Abstract
Background
The uremic milieu exposes chronic kidney disease (CKD) patients to premature ageing processes. The impact of renal replacement therapy (dialysis and renal transplantation [RTx]) or immunosuppressive treatment regimens on ageing biomarkers has scarcely been studied.
Methods
In this study telomere length in whole blood cells was measured in 49 dialysis patients and 47 RTx patients close to therapy initiation and again after 12 months. Forty-three non-CKD patients were included as controls.
Results
Non-CKD patients had significantly (P ≤ 0.01) longer telomeres than CKD patients. Telomere attrition after 12 months was significantly greater in RTx patients compared to dialysis patients (P = 0.008). RTx patients receiving mycophenolate mofetil (MMF) had a greater (P = 0.007) degree of telomere attrition compared to those treated with azathioprine. After 12 months, folate was significantly higher in RTx patients than in dialysis patients (P < 0.0001), whereas the opposite was true for homocysteine (P < 0.0001). The azathioprine group had lower levels of folate after 12 months than the MMF group (P = 0.003).
Conclusions
The associations between immunosuppressive therapy, telomere attrition, and changes in folate indicate a link between methyl donor potential, immunosuppressive drugs, and biological ageing. The hypothesis that the increased telomere attrition, observed in the MMF group after RTx, is driven by the immunosuppressive treatment, deserves further attention.
Patients with chronic kidney disease (CKD) demonstrate premature vascular ageing and a marked discrepancy between chronological and biological age.1,2 The uremic milieu affects the ageing of the immunological system, with T cells from end-stage renal disease (ESRD) patients reported to display shorter telomeres.3 Because CKD patients are susceptible to premature ageing, great care should be taken not to aggravate the ageing process further. Senescence and apoptosis both influence biological age and are associated with endothelial dysfunction and premature atherosclerosis,4-6 which can be induced by numerous factors. Oxidative stress and inflammation, both present in the uremic milieu, exacerbate cellular ageing.7 Because cells are exposed to pro-ageing factors, and as the number of cellular divisions increase, telomeres gradually shorten8,9 until the Hayflick limit is reached,10 triggering cellular senescence. Telomere length is thus frequently used for measuring biological age, and truncated telomeres have been associated with several chronic diseases, such as rheumatoid arthritis11 and cardiovascular disease (CVD).12 We have previously demonstrated that shorter telomeres are associated with inflammation, DNA damage, and premature mortality,13 and a study of patients with moderate CKD has shown that shorter telomeres associate with CVD.14
The methyl donor folate is important for maintaining DNA integrity, DNA methylation, and nucleotide biosynthesis.15,16 Folate deficiency leads to uracil misincorporation during DNA replication,17 resulting in DNA instability and increased risk of double strand breaks and erroneous DNA fusions.17 Low folate results in elevated homocysteine, which is associated with CVD.18 The effects of folate on telomere length have not been fully explored, but several scenarios are possible: (a) high folate promotes accelerated telomere attrition through increased cell division, (b) low folate results in unstable telomeres due to increased uracil content, (c) less folate results in genome hypomethylation. Although the canonical telomeric repeats do not contain any methylation sites, the methylation status of the subtelomeric region may regulate telomere length.19 Hypomethylation has been associated with increased telomere length,19 whereas DNA hypermethylation has been associated with inflammation and increased mortality in CKD.20 However, others have found that hyperhomocysteinemia, resulting in DNA hypomethylation, is associated with decreased telomere length.21-24 Thus, the links between folate and telomere attrition appear complex and context dependent.
The antimetabolites azathioprine (AZA) and mycophenolate mofetil (MMF) both act by inhibiting purine synthesis and cell proliferation.25-27 Purine synthesis involves the folate derivative tetrahydrofolate.28 Hence, it can be speculated that MMF and AZA treatment will result in high folate, which could impact DNA stability and telomere length. In addition, it has been proposed that immunosuppressive treatment could affect overall telomere length through the accumulation of senescent cells.29 However, data regarding possible associations between immunosuppressive therapy, folate, and telomere length are scarce. Nonetheless, it is of great clinical importance, as treatments that accelerate biological ageing are undesirable in this vulnerable patient population. Because inflammation,30 hyperhomocysteinemia,22 and oxidative stress31 promote accelerated telomere attrition, we hypothesized that normalization of these features after renal transplantation (RTx) may mitigate accelerated telomere attrition. Moreover, as MMF treatment is associated with lower homocysteine levels compared with AZA treatment,32 we hypothesized that different antimetabolites may contribute differently to telomere attrition after RTx.
MATERIALS AND METHODS
The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the ‘Declaration of Istanbul on Organ Trafficking and Transplant Tourism’. The study adheres to the Declaration of Helsinki, and the regional committee of ethics in Stockholm, Sweden provided ethical approval (approval numbers 008/98 and 2008/1748). Written informed consent was obtained from all participants.
Dialysis Patients
Patients were included at the Karolinska University Hospital, Sweden. The cohort has been described previously.33 Blood samples were collected between March 2004 and September 2009 in 49 patients close to dialysis initiation (baseline) and again after 12 months of dialysis therapy. Patients were treated by hemodialysis (n = 19) or peritoneal dialysis (n = 30). Basic patient characteristics are outlined in Table 1. None of the patients had been transplanted previously. Renal diagnoses included diabetic nephropathy (22.5%), chronic glomerulonephritis (20.5%), unknown cause (18.4%), adult polycystic kidney disease (12.2%), nephrosclerosis (10.2%), or other causes (16.2%). The most common forms of medication were vitamin D (88%), diuretics (87%), and phosphate binders (83%).
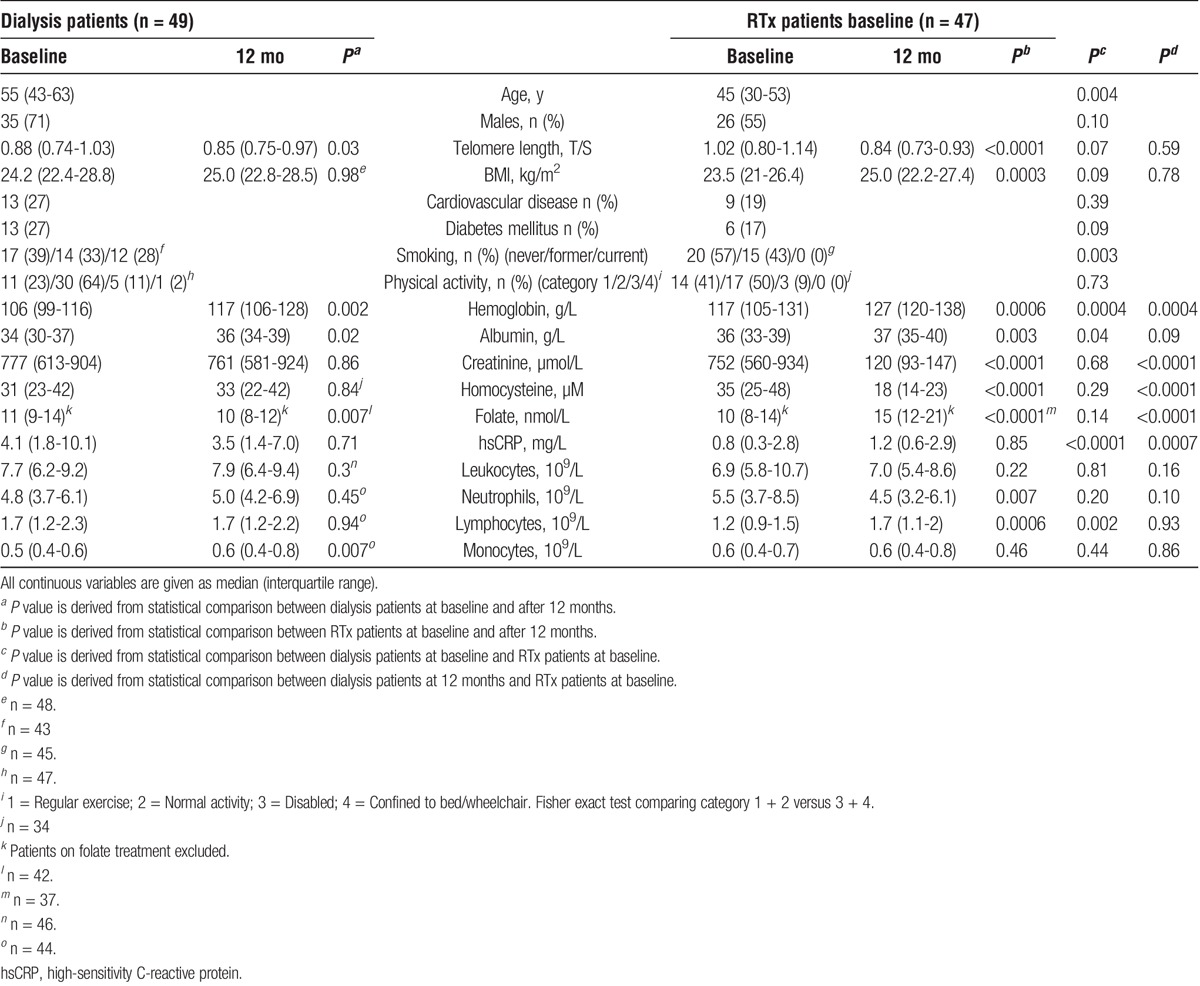
TABLE 1.
Basal demography and biochemical parameters of patients at baseline and after 12 months (mo) of renal replacement therapy
RTx Patients
Patients were included at the Karolinska University Hospital, Sweden. Blood samples were collected between March 2004 and February 2013 from 47 patients who underwent living donor (LD) RTx. Patient characteristics are outlined in Table 1. Thirty-three patients had undergone dialysis (17 hemodialysis and 16 peritoneal dialyses) before RTx (median duration 6 months). Ten patients underwent preemptive RTx, and 4 individuals had been transplanted previously (none of them received antithymoglobulin [ATG]). Seven patients (15%) experienced acute rejection post-RTx. Ten patients (21%) received rejection therapy. Two days before surgery, all patients displaying blood group compatibility received tacrolimus, prednisolone, and MMF. None of the patients received treatment with mammalian target of rapamycin inhibitors. None of the transplanted patients in the AZA group did receive MMF before the study. Patients displaying blood group incompatibility received rituximab 4 weeks before RTx, and MMF and prednisolone treatments were started 10 days before RTx. Solu-Medrol was administered on the day of RTx. Tacrolimus, prednisolone, and MMF were given similarly to the routine in blood group compatible patients from 1 day postsurgery. Renal diagnoses included chronic glomerulonephritis (40%), unknown cause (15%), adult polycystic kidney disease (11%), diabetic nephropathy (9%), nephrosclerosis (2%), or other causes (23%). The most common forms of medication after RTx were prednisolone (100%), tacrolimus (96%), and active vitamin D (89%). Forty-three patients (91.4%) received either AZA (n = 11) or MMF (n = 32) treatment. Immunosuppressive induction therapy, such as ATG or anti-CD25a monoclonal antibodies, was not part of the protocol for LD-RTx. Antipneumocystis pneumonia prophylaxis was only provided in RTx patients when S-Creatinine is greater than 200 μmol/L. Patients receiving antipneumocystis pneumonia prophylaxis did not receive folate supplementation.
Controls
Controls comprised 63 Glaswegian individuals without overt renal disease. The estimated glomerular filtration rate was greater than 60 mL/min for all individuals (when information was available). The median age was 58 years, median telomere length (T/S) was 1.13 and 71.4% of the group was male. Part of the cohort has been described previously.34
Telomere Length Measurement
DNA was isolated from whole blood samples using QIAamp DNA blood maxi kit (Qiagen, Hilden, Germany). DNA concentration and integrity were assessed by NanoDrop ND-1000 (NanoDrop, Wilmington, DE). All procedures were performed in accordance with manufacturers' protocols. Telomere length was measured during 2014 by quantitative PCR, following the method described by Cawthon.35 Each sample was analyzed in triplicate using primer sets specific for telomere length and a single-copy gene amplicon 36B4 (acidic ribosomal phosphoprotein). The average interassay coefficient of variation was 0.32% for telomere and 0.12% for 36B4, respectively. The relative T/S ratio (repeat copy number to single copy gene number) for each experimental sample was determined in relation to the control DNA sample. Telomere length was measured at baseline and after 12 months, and telomere attrition (ΔT/S) was calculated as: telomere length at 12 months − telomere length at baseline. Thus, the more negative the value, the larger the degree of attrition.
Statistical Analysis
Statistical analysis was performed using JMP 11.0.0 software (SAS Institute Inc., Cary, NC). Nonparametric tests were used for all analyses (Wilcoxon rank sum test or χ2 test/Fisher exact test for categorical data; Spearman rank correlation for continuous data). Wilcoxon matched-pairs signed rank test was used for paired analysis of baseline and 12-month data. When analysing folate data, patients with folate supplementation were excluded (folate ≥40 nmol/L; 3 dialysis patients and 8 RTx patients).
RESULTS
Patients and Controls
Although non-CKD patients (median 58 years) were significantly (P < 0.0001) older than dialysis (median 55 years) and RTx (median 45 years) patients, telomeres were longer in this group (1.13 T/S vs 0.88 T/S; P = 0.0007 and 1.13 T/S vs 1.02 T/S; P = 0.03, respectively). There was no significant difference in gender distribution between controls and dialysis patients (P = 1.00), or between controls and RTx patients (P = 0.08). Age was not significantly associated with telomere length in non-CKD patients (P = 0.84), dialysis patients at baseline (P = 0.25) or dialysis patients after 12 months (P = 0.12). In RTx patients, baseline telomere length showed a near-significant association with age (P = 0.06), which was significant after 12 months (ρ = −0.36; P = 0.01).
Comparison of Renal Replacement Therapy Modalities
RTx patients were significantly younger than dialysis patients (45 vs. 55 yrs; P = 0.004) (Table 1). At baseline, RTx patients had higher median haemoglobin (117 vs 106 g/L; P = 0.0004) and plasma albumin (36 vs 34 g/L; P = 0.04), and lower median high-sensitivity CRP (hsCRP) (0.8 vs 4.1 mg/L; P < 0.0001) and lymphocyte count (1.2 vs. 1.7 109/L; P = 0.002) (Table 1). The dialysis patient group included more former/current smokers at baseline than the RTx patient group (61% vs 43%; P = 0.003). After 12 months, RTx patients had higher median hemoglobin (127 vs 117 g/L; P = 0.0004) and folate (15 vs 10 nmol/L; P < 0.0001), and lower creatinine (120 vs. 761 μmol/L; P < 0.0001), homocysteine (18 vs 33 μM; (P < 0.0001) and hsCRP (1.2 vs 3.5 mg/L; P = 0.0007) compared to dialysis patients (Table 1).
Over 12 months, dialysis patients showed a small, but significant, decrease in telomere length (0.88 to 0.85 T/S; P = 0.03) and folate (11 to 10 nmol/L; P = 0.007), and a significant increase in median plasma albumin (34 to 36 g/L; P = 0.02), haemoglobin (106 to 117 g/L; P = 0.002) and monocyte count (0.5 to 0.6 109/L; P = 0.007). In the RTx patients, median creatinine (752 to 120 μmol/L; P < 0.0001), neutrophil count (5.5 to 4.5 109/L; P = 0.007) and homocysteine (35 to 18 μM; P < 0.0001) levels decreased over 12 months, while plasma albumin (36 to 37 g/L; P = 0.003), body mass index (BMI) (23.5 to 25.0 kg/m2; P = 0.0003), haemoglobin (117 to 127 g/L; P = 0.0006), lymphocyte count (1.2 to 1.7 109/L; P = 0.0006) and folate (10 to 15 nmol/L; P < 0.0001) increased. Telomere attrition in RTx patients (1.02 to 0.84 T/S; P < 0.0001) was significantly greater (−0.16 vs. −0.05; P = 0.008) than in dialysis patients (0.88 to 0.85 T/S; P = 0.03) (Figures 1A and B).
FIGURE 1.
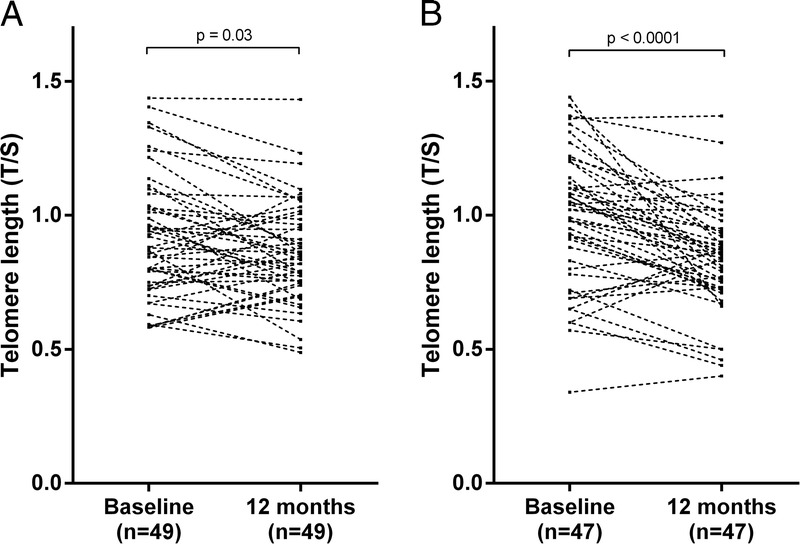
Change in telomere length during 12 months in patients receiving dialysis treatment (A), and in patients undergoing renal transplantation (B).
In RTx patients at baseline, telomere length was significantly associated with folate (P = 0.04, ρ = −0.33), homocysteine (P = 0.04; ρ = 0.29) (Figures 2A and B) and folate/homocysteine ratio (P = 0.04, ρ = −0.32). None of these associations were significant at 12 months or in dialysis patients at any time point. The folate/homocysteine ratio was significantly higher in dialysis patients than in RTx patients at baseline (0.35 vs 0.26; P = 0.02). However, after 12 months, the ratio was significantly higher in RTx patients (0.37 vs 1.00; P < 0.0001). Accordingly, the folate/homocysteine ratio increased in RTx patients over 12 months (P < 0.0001). Neither telomere attrition, nor telomere length at any time point, were associated with smoking status or physical activity in either patient group.
FIGURE 2.
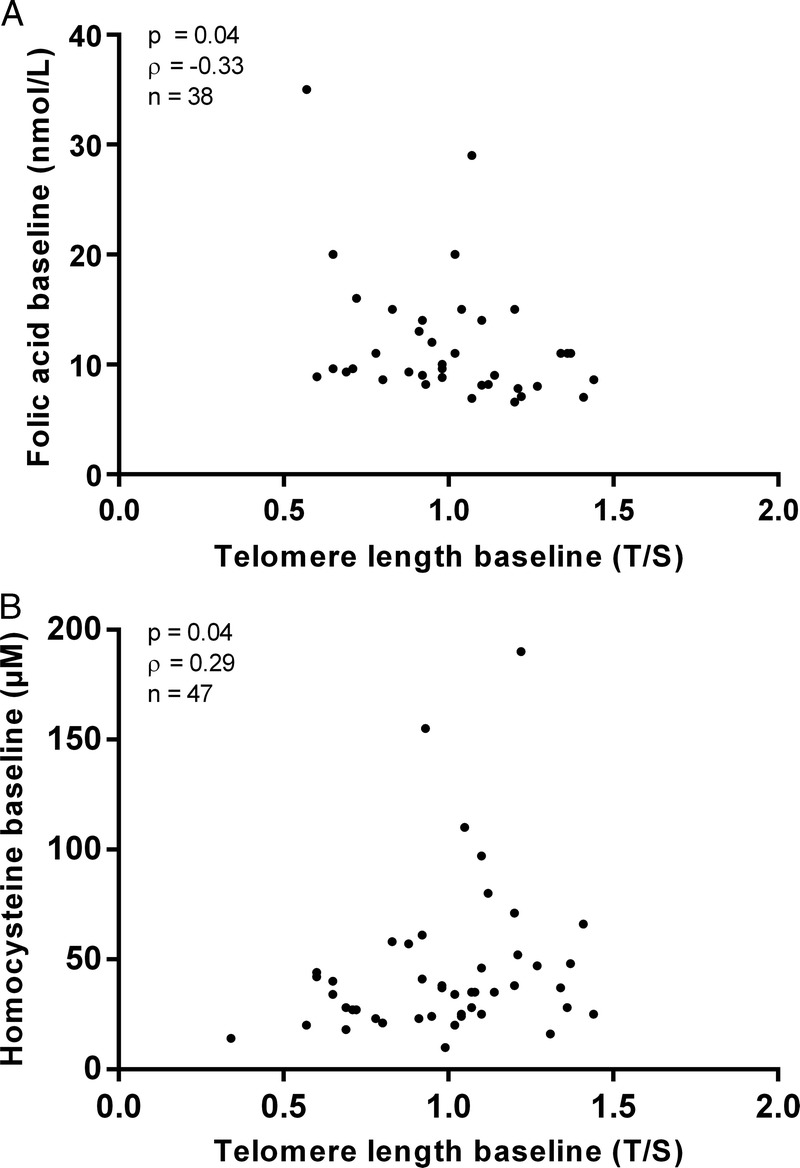
Association between baseline telomere length in renal transplant patients and folate at baseline (A); and between baseline telomere length and homocysteine at baseline (B). Two of the patients had homocystein levels >150 μM, which indicate an enzymatic abnormality of the folate pathway. Although these 2 outliers were not genotyped it is likely that they carry the methylenetetrahydrofolate reductase mutation.
Comparison Between AZA- and MMF-Treated RTx Patients
Forty-three RTx patients were divided into those receiving AZA (n = 11) and those receiving MMF (n = 32) (Table 2). The number of patients experiencing acute rejection was similar between the treatment groups (1 vs 5 patients, respectively; Fisher exact test P = 1.00). There were no significant differences between the groups, neither in age, gender, dialysis vintage before RTx, CVD prevalence, diabetes, and baseline folate levels, nor for BMI, telomere length and homocysteine levels at baseline and after 12 months. However, in patients treated with MMF, a significantly higher degree of telomere attrition was observed 12 months after RTx (−0.10 vs −0.19; P = 0.007) (Figure 3).
TABLE 2.
Properties of RTx patients receiving azathioprine versus those receiving mycophenolic acid
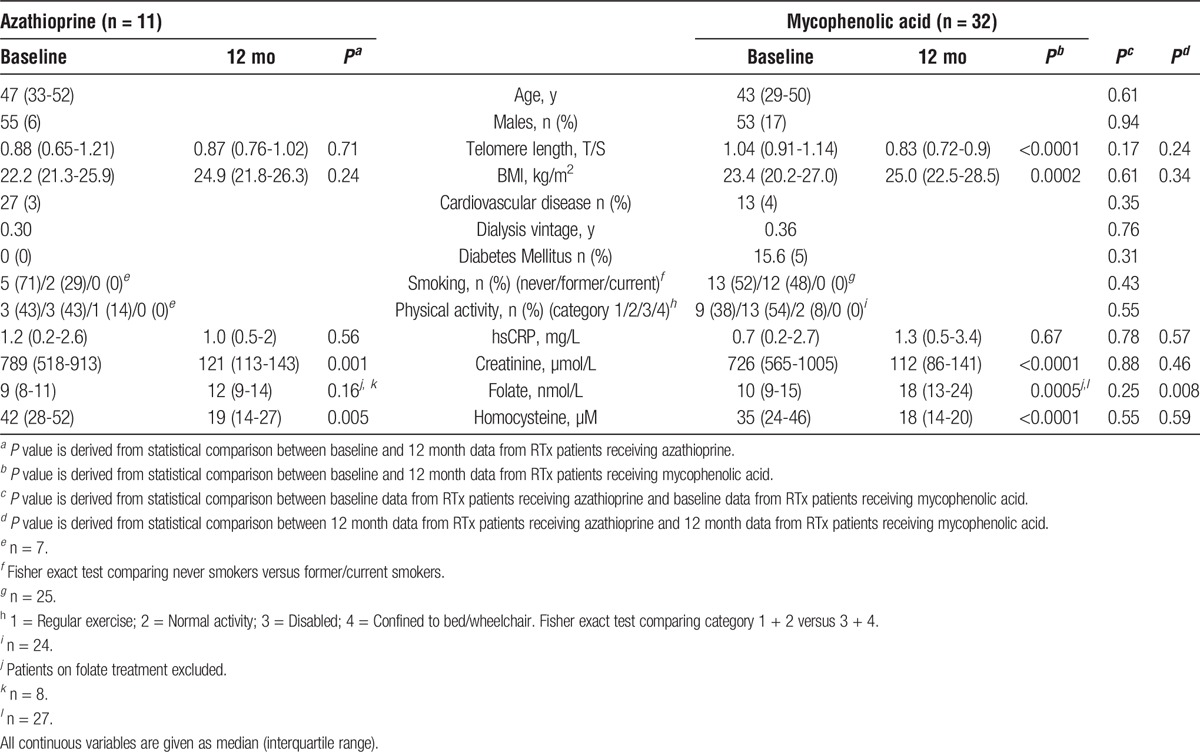
FIGURE 3.
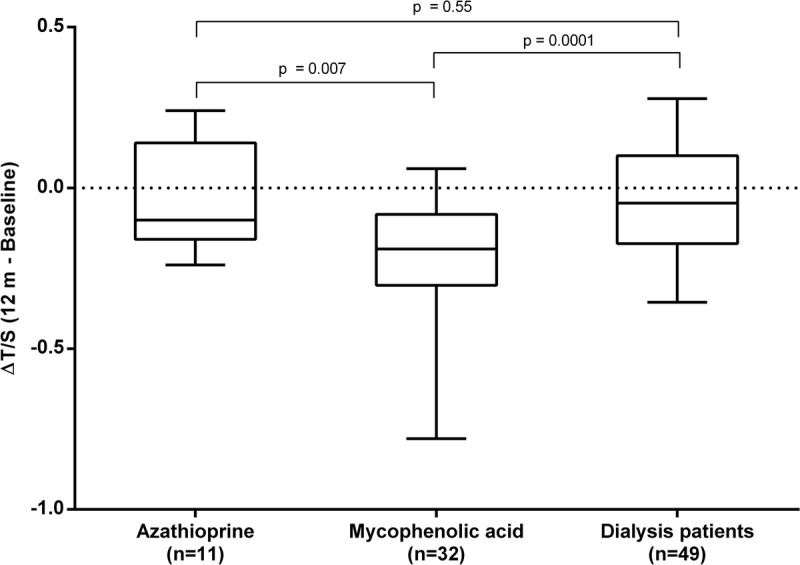
Comparison of change in telomere length (ΔT/S) during 12 months between renal transplant patients receiving azathioprine (n = 11) or mycophenolic acid (n = 32) and dialysis patients (n = 49).
Telomere attrition in RTx patients treated with AZA was similar to that of dialysis patients (P = 0.55). Although folate levels were similar between the groups at baseline, levels were significantly higher in patients receiving MMF compared to those receiving AZA after 12 months (P = 0.008) (Figure 4). Whereas MMF treatment was associated with a significant increase in median folate levels over 12 months (10 to 18 nmol/L; P = 0.0005), no changes in folate were observed in patients treated by AZA (9 to 12 nmol/L; P = 0.16). Neither telomere attrition, nor telomere length at any time point, were associated with smoking status or physical activity in either antimetabolite treatment group.
FIGURE 4.
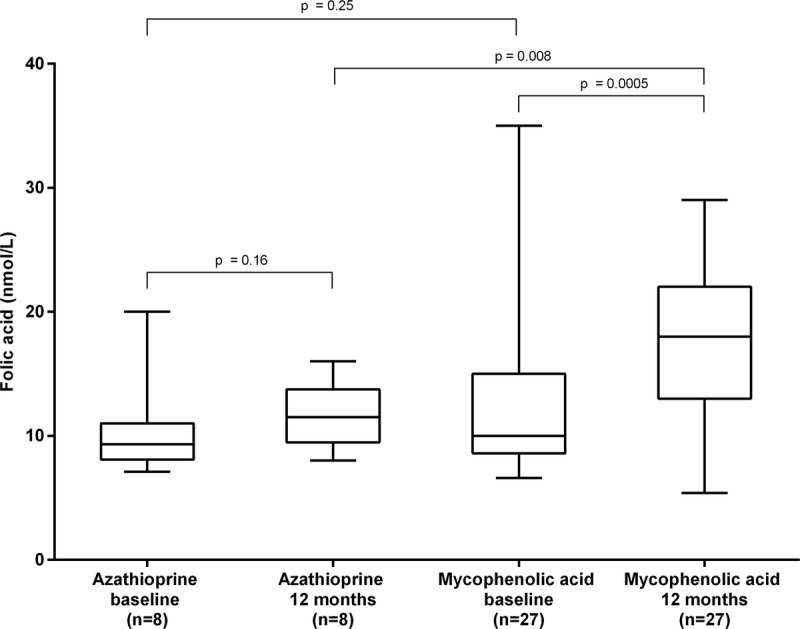
Levels of folate at baseline and after 12 months in renal transplant patients receiving azathioprine or mycophenolic acid.
DISCUSSION
Judging by the differences in telomere length, our data suggest that CKD patients are biologically older than the chronologically older non-CKD patients, supporting the hypothesis that CKD is a progeric state.1,2 Unexpectedly, telomere attrition was more pronounced 12 months after RTx than after 12 months of dialysis. Whereas immunosuppressive treatment with AZA was not associated with any significant change in telomere length after 12 months, MMF treatment was associated with increased telomere attrition.
We hypothesized that normalization of the prooxidative, proinflammatory, and hyperhomocysteinemic uremic milieu after RTx would be associated with less telomere attrition, as RTx improves long-term survival compared with dialysis treatment.36 Therefore, our finding of accelerated telomere attrition indicating accelerated aging processes after RTx appears surprising. However, the previous observations that both vascular calcification37 and immunological aging38 do not regress but rather continue to progress after normalization of the uremic milieu with RTx, indirectly support our observation of accelerated telomere attrition after RTx. In addition, Marechal et al37 found that a lower proportion of AZA-treated RTx patients experienced rapid vascular calcification progression compared to patients treated with MMF, thereby lending credibility to the potentially pro-ageing effects of MMF compared to AZA. Although the reasons for continuous biological aging despite normalization of the uremic milieu are unknown, a number of possibilities are worth considering. Because telomere attrition may vary with age,39 a decreased rate of attrition in the older dialysis patients could be a potential explanation. The significant increase in BMI after RTx could also promote telomere attrition, as increased BMI has been reported to associate with shorter telomeres.40 Although smoking, inflammation, and physical inactivity have been associated with shorter telomere length,40 we could not see any significant associations with telomere length or degree of telomere attrition in our data set. The physical trauma associated with surgery is a short-term biological stressor and might also impact aging biomarkers. Indeed, kidney grafts are exposed to oxidative, as well as replicative stress, both of which are associated with decreased telomere length.41,42 Additionally, in investigating longitudinal telomere length a regression toward the mean phenomenon cannot be excluded—hence, a replication study is desirable. Our data, however, suggest that immunosuppressive antimetabolite drugs may influence telomere attrition (and/or telomerase activity) after RTx. Whereas telomere attrition in AZA-treated patients did not differ from that in dialysis patients, markedly accelerated telomere attrition was observed in MMF-treated patients (Figure 3). Our finding accords with those of Meijers et al38 who report telomere attrition of naïve T cells in ESRD patients undergoing RTx with triple immunosuppression including MMF. As compared to AZA, MMF is regarded as a more potent immunosuppressant, which decreases the incidence of acute rejections.43,44 Because patients treated with MMF had significantly higher folate at 12 months than those treated with AZA, changes in folate may contribute to the difference in telomere attrition. Indeed, folate is known to be important for genomic stability.45 The link between higher folate and greater telomere attrition may appear counterintuitive because a decrease in folate has been associated with genomic instability.17,19,21,23 However, it has also been reported that elevated folate stimulates cell division,45 which could increase telomere attrition through its involvement in nucleotide synthesis. Indeed, Paul et al46 have reported decreasing telomere length with increasing folate, and a recent study has shown that folate deficiency results in telomere elongation.47 Because telomere length is inversely associated with plasma homocysteine, this suggests that it may be an intermediary in the relationship between homocysteine and CVD.22 However, although treatment with the two antimetabolite drugs was associated with markedly different folate levels, homocysteine levels did not change. Although the reason or reasons(s) for elevated levels of folic acid are not evident a previous in vitro study by Ignatescu et al32 showed a homocysteine lowering effect of MMF, indirectly supporting our observation. We report a positive correlation between telomere length and homocysteine at baseline in RTx patients. Since homocysteine levels are influenced by factors common in uraemia, such as inflammation and protein-energy wasting48 and low, not high, homocysteine predicts CVD, further studies are needed to reveal if, and how, DNA methylation impacts on telomere attrition in the uremic milieu. Although reports in the literature appear contradictory, homocysteine, folate and telomere attrition are consistently associated with each other.21-24,45-47 Thus, the relationship between them should be investigated more thoroughly, preferably in cohorts undergoing premature aging, such as CKD patients.
Strengths and limitations of the current study deserve mentioning. One strength is that the method used for telomere length analysis in the present study is documented to be reproducible and has a superior inter assay coefficient of variation (CV) to the flow cytometry measurements used in previous studies.49-52 The limitations include the lack of longitudinal data in non-CKD patients, which makes it impossible to compare the degree of telomere attrition to CKD patients. Nonetheless, the control data illustrated a significant difference in telomere length between ESRD patients and chronologically older patients supporting the concept of a major discrepancy between chronological and biological age in the uremic milieu. Another shortcoming is that dialysis patients and RTx patients were not matched for age and co-morbidity. By selection criteria, patients undergoing RTx are younger and have a lower CVD prevalence than patients receiving dialysis. It should be acknowledged that the RTx group was rather inhomogeneous and included a mix of clinical characteristics, such as preemptive RTx, a second RTx, a previous history of dialysis treatment and ABO blood group incompatibility. At baseline, 70% of the RTx patients had received short-term dialysis treatment (median period 6 months). While it could then be hypothesized that the baseline telomere lengths of RTx patients would be similar to those of dialysis patients after 12 months, we observed a significant difference between dialysis patients and MMF-treated RTx patients after 12 months. Although multivariate analysis would have been of interest to identify confounders, the sample size was too small to generate any reliable results. Hence, the study should be replicated in a larger cohort. Since ATG has been reported to accelerate immunological senescence,53 immunosuppressive induction therapy could also have contributed to the degree of telomere attrition. However, immunosuppressive induction therapy was not given before LD-RTx and the prevalence of acute rejection did not differ between the MMF and AZA groups. The lack of a significant correlation between age and telomere length in controls and dialysis patients is worth noting. However, telomere length may be more appropriate as a marker of biological, rather than chronological, age.54 The nonrandomized approach for treatment with AZA and MMF also limits the interpretation of the study. However, no significant baseline differences in demography, comorbidities, inflammation, telomere length or folate and homocysteine levels were observed between the two treatment groups. As the groups were small, replication studies are required, and mechanistic studies are needed to confirm any possible effects of immunosuppressive therapy on telomere length and folate. Finally, the lack of data on telomerase activity also limits the interpretation of the study.
In summary, this study confirms increased biological age in CKD patients when assessed by telomere length. We also confirm that RTx patients display a higher degree of telomere attrition over 12 months in comparison to dialysis patients. For the first time, we report that RTx patients on the anti-metabolite MMF have a greater degree of telomere attrition than those immunosuppressed with AZA. Our observations highlight the potential impact of immunosuppressive therapy on the process of biological ageing. Since long-term survival after RTx has improved,55 and a long life after RTx can be anticipated, the effects of immunosuppressive drugs on biological ageing need more attention.
Footnotes
Published online 16 November, 2016.
P.G.S and P.S. shared senior authorship.
None of the authors report any conflict of interest in regard to this study.
Research idea and study design: P.G.S., P.S.; data collection: K.L., D.M., L.W., H.C., T.Q., H.G., J.S., I.S., A.R.Q., P.B.; statistical analysis: K.L., D.M., A.R.Q.; data interpretation: K.L., L.N., P.G.S., P.S.; supervision: L.N., M.S., P.G.S., P.S.
REFERENCES
- 1.Kooman JP, Kotanko P, Schols AM, et al. Chronic kidney disease and premature ageing. Nat Rev Nephrol. 2014;10:732–742. [DOI] [PubMed] [Google Scholar]
- 2.Stenvinkel P, Larsson TE. Chronic kidney disease: a clinical model of premature aging. Am J Kidney Dis. 2013;62:339–351. [DOI] [PubMed] [Google Scholar]
- 3.Betjes MG, Langerak AW, van der Spek A, et al. Premature aging of circulating T cells in patients with end-stage renal disease. Kidney Int. 2011;80:208–217. [DOI] [PubMed] [Google Scholar]
- 4.Bhayadia R, Schmidt BM, Melk A, et al. Senescence-induced oxidative stress causes endothelial dysfunction. J Gerontol A Biol Sci Med Sci. 2016;71:161–169. [DOI] [PubMed] [Google Scholar]
- 5.Fyhrquist F, Saijonmaa O, Strandberg T. The roles of senescence and telomere shortening in cardiovascular disease. Nat Rev Cardiol. 2013;10:274–283. [DOI] [PubMed] [Google Scholar]
- 6.Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111:245–259. [DOI] [PubMed] [Google Scholar]
- 7.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. [DOI] [PubMed] [Google Scholar]
- 8.Olovnikov AM. Principle of marginotomy in template synthesis of polynucleotides. Dokl Akad Nauk SSSR. 1971;201:1496–1499. [PubMed] [Google Scholar]
- 9.Olovnikov AM. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973;41:181–190. [DOI] [PubMed] [Google Scholar]
- 10.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. [DOI] [PubMed] [Google Scholar]
- 11.Steer SE, Williams FM, Kato B, et al. Reduced telomere length in rheumatoid arthritis is independent of disease activity and duration. Ann Rheum Dis. 2007;66:476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzpatrick AL, Kronmal RA, Gardner JP, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165:14–21. [DOI] [PubMed] [Google Scholar]
- 13.Carrero JJ, Stenvinkel P, Fellstrom B, et al. Telomere attrition is associated with inflammation, low fetuin-A levels and high mortality in prevalent haemodialysis patients. J Intern Med. 2008;263:302–312. [DOI] [PubMed] [Google Scholar]
- 14.Raschenberger J, Kollerits B, Ritchie J, et al. Association of relative telomere length with progression of chronic kidney disease in two cohorts: effect modification by smoking and diabetes. Sci Rep. 2015;5:11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appling DR. Compartmentation of folate-mediated one-carbon metabolism in eukaryotes. FASEB J. 1991;5:2645–2651. [DOI] [PubMed] [Google Scholar]
- 16.Benesh FC, Carl GF. Methyl biogenesis. Biol Psychiatry. 1978;13:465–480. [PubMed] [Google Scholar]
- 17.Blount BC, Mack MM, Wehr CM, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997;94:3290–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wierzbicki AS. Homocysteine and cardiovascular disease: a review of the evidence. Diab Vasc Dis Res. 2007;4:143–150. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalo S, Jaco I, Fraga MF, et al. DNA methyltransferases control telomere length and telomere recombination in mammalian cells. Nat Cell Biol. 2006;8:416–424. [DOI] [PubMed] [Google Scholar]
- 20.Stenvinkel P, Karimi M, Johansson S, et al. Impact of inflammation on epigenetic DNA methylation—a novel risk factor for cardiovascular disease? J Intern Med. 2007;261:488–499. [DOI] [PubMed] [Google Scholar]
- 21.Bull CF, O'Callaghan NJ, Mayrhofer G, et al. Telomere length in lymphocytes of older South Australian men may be inversely associated with plasma homocysteine. Rejuvenation Res. 2009;12:341–349. [DOI] [PubMed] [Google Scholar]
- 22.Rane G, Koh WP, Kanchi MM, et al. Association between leukocyte telomere length and plasma homocysteine in a Singapore Chinese population. Rejuvenation Res. 2015;18:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards JB, Valdes AM, Gardner JP, et al. Homocysteine levels and leukocyte telomere length. Atherosclerosis. 2008;2008:271–277. [DOI] [PubMed] [Google Scholar]
- 24.Zhang D, Sun X, Liu J, et al. Homocysteine accelerates senescence of endothelial cells via DNA hypomethylation of human telomerase reverse transcriptase. Arterioscler Thromb Vasc Biol. 2015;35:71–78. [DOI] [PubMed] [Google Scholar]
- 25.Allison AC, Kowalski WJ, Muller CD, et al. Mechanisms of action of mycophenolic acid. Ann N Y Acad Sci. 1993;696:63–87. [DOI] [PubMed] [Google Scholar]
- 26.Elion GB. The George Hitchings and Gertrude Elion Lecture. The pharmacology of azathioprine. Ann N Y Acad Sci. 1993;685:400–407. [DOI] [PubMed] [Google Scholar]
- 27.Maltzman JS, Koretzky GA. Azathioprine: old drug, new actions. J Clin Invest. 2003;111:1122–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huennekens FM. Folic acid coenzymes in the biosynthesis of purines and pyrimidines. Vitam Horm. 1968;26:375–394. [DOI] [PubMed] [Google Scholar]
- 29.Childs BG, Durik M, Baker DJ, et al. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21:1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jurk D, Wilson C, Passos JF, et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun. 2014;2:4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurz DJ, Decary S, Hong Y, et al. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci. 2004;117:2417–2426. [DOI] [PubMed] [Google Scholar]
- 32.Ignatescu MC, Kletzmayr J, Fodinger M, et al. Influence of mycophenolic acid and tacrolimus on homocysteine metabolism. Kidney Int. 2002;61:1894–1898. [DOI] [PubMed] [Google Scholar]
- 33.Stenvinkel P, Heimbürger O, Paultre F, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–1911. [DOI] [PubMed] [Google Scholar]
- 34.Robinson MW, McGuinness D, Swann R, et al. Non cell autonomous upregulation of CDKN2 transcription linked to progression of chronic hepatitis C disease. Aging Cell. 2013;12:1141–1143. [DOI] [PubMed] [Google Scholar]
- 35.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. [DOI] [PubMed] [Google Scholar]
- 37.Marechal C, Coche E, Goffin E, et al. Progression of coronary artery calcification and thoracic aorta calcification in kidney transplant recipients. Am J Kidney Dis. 2012;59:258–269. [DOI] [PubMed] [Google Scholar]
- 38.Meijers RW, Litjens NH, de Wit EA, et al. Uremia-associated immunological aging is stably imprinted in the T-cell system and not reversed by kidney transplantation. Transpl Int. 2014;27:1272–1284. [DOI] [PubMed] [Google Scholar]
- 39.Frenck RW, Jr, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A. 1998;95:5607–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willeit P, Raschenberger J, Heydon EE, et al. Leucocyte telomere length and risk of type 2 diabetes mellitus: new prospective cohort study and literature-based meta-analysis. PLoS One. 2014;9:e112483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chkhotua A, Shohat M, Tobar A, et al. Replicative senescence in organ transplantation-mechanisms and significance. Transpl Immunol. 2002;9:165–171. [DOI] [PubMed] [Google Scholar]
- 42.Chkhotua AB, Abendroth D, Froeba G, et al. Up-regulation of cell cycle regulatory genes after renal ischemia/reperfusion: differential expression of p16(INK4a), p21(WAF1/CIP1) and p27(Kip1) cyclin-dependent kinase inhibitor genes depending on reperfusion time. Transpl Int. 2006;19:72–77. [DOI] [PubMed] [Google Scholar]
- 43.David KM, Morris JA, Steffen BJ, et al. Mycophenolate mofetil vs. azathioprine is associated with decreased acute rejection, late acute rejection, and risk for cardiovascular death in renal transplant recipients with pre-transplant diabetes. Clin Transplant. 2005;19:279–285. [DOI] [PubMed] [Google Scholar]
- 44.Oh JM, Wiland AM, Klassen DK, et al. Comparison of azathioprine and mycophenolate mofetil for the prevention of acute rejection in recipients of pancreas transplantation. J Clin Pharmacol. 2001;41:861–869. [DOI] [PubMed] [Google Scholar]
- 45.Moores CJ, Fenech M, O'Callaghan NJ. Telomere dynamics: the influence of folate and DNA methylation. Ann N Y Acad Sci. 2011;1229:76–88. [DOI] [PubMed] [Google Scholar]
- 46.Paul L, Jacques PF, Aviv A, et al. High plasma folate is negatively associated with leukocyte telomere length in Framingham Offspring cohort. Eur J Nutr. 2015;54:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bull C, Christensen H, Fenech M. Cortisol is not associated with telomere shortening or chromosomal instability in human lymphocytes cultured under low and high folate conditions. PLoS One. 2015;10:e0119367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suliman ME, Lindholm B, Barany P, et al. Homocysteine-lowering is not a primary target for cardiovascular disease prevention in chronic kidney disease patients. Semin Dial. 2007;20:523–529. [DOI] [PubMed] [Google Scholar]
- 49.Martin-Ruiz CM, Baird D, Roger L, et al. Reproducibility of telomere length assessment: an international collaborative study. Int J Epidemiol. 2015;44:1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rufer N, Brummendorf TH, Kolvraa S, et al. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med. 1999;190:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rufer N, Dragowska W, Thornbury G, et al. Telomere length dynamics in human lymphocyte subpopulations measured by flow cytometry. Nat Biotechnol. 1998;16:743–747. [DOI] [PubMed] [Google Scholar]
- 52.Zheng YL, Loffredo CA, Shields PG, et al. Chromosome 9 arm-specific telomere length and breast cancer risk. Carcinogenesis. 2009;30:1380–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crepin T, Carron C, Roubiou C, et al. ATG-induced accelerated immune senescence: clinical implications in renal transplant recipients. Am J Transplant. 2015;15:1028–1038. [DOI] [PubMed] [Google Scholar]
- 54.Mather KA, Jorm AF, Parslow RA, et al. Is telomere length a biomarker of aging? A review. J Gerontol A Biol Sci Med Sci. 2011;66:202–213. [DOI] [PubMed] [Google Scholar]
- 55.Legendre C, Canaud G, Martinez F. Factors influencing long-term outcome after kidney transplantation. Transpl Int. 2014;27:19–27. [DOI] [PubMed] [Google Scholar]


