Abstract
The objective of this study was to investigate the effects of long-term mental abacus calculation training (MACT) on children’s spatial attention orientation. Fifteen children with intensive MACT (MACT group) and 15 children without MACT (non-MACT group) were selected. The two groups of children were matched in age, sex, handedness, and academic grade. The participants were tested with a Posner spatial cueing task while their neural activities were recorded with a 32-channel electroencephalogram system. The participants’ behavior scores (reaction time and accuracy) as well as early components of event-related potential (ERP) during the tests were statistically analyzed. The behavioral scores showed no significant difference between the two groups of children, although the MACT group tended to have a shorter reaction time. The early ERP components showed that under valid cueing condition, the MACT group had significantly higher P1 amplitude [F(1, 28)=5.06, P<0.05, effective size=0.72] and lower N1 amplitude [F(1, 28)=6.05, P<0.05, effective size=0.82] in the occipital region compared with the non-MACT group. In the centrofrontal brain region, the MACT group had lower N1 amplitude [F(1, 28)=4.89, P<0.05, effect size=0.70] and longer N1 latency [F(1, 28)=6.26, P<0.05, effect size=0.80] than the non-MACT group. In particular, the MACT group also showed a higher centrofrontal P2 amplitude in the right hemisphere [F(1, 28)=4.82, P<0.05, effect size 0.81] compared with the left hemisphere and the middle location. MACT enhances the children’s spatial attention orientation, which can be detected in the early components of ERP.
Keywords: children, electroencephalogram, event-related potential, mental abacus calculation training, visuospatial attention
Introduction
Mental abacus calculation (MAC) is a type of mental calculation that is based on the abacus, through steps of actual and simulated bead-dialing training, then transforms into reflective bead dialing, and eventually forms visualized bead movements in the brain for calculation. Mental abacus calculation training (MACT) can induce changes in both brain structures 1,2 and degree of activation. Children with MACT show more activated visuospatial brain regions than those without MACT 3,4. The most significantly activated brain regions of MAC experts in additive operations include the posterior superior parietal (PSP), fusiform, frontal operculum, and bilateral middle frontal gyrus 5–7. Functional MRI studies have shown that when MAC experts recalled long-string numbers or performed complex computing tasks, the superior frontal sulcus and the superior parietal lobule were activated 2,8,9. According to Dehaene’s theory of three parietal circuits for number processing 10, the PSP lobule is responsible for spatial attention regulation and it is the brain region where the spatial attention orientation and number processing can co-act. Piazza and Dehaene 11 suggested that the PSP lobule is mainly responsible for space–time-related attention selection and is responsible for coordinating the bilateral horizontal segment of the intraparietal sulcus to cocomplete the number calculations. Thus, the activation of the superior parietal lobule in children with MACT indicates a close relationship between MAC and the spatial attention function.
In a previous study of the scaling effects of visuospatial attention in children with MACT on the basis of early components of event-related potential (ERP), we found that the posterior P1 amplitude in those children decreased as the target size increased, which was more apparent in the right cerebral hemisphere. The centrofrontal N1 amplitude increased as the target size increased. The centrofrontal P2 amplitude decreased as the target size increased 12. This study showed the feasibility of ERP in studying the impacts of MACT on children’s visuospatial attention.
Spatial orientation is an attention-related process to identify the location of the target. In MAC, spatial orientation is a necessary step for mentally simulating the bead movements and is essential to the trainees’ overall performance. The current study used ERP and a Posner cueing task 13 to investigate spatial orientation in children with MACT and matched controls without MACT. We focused on comparisons of the amplitudes and latencies of P1 and N1 components in the posterior brain region as well as those of N1 and P2 in the centrofrontal brain region between the two groups of children. Previous ERP studies of human attention have found that the posterior P1 and N1 components as well as the centrofrontal N1 component were significantly affected by attention, as indicated by their enhanced amplitudes. The P1 component in the occipital region is considered to originate from the extrastriate cortex 14,15. It might reflect the transmission process of visual signals from the striated cortex to the peripheral lateral striated cortex during the generation process of attention 16. N1 amplitude reflects the recognition process within the focus of attention and P2 is related to the scope of visual attention. Previous studies have shown that long-term MACT in childhood can alter the brain structurally 1,2 and/or functionally 3–6 as a result of neuroplasticity. Thus, we hypothesize that intensive MACT enhances the children’s spatial orientation, which can be detected in the early components of ERP including posterior P1/N1 and centrofrontal N1/P2.
Methods
Participants
Fifteen children with MACT (eight girls and seven boys, age 7.32±0.64 years) and 15 children without MACT (eight girls and seven boys, age 7.16±0.58 years) participated in the study. The two groups of participants were matched according to age, sex, and socioeconomic status of family at the time of enrollment. All of them were healthy elementary school first graders, right-handed, and had above average academic grades (grades in language and mathematics were both B or above). The participants had normal or corrected normal vision defined as decimal visual acuity of 1.0 or higher. The children in the MACT group were trained in MAC 3–4 h per week for 1.5 years (beginning from the third year of kindergarten), whereas the children in the non-MACT group had no MACT or related exercises. The study protocol was approved by the institutional review board (IRB) of Weifang Medical College. Written informed consent was obtained from the children’s parents or legal guardians.
Task
A Posner spatial cueing task 13,17 was used to assess the participants’ visuospatial attention function. As schematized in Fig. 1, the task followed an attention point-cue-target paradigm: first, a ‘+’ sign was displayed in the center of a computer screen for 500 ms as the attention point. Then, an arrow sign was displayed at the site of the attention point for 200 ms as a cue, which randomly pointed to the left or the right. After the cue disappeared for 600–800 ms, a 3-cm-diameter dot was presented on the left or the right side of the attention point for 1500 ms as the target stimulus. The participants were instructed to press a key to indicate the side of the target (‘F’ for left and ‘J’ for right) as quickly and accurately as possible, which was followed by a 500-ms interval before the next task. Thus, the total time of a task trial was 3800–4000 ms. The formal experiment consisted of 120 task trials, in which the trials with valid cues (the pointing direction of the arrow was consistent with the side of the target) accounted for 75% and the trials with invalid cues (the pointing direction of the arrow was opposite to the side of the target) accounted for 25%. The behavioral performance of each participant during the experiment was measured with reaction time (ms) and accuracy (% of correct responses over total).
Fig. 1.
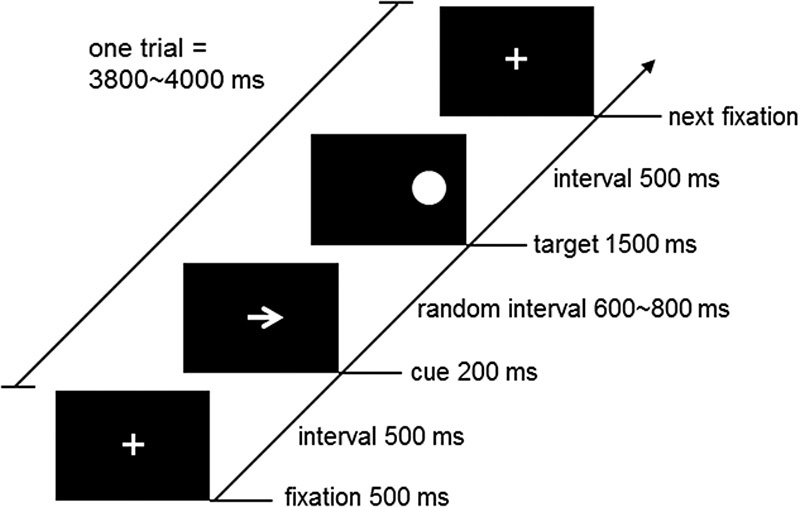
Schematic diagram of the Posner spatial cueing task. In the beginning, a ‘+’ sign was displayed in the center of a computer screen for 500 ms as the attention point. Then, an arrow sign was displayed at the attention point site for 200 ms as a cue, which randomly pointed to the left or right. After a random interval of 600–800 ms, a 3-cm-diameter dot was presented on the left or right side of the attention point for 1500 ms as the target stimulus. The participants were instructed to press a key to indicate the side of the target (‘F’ for left and ‘J’ for right) as quickly and as accurately as possible, which was followed by a 500-ms interval before the next task. Thus, the total time of a task trial was 3800–4000 ms.
The participants were seated 60 cm away from the computer screen at eye level and were asked to avoid eye blinking and body movements during the experiment (Fig. 2). All of the prompts and stimuli presented on the screen were white, whereas the background was black. Practice was allowed before the formal experiment to familiarize the participants with the task, which was observed by one of the experimenters (X.L.).
Fig. 2.

Real view of the experiment. The participants were seated 60 cm away from the computer screen at eye level to complete the Posner spatial cueing task. They were asked to avoid eye blinking and body movements during the experiment. A NeuroScan EEG/ERP system (SynAmps2; Compumedics USA) with a standard 32-channel cap for children was used to record the participants’ electroencephalogram signals during the experiment.
Electroencephalogram recording and analysis
The participants’ electroencephalogram (EEG) signals during the task were scalp-recorded using a NeuroScan EEG/ERP system (SynAmps2; Compumedics USA, Charlotte, North Carolina, USA) and a standard 32-channel cap for children (Quick-Caps; Compumedics USA). The sampling rate was 1000 Hz. The right mastoid was used as the reference electrode and the FZ point was used as the ground electrode according to the International 10–20 System of Electrode Placement. Two electrodes were placed at the lateral angles of both eyes to monitor the horizontal eye movements and another two electrodes were placed on the upper and lower orbits of the left eye to monitor the vertical eye movements. The scalp resistance was less than 5 KΩ for each electrode. The acquired data were then bandpass-filtered in a range of 0.05–40 Hz (Fig. 2).
Scan 4.3 signal processing package (Compumedics USA) was used to process all of the EEG data. First, the trials with significant artifacts attributed to eye movements were identified (>80 or <−80 µV) and removed. This resulted in an average of 57 artifact-free trials with valid cues and 15 artifact-free trials with invalid cues per participant. Then, the stimulus-induced ERPs per participant and types of cues were calculated in a time window of −200 to 500 ms in reference to the target onset, with a mean of −200 to 0 ms as the baseline. Because the number of artifact-free trials with invalid cues (∼15/participant) was too small, the stimulus-induced ERPs with invalid cues were excluded from further analysis. Usually, the early components of ERP usually referred to as the changes within 200 ms after stimulus. Previous studies 14,15 have indicated that the early components related to visuospatial attention processing include the temporal–occipital P1/N1, centrofrontal N1/P2, etc. In particular, O1, OZ, and O2 are the brain regions where visual signals arrive first. Thus, we selected three channels in the occipital lobe (O1, OZ, and O2) and six channels in the centrofrontal lobe (FC3, FCZ, FC4, F3, FZ, and F4) as the representative points. For the selected channels, the peak amplitudes and peak latencies of early ERP components were identified in the following time windows: posterior P1 50–160 ms, posterior N1 130–260 ms, centrofrontal N1 70–150 ms, and centrofrontal P2 140–260 ms.
Statistical analysis
Normality of the ERP data was initially examined using the Shapiro–Wilk test. SPSS Statistics 20.0 (IBM Inc., Armonk, New York, USA) was used for two-way duplicate analysis of variance toward the early ERP components in each of the two brain regions: the P1 and N1 components in the occipital region and the N1 and P2 in the centrofrontal region. In each brain region, the amplitudes and latencies of the corresponding early ERP components were analyzed with respect to two independent factors: the participant type (MACT and non-MACT) and hemispheric position (left hemisphere, middle, and right hemisphere). Similarly, the behavioral scores were analyzed with two independent factors: the participant type (MACT and non-MACT) and cue validity (valid cue and invalid cue). The Bonferroni–Holm correction 18 was applied for multiple comparisons. Statistical significance was defined by a corrected P-value less than 0.05 and an effect size more than 0.5.
Results
Behavioral scores
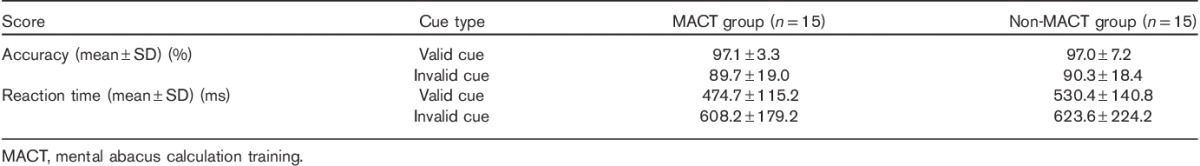
Table 1 summarizes the accuracy (%) and reaction time (ms) of children with/without MACT in completing the Posner spatial cueing task. For accuracy, the two groups of children showed no significant difference; however, the cue validity showed a significant main effect [F(1, 28)=5.33, P<0.05, effect size=0.52]. As expected, the accuracy under valid cues (97.0±4.3%) was higher than that under invalid cues (89.9±18.8%). The interactions between participant type and cue validity were not significant. For the reaction time, the difference between the two groups of children was also not significant, although the children with MACT (508.1±131.2 ms) tended to use shorter time than those without MACT (553.7±161.9 ms). The main effect of cue validity was significant [F(1, 28)=22.23, P<0.01, effect size=0.65]. As expected, the reaction time under valid cues (502.5±133.9 ms) was shorter than that under invalid cues (615.9±206.8 ms). The interactions between the participant type and validity of cues were not significant.
Table 1.
Accuracy and reaction time of children with/without mental abacus calculation training in completing the Posner spatial cueing task
Amplitudes and latencies of P1 and N1 in the occipital brain region
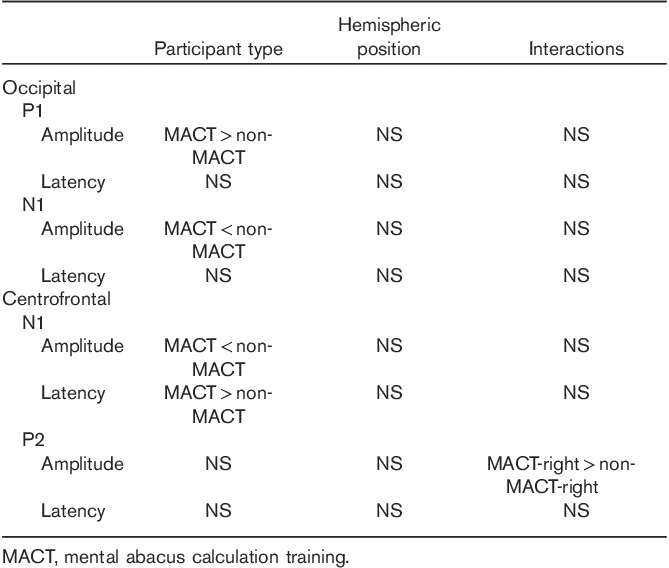
In the occipital region, the P1 amplitude showed that the main effect of participant type was significant [F(1, 28)=5.06, P<0.05, effect size=0.72]; the P1 amplitude of the MACT group (10.56±3.62 μV) was higher than that of the non-MACT group (7.96±2.65 μV) (Fig. 3). The main effect of the hemispheric position and the interactions between the participant type and hemispheric position were not significant. For P1 latency, there was no significant main effect or interaction in terms of participant type and hemispheric position.
Fig. 3.
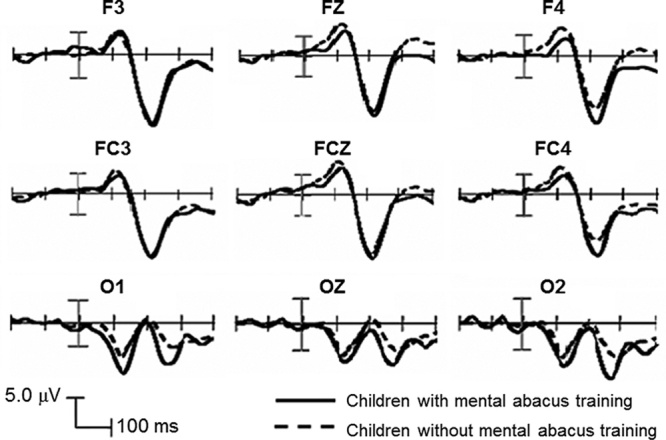
Centrofrontal and posterior ERP waveforms from the children with MACT (solid curves) and without MACT (dash curves). ERP, event-related potential; MACP, mental abacus calculation training.
The N1 amplitude in the occipital region showed a significant main effect of participant type [F(1, 28)=6.05, P<0.05, and effect size=0.82]. The absolute N1 amplitude of the MACT group (0.31±3.72 μV) was lower than that of the non-MACT group (3.65±3.85 μV) (Fig. 3). The effect of hemispheric position and the interactions between the participant type and hemispheric position were not significant. The N1 latency showed no significant main effect or interaction in terms of participant type and hemispheric position (Table 2).
Table 2.
Statistical findings in early event-related potential components between the children with and without mental abacus calculation training
Amplitudes and latencies of N1 and P2 in the centrofrontal brain region
In the centrofrontal region, the N1 amplitude showed that the main effect of participant type was significant [F(1, 28)=4.89, P<0.05, effect size=0.70]. However, the absolute N1 amplitude of the MACT group (6.28±4.96 μV) was lower than that of the non-MACT group (9.86±3.83 μV). The main effect of hemispheric position and the interactions between participant type and hemispheric position were not significant. For N1 latency, the main effect of participant type was significant [F(1, 28)=6.26, P<0.05, effect size=0.80]; the N1 latency of the MACT group (121.06±12.82 ms) was longer than that of the non-MACT group (110.81±8.73 ms) (Fig. 3). The main effect of hemispheric position and the interactions between participant type and hemispheric position were not significant.
The P2 amplitude in the centrofrontal lobe showed significant interactions between participant type and hemispheric position [F(2, 56)=5.87, P<0.01]. Further simple effect analysis showed that in the right hemisphere, the MACT group (16.35±7.02 μV) had a higher P2 amplitude than the non-MACT group (11.49±4.84 μV) [F(1, 28)=4.82, P<0.05, effect size=0.81] (Fig. 3). The main effects of participant type and hemispheric position were not significant. For P2 latency, neither the main effects of participant type and hemispheric position nor their interactions were significant (Table 2).
Discussion
This study investigated the impacts of MACT on children’s spatial attention orientation. Two groups of children, one with intensive MACT and the other without MACT or related exercise, were selected and matched in age, sex, socioeconomic status, handedness, and academic grade to explore their behavioral and ERP differences during the Posner spatial cueing task.
Previous studies 19–21 on visuospatial attention have shown that irrespective of the changes of cues as well as the shapes and locations of target stimuli, the Posner cueing effects are very stable. In this study, the cues were presented at the same location as the center of focus, which played the role of ‘endogenous orientation’. The behavioral data showed that for both groups of children, the valid cues led to a shorter reaction time and better accuracy than the invalid cues, which were consistent with the previous reports. These results indicate that both groups of children showed the cueing effects. The cue caused the participants to expect the side that the target stimuli would appear and then shifted their attention to that side. In case of a valid cue, the target indeed appeared on the expected side; therefore, the cue had a ‘priming’ effect that shortened the participants’ reaction time. In contrast, an invalid cue had an ‘antipriming’ effect that prolonged the participants’ reaction time.
The behavioral data also showed that the effects of MACT on the participants’ reaction time and accuracy were not significant. However, we did observe that the children with MACT had an overall shorter reaction time than those without MACT (Table 1). Such a difference was not statistically significant because of large individual variations. A previous study has suggested that the performance of the Posner spatial cueing task could be affected by personal characteristics 22. Therefore, these results were expected.
ERP studies of visuospatial attention have shown that the early ERP components are related to visual attention processing including the temporal–occipital P1 and N1 and centrofrontal N1 and P2 23,24. These studies led to the following question: while using a spatial cueing task, would there be any difference in the P1 and N1 amplitudes between the children with and without MACT? Our ERP results showed that under valid cues, the occipital P1 amplitude of the MACT group was significantly higher than that of the non-MACT group, whereas the occipital N1 amplitude of the MACT group was significantly lower than that of the non-MACT group. Conceptually, the amplitude of an ERP component reflects the total amount and/or synchronicity of activated neurons. Both are indicative of the degree of psychological load when the information is processed 25: the higher the ERP amplitude, the larger the amount and/or the stronger the synchronization of activated neurons involved in the information process. The occipital P1 component (O1, OZ, O2) represented the earliest stage in which the visual processing was modulated by the spatial attention cue 17. Therefore, in the case of valid cues, a higher occipital P1 amplitude in the MACT ground indicates that the trained children experienced greater neuronal activation of their visuospatial attention processing skills. The reduced N1 amplitude in the MACT group indicates that these children had a lower degree of neuronal activation in identifying the visual information compared with the children without MACT. These results provide evidence to support our primary hypothesis: MACT can enhance the children’s visuospatial attention processing as they are eased into the information identification within the attention focus. In other words, their long-time training is considered able to build up and facilitate the functional integration between the visuospatial network and the attention network 26.
The N1 component in the centrofrontal lobe could also be affected by attention. The N1 component mainly reflects an enhancement of feeling processing during the attention procedures as well as the procedures to identify and differentiate visual information 27, which is relatively more complicated 28. Our data showed under valid cues, the absolute N1 amplitude of the MACT group was lower than that of the non-MACT group. This finding is in accordance with the reduced amplitude of posterior N1 and further indicates that MACT facilities the information identification process. Subsequently, the neurons involved in the information identification and recognition are reduced and the latency is prolonged. These results provide evidence that intensive MACT can change the degree of activation in related brain regions 29,30.
The P2 component in the centrofrontal region reflected the detection of target feature and was correlated with the early identification of the target stimulus. The P2 component is considered to be largely affected by the visual attention scale 31; the changes in its amplitude reflect changes in visual attention concentration as well as regulatory mechanisms of the attention ‘quantity’ aspect. In this study, the MACT group showed a higher P2 amplitude in the right hemisphere of the frontal lobe than that shown in the non-MACT group, but there was no significant difference in the left hemisphere. These results indicated that MACT improved the children’s abilities to focus their attention on identifying the target, but only the right frontal region was affected.
A previous study by Buschkuehl et al. 29 has indicated that cognitive training could lead to changes in brain activation pattern, which compromises both increased and decreased degrees of activation. In this study, the children with intensive MACT showed increased amplitude in the occipital P1 component as well as decreased amplitudes in both occipital and centrofrontal N1 components, which are in line with the existing knowledge. In addition, it is generally believed that the right hemisphere is dominant for orienting of visuospatial attention 32. For the centrofrontal P2 component, we have found the MACT group had higher P2 amplitude than the non-MACT group in the right hemisphere. This finding indicates that the MACT might have strengthened the right hemisphere dominance in the spatial attention orientation process in the trained children.
Finally, two major limitations in this study should be noted. First, we did not measure the baseline from the two groups of participants before MACT began. However, the two groups were matched according to age, sex, socioeconomic status, handedness, and academic grade at the time of enrollment. We have a reason to believe that no significant group difference was present at the baseline. Second, the ERP components in this study were analyzed and presented channel-wise only. The actual source locations of these components could vary largely from their electrode positions. Over the past two decades, a variety of methods and software have been developed to estimate the sources of scalp-recorded potentials 33,34. Future study should consider using these methods and software for better source localization and visualization.
Conclusion
MACT enhances the children’s spatial attention orientation, which can be detected in the early components of ERP including posterior P1/N1 and centrofrontal N1/P2.
Acknowledgements
The authors thank all of the participants and their parents/legal guardians for their participation in the study.
This study was supported by the Provincial Natural Science Foundation of Shandong, China (no.ZR2011CM001) and the Provincial Adolescent Research Plan of Shandong, China (no. SDYSB150354).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Li Y, Wang Y, Hu Y, Liang Y, Chen F. Structural changes in left fusiform areas and associated fiber connections in children with abacus training: evidence from morphometry and tractography. Front Hum Neurosci 2013; 7:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu Y, Geng F, Tao L, Hu N, Du F, Fu K, Chen F. Enhanced white matter tracts integrity in children with abacus training. Hum Brain Mapp 2011; 32:10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du F, Chen F, Li Y, Hu Y, Tian M, Zhang H. Abacus training modulates the neural correlates of exact and approximate calculations in Chinese children: an fMRI study. Biomed Res Int 2013; 2013:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka S, Seki K, Hanakawa T, Harada M, Sugawara SK, Sadato N, et al. Abacus in the brain: a longitudinal functional MRI study of a skilled abacus user with a right hemispheric lesion. Front Psychol 2012; 28:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanakawa T, Honda M, Okada T, Fukuyama H, Shibasaki H. Neural correlates underlying mental calculation in abacus experts: a functional magnetic resonance imaging study. Neuroimage 2003; 19:296–307. [DOI] [PubMed] [Google Scholar]
- 6.Ku Y, Hong B, Zhou W, Bodner M, Zhou YD. Sequential neural processes in abacus mental addition: an EEG and fMRI case study. PLoS One 2012; 7:e36410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamada T, Iwaki S. Speed of mental addition in an abacus expert, estimated by eye movements and neural activities. Percept Mot Skills 2012; 115:1–6. [DOI] [PubMed] [Google Scholar]
- 8.Chen F, Hu Z, Zhao X, Wang R, Yang Z, Wang X, Tang X. Neural correlates of serial abacus mental calculation in children: a functional MRI study. Neurosci Lett 2006; 403 (1–2):46–51. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka S, Michimata C, Kaminaga T, Honda M, Sadato N. Superior digit memory of abacus experts:an event-related functional MRI study. NeuroReport 2002; 13:2187–2191. [DOI] [PubMed] [Google Scholar]
- 10.Dehaene S, Piazza M, Pinel P, Cohen L. Three parietal circuits for number processing. Cogn Neuropsychol 2003; 20 (3–6):487–506. [DOI] [PubMed] [Google Scholar]
- 11.Piazza M, Dehaene S. Gazzaniga MS. From number neurons to mental arithmetic: the cognitive neuroscience of number sense. The cognitive neurosciences, 3rd ed Cambridge, MA: MIT Press; 2004. 865–876. [Google Scholar]
- 12.Sun Y, Li X, Gao W, Xu G, Yang H, Liu X. Research of ERPs on the early process to visualspatial attention by mental abacus calculation children. Chinese J School Health 2012; 33:185–186. 189. [Google Scholar]
- 13.Posner MI. Orienting of attention. Exp Psychol 1980; 32:3–25. [DOI] [PubMed] [Google Scholar]
- 14.Di Russo F, Martinez A, Sereno MI, Pitzalis S, Hillyard SA. Cortical sources of the early components of the visual evoked potential. Hum Brain Mapp 2001; 15:95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milne E, Dunn SA, Freeth M, Rosas-Martinez L. Visual search performance is predicted by the degree to which selective attention to features modulates the ERP between 350 and 600 ms. Neuropsychologia 2013; 51:1109–1118. [DOI] [PubMed] [Google Scholar]
- 16.Heinze HJ, Luck SJ, Mangun GR, Hillyard SA. Visual event-related potentials index focused attention within bilateral stimulus arrays: Evidence for early selection. Electroencephalogr Clin Neurophysiol 1990; 7:511–527. [DOI] [PubMed] [Google Scholar]
- 17.Mangun GR, Hillyard SA. Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual-spatial priming. J Exp Psychol 1991; 17:1057–1074. [DOI] [PubMed] [Google Scholar]
- 18.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979; 6:65–70. [Google Scholar]
- 19.Hood BM, Willen JD, Driver J. Adults’ eyes trigger shifts of visual attention in human infants. Psychol Sci 1998; 9:131–134. [Google Scholar]
- 20.Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. J Abnorm Psychol 2002; 111:225–236. [DOI] [PubMed] [Google Scholar]
- 21.Driver J, Davis G, Ricciardelli P, Kidd P, Maxwell E, Baron-Cohen S. Gaze perception triggers reflexive visuospatial orienting. Vis cogn 1999; 6:509–540. [Google Scholar]
- 22.Pérez-Edgar K, Fox NA. A behavioral and electrophysiological study of children’s selective attention under neutral and affective conditions. J Cogn Dev 2005; 6:89–118. [Google Scholar]
- 23.Gao W, Wei J, Peng X, Wei X, Luo Y. Brain dynamic mechanisms on the visual attention scale with Chinese characters cues. Chinese Sci Bull 2002; 47:1644–1649. [Google Scholar]
- 24.Brignani D, Guzzon D, Marzi CA, Miniussi C. Attentional orienting induced by arrows and eye-gaze compared with an endogenous cue. Neuropsychologia 2009; 47:370–381. [DOI] [PubMed] [Google Scholar]
- 25.Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc Natl Acad Sci USA 1998; 95:781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Hu Y, Zhao M, Wang Y, Huang J, Chen F. The neural pathway underlying a numerical working memory task in abacus-trained children and associated functional connectivity in the resting brain. Brain Res 2013; 1539:24–33. [DOI] [PubMed] [Google Scholar]
- 27.Luck SJ. Multiple mechanisms of visual-spatial attention: recent evidence from human electrophysiology. Behav Brain Res 1995; 71 (1–2):113–123. [DOI] [PubMed] [Google Scholar]
- 28.Vogel EK, Luck SJ. The visual N1 component as an index of a discrimination process. Psychophysiology 2000; 37:190–203. [PubMed] [Google Scholar]
- 29.Buschkuehl M, Jaeggi SM, Jonides J. Neuronal effects following working memory training. Dev Cogn Neurosci 2012; 2 (Suppl 1):S167–S179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jolles DD, Crone EA. Training the developing brain: a neurocognitive perspective. Front Hum Neurosci 2012; 6:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emier M. An ERP study of sustained spatial attention to stimulus eccentricity. Biol Psychol 2000; 52:205–220. [DOI] [PubMed] [Google Scholar]
- 32.Barthélémy S, Boulinguez P. Orienting visuospatial attention generates manual reaction time asymmetries in target detection and pointing. Behav Brain Res 2002; 133:109–116. [DOI] [PubMed] [Google Scholar]
- 33.Pascual-Marqui RD. Review of methods for solving the EEG inverse problem. Int J Bioelectromagn 1999; 1:75–86. [Google Scholar]
- 34.Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol 2002; 24 (Suppl D):5–12. [PubMed] [Google Scholar]


