S, Middleton D, et al. Allele frequency net: a database and online repository for immune gene frequencies in worldwide populations. Nucleic Acids Res. 2011;39:D913–D919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiebe C, Ponchinco D, Blydt-Hansen TD, et al. Class II HLA epitope matching—a strategy to minimize de novo donor-specific antibody development and improve outcomes. Am J Transplant. 2013;13:3114–3122. [DOI] [PubMed] [Google Scholar]
- 11.ANZDATA Registry. 37th Report. Chapter 8: Transplantation. Australia and New Zealand Dialysis and Transplant Registry, Adelaide, Australia. 2015. Available at: http://www.anzdata.org.au.
- 12.Goodman RS, Taylor CJ, O'Rourke CM, et al. Utility of HLAMatchmaker and single-antigen HLA-antibody detection beads for identification of acceptable mismatches in highly sensitized patients awaiting kidney transplantation. Transplantation. 2006;81:1331–1336. [DOI] [PubMed] [Google Scholar]
- 13.Duquesnoy RJ, Takemoto S, de Lange P, et al. HLAmatchmaker: a molecularly based algorithm for histocompatibility determination. III. Effect of matching at the HLA-A,B amino acid triplet level on kidney transplant survival. Transplantation. 2003;75:884–889. [DOI] [PubMed] [Google Scholar]
- 14.Laux G, Mytilineos J, Opelz G. Critical evaluation of the amino acid triplet-epitope matching concept in cadaver kidney transplantation. Transplantation. 2004;77:902–907. [DOI] [PubMed] [Google Scholar]
- 15.Haririan A, Fagoaga O, Daneshvar H, et al. Predictive value of human leucocyte antigen epitope matching using HLAMatchmaker for graft outcomes in a predominantly African-American renal transplant cohort. Clin Transplant. 2006;20:226–233. [DOI] [PubMed] [Google Scholar]
- 16.Silva E, Alba A, Castro A, et al. Evaluation of HLA Matchmaker compatibility as predictor of graft survival and presence of anti-HLA antibodies. Transplant Proc. 2010;42:266–269. [DOI] [PubMed] [Google Scholar]
- 17.Sapir-Pichhadze R, Tinckam K, Quach K, et al. HLA-DR and -DQ eplet mismatches and transplant glomerulopathy: a nested case-control study. Am J Transplant. 2015;15:137–148. [DOI] [PubMed] [Google Scholar]
- 18.Lefaucheur C, Suberbielle-Boissel C, Hill GS, et al. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Am J Transplant. 2008;8:324–331. [DOI] [PubMed] [Google Scholar]
- 19.Adeyi OA, Girnita AL, Howe J, et al. Serum analysis after transplant nephrectomy reveals restricted antibody specificity patterns against structurally defined HLA class I mismatches. Transpl Immunol. 2005;14:53–62. [DOI] [PubMed] [Google Scholar]
- 20.Duquesnoy RJ. Human leukocyte antigen epitope antigenicity and immunogenicity. Curr Opin Organ Transplant. 2014;19:428–435. [DOI] [PubMed] [Google Scholar]
- 21.Lucas DP, Leffell MS, Zachary AA. Differences in immunogenicity of HLA antigens and the impact of cross-reactivity on the humoral response. Transplantation. 2015;99:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dankers MK, Heemskerk MB, Duquesnoy RJ, et al. HLAMatchmaker algorithm is not a suitable tool to predict the alloreactive cytotoxic T-lymphocyte response in vitro. Transplantation. 2004;78:165–167. [DOI] [PubMed] [Google Scholar]
- 23.Duquesnoy RJ, Kamoun M, Baxter-Lowe LA, et al. Should HLA mismatch acceptability for sensitized transplant candidates be determined at the high-resolution rather than the antigen level? Am J Transplant. 2015;15:923–930. [DOI] [PubMed] [Google Scholar]
- 24.Everly MJ, Rebellato LM, Haisch CE, et al. Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation. 2013;95:410–417. [DOI] [PubMed] [Google Scholar]
- 25.El-Awar N, Nguyen A, Almeshari K, et al. HLA class II DQA and DQB epitopes: recognition of the likely binding sites of HLA-DQ alloantibodies eluted from recombinant HLA-DQ single antigen cell lines. Hum Immunol. 2013;74:1141–1152. [DOI] [PubMed] [Google Scholar]
- 26.DeVos JM, Gaber AO, Knight RJ, et al. Donor-specific HLA-DQ antibodies may contribute to poor graft outcome after renal transplantation. Kidney Int. 2012;82:598–604. [DOI] [PubMed] [Google Scholar]
- 27.Ginevri F, Nocera A, Comoli P, et al. Posttransplant de novo donor-specific HLA antibodies identify pediatric kidney recipients at risk for late antibody-mediated rejection. Am J Transplant. 2012;12:3355–3362. [DOI] [PubMed] [Google Scholar]
- 28.Klitz W, Maiers M, Spellman S, et al. New HLA haplotype frequency reference standards: high-resolution and large sample typing of HLA DR-DQ haplotypes in a sample of European Americans. Tissue Antigens. 2003;62:296–307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


