Abstract
The results of simultaneous liver-kidney transplants in highly sensitized recipients have been controversial in terms of antibody-mediated rejection and kidney allograft outcomes. This case report provides a detailed and sophisticated documentation of histocompatibility and pathologic data in a simultaneous liver-kidney transplant performed in a recipient with multiple high-titered class I and II antidonor HLA antibodies and a strongly positive cytotoxic crossmatch. Patient received induction with steroids, rituximab, and eculizumab without lymphocyte depleting agents. The kidney transplant was delayed by 6 hours after the liver transplant to allow more time to the liver allograft to “absorb” donor-specific antibodies (DSA). Interestingly, the liver allograft did not prevent immediate antibody-mediated injury to the kidney allograft in this highly sensitized recipient. Anti-HLA single antigen bead analysis of liver and kidney allograft biopsy eluates revealed deposition of both class I and II DSA in both liver and kidney transplants during the first 2 weeks after transplant. Afterward, both liver and kidney allograft functions improved and remained normal after a year with progressive reduction in serum DSA values.
Clinical evidence suggests that the liver allograft exerts an immunoprotective effect from antibody-mediated injury on the kidney allograft in simultaneous liver kidney (SLK) deceased donor transplants when antidonor HLA antibodies are present at levels high enough to generate a positive crossmatch.1-3 Hyperacute rejection is generally not observed in the kidney allograft in SLK transplants performed in the face of a positive crossmatch.4 This protective effect is thought to be potentially due to HLA antibody absorption by the liver as preformed HLA donor-specific antibody (DSA) levels (especially class I) often decrease or disappear following SLK.4-6 It is important to note, however, that most of the experience with SLK transplants in patients with a positive crossmatch were not focused specifically on the patients with the highest degrees of sensitization. The data on SLK transplants in very highly sensitized recipients (ie, with very high preformed DSA levels) is scant and based on a few reports often lacking detailed immunocompatibility and pathology assessments. Some studies compared sensitized SLK recipients with nonsensitized SLK recipients and did not find any difference in antibody-mediated rejection (AMR) rates, kidney graft survival, and patient survival.3,5
Several studies have shown that acute kidney rejection incidence is reduced in SLK transplants compared to kidney transplants alone.3,7 This potential immunoprotective effect in SLK has been used to explain the better outcomes of SLK compared to kidney transplants after liver transplants.8 In many transplant centers, SLK are allocated based only on ABO compatibility without consideration of crossmatch results or level of HLA sensitization in the recipient.1,4,5,9
SLK outcomes have become increasingly relevant due to the rising number of SLK procedures following the introduction of the model for end-stage liver disease for liver allocation.10,11 In many instances, SLK candidates have significantly decompensated liver disease, tolerate desensitization treatments poorly, and often cannot wait for an optimally HLA matched donor. In addition, optimal induction protocols and early immunosuppressive treatments for highly sensitized SLK recipients have not been established. The aim of this report is to present a detailed evaluation of HLA antibody-mediated kidney and liver injury in a transplant recipient with extraordinarily high levels of preformed DSA treated with a novel immunosuppressive regimen including rituximab induction and eculizumab maintenance therapy.
CASE DESCRIPTION
A 64-year-old white woman presented with decompensated cirrhosis secondary to chronic hepatitis C, with concomitant idiopathic chronic kidney disease and a history of previous right radical nephrectomy for renal cell carcinoma. At the time of transplant, patient model for end-stage liver disease score was 40 (serum bilirubin, 16.6 mg/dL; international normalized ratio, 2.5), and she was on hemodialysis for oliguric renal failure. Pretransplant HLA antibody analysis revealed a calculated panel-reactive antibody (CPRA) at 1500 mean fluorescence intensity (MFI) cutoff of 100%, CPRA4000 of 100%, and CPRA8000 of 100%. A dilution analysis of single HLA antigen bead (SAB) microarray assay was necessary to titer accurately preformed anti-HLA antibodies because of the saturating levels of anti-HLA antibodies.12 The immunodominant anti-HLA class I antibody was A1 (14 100 MFI at a dilution titer of 1:4096). The immunodominant anti-HLA class II antibody was DR17 (8800 MFI at a titer of 1:1024). HLA sensitization was due to 2 previous pregnancies and previous blood transfusions.
A 38-year old blood type O deceased donor with normal liver and kidney function became available. Eight HLA antigens were mismatched (A1, B8, B35, Cw4, DR17, DR52, DQ2; DQA1*05, Table 1). Virtual crossmatch was positive with the following DSA: A1 at 14,100 MFI (1:4096), B35 at 6,700 MFI (1:1024), B8 at 11,200 MFI (1:32), DR17 at 22,100 MFI (1:32), DQ2 at 18,200 MFI (1:32), DR52 at 19,900 (1:32). Before liver transplant, both cytotoxic (titer = 1:1024) and flow cytometry crossmatches resulted positive. T-cell flow cytometry crossmatch was positive at 345 channels above the positive cutoff, B cell crossmatch was positive at 364 channels above the positive cutoff (1024 scale) (Table 2). Although histocompatibility testing revealed evidence of substantial presensitization, patient’s clinical conditions were deteriorating, therefore transplantation with this donor was deemed necessary for survival.
TABLE 1.
HLA phenotypes of donor and recipient
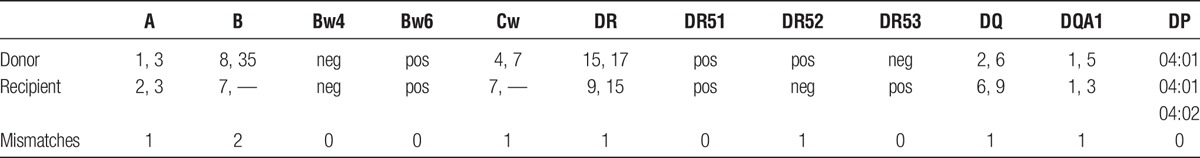
TABLE 2.
Crossmatch results before and 6 hours after liver transplant
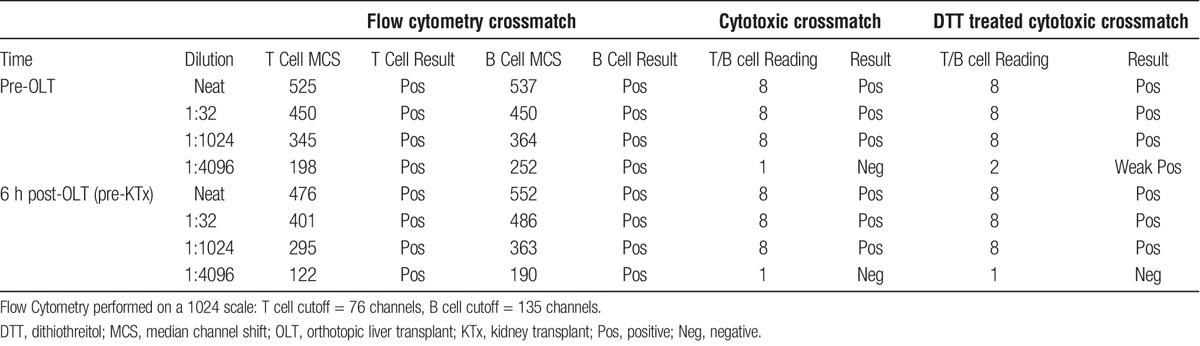
The liver transplant procedure was uneventful (estimated blood loss, 1250 mL), and the kidney transplant was deliberately delayed for 6 hours after liver allograft reperfusion to allow for the liver allograft absorption of DSA. Total cold ischemic times for the liver and the kidney were 6 and 13 hours, anastomotic times were 39 and 35 minutes, respectively. In consideration of her high DSA titers, patient received induction with rituximab (375 mg/m2) and methylprednisolone 500 mg during the anhepatic phase of liver transplant, and eculizumab (1200 mg) before reperfusion of the kidney allograft. After surgery, patient received tacrolimus (target through, 10 mg/mL), mycophenolate mofetil (500 mg by mouth twice a day), and a corticosteroid taper. Cytotoxic and flow cytometry crossmatches repeated 6 hours after liver transplant before the implantation of the kidney allograft, remained markedly positive: T-cell flow cytometry crossmatch (295 channels above the positive cutoff, 1:1024 scale), B cell crossmatch (363 channels above the positive cutoff, 1:1024 scale). DSA levels from SAB analysis are presented in Table 3.
TABLE 3.
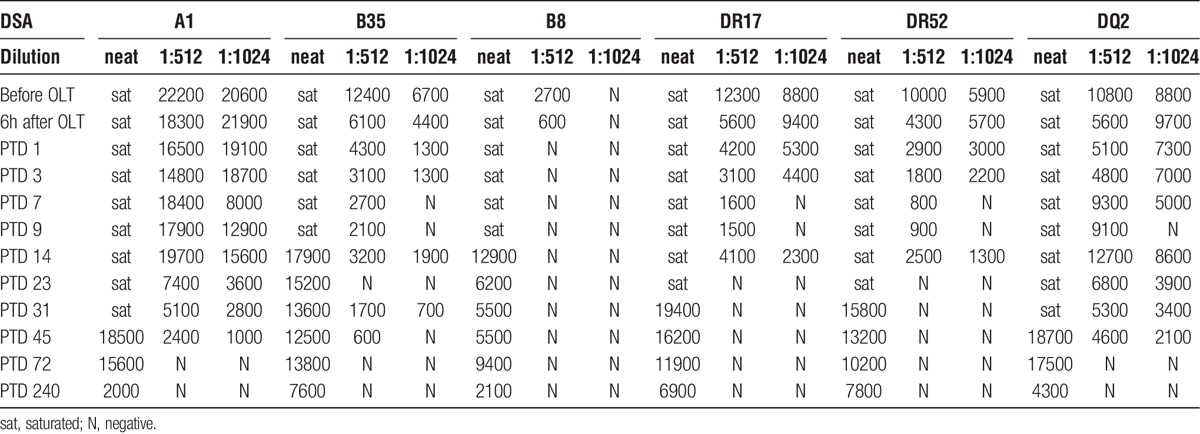
Anti-HLA donor-specific antibody titers before and after transplant (MFI)
During the initial postoperative period, patient's liver function was adequate with progressive decline in her transaminase levels (Figure 1). Renal allograft function was delayed with oliguric acute tubular necrosis for the first 4 posttransplant days. Continuous renal replacement therapy was continued for the first 4 days posttransplant to relieve volume overload and oliguria. Postreperfusion liver biopsy showed mild diffuse hepatocyte swelling and scattered neutrophilic infiltrates compatible with mild ischemia-reperfusion injury (Figure 2). Postreperfusion renal transplant biopsy showed mild acute tubular injury in the kidney with nonspecific focal glomerular C4d staining (Figure 3). Electron microscopy of the postreperfusion renal transplant biopsy showed degenerative changes in the endothelial cells of the capillaries with separation of the endothelial cells from the basement membrane (Figures 3J, K). On posttransplant day (PTD) 2, an open kidney transplant biopsy was performed and demonstrated strong, diffuse C4d staining of peritubular and glomerular capillaries with mild tubular injury and peritubular capillaritis consistent with acute AMR (Figure 3). A second dose of eculizumab (1200 mg) was given. Urine output improved on PTD 5 and her serum creatinine progressively decreased thereafter.
FIGURE 1.
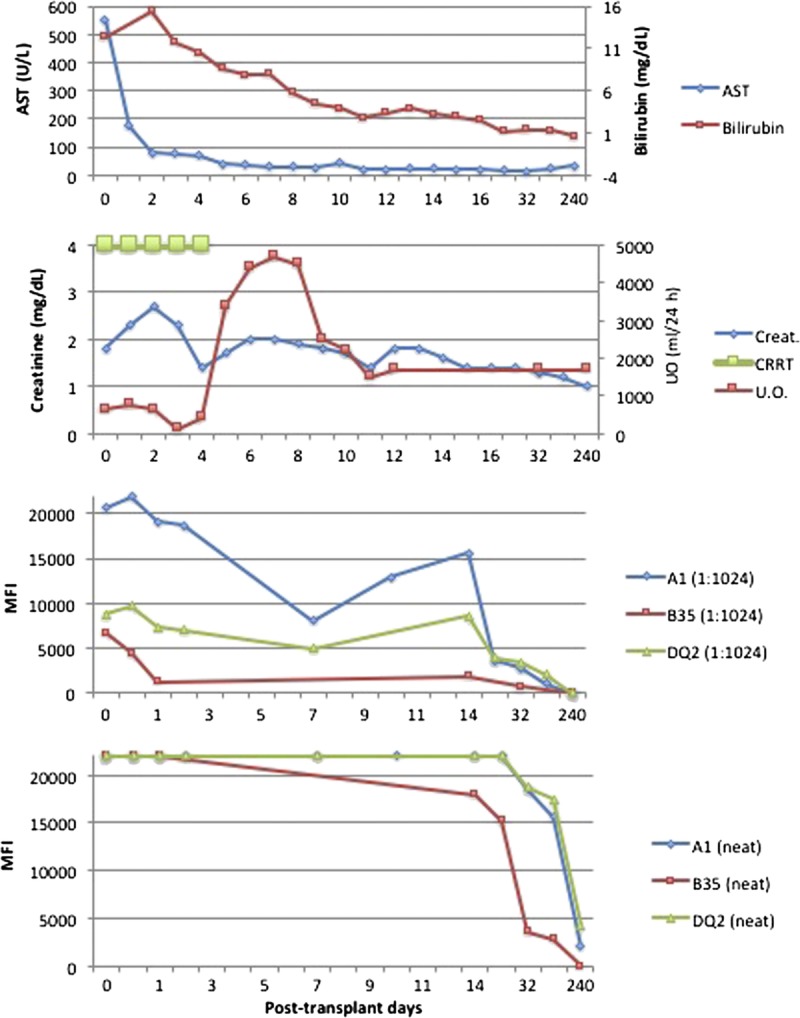
Serum AST, serum total bilirubin, serum creatinine, daily U.O., CRRT, DSA levels on neat and diluted sera (at 1:1024) after transplant. AST, aspartate aminotransferase; CRRT, continuous renal replacement therapy; U.O., urine output.
FIGURE 2.
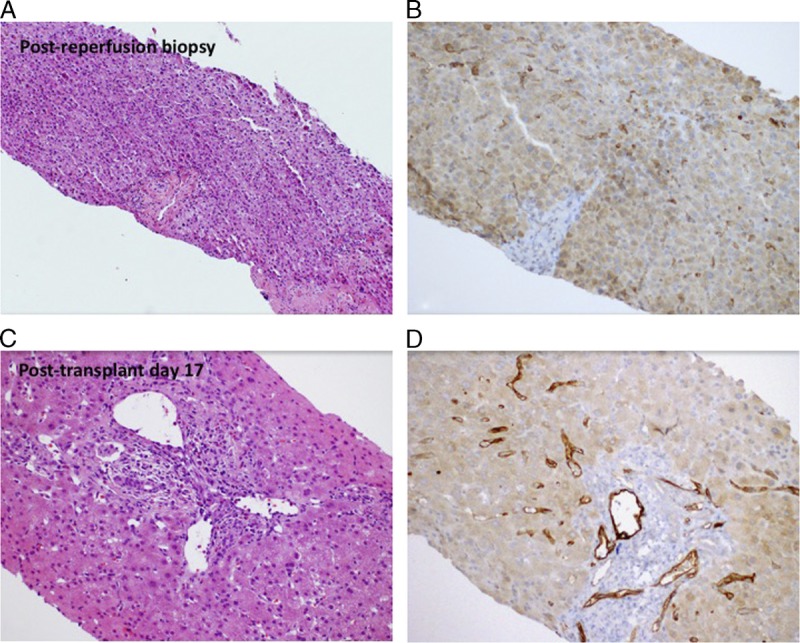
Liver transplant biopsies. Postreperfusion liver transplant biopsy shows (A) mild diffuse hepatocyte swelling with scattered neutrophilic inflammation consistent with mild preservation/reperfusion injury. C4d immunostain (B) exhibits weak focal positivity with hepatocellular background. On posttransplant day 17, liver biopsy shows (C) mixed inflammatory infiltrate in the portal tracts, including eosinophils, lymphocytes, and plasma cells. C4d immunostain (D) is now strongly positive in portal tract venules and sinusoids.
FIGURE 3.
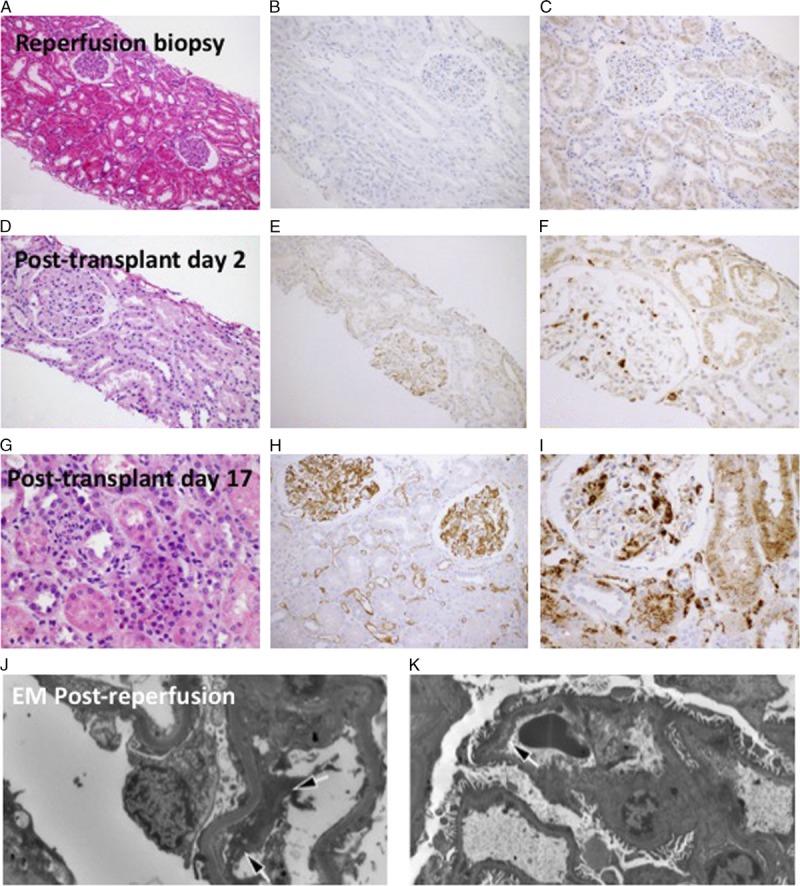
Kidney transplant biopsies. Postreperfusion kidney transplant biopsy shows (A) mild tubular dilatation without significant inflammatory infiltrates, (B) nonspecific focal glomerular C4d staining, and (C) scattered CD68-positive mononuclear cells in the interstitium and in the glomeruli. On PTD 2, the renal allograft biopsy demonstrates (D) mild tubular injury, (E) diffuse linear C4d staining along glomerular and peritubular capillaries, and (F) scattered CD-68-positive mononuclear cells in the interstitium and in the glomeruli. Renal allograft biopsy on PTD 17 shows (G) tubular dilatation with prominent cytoplasmic vacuoles, mild inflammatory infiltrates and capillaritis, (H) stronger C4d staining in glomerular and peritubular capillaries, (I) numerous CD68 positive cells. J, K, Electron microscopic examination of the postreperfusion kidney transplant biopsy obtained approximately 30 minutes postreperfusion. There are already degenerative changes in the endothelial cell layer of capillary loops with a significant separation of the endothelial cells from the basement membrane (arrows).
On PTD 13, a bilirubin elevation up to 3.9 mg/dL and alkaline phosphatase elevation up to 150 U/L were noted, while aspartate aminotransferase and alanine aminotransferase were normal. A liver biopsy was performed and demonstrated mild mixed inflammatory infiltrate in portal tracts, including frequent eosinophils, scattered lymphocytes and rare neutrophils and plasma cells (Figure 2). Eosinophils infiltrating portal venules were present. C4d immunostain showed strong positivity in the portal tract venules and periportal sinusoids. At the same time, the patient’s serum creatinine reached a level of 1.8 mg/dL without further improvement. Kidney allograft biopsy on PTD 17 showed strong and diffuse C4d staining involving peritubular and glomerular capillaries with mild tubular injury and peritubular capillaritis consistent with acute AMR (Figure 3). Class I and II DSA levels at that time were still very high (Figures 4, 5).
FIGURE 4.
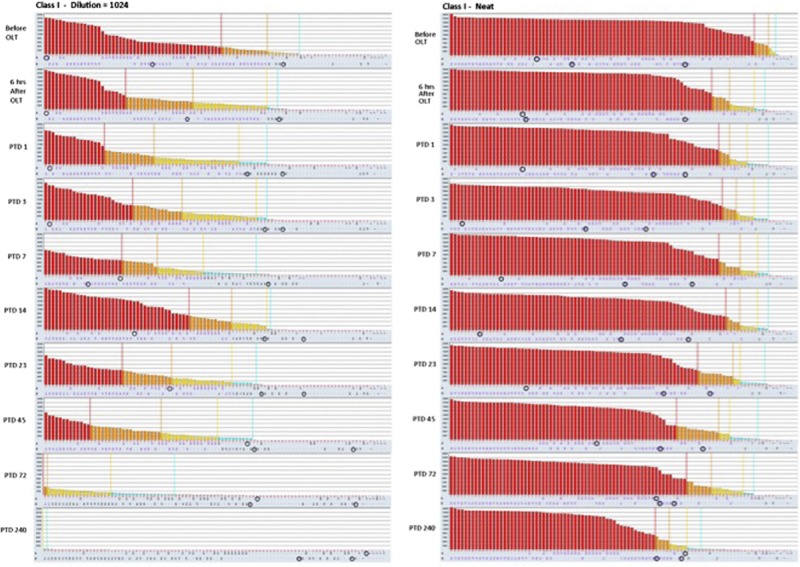
Class I HLA antibody histograms obtained from single antigen bead assay on diluted (1:1024) and neat sera. There is a progressive reduction in class I HLA antibody MFI values, especially a month after transplantation. The DSA specificities, marked by blue circles, migrate toward the right of the plots indicating a significant reduction in MFI values.
FIGURE 5.
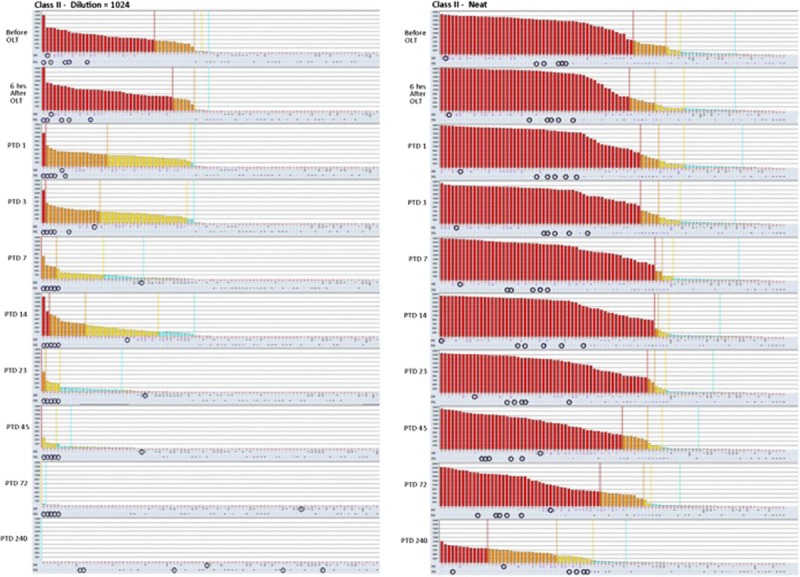
Class II HLA antibody histograms obtained from single antigen bead assay on diluted (1:1024) and neat sera.
HLA antibody elutions of the kidney and liver transplant biopsies were analyzed by SAB assay and demonstrated the presence of class I and II DSA in both liver and kidney biopsies (Table 4, Figure 6). As shown in Figure 6B, the deposition of HLA antibodies in the kidney transplant increased from PTD 2 to PTD 17 when compared to the HLA antibody deposition in the liver transplant. Despite the fact that most of serum DSA levels decreased (Figure 6C), the amount of DSA eluted in the kidney allograft increased.
TABLE 4.
Eluted liver and kidney transplant DSAs, serum DSA (MFI)
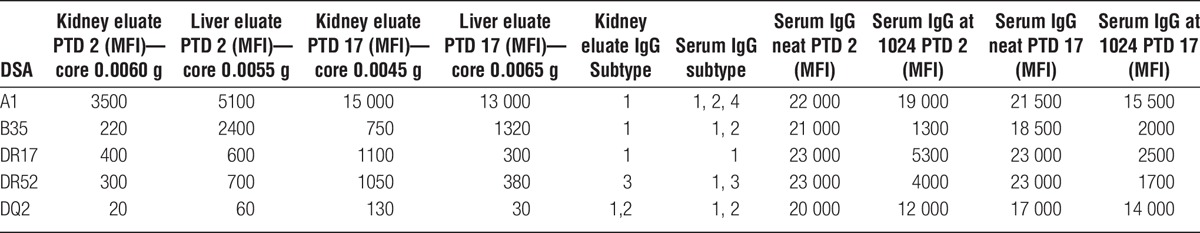
FIGURE 6.
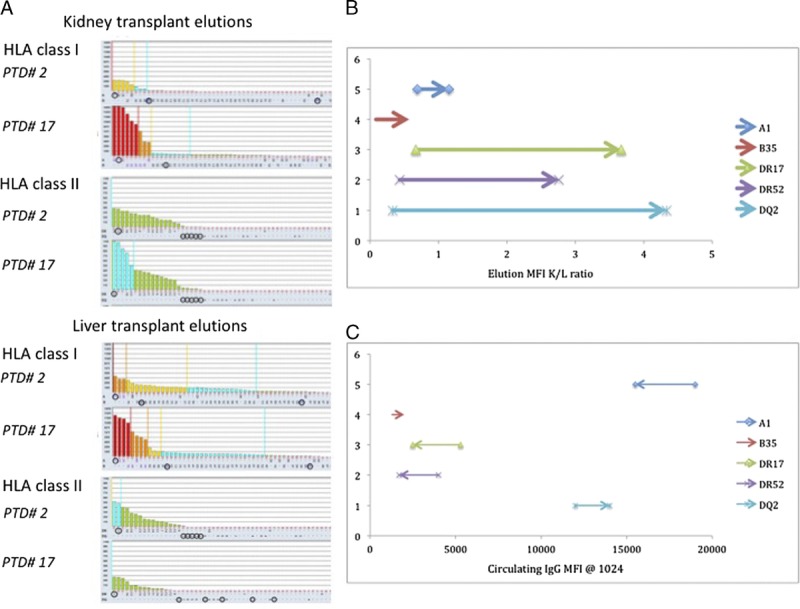
Eluted kidney and liver transplant HLA antibodies. There is a progressive reduction in class II HLA antibody MFI values after transplantation as demonstrated by the decreasing height of histograms. HLA antibody elutions of kidney and liver transplant biopsies were analyzed by single antigen bead assay. Both class I and II HLA antibody deposition in the kidney allograft increased from PTD 2 to PTD 17 (A). B, Variation on HLA antibody elutions (MFI) kidney-to-liver ratio based on kidney and liver biopsies on PTD 2 and PTD 17 (each arrow is oriented from the ratio at PTD 2 toward the ratio at PTD 17). C, Variation in circulating IgG DSA values (MFI) on PTD 2 and PTD 17 (each arrow is oriented from the value at PTD 2 toward the value at PTD 17).
Patient was treated with weekly doses of eculizumab (1200 mg) on PTD 11, 18, 25, and 32. During the following weeks, her liver function tests became normal, her creatinine decreased further. At her last clinic follow-up, 1 year after transplant, she was doing well with normal liver and kidney functions, total bilirubin was 0.2 mg/dL, baseline serum creatinine was 1.1 mg/dL, and without abnormal proteinuria (urine protein-to-creatinine ratio, 0.11). Figures 4 and 5 display the HLA antibody histograms obtained with SAB assays on neat and diluted sera after transplant. The analysis on diluted sera showed that the titers of both class I and II anti-HLA antibodies decreased progressively after transplantation. Furthermore, both class I and II DSA levels decreased more than the other anti-HLA antibodies. On PTD 240, patient still had high titers of anti-HLA antibodies on neat sera; however, all DSA decreased below 8000 MFI.
DISCUSSION
This case presents a detailed documentation of clinical, pathologic, and histocompatibility data using the state of the art testing with a level of documentation that supersedes previous reports of SLK transplants in highly sensitized recipients. The clinical and pathologic data in this case support the observation that terminal complement inhibition in this highly sensitized patient undergoing a simultaneous liver and kidney transplantation did not prevent either the kidney or the liver allograft from developing AMR. It is possible, however, that eculizumab mitigated kidney and liver allograft injury. Anti-HLA antibody SAB analysis of kidney and liver allografts eluates clearly demonstrated the deposition of both class I and II DSA in the liver and kidney allografts with strongly positive C4d staining in both the liver and kidney allografts. Previous studies have supported the concept of an immunoprotective effect by the liver in combined SLK transplants.7,13 This immunoprotection seemed to prevent renal transplant AMR even in “sensitized” SLK recipients.3,5 Clearly documented cases4,14 have indicated that early AMR may occur in “sensitized” SLK recipients indicating that the immunoprotective effect of the liver is not universal. The levels of DSA in our patient were extraordinarily high to the point that they saturated the single-antigen beads, and to obtain a measurable MFI titer, we had to dilute the recipient serum to 1:1024. These very high DSA levels may explain why clinical and pathologic signs of AMR developed in both the kidney and liver allografts.
A previous study showed that SLK can be performed safely in the face of a positive crossmatch. Repeated crossmatch testing performed on sera obtained 1 hour after liver transplantation revealing conversion to a negative results suggested that liver reduces HLA levels by absorption (although this evidence is indirect).1 In our patient, the kidney transplant was delayed by 6 hours to provide more time to the liver allograft to “absorb DSA“ from circulation. However, despite this delay, the repeat crossmatch 6 hours after liver transplant was still positive at the same titer. DSA levels as assessed by SAB dilutional analysis before and 6 hours after the liver transplant revealed that levels of both class I and II DSA were unchanged. The failure of the liver allograft to reduce DSA may have been due to the very high DSA levels, raising the possibility that the capacity of the liver allograft to reduce anti-HLA antibody levels may be limited in the presence of extremely high HLA antibody levels. In other words, if the immunoprotective effect of the liver is mediated by HLA antibody absorption, this HLA antibody absorption may have a limit and may not be adequate in extremely highly sensitized recipients.
The prospective DSA analysis 1 month after transplant showed a first significant decline in DSA levels class I and II when the DSA did no longer saturate the HLA SAB. In the following months, DSA levels declined further, but slowly. It was only after 8 months that DSA values fell below 10 000 MFI on neat sera. Previous studies have demonstrated different kinetics in the MFI decline between class I and class II antibodies.4,6 In the present case, DSA analysis did not show difference between class I and class II antibodies. However, DSA elution studies showed that the relative deposition class II HLA increases in the kidney transplant at higher rate than class I HLA (Figure 4A). Despite “the immunoprotective effect of the liver allograft,” the amount of class I and class II DSA eluded in the kidney transplant increased during the first 2 weeks after transplantation.
The immunosuppression protocols and induction treatments for SLK transplants have not been established, and there is no clear evidence supporting any specific protocol even in highly sensitized recipients. Analysis of the Scientific Registry of Transplant Recipients showed that only a minority of patients undergoing SLK usually receive lymphocyte-depleting agents as induction (14-19%), even among sensitized recipients.9 Several centers use only an interleukin-2 receptor antagonist, such as basiliximab for induction in SLK transplants.3,15,16 Our patient had a large amount of pre-formed anti-HLA donor-specific antibodies therefore, we targeted the antibody-mediated complement activation with eculizumab and B lymphocytes with rituximab.
The patient exhibited a significant degree of sensitization with high DSA levels and significant complement activation in both liver and kidney transplants as demonstrated by the C4d stains. Despite this significant C4d deposition, the patient developed only mild clinical signs of AMR in both liver and kidney allografts, which may have been due to eculizumab blockade of terminal activation. Eculizumab is a monoclonal antibody specific for the C5 convertase that has been used for terminal complement blockade in highly sensitized kidney transplant recipients. A case-control study showed that the use of eculizumab in sensitized kidney transplant recipients with initial positive crossmatch is associated with a lower incidence of AMR in the first 3 months after kidney transplant.17 In this study, patients treated with eculizumab still had kidney transplant biopsy with positive C4d stains but did not develop significant signs of tubular and capillary injury on light and electron microscopy.17 Despite high serum DSA and strong C4d stain on kidney and liver biopsy, our patient developed only mild histological signs of AMR and renal dysfunction. It appears reasonable that the treatment with eculizumab probably limited the terminal activation of the complement and the tissue injury related, therefore patient developed only mild histologic signs of tissue injury and mild renal and liver dysfunction.
This is the first report of the use of rituximab and eculizumab induction with eculizumab maintenance therapy for AMR in SLK recipient with good clinical results and acceptable toxicity. It is important to note that it is not clear how much eculizumab contributed to the successful outcome; however, this carefully studied case provides important insights into SLK transplantation in patients with very high DSA levels.
In conclusion, this case indicates that: (1) in the presence of extremely high preformed class I and II DSA, simultaneous liver transplant did not prevent AMR in the kidney transplant, even with a 6-hour delay between liver and kidney transplants from the same donor; (2) DSA levels may be high enough in some patients that the liver cannot protect the kidney by DSA absorption, therefore, the liver may have a threshold for absorbing antibodies in the cases of extremely elevated titers; (3) induction with rituximab and eculizumab therapy was well tolerated and might have minimized histologic and clinical findings of AMR with excellent intermediate-term results.
Footnotes
Published online 23 November, 2016.
The authors declare no funding or conflicts of interest.
S.E.W. participated in the study conceptualization, revision of all article drafts. F.P. participated in data collection, preparation of original article draft and revisions. S.S., M.C., T.D. participated in the critical article review. A.G. and P.B. participated in histocompatibility data analysis. D.W. and J.W. participated in pathology data analysis. S.T., J.R., R.A. participated in pharmacology data analysis, critical article review. M.R.S. and N.A. participated in critical article review.
REFERENCES
- 1.Morrissey PE, Gordon F, Shaffer D, et al. Combined liver-kidney transplantation in patients with cirrhosis and renal failure: effect of a positive cross-match and benefits of combined transplantation. Liver Transpl. 1998;4:363–369. [DOI] [PubMed] [Google Scholar]
- 2.Creput C, Durrbach A, Samuel D, et al. Incidence of renal and liver rejection and patient survival rate following combined liver and kidney transplantation. Am J Transplant. 2003;3:348–356. [DOI] [PubMed] [Google Scholar]
- 3.Hanish SI, Samaniego M, Mezrich JD, et al. Outcomes of simultaneous liver/kidney transplants are equivalent to kidney transplant alone: a preliminary report. Transplantation. 2010;90:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dar W, Agarwal A, Watkins C, et al. Donor-directed MHC class I antibody is preferentially cleared from sensitized recipients of combined liver/kidney transplants. Am J Transplant. 2011;11:841–847. [DOI] [PubMed] [Google Scholar]
- 5.Leca N, Warner P, Bakthavatsalam R, et al. Outcomes of simultaneous liver and kidney transplantation in relation to a high level of preformed donor-specific antibodies. Transplantation. 2013;96:914–918. [DOI] [PubMed] [Google Scholar]
- 6.O'Leary JG, Gebel HM, Ruiz R, et al. Class II alloantibody and mortality in simultaneous liver-kidney transplantation. Am J Transplant. 2013;13:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruiz R, Kunitake H, Wilkinson AH, et al. Long-term analysis of combined liver and kidney transplantation at a single center. Arch Surg. 2006;141:735–741; discussion 41–2. [DOI] [PubMed] [Google Scholar]
- 8.Simpson N, Cho YW, Cicciarelli JC, et al. Comparison of renal allograft outcomes in combined liver-kidney transplantation versus subsequent kidney transplantation in liver transplant recipients: analysis of UNOS Database. Transplantation. 2006;82:1298–1303. [DOI] [PubMed] [Google Scholar]
- 9.Askar M, Schold JD, Eghtesad B, et al. Combined liver-kidney transplants: allosensitization and recipient outcomes. Transplantation. 2011;91:1286–1292. [DOI] [PubMed] [Google Scholar]
- 10.Dube GK, Cohen DJ. Simultaneous liver and kidney transplantation. Curr Opin Nephrol Hypertens. 2007;16:547–553. [DOI] [PubMed] [Google Scholar]
- 11.Freeman RB, Jr, Wiesner RH, Harper A, et al. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8:851–858. [DOI] [PubMed] [Google Scholar]
- 12.Woodle ES, Shields AR, Ejaz NS, et al. Prospective iterative trial of proteasome inhibitor-based desensitization. Am J Transplant. 2015;15:101–118. [DOI] [PubMed] [Google Scholar]
- 13.Rana A, Robles S, Russo MJ, et al. The combined organ effect: protection against rejection? Ann Surg. 2008;248:871–879. [DOI] [PubMed] [Google Scholar]
- 14.Reichman TW, Marino SR, Milner J, et al. Acute humoral rejection in an ABO compatible combined liver-kidney transplant—the kidney is not always protected. Am J Transplant. 2009;9:1957–1960. [DOI] [PubMed] [Google Scholar]
- 15.Doyle MB, Subramanian V, Vachharajani N, et al. Results of simultaneous liver and kidney transplantation: a single-center review. J Am Coll Surg. 2016;223:193–201. [DOI] [PubMed] [Google Scholar]
- 16.Lunsford KE, Bodzin AS, Markovic D, et al. Avoiding futility in simultaneous liver-kidney transplantation: analysis of 331 consecutive patients listed for dual organ replacement. Ann Surg. 2016. [published online ahead of print May 26, 2016]. 10.1097/SLA.0000000000001801 [DOI] [PubMed]
- 17.Stegall MD, Diwan T, Raghavaiah S, et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011;11:2405–2413. [DOI] [PubMed] [Google Scholar]


