Abstract
There is an impelling need to develop effective therapeutic strategies for patients with retinal disorders. Gleaning from the large quantity of information gathered over the past two decades on the mechanisms governing degeneration of the retina, it is now possible to devise innovative therapies based on retinal gene transfer. Different gene-based approaches are under active investigation. They include strategies to correct the specific genetic defect in inherited retinal diseases, strategies to delay the onset of blindness independently of the disease-causing mutations and strategies to reactivate residual cells at late stages of the diseases. In this review, we discuss the status of application of these technologies, outlining the future therapeutic potential for many forms of retinal blinding diseases.
Keywords: Retinopathies, photoreceptors, RPE, gene therapy, adeno-associated virus (AAV), neuroprotection, optogenetics
Introduction
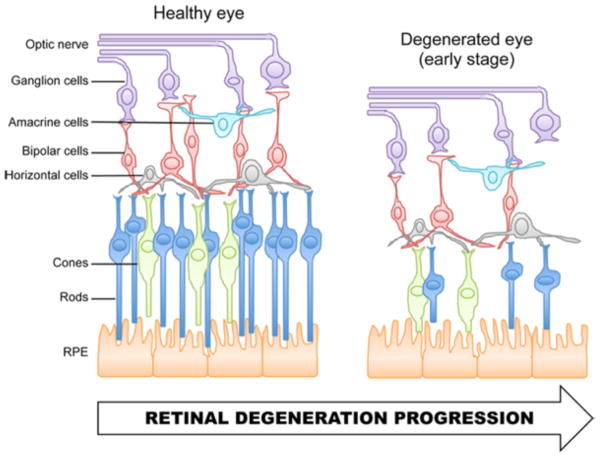
Retinal degenerative diseases are major causes of blindness worldwide. As a group, these diseases are characterized by the progressive loss of one or several cell types of the retina (Figure 1), resulting in irreversible vision loss. Retinal degenerations encompass multifactorial diseases such as age-related macular degeneration and glaucoma, as well as a large variety of monogenic ocular disorders, such as Leber congenital amaurosis (LCA) and retinitis pigmentosa. Currently, there is no cure for retinal degenerative diseases.
Figure 1.
Schematic representation of (A) the normal organization of the mammalian retina and (B) the subsequent cell loss that occurs during retinal degeneration. (A) The retina contains five major classes of neurons. The photoreceptors, rods and cones, transduce light stimuli into electrical signals. Rods allow dim-light vision whereas cones supply central acuity and color perception in high ambient light conditions. At the synaptic terminals of photoreceptors, the electrical signals are transferred onto horizontal and bipolar cells. Bipolar cells relay signals to amacrine and ganglion cells. Ganglion cells integrate these signals and carry the information to the brain through the optic nerve. (B) The retina is the site of various diseases. Although these diseases have different causes, they are often associated with a progressive degeneration of one or several types of retinal neurons, including the photoreceptors. RPE, retinal pigment epithelium.
Over the past two decades, the rapid elucidation of the molecular and genetic mechanisms involved in these diseases has led to the identification of novel targets for therapeutic development. Among the therapeutic approaches being developed to slow or stop disease progression in patients, ocular gene therapy demonstrates a lot of promise. Gene therapy is a strategy that takes advantage of the host protein synthesis machinery to locally produce therapeutic substances (Wang & Gao, 2014). It involves the transfer of nucleic acids into target cells to correct or supplant a mutated gene product or to introduce a new function that will alter the pathogenesis of the disease. Consequently, one significant advantage of gene therapy over traditional pharmacological approaches is that therapeutic benefits can be maintained over a long period of time without the need of repeated interventions. Moreover, the combination of appropriate gene delivery vectors and cell type specific promoters can limit expression of the therapeutic gene to the desired target cells, which is rarely possible with local drug delivery methods.
Due to its specific anatomical and physiological characteristics, the retina is particularly suited for gene transfer. Firstly, the retina is easily accessible for surgical injection and different injection routes can easily target either the inner retina (intravitreal injection) or the outer retina and the retinal pigment epithelium (RPE) (subretinal injection). Secondly, the retina is small and compartmentalized, which allows obtaining a therapeutic effect with a low gene/vector dose. Thirdly, the blood-retinal barrier confers immune-privilege to the tissue and also limits systemic spread of the virus. In addition, the characterization of small and large animal models recapitulating the clinical course of the corresponding human diseases has enabled rapid implementation of gene therapy strategies for dozens of ocular conditions.
Several strategies of gene therapy have been developed and tested in different animal models of retinal degenerations (Petit et al., 2016). These strategies include (i) gene-specific therapy approaches that aim at targeting the primary genetic defect in affected cells to cure the disease, and (ii) non gene-specific therapies that aim at either expanding the time of useful vision by modulating common degenerative secondary effects of the disease, or at exploiting the remaining cells to restore visual perception to the blind retina during the terminal stage of the degeneration. Here, we will review some of the studies that have developed these approaches of ocular gene therapy.
Gene-specific therapies for inherited retinal diseases
Gene replacement for recessive inherited retinal disorders
Most autosomal recessive and X-linked retinal degenerations are caused by the lack of a functional protein. Delivery of a normal copy of the defective gene to affected cells has significantly ameliorated the disease phenotype in various models of recessive retinal degenerations (Boye et al., 2013; Pang et al., 2012; Petit et al., 2016). Of particular note are studies demonstrating long-term correction of the visual defects in animal models of LCA type 2 (LCA2) (Cideciyan, 2010), a severe form of recessive inherited retinal disease.
LCA2 is caused by mutations in a gene coding for the retinal pigment epithelium protein (RPE65), an enzyme mainly expressed in RPE cells, which is critical for retinal activity (Moiseyev et al., 2005). Without a functioning RPE65 protein, RPE cells cannot recycle the visual chromophore required for light detection by photoreceptors (Redmond et al., 1998). LCA2 is marked by profound vision impairment at birth, which generally progresses to total blindness by mid-adulthood as photoreceptor cells degenerate. Unlike other forms of retinal dystrophy, retinal structure in LCA2 patients is relatively well preserved for decades after diagnosis (Cideciyan, 2010), which constitutes a favorable window of opportunity for gene therapy since the most important factor for visual rescue is the presence of photoreceptors.
Initial preclinical proof-of-concepts for LCA2 gene therapy used an adeno-associated virus (AAV) as a vector to deliver the human RPE65 cDNA (coding DNA) to the RPE cells. AAV is a particularly attractive vector for gene delivery as it is non-pathogenic; it elicits minimal immunogenicity and results in remarkably stable transduction of post-mitotic cells. Additionally, AAV vectors derived from a variety of different serotypes have been shown to mediate efficient and long-term transgene expression in different retinal cell types (Dinculescu et al., 2005; Vandenberghe & Auricchio, 2012); for example AAV2, 5, 8 and 9 efficiently transduce both RPE and photoreceptor cells after subretinal injection, whereas AAV4 specifically targets the RPE.
Subretinal injection of AAV2-RPE65 vectors in murine and canine models of LCA2 was able to overcome the defect in retinal activity caused by RPE65 deficiency (Cideciyan, 2010). Subsequent studies with other serotypes and promoters showed stable visual benefits (Acland et al., 2005; Annear et al., 2013; Bainbridge et al., 2015; Bennicelli et al., 2008; Cideciyan et al., 2013; Le Meur et al., 2007; Mowat et al., 2013; Narfstrom et al., 2003; Narfstrom et al., 2005) and demonstrated that photoreceptors can be protected from further degeneration in the vector-exposed area of the retina (Annear et al., 2013; Bainbridge et al., 2015; Mowat et al., 2013). On the basis of these observations, six dose-escalation phase I/II clinical trials of AAV2-RPE65 were initiated. In all of the studies reported to date, the unilateral subretinal injection of AAV2 was shown to be safe with no evidence of toxicity, serious inflammatory or immunologic responses. In addition, participants in all studies exhibited some improvements in aspects of visual function within weeks after treatment (Bainbridge et al., 2015; Bainbridge et al., 2008; Bennett et al., 2012; Bennett et al., 2016; Cideciyan et al., 2008; Cideciyan et al., 2013; Hauswirth et al., 2008; Jacobson et al., 2012; Jacobson et al., 2015; Maguire et al., 2008; Weleber et al., 2016). These remarkable improvements suggest that some subpopulations of photoreceptors remain capable of responding positively to the treatment despite advanced retinal degeneration. The findings were widely seen as a stunning success in translational gene therapy and have greatly expanded the interest for AAV-mediated applications in patients with retinal degenerations. The results opened the possibility of vector re-administration in the second untreated eye of the patients previously enrolled in the original phase I/II trial (Bennett et al., 2012; Bennett et al., 2016) and supported the development of a phase III study, which is now well underway. In addition, RPE65 trials were used as a benchmark for the clinical testing of gene therapies to combat other recessive forms of retinopathies (Carvalho & Vandenberghe, 2015; Petit et al., 2016), such as retinitis pigmentosa due to mutations in MERTK (Ghazi et al., 2016), or choroideremia caused by mutations in REP1 (Edwards et al., 2016; MacLaren et al., 2014).
Retinal gene replacement therapy is now progressing faster than ever with dozens of additional preclinical studies currently moving to translation (Petit et al., 2016). However, the main challenge over the next decades will not just be to translate more animal findings into clinical studies, but also to maximize treatment efficacy in patients. So far, contrary to the remarkable disease rescue obtained in RPE65-deficient dogs, the level of total functional rescue obtained in LCA2 patients after gene therapy was too low to make a detectable difference by full-field electroretinography (ERG), a measure of the function of the entire retina (Cideciyan, 2010). In addition, in two of the RPE65 trials, retinal degeneration continued, alongside a decline that started between 6 months to 3 years after treatment of the initial visual improvements (Bainbridge et al., 2015; Cideciyan et al., 2013; Jacobson et al., 2015). Several factors likely limited the quality of the treatment and thus the overall clinical benefits, including, the number of surviving RPE and photoreceptor cells at the time of treatment, the overall health of the remaining tissue and the limited levels of RPE65 expression. Of note, an AAV4-RPE65 trial was recently completed with the goal of achieving greater RPE65 expression in RPE cells.
The progressive nature of retinal degenerations may pose challenges for delivering long-term clinical benefits because the death of mutant cells often triggers neighboring healthy cells to die (Cepko & Vandenberghe, 2013). Studies in chimeric mice in which patches of mutant rods were intermingled with wild-type rods, showed that both dysfunctional and normal photoreceptors died, even when more than 40% of the retina is composed of normal photoreceptors (Huang et al., 1993; Kedzierski et al., 1998). Comparably in humans with retinitis pigmentosa, the death of rods, which constitute over 95% of the photoreceptors, invariably leads to the secondary degeneration of neighboring healthy cones (Punzo et al., 2012). Correcting the functional defect in a few cells may thus fail to halt the continued degeneration in the long-term. In such cases, an intravitreal approach, that would theoretically broadly distribute the vector throughout the retina, may offer a more appropriate mode of delivery. However, it is important to note that the percentage of dying cells required to initiate a bystander effect and how this effect propagates remains to be defined. Studies in zebrafish have elegantly shown that spatial cell density may be a key factor in the ability of photoreceptors to survive in a degenerating environment (Stearns et al., 2007). Interestingly, a recent study performed in a canine model of retinitis pigmentosa showed that the local correction of the genetic defect in ~50% of rods over a surface of 25–35% of the total retina completely stopped the degeneration process in the vector-exposed area for at least 30 months, despite the widespread degeneration in untreated areas (Pichard et al., 2016). Another study in a dog model of X-linked retinopathy showed that degeneration could be halted with a gene replacement approach, even when treatment occurs at late stages of the disease (Beltran et al., 2015). Notably, long-term follow-up of the treated dogs showed that while photoreceptors initially also degenerated in the vector-exposed area of the retina, islands of rescued cells remained as the disease progressed and retinal thickness was stabilized in the transduced area (Beltran et al., 2015). The prospect of the elucidation of mechanisms of retinal degenerations in this respect is fascinating because this knowledge may permit the identification of the best ‘targetable’ cells and the design of the most appropriate gene therapy intervention for each patient.
Gene-specific therapy for dominant inherited retinal disorders
Compared to recessive diseases, dominant diseases pose additional challenges to their treatment by gene therapy, as in several cases the aberrant protein not only displays an impaired function but also alters the expression or the function of the wild-type protein. Treating these conditions by gene therapy ideally requires the suppression of the dominant allele to diminish the toxicity while preserving the expression of the wild-type allele to ensure proper gene function after treatment. Mutation-specific suppression of the mutant gene has been achieved at the RNA level by AAV-mediated delivery of ribozymes and RNA interference in murine models of several forms of dominantly inherited retinopathies (Farrar et al., 2012). This approach, however, addresses only one pathogenic mutation at the time. Thus, it will likely not be possible to extend it to all patients because most disease genes present an extremely high degree of allelic heterogeneity and the number of patients that carry one specific mutation is very small. Specifically, rhodopsin (RHO)-linked autosomal dominant retinitis pigmentosa, the most common form of dominant retinopathy is caused by over 200 different mutations in the RHO gene alone. The time and cost of developing sequence-specific inhibitor for each mutation would be prohibitive, as the efficacy and safety of each new construct will need to be tested rigorously in preclinical and clinical trials. To circumvent this problem, progress has been made on ways to eliminate indiscriminately both mutated and normal RNA, while supplementing at the same time the cell with a normal RNA engineered to resist degradation. However, as a dual therapy, the gene suppression-and-replacement strategy requires optimization of each element separately. The approach must silence endogenous RNA to therapeutically relevant levels, and also, as it is the case for gene replacement therapy, drive sufficiently high levels of the resistant transgene to avoid the development of a more aggressive form of the disease. Transgenic mice expressing dominant RHO transgenes have been widely used for the development of mutation-independent approaches of gene therapy for autosomal dominant diseases (Farrar et al., 2012). To date, significant functional and structural benefits have been obtained in these murine models, using either a single dual-component AAV vector (Mao et al., 2012) or two separate AAV vectors (Millington-Ward et al., 2011), strengthening the validity of this approach. However, rhodopsin constitutes over 90% of the outer segment membrane proteins and recapitulating such high level of expression after gene transfer remains a major technical challenge for the future progress of this approach toward clinical application. In this regard, it is of note that gene transfer to the retina is improving quickly with the discovery of more efficient AAV serotypes and the construction of optimized promoters (Vandenberghe & Auricchio, 2012). For example, functional rescue of a murine model of RHO knockout has been recently achieved using a novel variant of AAV and an optimized murine RHO promoter (Palfi et al., 2015). Undoubtedly, new vectors will be used more extensively in the future in an attempt to optimize the treatment of dominant retinopathies and move gene suppression-and-replacement therapies to clinical reality.
Non gene-specific therapies for ocular diseases
Data from the early clinical trials of gene replacement therapy and the increasing number of proof-of-concept studies illustrate the very high potential of gene-specific therapy for the treatment of retinal degenerative diseases. It needs to be noted, however, that as we write, inherited retinopathies involve mutations in over 260 different genes, some of which are too large for the currently used vectors. To broaden the applicability of retinal gene therapy, generic approaches that work across genetic subtypes are being explored in parallel with therapies aimed at correcting the primary genetic defect.
Gene therapies modulating the secondary mechanisms of retinopathies
An alternative strategy to the correction of the primary genetic defect of the disease is to target the common causes or mechanisms concurring to photoreceptor degeneration in order to prolong the lifespan of the retinal cells and delay the onset of blindness. This approach offers the possibility to treat not only genetic diseases but also a range of more common multifactorial disorders. The idea for such an approach was based on the realization that in many forms of retinal diseases, the patterns of photoreceptor cell death are similar. For example, as mentioned above, as rods malfunction and die, there is always a secondary death of neighboring healthy cones. Interestingly, cone death always starts near the end of the rod death phase independently of the rate of rod death (Punzo et al., 2009), indicating a common pathologic cause and process.
Cones represent only 5% of all photoreceptors in humans, but their role in vision is essential. It is their loss that leads to the most damaging symptoms of retinal degenerations and potentially total blindness. One strategy to delay secondary cone death involves the generic protection of rods, even without rescuing their function, as this should inhibit the onset of secondary cone death. Similarly, gene therapies directed toward prolonging cone survival would enable the treatment of a very large number of individuals. Cones persist in most patients for a prolonged period of time before they die, indicating that slowing their death down further could result in an almost permanent cure.
Several therapies have been tested to delay rod photoreceptor death before loss of cones occurs. These therapies include the delivery of genes encoding anti-apoptotic factors to inhibit the execution of a cell death program in photoreceptors (Chinskey et al., 2014; Leonard et al., 2007; Zadro-Lamoureux et al., 2009), or the delivery of genes encoding neurotrophic factors to raise the threshold of cellular stress required for the initiation of apoptosis, such that rod death is delayed or less frequent. Overall, anti-apoptotic therapies have been shown to be less robust than neuroprotective ones, probably because they target the final step in the lifecycle of photoreceptor cells. In addition, it is now well accepted that different cell death mechanisms can be activated in photoreceptors and that such mechanisms often cooperate during cell death. Thus, photoreceptors that are physiologically ready to die may activate alternative cell death pathways to the one that is inhibited.
AAV-mediated delivery of genes coding for various neurotrophic trophic factors (e.g. erythropoietin derivatives, CNTF or GDNF) has resulted in effective protection of photoreceptors in rodent models of induced and inherited retinal degenerations (Bok et al., 2002; Colella et al., 2011; Fernandez-Sanchez et al., 2012; Leaver et al., 2006; Liang et al., 2001; Lipinski et al., 2015; MacLaren et al., 2014; Martin et al., 2003; Rex et al., 2004; Wu et al., 2002). However, it is worth noting that their mode of action is not always fully understood, which makes the translation of the approach into the clinic challenging. Such therapies could have possible pleiotropic effects, depending on the dose, delivery mode and/or the target cells, and thus be associated with undesirable side effects. Another concern is that significant rod degeneration often occurs in many patients before they first visit an ophthalmologist, resulting in missed opportunities to rescue the rods and indirectly prevent the loss of cones.
Several groups are currently trying to identify the mechanisms of cone death with the goal to directly prevent cone death. Recently, the conjunction of retinal biology, genetics and biochemistry has led to the realization that metabolic deregulation and oxidative stress are major causes for secondary cone death (Punzo et al., 2009; Punzo et al., 2012). Rods constitute 95% of the photoreceptors and their massive death may irreversibly compromise the ability of cones to efficiently uptake the glucose that is provided by the RPE. In parallel, as rod die, cones may be subjected to increased levels of oxygen and experience oxidative damage.
Cone death was delayed after transgenic (Usui et al., 2009) or viral-mediated (Xiong et al., 2015) overexpression of antioxidant factors in murine models of retinal degenerations, which supported the idea that oxidative stress contributes to cone death. Moreover, gene transfer of rod-derived cone viability factor (RdCVF), a truncated thioredoxin that mediates resistance against photo-oxidative damage (Cronin et al., 2010; Elachouri et al., 2015; Fridlich et al., 2009; Mei et al., 2016) and induces glucose uptake in vitro (Ait-Ali et al., 2015) promoted cone survival in two different models of retinopathy (Byrne et al., 2015). However, oxidative stress itself is unlikely to be the sole cause governing secondary cone death in retinal dystrophy. Recent findings that activation of metabolic genes downstream of mTORC1 in cones dramatically improved cone survival in mice, strongly suggest that the overall cause of cone death is nutrient shortage (Venkatesh et al., 2015). This discovery directly intersects with the increased oxidative stress in cones during degeneration, since cones mainly use glucose to fuel the biosynthesis of NADPH and NADPH regulates antioxidant activity of the cells. Based on these results, boosting cone metabolism emerges as a central theme to prevent the secondary death of cones. Gene therapy methods that enhance glucose uptake or utilization in cone photoreceptors are expected have great impact on the treatment of various forms of retinal degenerations (Venkatesh et al., 2015, Zieger and Punzo, 2016).
Optogenetics
Once most photoreceptors have degenerated, other strategies need to be employed if some form of light sensitivity is to be restored. Optogenetics is a particular form of gene therapy in which photosensitivity is conferred to the remaining retinal cells by means of transgenic expression of a light-sensitive protein. A variety of different optogenetic tools have been developed (Deisseroth, 2015). In most cases these tools are light sensitive ion channels. Once these genes are expressed in retinal neurons, it becomes possible to activate (depolarization) or silence (hyperpolarization) the cells upon light stimuli. This approach has been shown to restore light perception and basic visual-guided behavior in small and large animal models of retinal degenerations, either by reactivating remaining cones compromised during disease progression (Busskamp et al., 2012) or by turning inner retinal neurons into artificial photoreceptors (Bi et al., 2006; Cronin et al., 2014; Doroudchi et al., 2011; Lagali et al., 2008; Mace et al., 2015; Scalabrino et al., 2015; Thyagarajan et al., 2010).
The big challenges of the optogenetics field are to deliver sufficient light sensitivity and to specifically target certain retinal cell types. Progress is expected thanks to the possibility to act at the molecular level to increase the sensitivity and amplitude of the light currents produced by these light-sensitive channels (Deisseroth, 2015). Additionally, novel vectors and cell type specific promoters should greatly benefit the optogenetics approach (Cronin et al., 2014; Lu et al., 2016; Vandenberghe & Auricchio, 2012).
Conclusion
Gene therapy for the treatment of retinal diseases is an advancing field. Gene therapy clinical trials for several forms of retinopathies have now started and demonstrated that these approaches are both safe and efficient. In parallel, progress in our understanding of disease pathogenesis continues to expand the applicability of retinal gene therapy. The continued successful implementation of ocular gene therapy into the clinic heralds a time when the treatment of many other forms of blinding disorders, which until recently have been considered to be incurable, will be available.
Acknowledgments
We would like to thank Shun-Yun Cheng and Marina Zieger for critical reading of the manuscript. The following funding support is also gratefully acknowledged: the “Information Recherche Retinite Pigmentaire” financial support, the Fulbright/Fondation Mohanan Fellowship, the Fondation de France/Fondation Berthe Fouassier Fellowship, the “Association Francaise contre les Myopathies” Fellowship, and the National Eye Institute grant (EY023570).
Footnotes
Disclosure statement
The authors report no conflicts of interest.
References
- Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J, Dejneka NS, Pearce-Kelling SE, Maguire AM, Palczewski K, Hauswirth WW, Jacobson SG. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12(6):1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Ali N, Fridlich R, Millet-Puel G, Clerin E, Delalande F, Jaillard C, Blond F, Perrocheau L, Reichman S, Byrne LC, Olivier-Bandini A, Bellalou J, Moyse E, Bouillaud F, Nicol X, Dalkara D, Van Dorsselaer A, Sahel JA, Leveillard T. Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell. 2015;161(4):817–832. doi: 10.1016/j.cell.2015.03.023. [DOI] [PubMed] [Google Scholar]
- Annear MJ, Mowat FM, Bartoe JT, Querubin J, Azam SA, Basche M, Curran PG, Smith AJ, Bainbridge JW, Ali RR, Petersen-Jones SM. Successful gene therapy in older Rpe65-deficient dogs following subretinal injection of an adeno-associated vector expressing RPE65. Hum Gene Ther. 2013;24(10):883–893. doi: 10.1089/hum.2013.146. [DOI] [PubMed] [Google Scholar]
- Bainbridge JW, Mehat MS, Sundaram V, Robbie SJ, Barker SE, Ripamonti C, Georgiadis A, Mowat FM, Beattie SG, Gardner PJ, Feathers KL, Luong VA, Yzer S, Balaggan K, Viswanathan A, De Ravel TJ, Casteels I, Holder GE, Tyler N, Fitzke FW, et al. Long-term effect of gene therapy on Leber's congenital amaurosis. N Engl J Med. 2015;372(20):1887–1897. doi: 10.1056/NEJMoa1414221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358(21):2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Beltran WA, Cideciyan AV, Iwabe S, Swider M, Kosyk MS, Mcdaid K, Martynyuk I, Ying GS, Shaffer J, Deng WT, Boye SL, Lewin AS, Hauswirth WW, Jacobson SG, Aguirre GD. Successful arrest of photoreceptor and vision loss expands the therapeutic window of retinal gene therapy to later stages of disease. Proc Natl Acad Sci U S A. 2015;112(43):E5844–5853. doi: 10.1073/pnas.1509914112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J, Ashtari M, Wellman J, Marshall KA, Cyckowski LL, Chung DC, Mccague S, Pierce EA, Chen Y, Bennicelli JL, Zhu X, Ying GS, Sun J, Wright JF, Auricchio A, Simonelli F, Shindler KS, Mingozzi F, High KA, Maguire AM. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med. 2012;4(120):120ra115. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J, Wellman J, Marshall KA, Mccague S, Ashtari M, Distefano-Pappas J, Elci OU, Chung DC, Sun J, Wright JF, Cross DR, Aravand P, Cyckowski LL, Bennicelli JL, Mingozzi F, Auricchio A, Pierce EA, Ruggiero J, Leroy BP, Simonelli F, et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet. 2016 Jun 30; doi: 10.1016/S0140-6736(16)30371-3. Epud ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennicelli J, Wright JF, Komaromy A, Jacobs JB, Hauck B, Zelenaia O, Mingozzi F, Hui D, Chung D, Rex TS, Wei Z, Qu G, Zhou S, Zeiss C, Arruda VR, Acland GM, Dell'osso LF, High KA, Maguire AM, Bennett J. Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol Ther. 2008;16(3):458–465. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM, Pan ZH. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50(1):23–33. doi: 10.1016/j.neuron.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok D, Yasumura D, Matthes MT, Ruiz A, Duncan JL, Chappelow AV, Zolutukhin S, Hauswirth W, Lavail MM. Effects of adeno-associated virus-vectored ciliary neurotrophic factor on retinal structure and function in mice with a P216L rds/peripherin mutation. Exp Eye Res. 2002;74(6):719–735. doi: 10.1006/exer.2002.1176. [DOI] [PubMed] [Google Scholar]
- Boye SE, Boye SL, Lewin AS, Hauswirth WW. A comprehensive review of retinal gene therapy. Mol Ther. 2013;21(3):509–519. doi: 10.1038/mt.2012.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busskamp V, Picaud S, Sahel JA, Roska B. Optogenetic therapy for retinitis pigmentosa. Gene Ther. 2012;19(2):169–175. doi: 10.1038/gt.2011.155. [DOI] [PubMed] [Google Scholar]
- Byrne LC, Dalkara D, Luna G, Fisher SK, Clerin E, Sahel JA, Leveillard T, Flannery JG. Viral-mediated RdCVF and RdCVFL expression protects cone and rod photoreceptors in retinal degeneration. J Clin Invest. 2015;125(1):105–116. doi: 10.1172/JCI65654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho LS, Vandenberghe LH. Promising and delivering gene therapies for vision loss. Vision Res. 2015;111(Pt B):124–133. doi: 10.1016/j.visres.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko CL, Vandenberghe LH. Retinal gene therapy coming of age. Hum Gene Ther. 2013;24(3):242–244. doi: 10.1089/hum.2013.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinskey ND, Besirli CG, Zacks DN. Retinal cell death and current strategies in retinal neuroprotection. Curr Opin Ophthalmol. 2014;25(3):228–233. doi: 10.1097/ICU.0000000000000043. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV. Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retin Eye Res. 2010;29(5):398–427. doi: 10.1016/j.preteyeres.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, Pang JJ, Sumaroka A, Windsor EA, Wilson JM, Flotte TR, Fishman GA, Heon E, Stone EM, Byrne BJ, Jacobson SG, Hauswirth WW. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A. 2008;105(39):15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Jacobson SG, Beltran WA, Sumaroka A, Swider M, Iwabe S, Roman AJ, Olivares MB, Schwartz SB, Komaromy AM, Hauswirth WW, Aguirre GD. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci U S A. 2013;110(6):E517–525. doi: 10.1073/pnas.1218933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella P, Iodice C, Di Vicino U, Annunziata I, Surace EM, Auricchio A. Non-erythropoietic erythropoietin derivatives protect from light-induced and genetic photoreceptor degeneration. Hum Mol Genet. 2011;20(11):2251–2262. doi: 10.1093/hmg/ddr115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin T, Raffelsberger W, Lee-Rivera I, Jaillard C, Niepon ML, Kinzel B, Clerin E, Petrosian A, Picaud S, Poch O, Sahel JA, Leveillard T. The disruption of the rod-derived cone viability gene leads to photoreceptor dysfunction and susceptibility to oxidative stress. Cell Death Differ. 2010;17(7):1199–1210. doi: 10.1038/cdd.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin T, Vandenberghe LH, Hantz P, Juttner J, Reimann A, Kacso AE, Huckfeldt RM, Busskamp V, Kohler H, Lagali PS, Roska B, Bennett J. Efficient transduction and optogenetic stimulation of retinal bipolar cells by a synthetic adeno-associated virus capsid and promoter. EMBO Mol Med. 2014;6(9):1175–1190. doi: 10.15252/emmm.201404077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci. 2015;18(9):1213–1225. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinculescu A, Glushakova L, Min SH, Hauswirth WW. Adeno-associated virus-vectored gene therapy for retinal disease. Hum Gene Ther. 2005;16(6):649–663. doi: 10.1089/hum.2005.16.649. [DOI] [PubMed] [Google Scholar]
- Doroudchi MM, Greenberg KP, Liu J, Silka KA, Boyden ES, Lockridge JA, Arman AC, Janani R, Boye SE, Boye SL, Gordon GM, Matteo BC, Sampath AP, Hauswirth WW, Horsager A. Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Mol Ther. 2011;19(7):1220–1229. doi: 10.1038/mt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards TL, Jolly JK, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Black GC, Webster AR, Lotery AJ, Holder GE, Xue K, Downes SM, Simunovic MP, Seabra MC, Maclaren RE. Visual Acuity after Retinal Gene Therapy for Choroideremia. N Engl J Med. 2016 May 19; doi: 10.1056/NEJMc1509501. Epud ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elachouri G, Lee-Rivera I, Clerin E, Argentini M, Fridlich R, Blond F, Ferracane V, Yang Y, Raffelsberger W, Wan J, Bennett J, Sahel JA, Zack DJ, Leveillard T. Thioredoxin rod-derived cone viability factor protects against photooxidative retinal damage. Free Radic Biol Med. 2015;81:22–29. doi: 10.1016/j.freeradbiomed.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Farrar GJ, Millington-Ward S, Chadderton N, Humphries P, Kenna PF. Gene-based therapies for dominantly inherited retinopathies. Gene Ther. 2012;19(2):137–144. doi: 10.1038/gt.2011.172. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sanchez L, Lax P, Isiegas C, Ayuso E, Ruiz JM, De La Villa P, Bosch F, De La Rosa EJ, Cuenca N. Proinsulin slows retinal degeneration and vision loss in the P23H rat model of retinitis pigmentosa. Hum Gene Ther. 2012;23(12):1290–1300. doi: 10.1089/hum.2012.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlich R, Delalande F, Jaillard C, Lu J, Poidevin L, Cronin T, Perrocheau L, Millet-Puel G, Niepon ML, Poch O, Holmgren A, Van Dorsselaer A, Sahel JA, Leveillard T. The thioredoxin-like protein rod-derived cone viability factor (RdCVFL) interacts with TAU and inhibits its phosphorylation in the retina. Mol Cell Proteomics. 2009;8(6):1206–1218. doi: 10.1074/mcp.M800406-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazi NG, Abboud EB, Nowilaty SR, Alkuraya H, Alhommadi A, Cai H, Hou R, Deng WT, Boye SL, Almaghamsi A, Al Saikhan F, Al-Dhibi H, Birch D, Chung C, Colak D, Lavail MM, Vollrath D, Erger K, Wang W, Conlon T, et al. Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector: results of a phase I trial. Hum Genet. 2016;135(3):327–343. doi: 10.1007/s00439-016-1637-y. [DOI] [PubMed] [Google Scholar]
- Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, Jacobson SG. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19(10):979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PC, Gaitan AE, Hao Y, Petters RM, Wong F. Cellular interactions implicated in the mechanism of photoreceptor degeneration in transgenic mice expressing a mutant rhodopsin gene. Proc Natl Acad Sci U S A. 1993;90(18):8484–8488. doi: 10.1073/pnas.90.18.8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ, Peden MC, Aleman TS, Boye SL, Sumaroka A, Conlon TJ, Calcedo R, Pang JJ, Erger KE, Olivares MB, Mullins CL, Swider M, Kaushal S, Feuer WJ, Iannaccone A, et al. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol. 2012;130(1):9–24. doi: 10.1001/archophthalmol.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Roman AJ, Sumaroka A, Schwartz SB, Heon E, Hauswirth WW. Improvement and decline in vision with gene therapy in childhood blindness. N Engl J Med. 2015;372(20):1920–1926. doi: 10.1056/NEJMoa1412965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierski W, Bok D, Travis GH. Non-cell-autonomous photoreceptor degeneration in rds mutant mice mosaic for expression of a rescue transgene. J Neurosci. 1998;18(11):4076–4082. doi: 10.1523/JNEUROSCI.18-11-04076.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagali PS, Balya D, Awatramani GB, Munch TA, Kim DS, Busskamp V, Cepko CL, Roska B. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat Neurosci. 2008;11(6):667–675. doi: 10.1038/nn.2117. [DOI] [PubMed] [Google Scholar]
- Le Meur G, Stieger K, Smith AJ, Weber M, Deschamps JY, Nivard D, Mendes-Madeira A, Provost N, Pereon Y, Cherel Y, Ali RR, Hamel C, Moullier P, Rolling F. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther. 2007;14(4):292–303. doi: 10.1038/sj.gt.3302861. [DOI] [PubMed] [Google Scholar]
- Leaver SG, Cui Q, Plant GW, Arulpragasam A, Hisheh S, Verhaagen J, Harvey AR. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther. 2006;13(18):1328–1341. doi: 10.1038/sj.gt.3302791. [DOI] [PubMed] [Google Scholar]
- Leonard KC, Petrin D, Coupland SG, Baker AN, Leonard BC, Lacasse EC, Hauswirth WW, Korneluk RG, Tsilfidis C. XIAP protection of photoreceptors in animal models of retinitis pigmentosa. PLoS One. 2007;2(3):e314. doi: 10.1371/journal.pone.0000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FQ, Dejneka NS, Cohen DR, Krasnoperova NV, Lem J, Maguire AM, Dudus L, Fisher KJ, Bennett J. AAV-mediated delivery of ciliary neurotrophic factor prolongs photoreceptor survival in the rhodopsin knockout mouse. Mol Ther. 2001;3(2):241–248. doi: 10.1006/mthe.2000.0252. [DOI] [PubMed] [Google Scholar]
- Lipinski DM, Barnard AR, Singh MS, Martin C, Lee EJ, Davies WI, Maclaren RE. CNTF Gene Therapy Confers Lifelong Neuroprotection in a Mouse Model of Human Retinitis Pigmentosa. Mol Ther. 2015;23(8):1308–1319. doi: 10.1038/mt.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Ganjawala TH, Ivanova E, Cheng JG, Troilo D, Pan ZH. AAV-mediated transduction and targeting of retinal bipolar cells with improved mGluR6 promoters in rodents and primates. Gene Ther. 2016 doi: 10.1038/gt.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace E, Caplette R, Marre O, Sengupta A, Chaffiol A, Barbe P, Desrosiers M, Bamberg E, Sahel JA, Picaud S, Duebel J, Dalkara D. Targeting channelrhodopsin-2 to ON-bipolar cells with vitreally administered AAV Restores ON and OFF visual responses in blind mice. Mol Ther. 2015;23(1):7–16. doi: 10.1038/mt.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, Clark KR, During MJ, Cremers FP, Black GC, Lotery AJ, Downes SM, Webster AR, Seabra MC. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383(9923):1129–1137. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell'osso L, Hertle R, Ma JX, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358(21):2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, Gorbatyuk MS, Rossmiller B, Hauswirth WW, Lewin AS. Long-term rescue of retinal structure and function by rhodopsin RNA replacement with a single adeno-associated viral vector in P23H RHO transgenic mice. Hum Gene Ther. 2012;23(4):356–366. doi: 10.1089/hum.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KR, Quigley HA, Zack DJ, Levkovitch-Verbin H, Kielczewski J, Valenta D, Baumrind L, Pease ME, Klein RL, Hauswirth WW. Gene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2003;44(10):4357–4365. doi: 10.1167/iovs.02-1332. [DOI] [PubMed] [Google Scholar]
- Mei X, Chaffiol A, Kole C, Yang Y, Millet-Puel G, Clerin E, Ait-Ali N, Bennett J, Dalkara D, Sahel JA, Duebel J, Leveillard T. The Thioredoxin Encoded by the Rod-Derived Cone Viability Factor Gene Protects Cone Photoreceptors Against Oxidative Stress. Antioxid Redox Signal. 2016;24(16):909–923. doi: 10.1089/ars.2015.6509. [DOI] [PubMed] [Google Scholar]
- Millington-Ward S, Chadderton N, O'reilly M, Palfi A, Goldmann T, Kilty C, Humphries M, Wolfrum U, Bennett J, Humphries P, Kenna PF, Farrar GJ. Suppression and replacement gene therapy for autosomal dominant disease in a murine model of dominant retinitis pigmentosa. Mol Ther. 2011;19(4):642–649. doi: 10.1038/mt.2010.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci U S A. 2005;102(35):12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat FM, Breuwer AR, Bartoe JT, Annear MJ, Zhang Z, Smith AJ, Bainbridge JW, Petersen-Jones SM, Ali RR. RPE65 gene therapy slows cone loss in Rpe65-deficient dogs. Gene Ther. 2013;20(5):545–555. doi: 10.1038/gt.2012.63. [DOI] [PubMed] [Google Scholar]
- Narfstrom K, Katz ML, Bragadottir R, Seeliger M, Boulanger A, Redmond TM, Caro L, Lai CM, Rakoczy PE. Functional and structural recovery of the retina after gene therapy in the RPE65 null mutation dog. Invest Ophthalmol Vis Sci. 2003;44(4):1663–1672. doi: 10.1167/iovs.02-0595. [DOI] [PubMed] [Google Scholar]
- Narfstrom K, Vaegan, Katz M, Bragadottir R, Rakoczy EP, Seeliger M. Assessment of structure and function over a 3-year period after gene transfer in RPE65−/− dogs. Doc Ophthalmol. 2005;111(1):39–48. doi: 10.1007/s10633-005-3159-0. [DOI] [PubMed] [Google Scholar]
- Palfi A, Chadderton N, O'reilly M, Nagel-Wolfrum K, Wolfrum U, Bennett J, Humphries P, Kenna P, Millington-Ward S, Farrar J. Efficient gene delivery to photoreceptors using AAV2/rh10 and rescue of the Rho(−/−) mouse. Mol Ther Methods Clin Dev. 2015;2:15016. doi: 10.1038/mtm.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Lei L, Dai X, Shi W, Liu X, Dinculescu A, Mcdowell JH. AAV-mediated gene therapy in mouse models of recessive retinal degeneration. Curr Mol Med. 2012;12(3):316–330. doi: 10.2174/156652412799218877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit L, Khanna H, Punzo C. Advances in Gene Therapy for Diseases of the Eye. Hum Gene Ther. 2016 Jun 13; doi: 10.1089/hum.2016.040. Epud ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichard V, Provost N, Mendes-Madeira A, Libeau L, Hulin P, Tshilenge KT, Biget M, Ameline B, Deschamps JY, Weber M, Le Meur G, Colle MA, Moullier P, Rolling F. AAV-mediated Gene Therapy Halts Retinal Degeneration in PDE6beta-deficient Dogs. Mol Ther. 2016 May 24; doi: 10.1038/mt.2016.37. Epud ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009;12(1):44–52. doi: 10.1038/nn.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punzo C, Xiong W, Cepko CL. Loss of daylight vision in retinal degeneration: are oxidative stress and metabolic dysregulation to blame? J Biol Chem. 2012;287(3):1642–1648. doi: 10.1074/jbc.R111.304428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20(4):344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- Rex TS, Allocca M, Domenici L, Surace EM, Maguire AM, Lyubarsky A, Cellerino A, Bennett J, Auricchio A. Systemic but not intraocular Epo gene transfer protects the retina from light-and genetic-induced degeneration. Mol Ther. 2004;10(5):855–861. doi: 10.1016/j.ymthe.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Scalabrino ML, Boye SL, Fransen KM, Noel JM, Dyka FM, Min SH, Ruan Q, De Leeuw CN, Simpson EM, Gregg RG, Mccall MA, Peachey NS, Boye SE. Intravitreal delivery of a novel AAV vector targets ON bipolar cells and restores visual function in a mouse model of complete congenital stationary night blindness. Hum Mol Genet. 2015;24(21):6229–6239. doi: 10.1093/hmg/ddv341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns G, Evangelista M, Fadool JM, Brockerhoff SE. A mutation in the cone-specific pde6 gene causes rapid cone photoreceptor degeneration in zebrafish. J Neurosci. 2007;27(50):13866–13874. doi: 10.1523/JNEUROSCI.3136-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyagarajan S, Van Wyk M, Lehmann K, Lowel S, Feng G, Wassle H. Visual function in mice with photoreceptor degeneration and transgenic expression of channelrhodopsin 2 in ganglion cells. J Neurosci. 2010;30(26):8745–8758. doi: 10.1523/JNEUROSCI.4417-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui S, Komeima K, Lee SY, Jo YJ, Ueno S, Rogers BS, Wu Z, Shen J, Lu L, Oveson BC, Rabinovitch PS, Campochiaro PA. Increased expression of catalase and superoxide dismutase 2 reduces cone cell death in retinitis pigmentosa. Mol Ther. 2009;17(5):778–786. doi: 10.1038/mt.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe LH, Auricchio A. Novel adeno-associated viral vectors for retinal gene therapy. Gene Ther. 2012;19(2):162–168. doi: 10.1038/gt.2011.151. [DOI] [PubMed] [Google Scholar]
- Venkatesh A, Ma S, Le YZ, Hall MN, Ruegg MA, Punzo C. Activated mTORC1 promotes long-term cone survival in retinitis pigmentosa mice. J Clin Invest. 2015;125(4):1446–1458. doi: 10.1172/JCI79766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Gao G. State-of-the-art human gene therapy: part I. Gene delivery technologies. Discov Med. 2014;18(97):67–77. [PMC free article] [PubMed] [Google Scholar]
- Weleber RG, Pennesi ME, Wilson DJ, Kaushal S, Erker LR, Jensen L, Mcbride MT, Flotte TR, Humphries M, Calcedo R, Hauswirth WW, Chulay JD, Stout JT. Results at 2 Years after Gene Therapy for RPE65-Deficient Leber Congenital Amaurosis and Severe Early-Childhood-Onset Retinal Dystrophy. Ophthalmology. 2016 doi: 10.1016/j.ophtha.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Wu WC, Lai CC, Chen SL, Xiao X, Chen TL, Tsai RJ, Kuo SW, Tsao YP. Gene therapy for detached retina by adeno-associated virus vector expressing glial cell line-derived neurotrophic factor. Invest Ophthalmol Vis Sci. 2002;43(11):3480–3488. [PubMed] [Google Scholar]
- Xiong W, Maccoll Garfinkel AE, Li Y, Benowitz LI, Cepko CL. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J Clin Invest. 2015;125(4):1433–1445. doi: 10.1172/JCI79735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadro-Lamoureux LA, Zacks DN, Baker AN, Zheng QD, Hauswirth WW, Tsilfidis C. XIAP effects on retinal detachment-induced photoreceptor apoptosis [corrected] Invest Ophthalmol Vis Sci. 2009;50(3):1448–1453. doi: 10.1167/iovs.08-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieger M, Punzo C. Improved cell metabolism prolongs photoreceptor survival upon retinal-pigmented epithelium loss in the sodium iodate induced model of geographic atrophy. Oncotarget. 2016;7(9):9620–9633. doi: 10.18632/oncotarget.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]