Abstract
1,25-Dihydroxyvitamin D3 [1,25(OH)2D] has been shown to inhibit development of dextran sodium sulfate (DSS)-induced colitis in mice but can also cause hypercalcemia. The aim of this study was to evaluate whether β-glucuronides of vitamin D could deliver 1,25(OH)2D to the colon to ameliorate colitis while reducing the risk of hypercalcemia. Initial studies demonstrated that bacteria residing in the lower intestinal tract were capable of liberating 1,25(OH)2D from 1,25-dihydroxyvitamin D3-25-β-glucuronide [β-gluc-1,25(OH)2D]. We also determined that a much greater upregulation of the vitamin D-dependent 24-hydroxylase gene (Cyp24) was induced in the colon by treatment of mice with an oral dose of β-gluc-1,25(OH)2D than 1,25(OH)2D, demonstrating targeted delivery of 1,25(OH)2D to the colon. We then tested β-glucuronides of vitamin D in the mouse DSS colitis model in two studies. In mice receiving DSS dissolved in distilled water and treated with 1,25(OH)2D or β-gluc-1,25(OH)2D, severity of colitis was reduced. Combination of β-gluc-1,25(OH)2D with 25-hydroxyvitamin D3-25-β-glucuronide [β-gluc-25(OH)D] resulted in the greatest reduction of colitis lesions and symptoms in DSS-treated mice. Plasma calcium concentrations were lower in mice treated with β-gluc-1,25(OH)2D alone or in combination with β-gluc-25(OH)D than in mice treated with 1,25(OH)2D, which were hypercalcemic at the time of death. β-Glucuronides of vitamin D compounds can deliver 1,25(OH)2D to the lower intestine and can reduce symptoms and lesions of acute colitis in this model.
Keywords: 1,25-dihydroxyvitamin D3; 1,25-dihydroxyvitamin D3-25-β-glucuronide; 24-hydroxylase; dextran sodium sulfate
crohn's disease and ulcerative colitis are forms of inflammatory bowel disease (IBD) affecting the lower ileum and colon. IBD, especially Crohn's disease, is often considered an autoimmune disease, characterized by lower intestinal tract innate immune cells that inappropriately secrete proinflammatory cytokines in response to commensal bacteria residing in the lower intestinal tract. Lower intestinal tract Th1 lymphocytes react by secreting IL-2, IL-12, IL-17, TNF-α, and IFN-γ, inducing excessive inflammation in response to bacterial antigens.
Epidemiological evidence suggests that geographical location, specifically distance from the equator, is associated with increased incidence of Crohn's disease (17). One possible explanation for this epidemiological observation is that living closer to the equator increases exposure to the sun's UV-B rays, which may promote cutaneous vitamin D synthesis (21). In support of this, <22% of patients recently diagnosed with Crohn's disease had optimal serum 25-hydroxyvitamin D3 [25(OH)D] levels, defined as ≥75 nmol/l (14). In a recent clinical trial, treatment of Crohn's patients with vitamin D3 at 1,200 IU/day caused a small reduction in the risk of relapse (P < 0.08). Plasma 25(OH)D concentrations were increased from 31 to 96 nmol/l by this treatment (10).
Since dietary vitamin D absorption is often suboptimal in Crohn's patients, previous work in such patients often focused on the endocrine actions of vitamin D on calcium homeostasis and bone health. The endocrine effects of vitamin D are mediated by renal production of the hormone 1,25-dihydroxyvitamin D3 [1,25(OH)2D], produced from the vitamin D metabolite 25(OH)D, which is synthesized by the liver. In addition to its endocrine actions, vitamin D plays an autocrine/paracrine role in the differentiation and regulation of cell function. Circulating 25(OH)D is taken up by many cells of the body and converted to 1,25(OH)2D by cells that express the 25-hydroxyvitamin-D3 1α-hydroxylase (1α-hydroxylase) enzyme. The 1,25(OH)2D produced in this way can act directly within the cell or diffuse to neighboring cells and bind to vitamin D receptors (VDRs) to regulate transcription of genes. VDRs are widely expressed in the epithelial cells and the immune cells in the colon, including activated T lymphocytes, and antigen-presenting macrophages and dendritic cells. The 1,25(OH)2D produced within the cell binds to the VDR and regulates transcription of a wide variety of genes in epithelial and innate immune cells that may be of benefit to the health of the lower intestine.
Several studies have demonstrated a direct therapeutic effect of vitamin D and 1,25(OH)2D in mouse models of IBD. Cantorna et al. (2) performed a series of elegant studies demonstrating that >50% of vitamin D-deficient IL-10 knockout (IL-10 KO) mice died of IBD by 8 wk of age. Vitamin D-sufficient IL-10 KO mice showed no IBD symptoms through 9 wk of age. Treatment of vitamin D-deficient IL-10 KO mice with as little as 12 pmol (5 ng) of 1,25(OH)2D per day reversed vitamin D deficiency and prevented development of IBD-associated lesions. Diet supplementation with 480 pmol (200 ng) of 1,25(OH)2D per day blocked further development of IBD in IL-10 KO mice (2). Further studies demonstrated that treatment of vitamin D-deficient IL-10 KO mice with 48 pmol (20 ng) of 1,25(OH)2D per day reduced expression of TNF-α, TNF receptor superfamily 1A, and TNF-α-induced protein 2 in the colon, while reducing or preventing the onset of IBD. Interestingly, this benefit was only observed in mice fed a calcium-sufficient diet (29). The absence of 1,25(OH)2D action on its receptor in lower intestinal cells permits higher numbers of activated dendritic cells to exist in the intestine and may permit autoreactive T cells to develop (5, 27). Although administration of 1,25(OH)2D can ameliorate induced IBD in mice, effective doses may cause hypercalcemia. Another strategy that has been used is treatment of IBD with VDR agonists with low calcemic index. Laverny et al. (13) demonstrated that intrarectal administration of one such compound, 1α,25(OH)2-16-ene-20-cyclopropylvitamin D, had beneficial effects in the dextran sodium sulfate (DSS) mouse model, without causing hypercalcemia. Recently, Miheller et al. (18) reported that two 0.25-μg doses of 1,25(OH)2D per day did not cause hypercalcemia and did improve the Crohn's disease activity index in Crohn's patients 6 wk after treatment.
Treatment with 1,25(OH)2D induces a 25-hydroxyvitamin D 24-hydroxylase (Cyp24) enzyme in target tissues, which speeds the catabolism of 1,25(OH)2D. Over time, larger doses of 1,25(OH)2D must be given to achieve the same effect. IBD itself results in upregulation of the Cyp24 enzyme in the proximal colon of mice induced to develop IBD with DSS (15). Froicu and Cantorna (5) demonstrated that administration of 120 pmol (50 ng) of 1,25(OH)2D rectally, every other day, ameliorated symptoms of IBD in the colons of DSS-treated mice and did not cause hypercalcemia. While 1,25(OH)2D suppositories may be possible, it may be difficult to deliver 1,25(OH)2D to the ileum, which is also commonly affected in Crohn's disease.
We have conjugated glucuronic acid to the 25-carbon position of the 1,25(OH)2D and 25(OH)D molecules in a β-linkage to form 1,25-dihydroxyvitamin D3-25-β-glucuronide [β-gluc-1,25(OH)2D] (Fig. 1) and 25-hydroxyvitamin D3-25-β-glucuronide [β-gluc-25-(OH)D]. The addition of the glucuronide makes the compounds water-soluble. They are also essentially inactive until acted upon by a β-glucuronidase (20). Rambeck et al. (22) reported that glycosidic forms of 1,25(OH)2D had <1% of the intestinal VDR binding activity of the aglycone 1,25(OH)2D. It is believed that little of the glycosidic forms of vitamin D compounds can be absorbed across the intestine (20). However, the possibility cannot be ruled out, as we have no assay sensitive enough to detect the glycosidic forms in blood. When administered orally, the β-glucuronides of the vitamin D compounds are converted to the active vitamin D moiety upon hydrolysis of the β-glucuronide by β-glucuronidase produced by bacteria in the lower intestinal tract. This should allow targeted delivery of 1,25(OH)2D to the ileum/colon cells affected by IBD. Cleavage of the β-gluc-1,25(OH)2D to free 1,25(OH)2D in the colon would also reduce systemic absorption of the 1,25(OH)2D compared with absorption of 1,25(OH)2D from the duodenum. Rectally administered 1,25(OH)2D is less hypercalcemic than orally administered 1,25(OH)2D, perhaps because of reduced systemic absorption (5, 13).
Fig. 1.
Chemical structure of 1,25-dihydroxyvitamin D3-25-β-glucuronide [β-gluc-1,25(OH)2D].
Destruction of 1,25(OH)2D by upregulation of the Cyp24 enzyme within IBD target cells (15) necessitates administration of very high doses of 1,25(OH)2D to observe a benefit. In vitro, the effects of 1,25(OH)2D can be enhanced if cells are also treated with a competitive inhibitor of Cyp24, such as 25(OH)D or 24,25-dihydroxyvitamin D3 [24,25(OH)2D] (24). Using these prodrugs, we postulated that it would be possible to deliver a therapeutic dose of 1,25(OH)2D to the ileum/colon, along with enough 25(OH)D (in the form of the glucuronide) to competitively inhibit 24-hydroxylation of 1,25(OH)2D, thus potentiating 1,25(OH)2D effectiveness. This could allow consistent effects on the colon with doses of vitamin D compounds that might otherwise be systemically toxic.
MATERIALS AND METHODS
General
The β-25-monoglucuronides of 1,25(OH)2D and 25(OH)D were synthesized from the corresponding provitamin D or its derivatives using the Koenigs-Knorr reaction (25). Their structure was confirmed by Fourier transform infrared microscopy and NMR. β-Gluc-1,25(OH)2D (mol wt 592.76) was purified by HPLC to >97% purity, and β-gluc-25(OH)D (mol wt 576.7) was purified by HPLC to >90% purity; 1,25(OH)2D (mol wt 416.64), 25(OH)D (mol wt 400.6), and 24,25(OH)2D (mol wt 416.64) were >98% pure (Sigma Aldrich, St. Louis, MO). Quantitation of vitamin D compounds in ethanol solutions utilized to prepare each diet treatment was based on absorbance at 265 nm (using a molar extinction coefficient of 18,300 absorbance units·mol−1·l−1).
Male C57BL/6 mice (Jackson Labs, Bar Harbor, ME) were fed Teklad 2018 rodent diet based on wheat, corn, and soybean meal and containing 1% calcium, 0.7% phosphorus, and 1.5 IU of vitamin D3 per gram (Harlan Labs, Madison, WI). Sprague-Dawley rats (Harlan Labs) were fed Teklad 2018 diet prior to harvest of their intestinal tissue. Animals were housed in individual wire-bottom cages to reduce coprophagy. The room was maintained at 24–26°C with a 12:12-h light-dark cycle. All procedures performed on the animals were submitted to and approved by the Iowa State University Institutional Animal Care and Use Committee (protocols 4-09-6378-M and 10-08-6651-R).
In Vitro Assessment of β-Glucuronidase Activity of Intestine Contents
In most mammals, there are relatively few bacteria in the duodenum, and, generally, bacterial species such as Bacteroides sp. in the lower intestinal tract are capable of cleaving β-glycosidic linkages (9). Rats were chosen to test the ability of the glucuronides of vitamin D to be cleaved in different sections of the intestinal tract. The microbes found in rat intestine are similar to those in mouse intestine, and the amount of intestinal lumen material that could be obtained from the rat intestines allowed for larger incubations of material. The proximal 25 cm of duodenum/jejunum, the caudal 12 cm of ileum, and the cranial 12 cm of colon were removed from 18 Sprague-Dawley rats, and the contents of the lumen from each section were flushed with 3 ml of distilled water, collected, and pooled. Three-milliliter aliquots of intestinal contents were placed into tubes in duplicate. To some tubes, 3.53 pmol of β-gluc-1,25(OH)2D were added, and the contents were incubated at 37°C for 0, 1, 3, or 6 h. To other tubes, 0.36 pmol of 1,25(OH)2D was added, and the contents were incubated for 0 or 6 h (Table 1). The 1,25(OH)2D-containing tubes served as a control to confirm the ability to extract and detect 1,25(OH)2D in this material and to determine if any degradation of 1,25(OH)2D would occur. Tritiated 1,25(OH)2D [2,200 disintegrations/min of 156 Ci/mmol 1,25(OH)2D; Amersham-GE Healthcare, Piscataway, NJ] was added to each tube to assess extraction efficiency. Acetonitrile (3 ml) was added to each tube after incubation to end all enzymatic activity and to extract the 1,25(OH)2D that was liberated. The preparations were cleaned using a 0.5-g C18OH solid phase extraction column followed by an analytical Zorbax Sil HPLC column (both purchased from Varian, Lexington, MA). β-Gluc-1,25(OH)2D, being more water-soluble than 1,25(OH)2D, does not coelute with 1,25(OH)2D on the C18OH or Zorbax Sil column. Samples were analyzed for 1,25(OH)2D content by RIA (Heartland Assays) (8).
Table 1.
β-Glucuronidase activity of duodenum and ileal contents of rats and amount of 1,25(OH)2D liberated
| Origin of Lumen Contents | Incubation Time, h | Vitamin D Form | Added Amount, pmol | Measured 1,25(OH)2D, pmol |
|---|---|---|---|---|
| Duodenum | 0 | 1,25(OH)2D | 0.36 | 0.23 ± 0.06 |
| Duodenum | 6 | 1,25(OH)2D | 0.36 | 0.28 ± 0.02 |
| Duodenum | 0 | β-Gluc-1,25(OH)2D | 3.54 | 0.07 ± 0.12 |
| Duodenum | 6 | β-Gluc-1,25(OH)2D | 3.54 | 0.25 ± 0.02 |
| Ileum | 0 | 1,25(OH)2D | 0.36 | 0.31 ± 0.02 |
| Ileum | 6 | 1,25(OH)2D | 0.36 | 0.31 ± 0.03 |
| Ileum | 0 | β-Gluc-1,25(OH)2D | 3.54 | 0.09 ± 0.01 |
| Ileum | 1 | β-Gluc-1,25(OH)2D | 3.54 | 3.18 ± 0.67 |
| Colon | 0 | 1,25(OH)2D | 0.36 | 0.22* |
| Colon | 6 | 1,25(OH)2D | 0.36 | 0.17 ± 0.02 |
| Colon | 0 | β-Gluc-1,25(OH)2D | 3.54 | 0.91 ± 0.51 |
| Colon | 1 | β-Gluc-1,25(OH)2D | 3.54 | 2.31 ± 0.41 |
Values are means ± SE; n = 2. β-Glucuronidase activity was assessed by cleavage of 1,25-dihydroxyvitamin D3-25-β-glucuronide [β-gluc-1,25(OH)2D] following in vitro incubation of β-gluc-1,25(OH)2D or 1,25(OH)2D (as assay internal control) for 0, 1, or 6 h.
Duplicate lost to analysis.
In Vivo Assessment of β-Gluc-1,25(OH)2D vs. 1,25(OH)2D on Cyp24 Gene Expression in Colon and Duodenum
Our goal was to determine if β-gluc-1,25(OH)2D could be used to target delivery of 1,25(OH)2D activity to the lower intestine. These acute studies allowed us to determine the site of action of a single dose. Two studies were conducted in mice to investigate the relative activity of β-gluc-1,25(OH)2D and 1,25(OH)2D on colon and duodenum, with Cyp24 expression utilized as an indicator of action of the secosteroid on the tissues. Study 1 was a dose-titration study. Ten-week-old ad libitum-fed male C57BL/6 mice received a single oral dose (6, 12, 24, or 48 pmol) of 1,25(OH)2D or β-gluc-1,25(OH)2D suspended in 50 μl of peanut oil (4 mice/treatment). Mice were euthanized 6 h later, blood was collected into heparinized tubes, and the plasma samples were analyzed for 1,25(OH)2D content by RIA, as described above. Because β-gluc-1,25(OH)2D is more water-soluble than 1,25(OH)2D and, therefore, elutes with the methanol wash of the 0.5-g C18OH solid phase extraction column, it is possible to measure only 1,25(OH)2D in the samples.
A 1-cm section of duodenum (2–3 cm from the pylorus) and a 1-cm section of colon (2–3 cm from the cecum) were obtained from each mouse for mRNA analysis. Tissue samples were flushed with ice-cold phosphate-buffered saline and immediately homogenized in 1 ml of TRIzol reagent (Invitrogen, Carlsbad, CA). Samples were kept frozen at −86°C prior to processing for RNA.
Study 2 was a time-course study. Similarly maintained mice were treated with a single oral 24-pmol dose of 1,25(OH)2D or β-gluc-1,25(OH)2D suspended in 50 μl of peanut oil (5 mice/treatment). Mice were euthanized 1, 3, 6, and 24 h after treatment, and plasma and tissues were harvested as described above.
Each TRIzol homogenate was thawed at room temperature, and 500 μl were placed in a clean microfuge tube, mixed thoroughly with 100 μl of chloroform for 15 s, and then centrifuged at 12,000 g for 15 min at 4°C. The upper aqueous phase was removed and mixed with 0.93 volumes of 75% ethanol. The mixture was applied to an RNeasy spin column (Qiagen, Germantown, MD) and processed as described by the manufacturer, except an additional wash with 2 M NaCl-2 mM EDTA (pH 4.0) was included (3). RNA was eluted in 50 μl of water, and the concentration was obtained by UV spectrometry. One microgram of RNA was used as a template for production of cDNA in a 20-μl reaction volume using random hexamers and SuperScript III as described by the manufacturer (Invitrogen). The samples were diluted to 100-μl final volume with Tris·HCl-EDTA buffer and stored at −20°C prior to PCR analysis.
Quantitative real-time PCR was performed using a Stratagene Mx3005p cycler (Stratagene, La Jolla, CA) and PerfeCTa SYBR Green FastMix ROX reagent (Quanta Biosciences, Gaithersburg, MD). Amplification of target cDNAs was accomplished with the following primers (synthesized by Integrated DNA Technologies, Coralville, IA): Cyp24 [5′-CACACGCTGGCCTGGGACAC (forward) and 5′-GGAGCTCCGTGACAGCAGCG (reverse)] and GAPDH [5′-GAAGGTCGGTGTGAACGGATTTGGC (forward) and 5′-TTGATGTTAGTGGGGTCTCGCTCCTG (reverse)]. Aliquots (8.3 ng) of cDNA were amplified under the following conditions: 95°C for 30 s, followed by 45 cycles of 95°C for 1 s and 57°C for 30 s. All reactions were performed in duplicate, with four or five animals per treatment, and Cyp24 target gene expression was estimated using the cycle threshold (ΔCT) method relative to GAPDH expression, as described previously (6).
Testing the Effect of Vitamin D Compounds on DSS-Induced Colitis in Mice
Diet treatments were prepared so that the daily dose of each desired vitamin D compound was delivered in 3.5 g of Teklad 2018 diet. Accordingly, the appropriate amount of each vitamin D compound dissolved in 40 ml of ethanol was thoroughly mixed into the finely ground diet. The diets were left uncovered at room temperature overnight with occasional stirring to allow the ethanol to evaporate. The diets were stored at 4°C between feedings. The dietary treatments began 4 days prior to initiation of colitis with DSS administered in drinking water. The diets were fed at a rate of 3.5 g/day, and any diet not consumed in 24 h was removed and weighed to determine feed refusal (data not shown). Mice were administered 2.5% DSS dissolved in distilled water ad libitum for 7 days. DSS (mol wt 35,000–50,000) was obtained from MP Biomedicals (Solon, OH).
Study 1.
Groups of 10-wk-old mice (9 mice/group) were randomly assigned to the different treatments. One group of mice was allowed to drink normal water (No DSS), while all other groups of mice received DSS in their drinking water. Of the groups that received DSS in their drinking water, one group received no vitamin D compounds added to the diet (DSS only). Other groups received treatments consisting of 1,25(OH)2D incorporated into the diet to supply 24 or 120 pmol/day. β-Gluc-1,25(OH)2D was incorporated into the diet to supply 24, 120, or 600 pmol/day. The final three treatments consisted of β-gluc-25(OH)D at 8.67 nmol/day by itself or combined with 24 or 120 pmol of β-gluc-1,25(OH)2D per day. The β-gluc-25(OH)D treatment was included to try to competitively inhibit the 24-hydroxylase in the colon and potentiate the effects of 1,25(OH)2D in the colon.
Study 2.
Six mice (14 wk of age) were assigned to each treatment, which consisted of No DSS, DSS only, and DSS + 171 pmol of β-gluc-1,25(OH)2D per day. This dose of β-gluc-1,25(OH)2D was higher than that found to be effective in study 1.
Animals were weighed daily and monitored clinically for bleeding from the rectum and general signs of morbidity. Blood loss from the rectum was scored as follows on the day of necropsy: 0 for no blood, 1 for a single spot of blood in the cage, 2 for <10 spots of blood in the cage, and 3 for >10 spots of blood in the cage. One mouse in the treatment group receiving 600 pmol of β-gluc-1,25(OH)2D per day in study 1 failed to finish the trial, as it developed severe bloody diarrhea and weight loss, necessitating euthanasia on day 7 of DSS administration. Its tissues were collected, and data from this mouse are retained in the study results. After 7 days of consuming DSS in their drinking water, the mice were offered regular tap water for 24 h and euthanized by guillotine while under isoflurane anesthesia.
Blood was collected from the cervical stump into heparinized tubes, and each mouse's plasma was harvested and frozen at −86°C until it was analyzed for calcium content by a colorimetric assay (Arsenazo III, Pointe Scientific, Canton, MI). Plasma 25(OH)D and 1,25(OH)2D concentrations were determined by RIA on pooled samples of plasma collected from the mice (Heartland Assays) (7, 8). In study 1, each pooled sample consisted of 50 μl of plasma from four or five mice, resulting in two samples per treatment. In study 2, each pooled sample consisted of 100 μl of plasma from three mice, resulting in three samples per treatment. Because of the small number of samples, the results of the vitamin D metabolite determinations were not subjected to statistical analysis.
The entire colon and rectum of each mouse was removed intact, and the length was recorded. The colon/rectum was cut in half, and the caudal half was discarded. The caudal 1 cm of the remaining half was fixed in formalin for histopathological analysis. The fixed colon section was stained with hematoxylin-eosin for microscopic histopathological evaluation by a veterinary pathologist (J. S. Haynes) blinded to the treatments. Each tissue section received a score of 0–4, from no lesion to severe extensive lesion, for each of three criteria: 1) the degree of erosion/ulceration of colon mucosa, 2) the degree of infiltration of the tissues by inflammatory cells, and 3) the degree of submucosal edema. The sum of the score of each of these criteria constitutes the histopathological score for the tissue, with a 0 score representing normal tissue based on all criteria and the worst possible outcome being a score of 12.
A second 1-cm piece of colon tissue cranial to the section used for histopathological analysis was obtained from each mouse for isolation of mRNA. In study 1, the tissues were flash-frozen. Unfortunately, the RNA isolated from these tissues was degraded. In study 2, the tissues were immediately placed into TRIzol and homogenized as described above for the dose and time-course studies on effects of a single dose of the vitamin D compounds. This method successfully preserved the RNA. Quantitative real-time PCR was performed and quantitated as described above. Amplification of target cDNAs for Cyp24, TNF-α, and cadherin 1 was accomplished with the following primers: TNF-α [5′-AGGGGCCACCACGCTCTTCT (forward) and 5′-CCACTCCAGCTGCTCCTCCACT (reverse)] and cadherin 1 [5′-TGGAGGGATCCTCGCCCTGC (forward) and 5′-CATCCAGGCCCCTGTGCAGC (reverse)].
Statistical Analysis
Data were analyzed by one-way ANOVA (SAS, Cary, NC). When ANOVA suggested a significant difference (P < 0.05), post hoc comparison of individual means was conducted using Fisher's test of least significant difference. Results from studies of the mice treated with DSS to induce inflammation of the lower bowel are presented as treatment differences from the positive (No DSS) or negative (DSS only) control means and are considered significant at P < 0.05, unless otherwise noted. Results of several of the treatments from study 1 with no significant effects are not presented in Tables 2 and 3 to improve clarity of presentation, but any significant effects attributable to these treatments are discussed in the text.
Table 2.
Effect of vitamin D compounds on progression of colitis in mice with DSS-induced colon IBD in study 1
| Treatment | Fecal Blood Score | Colon Length, cm | Colon Lesion Score | Change in Body Weight, g |
|---|---|---|---|---|
| No DSS | 0 ± 0* | 6.89 ± 0.37* | 0.55 ± 0.18* | 1.91 ± 0.31 |
| DSS only | 0.89 ± 0.34 | 5.22 ± 0.20 | 9.33 ± 0.62 | −1.03 ± 0.71† |
| DSS + 120 pmol 1,25(OH)2D | 0.22 ± 0.12* | 5.74 ± 0.17 | 5.89 ± 0.54* | −0.96 ± 0.51† |
| DSS + 120 pmol β-gluc-1,25(OH)2D | 0.22 ± 0.08* | 5.41 ± 0.15 | 6.33 ± 0.74* | −0.55 ± 0.38† |
| DSS + 120 pmol β-gluc-1,25(OH)2D + 8.67 nmol β-gluc-25(OH)D | 0.11 ± 0.11* | 6.22 ± 0.19* | 6.78 ± 0.98* | 0.33 ± 0.44 |
Values are means ± SE; n = 9 mice per treatment. IBD, inflammatory bowel disease. Mice received diets containing treatments that included 1,25(OH)2D, β-gluc-1,25(OH)2D, and 25-hydroxyvitamin D3-25-β-glucuronide [β-gluc-25(OH)D]. Diet treatments began 4 days prior to initiation of dextran sodium sulfate (DSS)-water and continued until the animals were euthanized. DSS-water was administered for 7 days, and mice were euthanized on day 8. Fecal blood was scored on a scale of 0–3, where 0 = no blood and 3 = multiple blood spots in the cage. Colon lesions were scored on a scale of 0–12, where 0 = no lesions and 12 = severe erosion, hemorrhage, and submucosal edema. Change in body weight = body weight on day 8 of DSS period − average body weight over the 3 days prior to initiation of DSS treatment.
Significantly different from DSS only (P < 0.05).
Significantly different from No DSS (P < 0.05).
Table 3.
Effect of vitamin D compounds on plasma calcium and plasma vitamin D metabolite concentrations in mice with DSS-induced colon IBD in study 1
| Treatment | Calcium, mg/dl | 25(OH)D, nmol/l | 1,25(OH)2D, pmol/l |
|---|---|---|---|
| No DSS | 9.54 ± 0.11 | 77 ± 2.5 | 223 ± 26 |
| DSS only | 8.44 ± 0.36* | 45 ± 2.5 | 182 ± 72 |
| DSS + 120 pmol 1,25(OH)2D | 11.58 ± 0.18* | 42 ± 2.5 | 389 ± 77 |
| DSS + 120 pmol β-gluc-1,25(OH)2D | 10.12 ± 0.30 | 40 ± 2.5 | 255 ± 60 |
| DSS + 120 pmol β-gluc-1,25(OH)2D + 8.67 nmol β-gluc-25(OH)D | 10.26 ± 0.22 | 188 ± 2.5 | 507 ± 137 |
Values are means ± SE; n = 9 mice per treatment. Mice received diets containing treatments that included 1,25(OH)2D, β-gluc-1,25(OH)2D, and β-gluc-25(OH)D. Diet treatments began 4 days prior to initiation of DSS-water and continued until the animals were euthanized. DSS-water was administered for 7 days, and mice were euthanized on day 8. 25(OH)D and 1,25(OH)2D represent vitamin D metabolite concentrations of pooled plasma. Each pooled sample consisted of 50 μl of plasma from 4 or 5 mice, resulting in 2 samples per treatment. No statistical testing applied to these data.
Significantly different from No DSS, P < 0.05.
RESULTS
Ileal and Colon, but not Duodenal, Contents Have High Levels of β-Glucuronidase Activity
To determine the β-glucuronidase activity capable of liberating a vitamin D aglycone from the prodrug form of the vitamin D compound in various parts of the small intestine, β-glucuronidase activity was tested in contents from intestinal subsections of rats. The assay system recovered ≥60% of the added 1,25(OH)2D from intestinal content incubations at time 0, and the level of 1,25(OH)2D did not change with time of incubation, suggesting that the 1,25(OH)2D was not degraded during the course of the incubations and that the assay was reasonably able to detect 1,25(OH)2D from this type of sample (Table 1). When β-gluc-1,25(OH)2D was incubated with duodenal contents, <5% of the compound was converted to the aglycone 1,25(OH)2D, even after 6 h of incubation. When β-gluc-1,25(OH)2D was incubated with the ileal contents, >85% was cleaved to free the aglycone 1,25(OH)2D within 1 h of incubation. Colon contents liberated 65% of the 1,25(OH)2D within 1 h, and the amount of 1,25(OH)2D recovered increased slightly at 3 and 6 h for ileal and colon contents (data not shown). The results shown in Table 1 demonstrate that the upper small intestine of rats is unlikely to contain sufficient β-glucuronidase activity to cause release of substantial amounts of 1,25(OH)2D in the upper small intestine. On the other hand, β-gluc-1,25(OH)2D is likely to be rapidly cleaved upon entry to the ileum and colon, where substantial numbers of bacteria reside that are capable of producing β-glucuronidase.
β-Gluc-1,25(OH)2D vs. 1,25(OH)2D Effects on Cyp24 Gene Expression in Colon and Duodenum
A prominent action of 1,25(OH)2D on its target tissues is induction of the mRNA for the Cyp24 enzyme. The effect of increasing doses of β-gluc-1,25(OH)2D and 1,25(OH)2D on Cyp24 expression in the colon and duodenum relative to untreated control mice is presented in Fig. 2, A and B. At the highest dose of 1,25(OH)2D (48 pmol), Cyp24 expression in the colon increased ∼4.8 ± 4 fold 6 h after treatment. The equimolar dose of β-gluc-1,25(OH)2D caused a >400-fold increase in colon Cyp24 expression. Even at the 12-pmol dose, β-gluc-1,25(OH)2D increased Cyp24 expression in the colon 60-fold, which was ∼20 times greater than the response from the equimolar dose of 1,25(OH)2D. As expected, 1,25(OH)2D was able to strongly induce Cyp24 gene expression in the duodenums of the same mice 6 h after oral dosing, with maximal (>1,000-fold) induction at the highest dose (48 pmol) evaluated. Although β-gluc-1,25(OH)2D induced Cyp24 gene expression in the duodenums of mice, it was consistently less effective than the analogous dose of 1,25(OH)2D. Plasma 1,25(OH)2D concentration was not significantly increased at 6 h by 6 pmol of 1,25(OH)2D or β-gluc-1,25(OH)2D (Fig. 2C). Higher doses of either compound resulted in higher levels of 1,25(OH)2D in the blood. At the 48-pmol dose, β-gluc-1,25(OH)2D resulted in higher blood 1,25(OH)2D at the time of euthanasia, which was 6 h after treatment in this study. Plasma calcium concentrations were similar to control mouse plasma calcium concentrations in all treatment groups, which likely reflects the short duration of the experiment.
Fig. 2.
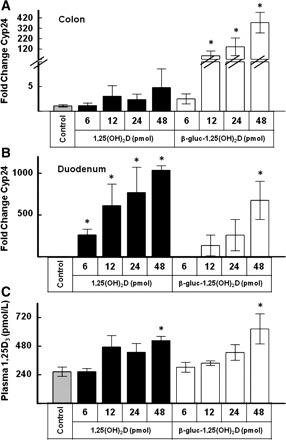
Effect of increasing equimolar doses (6, 12, 24, or 48 pmol) of 1,25-dihydroxyvitamin D3 [1,25(OH)2D] or 1,25-dihydroxyvitamin D3-25-β-glucuronide [β-gluc-1,25(OH)2D] administered orally on colon and duodenum expression of 25-hydroxyvitamin D 24-hydroxylase (Cyp24) and plasma concentrations of 1,25(OH)2D. Values are means ± SE; n = 4 mice/time point. Mice were killed 6 h after treatment. *Significantly different from control (P < 0.05).
When 24 pmol of β-gluc-1,25(OH)2D or 1,25(OH)2D were administered orally and the animals were killed at intervals following treatment, the highest levels of expression of Cyp24 in the colon and duodenum were observed 3 or 6 h after treatment (Fig. 3, A and B). In colon tissue, β-gluc-1,25(OH)2D treatment caused a ∼700-fold increase in Cyp24 expression compared with control mice at 6 h, whereas 1,25(OH)2D caused only a ∼5-fold increase. In the duodenum, the relative effects of 1,25(OH)2D and β-gluc-1,25(OH)2D were reversed. Compared with control mice, 1,25(OH)2D treatment steadily increased Cyp24 expression in the duodenum from 1 h (350-fold induction) to 3 h (1,600-fold induction) to 6 h (>2,500-fold). In contrast, the effects of β-gluc-1,25(OH)2D on Cyp24 expression peaked at 3 h in the duodenum, with a 1,300-fold increase, and fell to a 500-fold increase at 6 h. The effects of 1,25(OH)2D and β-gluc-1,25(OH)2D on Cyp24 gene expression in both tissues was similar to control mouse levels 24 h after treatment.
Fig. 3.
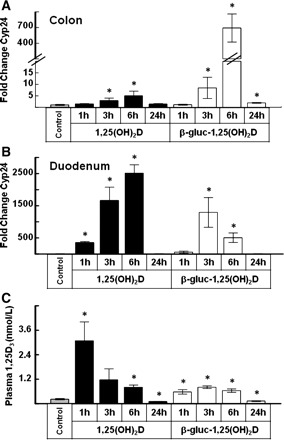
Colon and duodenum Cyp24 expression and plasma 1,25(OH)2D concentrations in mice (n = 5 mice/time point) receiving an oral dose of 24 pmol of 1,25(OH)2D or β-gluc-1,25(OH)2D. Mice were killed at 1, 3, 6, or 24 h after treatment. *Significantly different from control (P < 0.05).
Plasma concentrations of 1,25(OH)2D peaked at 1 h following oral treatment with 24 pmol at 1,280 pg/ml, a ∼14-fold increase over control mouse plasma 1,25(OH)2D (Fig. 3C). In contrast, the average plasma 1,25(OH)2D concentration in mice treated with 24 pmol of β-gluc-1,25(OH)2D peaked ∼3 h after treatment at 325 pg/ml, a level that was only 3.5-fold greater than control levels. By 24 h after treatment, plasma 1,25(OH)2D concentration in 1,25(OH)2D- and β-gluc-1,25(OH)2D-treated mice was slightly below that in control animals. Plasma calcium concentrations were similar to control mouse plasma calcium concentrations at all time points in both treatment groups.
Taken together, studies 1 and 2 demonstrate that oral administration of β-gluc-1,25(OH)2D has a greater effect on colon tissue and a lesser effect on duodenum than does the native hormone. Oral administration of β-gluc-1,25(OH)2D also causes a much lower increase in plasma concentration of 1,25(OH)2D than does the equimolar dose of 1,25(OH)2D. However, the time at which each drug causes peak levels of 1,25(OH)2D in the blood differs. As expected, the highest plasma concentrations of 1,25(OH)2D occur shortly after oral administration of 1,25(OH)2D, and concentrations decline thereafter due to rapid metabolism of 1,25(OH)2D in the mouse. However, there is a delay in the time to peak 1,25(OH)2D concentrations in mice receiving β-gluc-1,25(OH)2D. This likely represents the amount of time required for the compound to reach the ileum and to be converted to 1,25(OH)2D and then to be absorbed into the circulation. Quantitative real-time PCR was also used to examine changes in expression of the VDR and calbindin-D9k, both of which are commonly reported to be upregulated by 1,25(OH)2D. We could not detect changes in expression of these genes in these tissues (data not shown). These animals were vitamin D-replete; most studies demonstrating strong upregulation of these proteins are done using vitamin D-deficient animals. Our studies were completed <24 h after treatment. Many of the studies demonstrating a VDR or calbindin-D9k response require repeated doses of 1,25(OH)2D over >24 h to see significant upregulation in the animal's tissues. Also, when VDR expression is upregulated, it may be just a three- to fourfold increase (23).
Effect of Vitamin D Compounds on DSS-Induced Colitis
Study 1.
Control mice that did not receive DSS gained 1.91 g body wt during the 8 days of the IBD induction period and had no blood in their feces, and their colon length was 6.89 ± 0.37 cm. Their plasma calcium was 9.54 ± 0.11 mg/dl. In the mice treated with DSS alone, frank blood began to appear in the feces ∼6 days after the start of addition of DSS to their drinking water, and at the time of death these animals had an average fecal blood score of 0.89 ± 0.34 and colon length of 5.22 ± 0.20 cm and had lost 1.03 g body wt (4.7% of initial body weight) during the 8 days following the start of addition of DSS to their drinking water (Tables 2 and 3). Their histopathology colon lesion score was 9.33 ± 0.62. The DSS-treated mice were significantly hypocalcemic at the time of death, with plasma calcium of 8.44 ± 0.36 mg/dl. Severe inflammatory processes often result in high levels of IL-1 and other cytokines, which cause a decline in blood calcium level via mechanisms that may involve vitamin D, parathyroid hormone, or calcitonin (19, 28).
Mice receiving DSS in their drinking water and treated with 1,25(OH)2D or β-gluc-1,25(OH)2D at 120 pmol/day had reduced fecal blood scores and reduced colon lesion scores. Colon length and weight loss in both groups were not statistically improved over those in the mice receiving DSS only. However, treatment of the mice with 120 pmol of 1,25(OH)2D per day also resulted in severe hypercalcemia (11.58 ± 0.18 mg Ca/dl), while feeding 120 pmol of β-gluc-1,25(OH)2D per day did not (10.12 ± 0.30 mg Ca/dl; Tables 2 and 3).
By itself, 8.67 nmol/day of β-gluc-25(OH)D was ineffective in preventing weight loss, improving fecal blood scores or colon length, or reducing histological lesion score (data not shown). However, 120 pmol of β-gluc-1,25(OH)2D + 8.67 nmol of β-gluc-25(OH)D significantly reduced fecal blood score, improved colon lesion score, and prevented DSS-induced weight loss. This was the only treatment that significantly improved colon length over that observed in mice receiving DSS only. Plasma calcium was not significantly elevated above that of control mice by 120 pmol of β-gluc-1,25(OH)2D + 8.67 nmol of β-gluc-25(OH)D (10.26 ± 0.22 mg Ca/dl).
Lower doses (24 pmol) of either vitamin D compound were largely ineffective in preventing negative effects of DSS in the mice. In mice treated with 24 pmol of 1,25(OH)2D/day, colon length, colon lesion score, and weight loss during DSS treatment were not significantly improved over mice receiving DSS only. Mice fed DSS in their drinking water and treated with 24 pmol of β-gluc-1,25(OH)2D per day, with or without concurrent treatment with 8.67 nmol/day of β-gluc-25(OH)D, fared somewhat better. Their colon lesion scores were significantly lower and they lost less weight than mice treated with DSS alone, but colon length and fecal blood score were not improved (data not included in Tables 2 and 3 for clarity of presentation). In DSS-treated mice, 600 pmol of β-gluc-1,25(OH)2D per day was of no benefit in reducing IBD symptoms or lesions and caused significant hypercalcemia (10.72 ± 0.33 mg Ca/dl). Feed intake was significantly reduced in this group as early as day 4 of DSS treatment [day 8 of treatment with 600 pmol of β-gluc-1,25(OH)2D per day; data not shown]. One mouse in this group needed to be euthanized on day 7 of DSS treatment because of severe IBD and weight loss.
On the basis of the pooled samples of plasma, a qualitative assessment of plasma vitamin D metabolite concentrations is presented. Plasma 25(OH)D concentrations were decreased in animals receiving DSS in their drinking water compared with No-DSS controls. The diarrhea in affected mice, although not severe, may have interfered with the enterohepatic circulation of 25(OH)D. In mice receiving β-gluc-25(OH)D in their diet, plasma 25(OH)D concentrations were greatly increased. Plasma 1,25(OH)2D concentrations were increased in animals receiving 120 pmol of 1,25(OH)2D per day and in mice receiving 600 pmol of β-gluc-1,25(OH)2D per day, but not in mice receiving 120 pmol of β-gluc-1,25(OH)2D per day. When combined with β-gluc-25(OH)D, plasma 1,25(OH)2D concentrations increased in mice receiving 120 pmol of β-gluc-1,25(OH)2D per day.
Study 2.
All mice completed study 2, and the degree of colitis, based on body weight loss, histopathological colon lesion score, and colon length, experienced by the DSS-only group was milder than in the mice that completed study 1. Compared with mice not receiving DSS in their drinking water, DSS-only mice had significantly more blood in their feces, shorter colon length, and lost more body weight (Table 4). Fecal blood score was 1.33 ± 0.49 in DSS-only mice. The treatment consisting of 171 pmol of β-gluc-1,25(OH)2D per day (a 42% increase in the dose found to be effective in study 1) significantly reduced fecal blood score (0.33 ± 0.33, P < 0.05) and body weight loss (P < 0.075) compared with DSS-only mice. In addition, colon length of the mice receiving 171 pmol of β-gluc-1,25(OH)2D per day (7.40 ± 0.22 cm) was not statistically different from that of No-DSS control mice (7.57 ± 0.34 cm) and was statistically improved compared with DSS-only mice (6.68 ± 0.19 cm, P < 0.075). Plasma calcium of DSS-only mice was lower than that of no-DSS mice (P < 0.075), but none of the vitamin D treatments caused a significant increase in plasma calcium concentration (P > 0.075; Table 5). Histopathology colon lesion score for the DSS-only mice was much lower than in study 1 (3.67 vs. 9.33 in study 2), indicating that a milder inflammation was induced by DSS in this trial. Treatment with 171 pmol of β-gluc-1,25(OH)2D per day did not alter colon lesion score, although fecal blood score and colon length were improved over DSS-only mice.
Table 4.
Effect of vitamin D compounds on progression of colitis in mice with DSS-induced colon IBD in study 2
| Treatment | Body Weight Change, g | Colon Length, cm | Fecal Blood Score | Colon Lesion Score |
|---|---|---|---|---|
| No DSS | 0.012 ± 0.011 | 7.56 ± 0.33† | 0 ± 0† | 0.17 ± 0.17 |
| DSS only | −0.092 ± 0.016* | 6.68 ± 0.19* | 1.33 ± 0.49* | 3.67 ± 0.33* |
| DSS + 171 pmol β-gluc-1,25(OH)2D | −0.052 ± 0.019* | 7.40 ± 0.21† | 0.33 ± 0.33† | 5.33 ± 0.80* |
Values are means ± SE; n = 6 mice per treatment. Mice received diets containing treatments that included carrier or 171 pmol of β-gluc-1,25(OH)2D. Diet treatments began 4 days prior to initiation of DSS-water and continued until the animals were euthanized. DSS-water was administered for 7 days, and mice were euthanized on day 8. Body weight change, fecal blood score, and histopathology colon lesion score are described in Table 2 footnote.
Significantly different from No DSS (P < 0.05).
Significantly different from DSS only (P < 0.075).
Table 5.
Effect of vitamin D compounds on plasma calcium and vitamin D metabolite concentrations in mice with DSS-induced colon IBD in study 2
| Treatment | Plasma Calcium, mg/dl | Plasma 25(OH)D, nmol/l | Plasma 1,25(OH)2D, pmol/l |
|---|---|---|---|
| No DSS | 9.46 ± 0.07 | 117 ± 2.5 | 259 ± 17 |
| DSS only | 8.90 ± 0.27* | 70 ± 2.5 | 120 ± 17 |
| DSS + 171 pmol β-gluc-1,25(OH)2D | 9.84 ± 0.20 | 42 ± 12 | 504 ± 63 |
Values are means ± SE; n = 6 mice per treatment. Mice received diets containing treatments that included carrier or 171 pmol of β-gluc-1,25(OH)2D. Diet treatments began 4 days prior to initiation of DSS-water and continued until the animals were euthanized. DSS-water was administered for 7 days, and mice were euthanized on day 8. Plasma 25(OH)D and 1,25(OH)2D represent vitamin D metabolite concentrations in pooled plasma. Each pooled sample consisted of 100 μl of plasma from 2 mice, resulting in 3 samples per treatment. No statistical testing applied to these data.
Significantly different from No DSS (P < 0.075).
Plasma 25(OH)D concentrations were decreased in animals receiving DSS in their drinking water, similar to the animals receiving DSS in study 1. Plasma 1,25(OH)2D concentrations were decreased in mice fed DSS in their drinking water and increased in mice receiving 171 pmol of β-gluc-1,25(OH)2D per day.
Gene expression data of colon tissue from No-DSS, DSS-only, and DSS + 171 pmol of β-gluc-1,25(OH)2D per day groups are presented in Fig. 4. There was a trend toward increased Cyp24 expression in the colon of DSS mice compared with no-DSS controls, but this difference was not statistically significant. However, mice receiving 171 pmol of β-gluc-1,25(OH)2D per day exhibited a ∼200-fold increase in Cyp24 expression. There was no statistically significant decrease in expression of the epithelial tight cell junction protein cadherin 1 in DSS-only mice, and treatment with β-gluc-1,25(OH)2D did not increase cadherin 1 expression. In mice receiving DSS in their drinking water, TNF-α expression was significantly increased, and administration of β-gluc-1,25(OH)2D did not reduce TNF-α expression.
Fig. 4.
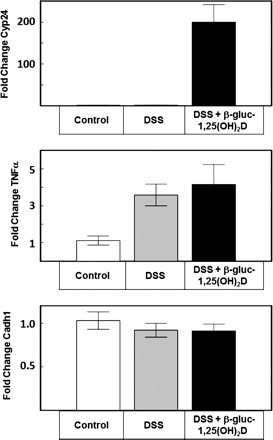
Colon expression of Cyp24, TNF-α, and cadherin 1 (Cadh1) in mice from study 2. Control mice received no dextran sodium sulfate (DSS) in their drinking water, and DSS mice received 2.5% DSS their drinking water for 7 days. DSS + β-gluc-1,25(OH)2D mice received DSS in their drinking water and 171 pmol of β-gluc-1,25(OH)2D mixed with their diet each day. Mice were killed 8 days after initiation of DSS to their drinking water.
DISCUSSION
Conjugating a glucuronide to the 1,25(OH)2D or 25(OH)D molecules in a β-conformation link makes the compounds water-soluble, but, more importantly, they are biologically inert until the glucuronide moiety is cleaved, leaving the aglycone vitamin D compound. As these studies demonstrate, glucuronidase activity in the upper small intestine is unlikely to allow formation of significant amounts of the aglycone vitamin D compounds. However, the colon and ileum possess substantial numbers of bacteria, which are able to produce β-glucuronidase, and nearly all the glucuronide is cleaved from the vitamin D compounds very rapidly. Administration of a single oral dose of 24 pmol of 1,25(OH)2D had a profound impact on duodenal Cyp24 expression that persisted for ≥6 h but was essentially gone by 24 h. The colon responded to oral administration of 24 pmol of 1,25(OH)2D with a fivefold increase in colon Cyp24 expression. In contrast, the equimolar amount of β-gluc-1,25(OH)2D administered orally was much less able to upregulate duodenal Cyp24 expression than 1,25(OH)2D. β-Gluc-1,25(OH)2D was able to upregulate colon Cyp24 expression ∼700-fold by 6 h after treatment, >100 times as active on colon tissues as the native hormone. The effect of the vitamin D compounds at these doses on Cyp24 was short-lived, as Cyp24 essentially returned to basal levels by 24 h after treatment.
Not only did Cantorna et al. (2) demonstrate that correction of vitamin D deficiency improved resistance to IBD in IL-10 KO mice, in subsequent studies, Froicu and Cantorna (5) demonstrated that DSS-induced IBD could be ameliorated in vitamin D-replete genetically normal animals by treatment with orally or rectally administered 1,25(OH)2D. Our data corroborate these observations, in that feeding mice 120 pmol of 1,25(OH)2D per day reduced lesions and symptoms of IBD in the DSS-treated mouse model. Unlike the mice in the study of Froicu and Cantorna, the mice fed 120 pmol of 1,25(OH)2D per day in our study developed rather severe hypercalcemia (11.58 mg Ca/dl). The lower dose of 24 pmol of 1,25(OH)2D used in our study did not cause hypercalcemia but resulted in little amelioration of IBD.
Daily feeding of 24 pmol of β-gluc-1,25(OH)2D improved a few aspects of IBD in the DSS-treated mice, but this effect was modest at best. Feeding five or seven times this amount of β-gluc-1,25(OH)2D (120 or 171 pmol/day) resulted in a modest improvement in lesions of IBD compared with the lower dose. A β-gluc-1,25(OH)2D dose of 600 pmol/day proved to be toxic: it was associated with hypercalcemia (10.72 mg Ca/dl), and the mice consumed less diet, lost more body weight than animals subjected to the other treatments, and had IBD that was nearly identical to the animals receiving DSS only. Although it is less hypercalcemic than the native hormone 1,25(OH)2D, β-gluc-1,25(OH)2D can induce hypercalcemia.
One complication from the use of 1,25(OH)2D and its analogs in treating disease is that the treatment induces Cyp24 expression. Upon translation, this enzyme will speed the catabolism of 1,25(OH)2D, limiting the effectiveness of 1,25(OH)2D. Both 25(OH)D and 24,25(OH)2D have been used as competitive inhibitors of 24-hydroxylase in vitro (24). When β-gluc-25(OH)D was administered at 8.67 nmol/day along with β-gluc-1,25(OH)2D at 120 pmol/day to DSS-treated mice, there was a substantial improvement in IBD compared with treatment with 120 pmol of β-gluc-1,25(OH)2D alone. β-Gluc-25(OH)D by itself was ineffective against IBD. We interpret this to mean that β-gluc-25(OH)D was converted to 25(OH)D in the colon and competitively inhibited colon 24-hydroxylase, allowing a longer persistence of anti-inflammatory action by the 1,25(OH)2D liberated from the β-gluc-1,25(OH)2D in the colon.
Several attributes of 1,25(OH)2D may help explain how administration of 1,25(OH)2D could ameliorate IBD. A characteristic of Crohn's disease is the inappropriate expression of the proinflammatory Th1 and Th17 cytokines by T cells in response to bacterial antigens. Vitamin D and its metabolites may influence this process. The active metabolite, 1,25(OH)2D, causes T cell differentiation to shift from Th1- and Th17-predominant phenotypes toward a Th2 phenotype (1) and downregulates gut-homing receptors expressed by T cells that migrate selectively to the intestinal tract (26). In support of this, vitamin D deficiency exacerbates symptoms of colitis in IL-10 KO mice (2), and VDR knockout mice are ultrasensitive to DSS-induced IBD (5).
Some Crohn's patients are known to have a genetic variant of the NOD2 gene, which is a pattern recognition receptor that recognizes bacterial peptidoglycans. It appears that the innate immune cells, such as monocytes and macrophages, of these patients fail to recognize certain bacterial cell wall antigens, and thus the cells are unable to initiate a cascade of events leading to production of antimicrobial peptides that might keep “inflammation-triggering” bacteria populations in check. NOD2 and Toll-like receptor pathways also stimulate the innate immune cell's ability to perform 1α-hydroxylation of 25(OH)D (16). Colon cells are also capable of producing 1,25(OH)2D. Although the role of recognition of pathogen-associated molecular patterns in control of colon mucosa cell 1α-hydroxylase is unclear (12), one can speculate that patients with defective NOD2 may not be producing adequate 1,25(OH)2D within their immune cells, even if adequately supplied with the 25(OH)D precursor. It has recently been demonstrated that exogenous treatment of normal human macrophages with 1,25(OH)2D can circumvent problems with endogenous production of 1,25(OH)2D due to inadequate 25(OH)D and results in production of antimicrobial peptides such as cathelicidin (16). Cathelicidin's ability to kill Mycobacteria may be critical to preventing IBD, especially if a major factor precipitating IBD proves to be the presence of Mycobacterium avium sp. paratuberculosis in the gut. Finally, the intestinal epithelial barrier, consisting of epithelial cells and intercellular tight junctions, prevents microorganisms, toxins, and luminal antigens from entering the body. Impaired barrier function is a common finding in patients with IBD (4). Studies in mice demonstrate that 1,25(OH)2D acts on enterocytes to increase expression of junction proteins to maintain the integrity of the intestinal mucosal barrier and allow rapid repair of damage to enterocyte tight junctions, which may facilitate recovery from a bout of IBD (11). In our study, there appeared to be some loss of cadherin 1 as a result of DSS treatment, but this was not statistically significant, and, more importantly, we could not demonstrate an increase in cadherin 1 expression following treatment with β-gluc-1,25(OH)2D.
Utilizing β-glucuronide forms of 1,25(OH)2D and 25(OH)D allows delivery of 1,25(OH)2D and 25(OH)D to the colon in amounts that stimulate colon gene expression to a much higher degree than is possible with the native hormone, 1,25(OH)2D. These compounds, at the doses that proved effective in this mouse model, also avoid the development of severe hypercalcemia observed with native 1,25(OH)2D. In this mouse model of IBD, the amelioration of IBD provided by β-gluc-1,25(OH)2D + β-gluc-25(OH)D was superior to that achieved with β-gluc-1,25(OH)2D alone. We believe that this is due to competitive inhibition of the 24-hydroxylase enzyme, prolonging the action of 1,25(OH)2D within the colon. It is also possible that provision of high amounts of 25(OH)D to the colon may also provide sufficient substrate to drive colon 1α-hydroxylase and enhance 1,25(OH)2D production within the epithelial and immune cells of the colon.
GRANTS
This work was supported in part by grants from the Grow Iowa Values Fund.
DISCLOSURES
J. Goff and R. Horst have filed use patents for the glucuronides of vitamin D thru Glycomyr, a company that they own jointly. This company manufactured the glucuronide forms of the vitamin D compounds and made them available for this study.
AUTHOR CONTRIBUTIONS
J.P.G. and N.J.K. are responsible for conception and design of the research; J.P.G., N.J.K., J.S.H., and R.L.H. performed the experiments; J.P.G. and N.J.K. analyzed the data; J.P.G., N.J.K., and J.S.H. interpreted the results of the experiments; J.P.G. drafted the manuscript; J.P.G. and N.J.K. edited and revised the manuscript; J.P.G., N.J.K., J.S.H., and R.L.H. approved the final version of the manuscript; N.J.K. prepared the figures.
ACKNOWLEDGMENTS
The authors thank Cathy Martens, Derrel Hoy, Blaine Nicks, Courtney Blake, and Matt Brewer for technical help. The authors also acknowledge the support of Heartland Assays for determining 1,25(OH)2D concentrations in our samples.
REFERENCES
- 1. Baeke F, van Etten E, Gysemans C, Overbergh L, Mathieu C. Vitamin D signaling in immune-mediated disorders: evolving insights and therapeutic opportunities. Mol Aspects Med 29: 376–387, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr 130: 2648–2652, 2000. [DOI] [PubMed] [Google Scholar]
- 3. Das A, Spackman E, Pantin-Jackwood MJ, Suarez DL. Removal of real-time reverse transcription polymerase chain reaction (RT-PCR) inhibitors associated with cloacal swab samples and tissues for improved diagnosis of avian influenza virus by RT-PCR. J Vet Diagn Invest 21: 771–778, 2009. [DOI] [PubMed] [Google Scholar]
- 4. Fasano A. Physiological, pathological, and therapeutic implications of zonulin-mediated intestinal barrier modulation: living life on the edge of the wall. Am J Pathol 173: 1243–1252, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol 8: 5, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25: 386–401, 2001. [DOI] [PubMed] [Google Scholar]
- 7. Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol 282: 174–186, 1997. [DOI] [PubMed] [Google Scholar]
- 8. Hollis BW, Horst RL. The assessment of circulating 25(OH)D and 1,25(OH)2D: where we are and where we are going. J Steroid Biochem Mol Biol 103: 473–476, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jain A, Gupta Y, Jain SK. Perspectives of biodegradable natural polysaccharides for site-specific drug delivery to the colon. J Pharm Pharm Sci 10: 86–128, 2007. [PubMed] [Google Scholar]
- 10. Jorgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, Bartels LE, Kelsen J, Christensen LA, Dahlerup JF. Clinical trial: vitamin D3 treatment in Crohn's disease—a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther 32: 377–383, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 294: G208–G216, 2008. [DOI] [PubMed] [Google Scholar]
- 12. Lagishetty V, Chun RF, Liu NQ, Lisse TS, Adams JS, Hewison M. 1α-Hydroxylase and innate immune responses to 25-hydroxyvitamin D in colonic cell lines. J Steroid Biochem Mol Biol 121: 228–233, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laverny G, Penna G, Vetrano S, Correale C, Nebuloni M, Danese S, Adorini L. Efficacy of a potent and safe vitamin D receptor agonist for the treatment of inflammatory bowel disease. Immunol Lett 131: 49–58, 2010. [DOI] [PubMed] [Google Scholar]
- 14. Leslie WD, Miller N, Rogala L, Bernstein CN. Vitamin D status and bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD Cohort Study. Am J Gastroenterol 103: 1451–1459, 2008. [DOI] [PubMed] [Google Scholar]
- 15. Liu N, Nguyen L, Chun RF, Lagishetty V, Ren S, Wu S, Hollis B, DeLuca HF, Adams JS, Hewison M. Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology 149: 4799–4808, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311: 1770–1773, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 126: 1504–1517, 2004. [DOI] [PubMed] [Google Scholar]
- 18. Miheller P, Muzes G, Hritz I, Lakatos G, Pregun I, Lakatos PL, Herszenyi L, Tulassay Z. Comparison of the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn's disease patients. Inflamm Bowel Dis 15: 1656–1662, 2009. [DOI] [PubMed] [Google Scholar]
- 19. Muller B, Becker KL, Kranzlin M, Schachinger H, Huber PR, Nylen ES, Snider RH, White JC, Schmidt-Gayk H, Zimmerli W, Ritz R. Disordered calcium homeostasis of sepsis: association with calcitonin precursors. Eur J Clin Invest 30: 823–831, 2000. [DOI] [PubMed] [Google Scholar]
- 20. Nagubandi S, Kumar R, Londowski JM, Corradino RA, Tietz PS. Role of vitamin-D glucosiduronate in calcium homeostasis. J Clin Invest 66: 1274–1280, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peyrin-Biroulet L, Oussalah A, Bigard MA. Crohn's disease: the hot hypothesis. Med Hypotheses 73: 94–96, 2009. [DOI] [PubMed] [Google Scholar]
- 22. Rambeck WA, Weiser H, Meier W, Labler L, Zucker H. Biological activity of the 3 mono-β-d-glucopyranosides of 1,25-dihydroxycholecalciferol. Int J Vitam Nutr Res 55: 263–267, 1985. [PubMed] [Google Scholar]
- 23. Reinhardt TA, Horst RL. Parathyroid hormone down-regulates 1,25-dihydroxyvitamin D receptors (VDR) and VDR messenger ribonucleic acid in vitro and blocks homologous up-regulation of VDR in vivo. Endocrinology 127: 942–948, 1990. [DOI] [PubMed] [Google Scholar]
- 24. Reinhardt TA, Horst RL. Self-induction of 1,25-dihydroxyvitamin D3 metabolism limits receptor occupancy and target tissue responsiveness. J Biol Chem 264: 15917–15921, 1989. [PubMed] [Google Scholar]
- 25. Shimada K, Sugaya K, Kaji H, Nakatani I, Mitamura K, Tsutsumi N. Syntheses and enzymatic hydrolysis of 25-hydroxyvitamin D monoglucuronides. Chem Pharm Bull 43: 1379–1384, 1995. [Google Scholar]
- 26. Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. DCs metabolize sunlight-induced vitamin D3 to “program” T cell attraction to the epidermal chemokine CCL27. Nat Immunol 8: 285–293, 2007. [DOI] [PubMed] [Google Scholar]
- 27. Strauch UG, Obermeier F, Grunwald N, Dunger N, Rath HC, Scholmerich J, Steinmeyer A, Zugel U, Herfarth HH. Calcitriol analog ZK191784 ameliorates acute and chronic dextran sodium sulfate-induced colitis by modulation of intestinal dendritic cell numbers and phenotype. World J Gastroenterol 13: 6529–6537, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zaloga GP, Chernow B. The multifactorial basis for hypocalcemia during sepsis. Studies of the parathyroid hormone-vitamin D axis. Ann Intern Med 107: 36–41, 1987. [DOI] [PubMed] [Google Scholar]
- 29. Zhu Y, Mahon BD, Froicu M, Cantorna MT. Calcium and 1α,25-dihydroxyvitamin D3 target the TNF-α pathway to suppress experimental inflammatory bowel disease. Eur J Immunol 35: 217–224, 2005. [DOI] [PubMed] [Google Scholar]


