Abstract
Chimeric antigen receptors (CARs) are used to redirect effector cell specificity to selected cell surface antigens. Using CARs, antitumor activity can be initiated in patients with no prior tumor specific immunity. Although CARs have shown promising clinical results, the technology remains limited by the availability of specific cognate cell target antigens. To increase the repertoire of targetable tumor cell antigens we utilized the immune system of the sea lamprey to generate directed variable lymphocyte receptors (VLRs). VLRs serve as membrane bound and soluble immune effectors analogous but not homologous to immunoglobulins. They have a fundamentally different structure than immunoglobulin (Ig)-based antibodies while still demonstrating high degrees of specificity and affinity. To test the functionality of VLRs as the antigen recognition domain of CARs, two VLR-CARs were created. One contained a VLR specific for a murine B cell leukemia and the other contained a VLR specific for the human T cell surface antigen, CD5. The CAR design consisted of the VLR sequence, myc-epitope tag, CD28 transmembrane domain, and intracellular CD3ζ signaling domain. We demonstrate proof of concept, including gene transfer, biosynthesis, cell surface localization, and effector cell activation for multiple VLR-CAR designs. Therefore, VLRs provide an alternative means of CAR-based cancer recognition.
Introduction
Chimeric antigen receptors (CARs) provide a method by which immune effector cells can be redirected to recognize specific antigens displayed on tumor cells in a process that is not reliant on the major histocompatibility complex.1–4 Since its inception over 25 years ago, CAR technology has had significant advancements, culminating in the breakthrough success of CAR T-cell targeting of the B cell-specific antigen, CD19, in several B cell lymphomas.5–8 With CAR therapy expanding rapidly in its application and design, there is an increasing need to expand the number and variety of tumor cell targets available for CAR recognition. There remain difficulties, however, in the identification and implementation of antibodies against these new tumor cell antigens as studies have revealed significant unintended effects.6–13 Many of these side effects arise from either CARs acting off-target, recognizing an antigen or protein similar to the intended target, or on-target but off-tumor effects, where the target antigen is also found on other, nontumor cells.6–13 Thus, improving the impact of CAR technology requires the identification and utilization of a larger repertoire of antigen binding elements, as the majority of successful CAR trials have made use of only a handful of CAR targets. As a means of increasing the potential repertoire of antigens that may be recognized using a CAR complex, we proposed the use of variable lymphocyte receptors (VLRs) as the antigen binding domain.14–17 The advantages of VLRs specifically for CAR technologies are multifaceted including (i) their single chain nature, which enables one-step cloning/screening using any available high throughput surface expression technology, (ii) the evolutionary distance between human and lamprey self-proteins, which presumably facilitates greater diversity in antigen recognition due to a lower degree of self-tolerance based inhibition, and (iii) their unique geometry, which enables distinct binding interaction compared with binding through single chain variable fragments (scFvs). Collectively, these properties provide a platform by which the antigen binding elements of the CAR complex can be expanded to encompass a unique array of clinically-relevant antigens.
VLRs represent the functional component of the lamprey adaptive immune system. They differ significantly in structure compared with Ig-based antibodies of jawed vertebrates, but are analogous in function and have been shown to be capable of recognizing and binding as wide and diverse an array of antigens as conventional antibodies.14–17 The difference in structure is due to the divergence of lampreys and hagfish from the common vertebrate lineage ~450 million years ago, leading to two distinct but equally adaptable immune systems. While antibodies are produced by a Recombination-activating gene (RAG)-dependent recombination process, VLRs are RAG-independent and formed by a rearrangement of the germ line gene in the lamprey immune cells.14–17 Although, lampreys seem to lack lymph nodes and a thymus, they do contain lymphoid and myeloid cells found in the blood and tissues. Lamprey lymphocytes are comprised of both T-like cells and B-like cells that produce VLR-A and VLR-B, respectively. A third cell type, somewhat analogous to the δγ T-cell lineage produce VLR-C.18,19 Our work herein has focused exclusively with VLR-B produced from the lamprey B-like cells. In these cells, VLRs are generated through assembly of leucine-rich repeat (LRR) cassettes, forming the mature VLR gene shown in Figure 1a. The diversity in the VLR structure comes from the process of gene assembly in which a series of LRR cassettes flanking the incomplete VLR gene are spliced into the several distinct locations in this gene in a variable manner.14–17 Each LRR element is incorporated only once, with the exception of the LRRV elements. These segments can vary in number from 0 to 8 LRRs in the mature gene. Each additional LRR introduced increases the surface area by ~220 Å2.14–17 The result of this process is a complete VLR-B gene capable of binding antigen.
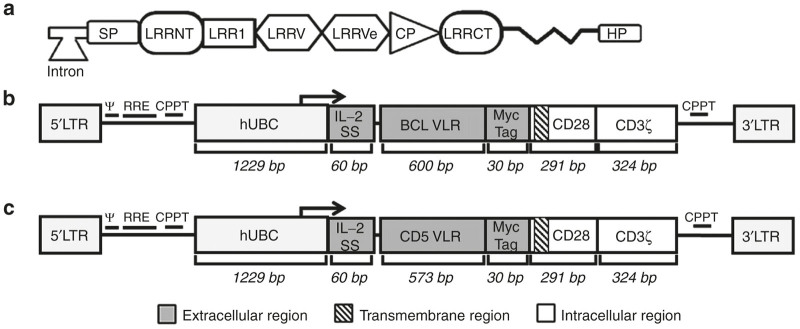
Figure 1.
Schematic of chimeric antigen receptor (CAR) structures containing the BCL or CD5 variable lymphocyte receptor (VLR) is shown along with the structure of the mature VLR gene. (a) A depiction of the mature VLR gene which is assembled from a series of leucine rich repeat (LRR) cassettes spliced from the germ line cell to the mature B-like cell in a combinatorial fashion. SP, signal peptide; LRRNT, N-terminal leucine-rich repeat; LRRV, variable leucine-rich repeats; LRRVe, end leucine-rich repeat; CP, connecting peptide; LRRCT, C-terminal leucine-rich repeat; HP - hydrophobic peptide. (b) The structure of the BCL-CAR transgene includes a 5′ long terminal repeat (LTR), human ubiquitin C promoter (hUBC), an interleukin-2 signal peptide (IL-2 SP), the BCL VLR, a myc epitope tag, the CD28 region, the CD3-ζ intracellular domain and a 3′ LTR. (c) The structure of the CD5-VLR-CAR transgene.
Antigen specific VLRs can be obtained from immunizing lampreys with a specific target antigen or cell type (i.e., immunogen). Immunized animals are then screened for serum antigen-reactive VLR positivity prior to lymphocyte harvesting and cDNA library generation. Due to the conserved nature of the terminal VLR gene sequences, it is possible to amplify the entire VLR cDNA repertoire in a single reaction, which can be then cloned into a surface display expression system for high-throughput screening and candidate VLR discovery.20,21 This system allows for both positive selection of immunogen reactive VLRs as well as negative screening for detection of off-target reactivity (e.g., homologous proteins) and on-target, off-tumor reactivity (e.g., normal tissue or cells), Candidate VLRs that meet the screening criteria then can be engineered in place of the scFv on first, second or third generation CAR scaffolds, with the VLR replacing the scFv as the antigen binding region.
The primary impetus for developing VLRs as a replacement for the scFv of traditional CARs is that the VLR has been shown to bind antigen in a manner distinct from that of Ig-based antibodies. This difference in binding is due to the structural differences found between VLRs and the traditional monoclonal antibodies from which scFvs are derived. Based on crystal structures, VLRs are known to have an overall crescent shape with β-strands and a C-terminal loop found on the convex portion of the structure.14–17,22–26 These regions have been shown to function as the antigen binding portion of the VLR and are able to expand in size with the incorporation of additional LRRV cassettes. Because of this difference in structure, VLRs generated against the same antigen may bind to dissimilar and distinct epitopes and at different affinities compared with conventional antibodies.22–25 This variation in binding sets VLRs apart from Ig antibodies as a method for directing CAR T cells to previously unavailable antigen epitopes. Herein as proof of concept, we examine the feasibility and utility of two distinct VLR-based CARs.
Results
Construction of lamprey VLR-based CARs
To generate the CAR constructs, sequences were used for a VLR previously shown to be specific for the B-cell receptor of the mouse tumor cell line, BCL (BCL1-3B3), and a VLR shown to be specific for the human T-cell surface protein CD5. The BCL VLR is 600 bp and contains one LRR in the LRRV element. The sequence for the CD5 VLR was generated using the previously published protein sequence, and the cDNA sequence designed to express the VLR was codon optimized for human cell expression.26 The portion of the VLR sequence used in our CAR constructs spanned from the beginning of the LRRNT element and included all sequences through the LRRCT element (Figure 1a). The same portion of the CD5 VLR sequence was used and totaled 573 bps. The two VLR sequences were cloned into the CAR construct as shown (Figure 1b,c). The CAR cassette was a second generation CAR composed of an N-terminal interleukin-2 (IL-2) signal peptide followed by the VLR antigen binding domain, a myc tag for cell surface identification, the transmembrane and intracellular domains of CD28, and the intracellular signaling domain of CD3ζ.
VLR-CAR T-cell expression and antigen-specific activation
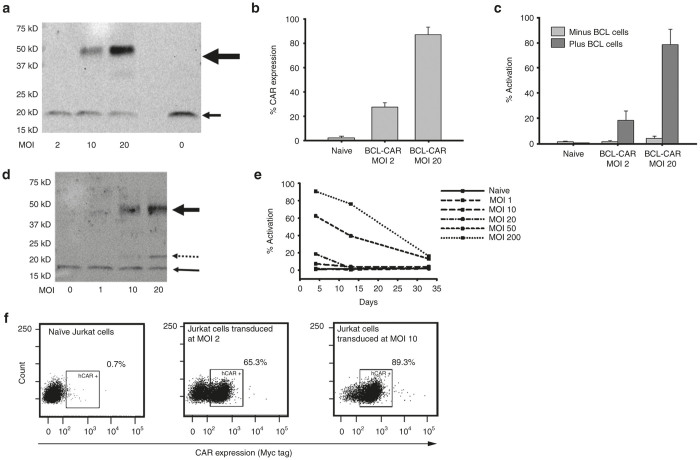
High-titer, recombinant, self-inactivating lentiviral vectors encoding the anti-BCL and anti-CD5 VLR-CARs were generated (Figure 1b,c) at titers of ~1 × 108 HEK 293 transducing units (HEK-293 TU/ml) and used to transduce the Jurkat T cell line. Quantitative polymerase chain reaction confirmed the successful transduction of these cells. A dose response was observed whereby integrated proviral vector copy number increased directly with multiplicity of infection (MOI) up to a vector copy number of 21 at an MOI of 20 (data not shown). As a means to assess VLR-CAR protein expression in the transduced Jurkat cells, western blot analysis was performed on whole cell lysates. VLR-CAR protein was detected using an anti-CD3-ζ antibody. Proteins of ~47 and 48 kDa were observed, which correspond to the predicted sizes of the anti-BCL (Figure 2a) and anti-CD5 (Figure 2d) VLR-CARs, respectively, as well as an 18 kDa band, which corresponds to the molecular weight of the endogenously expressed CD3-ζ protein known to be expressed in the Jurkat cell line. To confirm the cell-surface expression of the anti-BCL VLR-CAR, transduced Jurkat cells were stained with an anti-myc antibody, allowing for CAR detection by flow cytometry (Figure 2b). The BCL-VLR-CAR was expressed on the surface of the transduced cells and showed a dose dependent response to vector MOI. The transduced cells also showed persistent surface CAR expression for at least 3 months, with cell viability remaining over 85% (Supplementary Figure S1; day 5 and Figure 2f; day 35), demonstrating the durability of the VLR-CAR expression.
Figure 2.
VLR-CAR expression and activation of Jurkat cells when cocultured with cells expressing the target antigen. (a) Western blot using anti-CD3æ antibody showing BCL-VLR-CAR protein expression in whole cell lysates of Jurkat cells transduced at MOIs from 2 to 20. The bold arrow shows BCL-VLR-CAR and the thin arrow shows endogenously expressed CD3æ. (b) Flow analysis of myc expression on the surface of naive and transduced Jurkat cells at MOIs of 2 and 20. (c) Flow analysis of activation (CD69 expression) in naive Jurkat cells and Jurkat cells transduced with BCL-VLR-CAR in the absence or presence of BCL cells. Naive and transduced Jurkat cells not cocultured with BCL showed minimal activation. (d) Western blot using anti-CD3æ antibody showing increased CD5-VLR-CAR expression with increasing MOI. The bold arrow shows CD5-VLR-CAR, the thin arrow shows endogenously expressed CD3æ, and the dashed arrow shows a possible breakdown product of the CD5-VLR-CAR, which is not observed with the BCL-VLR-CAR. (e) Increased activation is observed in Jurkat cells transduced at increased MOIs with the CD5-VLR-CAR, but activation subsequently decreases over time in all transduction conditions. (f) VLR-CAR expression 35 days after transduction at MOIs of 0, 2, and 10. Values within the figures show the percentage of VLR-CAR positive cells. CAR, chimeric antigen receptor; MOI, multiplicity of infection; VLR, variable lymphocyte receptor.
Next, we assessed VLR-CAR functionality by demonstrating effector T cell activation following engagement of the VLR by the target antigen. First, BCL-specific VLR-CAR expressing Jurkat T cells were incubated with target BCL cells. Induction of CD69 expression mediated through the VLR-CAR was demonstrated with activation ranging ~20–80% of the total effector cell population, depending on the initial transduction MOI (Figure 2c and Supplementary Figure S2). Given their inherent surface expression of CD5, we hypothesized that anti-CD5-VLR-CAR expression in Jurkat T cells would result in activation of the transduced cells due to interaction with self or neighboring CD5 on Jurkat T cells. Cells were transduced at varying MOIs ranging from 1 to 200, and green fluorescence protein (GFP) lentivirus was used as a control. Again, western blot analysis using a CD3ζ antibody demonstrated increasing CAR expression with increasing MOI (Figure 2d). To determine if CD5-VLR-CAR expression resulted in T cell activation, transduced Jurkat T cells were stained with the T-cell activation marker CD69 and analyzed by flow cytometry (Supplementary Figure S3). Increased activation of Jurkat T cells was observed after CD5-VLR-CAR transduction and the degree of activation was higher with increasing MOI (Figure 2e). No activation was observed in the GFP transduced control group (Supplementary Figure S3). Unlike BCL-VLR-CAR modified cells, the percentage of activated Jurkat T cells decreased over time and returned to baseline after ~5 weeks in culture (Figure 2e). During this time there was also a decrease in copy number, which correlated to the expected decrease in activation, as activated Jurkat cells expanded at a slower rate compared with nonactivated cells (data not shown). Therefore, we speculate that this decrease in Jurkat T cell activation and vector copy number resulted from the faster division rate of nonmodified versus modified (i.e., VLR-CAR expressing) cells and activation-induced cell death resulting from a continuous activation of the transduced cell population via self and neighboring CD5 interactions, neither of which were observed using the anti-BCL VLR-CAR or GFP-modified cells.
VLR-CAR NK-cell effector cytotoxicity
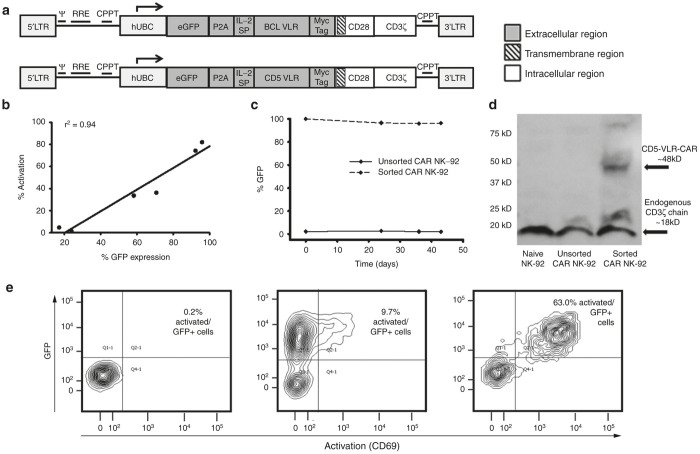
Although Jurkat T cell are capable of CAR-based activation they do not possess cytotoxic effector functionality. Therefore, as a final proof of concept validation test for the VLR-CAR technology platform, next we examined their ability to direct selective effector cell recognition and killing of target cells expressing the cognate VLR antigen. For this study, the well characterized cytotoxic human NK cell line, NK-92, was used. NK-92 is an IL-2 dependent immortalized cell line that has maintained its cytotoxic capabilities.27 These cells have been studied both preclinically, and now clinically as a stand-alone anticancer therapeutic.28,29 In addition, these cells do not display CD5 on their surface, which allows for expression of the CD5-VLR-CAR without self-activation and killing of transduced cells. Given the poor transduction efficiency of NK-92 cells (<10%), a construct incorporating a GFP P2A sequence was created (Figure 3a). This allowed for expression of both GFP protein and the CD5-VLR-CAR protein from a single transgene, which enabled selection of the CAR expressing NK-92 cell population by flow sorting. Initially, this construct was tested using Jurkat cells to confirm VLR-CAR expression and function. Modified Jurkat cells expressing the GFP-p2A-CD5-VLR-CAR construct showed a positive correlation between GFP expression and activation, thus verifying functionality of the new construct (Figure 3b and Supplementary Figure S4). By implementing this construct, we were able to generate a uniform population of anti-CD5 VLR-CAR expressing NK-92 cells by flow sorting for the GFP positive cells. After sorting, the percentage of GFP positive NK-92 cells increased from <5% to >95% (Supplementary Figure S5a). The VLR-CAR positive NK-92 cell population was then expanded in culture for several weeks and, in contrast to the genetically-modified Jurkat T cells, showed sustained GFP expression for a period of >40 days (Figure 3c). Quantitative polymerase chain reaction demonstrated an average of 2.3 transduced gene copies/cell in the sorted/expanded cells and western blotting confirmed CAR expression using a CD3ζ antibody (Figure 3d).
Figure 3.
Cassettes coexpressing GFP and VLR-CARs show a correlation between expression and activation. (a) The eGFP P2A CAR construct with either BCL or CD5-VLR. (b) Jurkat cells were transduced at various MOIs and a direct correlation between GFP expression and activation is observed in the eGFP P2A CD5-VLR-CAR transduced Jurkat cells, demonstrating dual expression of both proteins and activation of only VLR-expressing cells. (c) NK-92 cells were transduced with the GFP-p2a-CD5-VLR-CAR and sorted for GFP expressing cells. The isolated and expanded cells were followed over time for GFP expression. (d) Western blot using anti-CD3æ antibody on whole cell lysates of NK-92 cells shows the presence of CD5-VLR-CAR protein in the sorted cells. (e) Activation of eGFP P2A BCL-VLR-CAR transduced Jurkat cells when cocultured with target BCL cells. Naive Jurkat cells showed no activation when cocultured with BCL cells (left panel), cells modified but without coculture showed minimal activation (middle panel), and transduced cells cocultured with BCL cells (right panel) showed robust levels of activation. CAR, chimeric antigen receptor; eGFP, enhanced green fluorescence protein; MOI, multiplicity of infection; VLR, variable lymphocyte receptor.
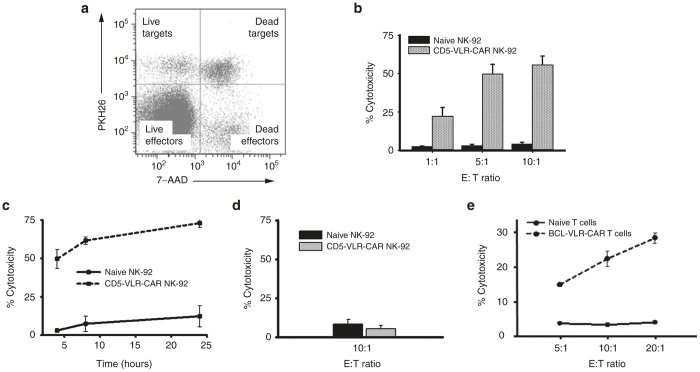
GFP+-selected, and thus CD5-VLR-CAR expressing, NK-92 cells were used to assess cytotoxicity against the CD5+ T-ALL cell line, CCRF-CEM, which is representative of a common T-ALL with robust CD5+ expression (Supplementary Figure S5b). To assess cytotoxic potential, CD5-VLR-CAR NK-92 effector (E) cells were cultured with CCRF-CEM target (T) cells in varying E:T ratios. Cytotoxicity was determined via uptake of 7-AAD, a marker for cell death, into target cells that also were prelabeled with PKH26 allowing for easy distinction from the GFP+ effector cells (Figure 4a).30 This shift indicates a significant increase in cytotoxicity observed with the CD5-VLR-CAR expressing NK-92 cells compared with naive NK-92 cells, even at the lowest E:T ratios (P < 0.01 for all cell groups) (Figure 4b). The observed percent killing increased with incubation time up to 24 hours (Figure 4c). As a negative control, CD5-VLR CAR expressing NK-92 cells were cultured with the CD5 negative B-ALL cell line, 697. No increase in target cell killing was observed with these cells (Figure 4d).
Figure 4.
BCL and CD5 VLR-CAR constructs significantly increase the cytotoxic potential of effector cells when cultured with their target cells. (a) Representation of flow cytometry cytotoxicity assay using PKH26 and 7-AAD. Target cells were labeled with PKH26, while effector cells were unlabeled. Cell death was assessed using 7-AAD (b) Transduced, sorted, and expanded NK-92 cells were cocultured with CCRF-CEM cells. Significant increase in cytotoxicity (P < 0.01) is observed against CD5-positive CCRF-CEM cells using CD5-VLR-CAR expressing NK-92 cells compared with naive NK-92 cells in a 4-hour assay. (c) A cytotoxicity assay was performed over a 24 hours’ time course using CD5-VLR-Car expressing NK92 cells and compared with naive cells, which demonstrates continued target cell killing over time at a 5:1 E:T ratio. (d) No increase in cytotoxicity against CD5-negative 697 (B-ALL) cells is observed using CAR-expressing NK-92 cells compared with naive NK-92 cells. (e) Cytotoxicity assay performed by coculture of naive or BCL-VLR-CAR transduced primary T cells (E) and target BCL cells (T) for 4 hours at various E:T ratios, which show increased killing at higher E:T ratios. CAR, chimeric antigen receptor; VLR, variable lymphocyte receptor.
To demonstrate the ability of the BCL-VLR-CAR to direct target cell killing, a similar GFP-p2a-VLR CAR construct was generated (Figure 3a). GFP+/BCL-VLR-CAR expressing Jurkat T cells were not activated unless cultured in the presence of BCL cells (Figure 3e and Supplementary Figure S6). To extend these VLR-CAR effector cell line findings to primary human cells, we expanded CD3+ T cells from frozen peripheral blood mononuclear cells for 3 days prior to transduction with VLR-CAR encoding lentiviral vector at MOI of 20, twice on consecutive days. Assessment of cytotoxic potential again was determined by coculture with BCL cell targets. Flow cytometric analysis showed a significant increase in target cell killing in the transduced T-cell culture compared with naive T cells, and this increase was observed for all E:T ratios tested (Figure 4e). These results show that CARs constructed using two different VLRs, one against a myeloid lineage and another one against a lymphoid lineage, effectively redirect immunocompetent cells to tumor associated antigens.
Discussion
CAR technology is progressing at an extremely rapid pace and its applications are expanding quickly, as new strategies are being applied to achieve tumor specific cell killing. The promising results in human trials involving CARs has fueled increasing interest in the technology and led to improvements in the design of CAR transgenes that have enhanced cell signaling as well as the lifespan of CAR-effector cells. A limitation persists in these approaches, however, as the types of tumor cell target antigens capable of being recognized by the CAR-effector cell still remains limited. Nearly all CAR designs thus far have employed scFvs as the antigen recognition region of the CAR. Our results provide proof of concept for a unique method of replacing the scFv with a VLR, thus increasing the number and diversity of targetable antigens. VLRs, as the functional unit of the lamprey adaptive immune system, allow for antigen engagement in a manner geometrically dissimilar to Ig, enabling the recognition of antigen epitopes not typically available to Ig. This results in an expanded selection of tumor cell target antigens/epitopes available for CAR design and application. VLR-CARs can be efficiently expressed on effector cells, and these VLR-CARs are capable of redirecting the effector cells to recognize a specific target cell. Expression of the VLR-CAR on the effector cell improved target cell killing for both BCL directed and CD5 directed CARs. The results provided herein indicate a potentially unique and effective method for activating CAR-effector cells as well as a method by which the array of CAR targeting domains can be expanded.
The VLR, as a component of immunity, arose in agnathans, jawless vertebrates, >500 million years ago. The only extant species known to use the VLR-based immune system are lampreys and hagfish. The VLR genes in these two species are produced in a RAG-independent manner that involves assembly of LRR cassettes into the germ line gene of lamprey and hagfish immune effector cells.14–17 The VLRs produced by this method represent the functional component of the lamprey and hagfish adaptive immune system. This VLR-based immune system has been shown in several studies to be equally as competent as the immunoglobulin-based immune system found in other vertebrate species, and is capable of >1015 unique variations in the VLR gene.14–17,23 The overall VLR structure is a crescent shape with β-strands lining the convex portion of the molecule, which along with the C-terminal loop provide the binding surface for antigen.14–17,22–26 This structure differs significantly from that of antibodies and their corresponding scFvs used in the conventional CAR design. An example of the unique binding methods of VLRs has been described previously with glycan binding VLRs. In comparison with Ig-based antibodies where the protein surface is commonly found to contain long grooves that can bind the oligosaccharide chains,31,32 VLRs bind this same molecule but through recognition of different regions, in a manner wherein the antigen is sandwiched between the loop created by one of the LRRs and the concave surface of the VLR formed from the β-strands.25 In this example, the VLR structure forces the bound oligosaccharide to bend as a way of adapting to the concave structure of the VLR, while still allowing it to retain contact with the protein. This contrasts with the carbohydrate binding surfaces of antibodies that are generally found to be flat or convex.25 Therefore, it is becoming clear that the structural differences between VLRs and antibodies impact antigen binding, which can lead to the ability of the VLR to recognize different antigen epitopes as well as impact antigen binding affinity compared with the corresponding antibodies.
A potential concern needing to be addressed is the immunogenicity of the VLR-CAR construct. LRRs that form the core structure of VLRs are seen in the human innate immune system as part of the extracellular domains of human Toll-like receptors.33,34 Therefore, the similar homology of LRRs in human Toll-like receptors may decrease their immunogenicity. However, this remains an obvious and important concern, and several options are being explored to determine the degree of VLR immunogenicity in the setting of CAR technologies and delivery and also to determine issues that may arise from introducing VLRs into mammals.
Overall, our results demonstrate VLRs can be used in place of the scFv to serve the function of antigen recognition in a CAR complex. The functionality of the VLR was initially demonstrated in Jurkat cells transduced with two different VLR sequences, a BCL-VLR and a CD5-VLR. CAR protein expression and CAR cell surface expression was confirmed in these cells, indicating a functional CAR design. The BCL-VLR-CAR was shown to be capable of effector cell activation when cultured in the presence of target BCL cells. Similar results were shown with a CD5-VLR transduced into multiple lineages of immunocompetent cells. Importantly, it was also demonstrated that the VLR-CARs activated effector cells specifically against their respective antigen-expressing target cells. These studies establish the potential of VLRs as the antigen recognition region of the CAR construct and position VLRs as a viable method for redirecting target cells using CAR technologies.
Materials and Methods
Cell lines
Jurkat, BCL1-3B3 (BCL), and CCRF-CEM cell lines were obtained from American Type Culture Collection (ATCC, Manassas, VA). The 697 B-ALL cell line was kindly provided by Douglas Graham (Aflac Cancer Center, Atlanta, GA). The primary culture media for the Jurkat, BCL1-3B3 (BCL), CCRF-CEM, and 697 cell lines was Roswell Park Memorial Institute (RPMI) (Corning, Manassas, VA) with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. For NK-92 cells, AIMV (Thermo Fisher Scientific, Waltham, MA) was used with 20% FBS, 1% penicillin/streptomycin and 1,000 U/ml recombinant IL-2 (Peprotech, Rocky Hill, NJ).
Cloning the VLR-CAR sequences
The BCL-VLR sequence was obtained from the laboratory of Max Cooper (Emory University, Atlanta, GA). The CD5 VLR cDNA sequence was generated from a published protein sequence of a VLR targeting CD526 and then codon optimized for human cell expression. The VLR sequence was then cloned into a vector containing the remaining necessary components for CAR production, which were obtained by gene synthesis from Genewiz (South Plainfield, NJ).
Generation of VLR-CAR encoding lentivirus
Recombinant HIV lentivirus was produced using a four plasmid system. Briefly, the expression plasmid encoding the VLR-CAR constructs as well as packaging plasmids containing the gag, pol, and envelope genes were transiently transfected into HEK-293T cells by calcium phosphate transfection. Cells were cultured in Dulbecco’s modified essential medium (DMEM, Thermo Fisher Scientific) supplemented with 10% FBS and 1% penicillin–streptomycin. Twenty-four hours after transfection, the cell culture medium was replaced with fresh medium. At 48, 72, and 96 hours the viral supernatant was collected, filtered through a 0.22 μm filter and stored at −80°C. After the final virus collection, the supernatant was pooled and concentrated overnight via centrifugation at 10,000g at 4°C. Pelleted virus was then resuspended in StemPro media (Thermo Fisher Scientific). Titering was performed on HEK-293T cells using quantitative polymerase chain reaction. Titer of the concentrated virus was found to be ~1 × 108 TU/ml.
Lentiviral transduction of cell lines
Transduction of recombinant HIV lentiviral particles was carried out by incubating cells with virus in appropriate culture medium supplemented with 6 μg/ml polybrene (EMD Millipore, Billerica, MA). Twenty-four hours after transduction, culture medium was replaced with fresh medium. The transduced cells were then cultured for at least 3 days before being used for downstream applications.
Western blotting with CD3ζ antibody
Cells were lysed using radioimmunoprecipitation assay buffer (Sigma-Aldrich, St. Louis, MO). Cell lysates were clarified by centrifugation and protein was quantified using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Equal quantities of protein were loaded and cell lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and transferred to a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA). The protein-loaded and blocked membrane was incubated with an anti-CD3-ζ mAb (1:500) followed by a horse radish peroxide (HRP)-labeled goat antimouse secondary Ab (1:2500) (BioLegend, San Diego, CA).
Flow cytometry analysis and sorting
Analysis was done using a BD fluorescence-activated cell sorting (FACS) Canto II Flow Cytometer and BD LSRII Flow Cytometer (BD Biosciences, San Jose, CA). Data was analyzed using the BD FACSDiva software. Antibodies used included anti-CD69 APC-Cy7, anti-CD5 PerCP/Cy5.5, antihuman CD45 PE (BD Biosciences) and anti-myc tag fluorescein isothiocyanate (FITC,Abcam, Cambridge, MA). For the cytotoxicity studies, target cells were stained with the membrane dye PKH26 and cell death was assessed using 7-AAD (described below). Flow sorting for GFP was performed using a BD FACS Aria II Cell Sorter (BD Biosciences).
Expansion and lentiviral transduction of primary human T cells
Commercially available human peripheral blood mononuclear cells were purchased (AllCells, Catalog # PB004F, Alameda, CA). After thawing and washing, a pan T-cell isolation was performed using the manufacturer’s recommendations (MACS Catalog #: 130-096-535, Miltinyi Biotec, San Diego, CA). Cells were then incubated with activation/expansion beads (MACS Catalog #: 130-091-441, Miltinyi Biotec) and cultured for 24 hours after which 100 U/ml IL-2 was added. Cells were stimulated with beads and IL-2 for 3 days prior to transduction. For transduction, 2 × 106 T cells were plated in complete RPMI with IL-2 in each well of a 6-well plate. Virus (300 µl) was added at an MOI of ~20. Media was changed after 24 hours and the cells were replated and transduced for the second time. Twenty-four hours after the second transduction, the media was changed to complete RPMI and IL-2 was added at 100 IU/ml. The cells were then allowed to expand for 12 days.
Coculture assay using Jurkat and BCL cells
The coculture assay was performed by incubating 50,000 transduced or naive Jurkat cells in a 1:1 ratio with the target BCL cells. This culture was incubated for 4 hours at 37°C and then stained using antibodies for human CD45, the myc epitope tag, and CD69. Cells were then analyzed via flow cytometry to determine levels of activation in the transduced and naive cell groups.
Cytotoxicity assay
Target cells were labeled with membrane dye PKH26 using the manufacturer’s protocol (Sigma-Aldrich). Effector cells were left unstained. Effector (E) and target (T) cells were counted and viability assessed using trypan blue. Labeled target cells were mixed with effector cells in 12 × 75 mm FACS tubes or round bottom 96-well plates at E:T ratios ranging from 0:1 to 20:1 in a total volume of 200 µl. Target cells (20,000) were plated per tube/well along with the corresponding number of effector cells. The cell mixture was incubated for 4 hours at 37°C in 5% CO2. After incubation, cells were washed and stained with 7-AAD (BD Biosciences). Flow cytometry analysis was then performed to assess 7-AAD positive cells. All experiments were performed in triplicate. To calculate specific cytotoxicity, the number of spontaneously lysed target cells in the absence of effector cells was subtracted from the number of dead target cells, which were identified as PKH26 and 7-AAD double positive in the measured sample.
Statistical analysis
Unpaired two-tailed Student’s t-test was used to determine statistical significance. All P-values were calculated with SigmaPlot, version 13.0 (Systat Software, Chicago, IL).
Acknowledgments
We thank Max Cooper and Brant Herrin at Emory University for providing us with the BCL-VLR construct used in some experiments and for outstanding discussions regarding lamprey immunology. This work was supported by grants from the National Institute of Health (NIH 1R43CA192710-01), CURE Childhood Cancer, and the Georgia Research Alliance. All flow cytometry experiments were done with equipment in the Emory+Children’s Pediatric Research Center Flow Cytometry Core.
The first two authors contributed equally to this work.
References
- Curran, KJ, Pegram, HJ and Brentjens, RJ (2012). Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. J Gene Med 14: 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus, A and Eshhar, Z (2014). Allogeneic chimeric antigen receptor-modified cells for adoptive cell therapy of cancer. Expert Opin Biol Ther 14: 947–954. [DOI] [PubMed] [Google Scholar]
- Jensen, MC and Riddell, SR (2015). Designing chimeric antigen receptors to effectively and safely target tumors. Curr Opin Immunol 33: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, S, Maus, MV and Porter, DL (2016). Chimeric antigen receptor T cell therapy: 25 years in the making. Blood Rev 30: 157–167. [DOI] [PubMed] [Google Scholar]
- Porter, DL, Levine, BL, Kalos, M, Bagg, A and June, CH (2011). Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365: 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila, ML, Riviere, I, Wang, X, Bartido, S, Park, J, Curran, K et al. (2014). Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6: 224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude, SL, Frey, N, Shaw, PA, Aplenc, R, Barrett, DM, Bunin, NJ et al. (2014). Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371: 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, DW, Kochenderfer, JN, Stetler-Stevenson, M, Cui, YK, Delbrook, C, Feldman, SA et al. (2015). T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385: 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop, HE (2010). Safer CARS. Mol Ther 18: 661–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, RA, Yang, JC, Kitano, M, Dudley, ME, Laurencot, CM and Rosenberg, SA (2010). Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 18: 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers, CH, Sleijfer, S, van Steenbergen, S, van Elzakker, P, van Krimpen, B, Groot, C et al. (2013). Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther 21: 904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casucci, M, Hawkins, RE, Dotti, G and Bondanza, A (2015). Overcoming the toxicity hurdles of genetically targeted T cells. Cancer Immunol Immunother 64: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, G and Eshhar, Z (2016). Therapeutic potential of T cell chimeric antigen receptors (CARs) in cancer treatment: counteracting off-tumor toxicities for safe CAR T cell therapy. Annu Rev Pharmacol Toxicol 56: 59–83. [DOI] [PubMed] [Google Scholar]
- Herrin, BR and Cooper, MD (2010). Alternative adaptive immunity in jawless vertebrates. J Immunol 185: 1367–1374. [DOI] [PubMed] [Google Scholar]
- Mariuzza, RA, Velikovsky, CA, Deng, L, Xu, G and Pancer, Z (2010). Structural insights into the evolution of the adaptive immune system: the variable lymphocyte receptors of jawless vertebrates. Biol Chem 391: 753–760. [DOI] [PubMed] [Google Scholar]
- Boehm, T, McCurley, N, Sutoh, Y, Schorpp, M, Kasahara, M and Cooper, MD (2012). VLR-based adaptive immunity. Annu Rev Immunol 30: 203–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara, M and Sutoh, Y (2014). Two forms of adaptive immunity in vertebrates: similarities and differences. Adv Immunol 122: 59–90. [DOI] [PubMed] [Google Scholar]
- Hirano, M, Guo, P, McCurley, N, Schorpp, M, Das, S, Boehm, T et al. (2013). Evolutionary implications of a third lymphocyte lineage in lampreys. Nature 501: 435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishishita, N and Nagawa, F (2014). Evolution of adaptive immunity: implications of a third lymphocyte lineage in lampreys. Bioessays 36: 244–250. [DOI] [PubMed] [Google Scholar]
- Tasumi, S, Velikovsky, CA, Xu, G, Gai, SA, Wittrup, KD, Flajnik, MF et al. (2009). High-affinity lamprey VLRA and VLRB monoclonal antibodies. Proc Natl Acad Sci USA 106: 12891–12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, G, Tasumi, S and Pancer, Z (2011). Yeast surface display of lamprey variable lymphocyte receptors. Methods Mol Biol 748: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velikovsky, CA, Deng, L, Tasumi, S, Iyer, LM, Kerzic, MC, Aravind, L et al. (2009). Structure of a lamprey variable lymphocyte receptor in complex with a protein antigen. Nat Struct Mol Biol 16: 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrin, BR, Alder, MN, Roux, KH, Sina, C, Ehrhardt, GR, Boydston, JA et al. (2008). Structure and specificity of lamprey monoclonal antibodies. Proc Natl Acad Sci USA 105: 2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchdoerfer, RN, Herrin, BR, Han, BW, Turnbough, CL Jr, Cooper, MD and Wilson, IA (2012). Variable lymphocyte receptor recognition of the immunodominant glycoprotein of Bacillus anthracis spores. Structure 20: 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, M, Velikovsky, CA, Yang, X, Siddiqui, MA, Hong, X, Barchi, JJ Jr et al. (2013). Recognition of the Thomsen-Friedenreich pancarcinoma carbohydrate antigen by a lamprey variable lymphocyte receptor. J Biol Chem 288: 23597–23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, C, Ali, S, St-Germain, J, Liu, Y, Yu, X, Jaye, DL et al. (2012). Purification and identification of cell surface antigens using lamprey monoclonal antibodies. J Immunol Methods 386: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, JH, Maki, G and Klingemann, HG (1994). Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 8: 652–658. [PubMed] [Google Scholar]
- Suck, G, Odendahl, M, Nowakowska, P, Seidl, C, Wels, WS, Klingemann, HG et al. (2016). NK-92: an ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol Immunother 65: 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkins, R, Burgess, A, Kerbel, R, Wels, WS and Hynynen, K (2016). Early treatment of HER2-amplified brain tumors with targeted NK-92 cells and focused ultrasound improves survival. Neuro Oncol 18: 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-MacAry, AE, Ross, EL, Davies, D, Laylor, R, Honeychurch, J, Glennie, MJ et al. (2001). Development of a novel flow cytometric cell-mediated cytotoxicity assay using the fluorophores PKH-26 and TO-PRO-3 iodide. J Immunol Methods 252: 83–92. [DOI] [PubMed] [Google Scholar]
- Bundle, DR, Eichler, E, Gidney, MA, Meldal, M, Ragauskas, A, Sigurskjold, BW et al. (1994). Molecular recognition of a Salmonella trisaccharide epitope by monoclonal antibody Se155-4. Biochemistry 33: 5172–5182. [DOI] [PubMed] [Google Scholar]
- Vyas, NK, Vyas, MN, Chervenak, MC, Johnson, MA, Pinto, BM, Bundle, DR et al. (2002). Molecular recognition of oligosaccharide epitopes by a monoclonal Fab specific for Shigella flexneri Y lipopolysaccharide: X-ray structures and thermodynamics. Biochemistry 41: 13575–13586. [DOI] [PubMed] [Google Scholar]
- Takeda, K and Akira, S (2005). Toll-like receptors in innate immunity. Int Immunol 17: 1–14. [DOI] [PubMed] [Google Scholar]
- Bell, JK, Mullen, GE, Leifer, CA, Mazzoni, A, Davies, DR and Segal, DM (2003). Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol 24: 528–533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.