Abstract
Background
Cetuximab in combination with docetaxel was examined in chemotherapy-refractory/resistant patients with advanced nonsmall-cell lung cancer (NSCLC) to determine response rate, survival, safety, and pharmacokinetics (PK).
Methods
Patients had evidence of epidermal growth factor receptor (EGFR) expression (≥1 +) and tumor progression during or disease recurrence within 3 months after chemotherapy. Cetuximab was administered weekly (400 mg/m2 initial; 250 mg/m2 thereafter). Docetaxel was administered every 3 weeks (75 mg/m2). A response in 3 of the first 21 patients was required to continue accrual to the target sample size of 50 patients.
Results
Confirmed responses included 1 complete response (1.8%), 10 partial responses (18.2%), and 20 with stable disease (36.4%). The response rate was 20% (95% confidence interval [CI], 10.4% to 33.0%) and median time to disease progression was 104 days. There were no differences in PK parameters of docetaxel alone or with cetuximab. The most common grade 3 of 4 adverse events were leukopenia (27.3%) and acne (21.8%). Four patients (7.3%) discontinued due to allergic reaction. The median overall survival (OS) was 7.5 months with a 1-year survival of 35%.
Conclusions
Cetuximab in combination with docetaxel was well tolerated. The response rate supports more definitive evaluation of this combination in the second-line setting.
Keywords: Cetuximab, NSCLC, EGFR, chemotherapy-refractory, docetaxel
The majority of patients with nonsmall-cell lung cancer (NSCLC) initially present with locally advanced or metastatic disease, and treatment with cisplatin-based chemotherapy in this population yields a median survival of 6 to 10 months.1-3 After failure of first-line chemotherapy, overall prognosis is especially poor. In patients who receive best supportive care in this setting, median survival time is 4.6 months with a 1-year survival rate of 11%.4 The role of salvage therapy after first-line treatment of NSCLC was first established by Fossella et al who compared docetaxel 100 and 75 mg/m2 with a control regimen of vinorelbine or ifosamide (overall response, 10.8% and 6.7% vs .8%).5 Docetaxel, when compared with best supportive care, increased response rates (time to progression, 10.6 vs 6.7 weeks), overall survival (OS; median survival, 7.0 vs 4.6 months), and quality of life in the randomized phase 3 study by Shepherd et al,4 and it was the first cytotoxic agent approved in this setting. Since then, other treatment options have become available for previously treated NSCLC patients including pemetrexed (similar efficacy results when compared with docetaxel)6 and erlotinib (response rate vs placebo, 8.9% vs <1%; progression-free survival, 2.2 vs 1.8 months, hazard ratio [HR] = .61; OS, 6.7 vs 4.7 months, HR = .70; P < .001 for all).7 These salvage lung studies all reported response rates of less than 10% and 1-year survivals of approximately 30%.
The epidermal growth factor receptor (EGFR) has become a promising target for anti-cancer therapy, specifically lung cancers, which frequently exhibit EGFR overexpression.8-10 EGFR targeted therapies lead to inhibition of cell cycle progression, induction of apoptosis, and impairment of tumor growth.11
Cetuximab is a recombinant DNA-derived, chimerized monoclonal antibody that blocks the binding of EGF or TGF-α to the receptor. Cetuximab inhibits ligand-induced receptor phosphorylation and stimulates receptor internalization. It may also trigger antibody-dependent cellular cytotoxicity. Numerous studies have demonstrated that cetuximab monotherapy effectively inhibits proliferation of EGFR-positive tumor cells in vitro and tumor growth in xenograft models.12-16 Furthermore, it has been shown to increase sensitivity to chemotherapy in vitro17 and to reverse resistance to chemotherapy, both in preclinical models18 and in a randomized phase 3 study of patients with advanced colorectal cancer.19 Based on the tolerability of cetuximab and the efficacy of docetaxel as second-line therapy, this trial was conducted to investigate the efficacy and tolerability of the combination in patients with NSCLC for whom front-line chemotherapy has failed. A patient population with chemotherapy refractory or resistant disease (and thus expected to have a poor outcome to salvage cytotoxic treatment alone) was specifically selected for this study.
Materials and Methods
Study Design
We conducted a multicenter, open-label, nonrandomized Phase 2 trial for patients with recurrent or progressive NSCLC. The trial opened in May 2001 and closed in May 2006. In all, 55 patients were entered from 3 participating institutions in the United States. The primary objective was to determine the response rate of cetuximab in combination with docetaxel in patients with recurrent or progressive NSCLC within 3 months of receiving a cytotoxic chemotherapy regimen. Secondary objectives were to assess the safety profile of the cetuximab/docetaxel combination, determine the duration of response and overall survival, and evaluate the effects of cetuximab on the PK of docetaxel.
Patient Eligibility
Patient eligibility requirements were as follows: histologically or pathologically proven recurrent or progressive NSCLC; unidimensionally measurable NSCLC; Karnofsky performance score (KPS) ≥60; progressive disease within 3 months after discontinuing 1 cytotoxic chemo-therapy regimen; signed informed consent; adequate hematologic, hepatic, and renal function; immunohisto-chemical evidence of EGFR expression (≥1 +); and be ≥18 years of age. EGFR expression was determined by a central laboratory (Impath Labs) and was considered ≥1 + if ≥10% of tumor cells presented any degree of staining. Exclusion criteria were as follows: pregnancy or lactation; prior anti-EGFR antibody therapy or small molecule therapy; prior docetaxel therapy; prior chemo-therapy or major thoracic or abdominal surgery within 30 days before the first infusion of cetuximab; wide field radiation therapy within 4 weeks before the first infusion of cetuximab; history of uncontrolled angina, arrhythmias, or congestive heart failure; uncontrolled seizure disorder, active neurological disease or ≥grade 2 neuropathy; and any investigational agent within 30 days of study entry.
This study was conducted in accordance with current good clinical practices (GCPs) and International Conference on Harmonization (ICH) recommendations, as well as all applicable local, state, and federal regulations and guidelines regarding the conduct of clinical trials. In addition, this study was conducted in accordance with the ethical principles included in the Recommendations Guiding Physicians in Biomedical Research Involving Human Subjects adopted by the 18th World Medical Assembly, Helsinki, Finland, June 1964, and amended by the 29th World Medical Assembly, Tokyo, Japan, October 1975, the 35th World Medical Assembly, Venice, Italy, October 1983, the 41st World Medical Assembly, Hong Kong, September 1989, and the 48th General Assembly, Somerset West, Republic of South Africa, October 1996.
Treatment
Enrolled patients received cetuximab weekly in combination with docetaxel every 3 weeks. Cetuximab was manufactured and supplied by ImClone Systems Incorporated (Branchburg, NJ), and was administered by intravenous (IV) infusion at an initial dose of 400 mg/m2 (over 120 minutes) followed by weekly maintenance doses of 250 mg/m2 (over 60 minutes). Cetuximab premedication comprised diphenhydramine hydrochloride 50 mg IV. Docetaxel (75 mg/m2) was given as a 1-hour IV infusion repeated every 21 days. Docetaxel premedication comprised corticosteroids for 3 days (starting 1 day before docetaxel infusion) and antiemetics per each institution's protocol. Cetuximab was administered on Days 1, 8, and 15 of each treatment cycle. Docetaxel was given 1 hour after the completion of the cetuximab infusion on Day 1 of each treatment cycle. For patients undergoing docetaxel PK studies only for cycle 1, cetuximab was started on Day 2.
Cetuximab was permanently discontinued if the patient's treatment was delayed for more than 2 consecutive weeks because of grade 3 skin reactions or if grade 3 skin reaction occurred a fourth time, but there was no change in the cetuximab weekly schedule for docetaxel-related toxicity. Two cetuximab dose reductions (to 200 and 150 mg/m2/week) were allowed for repeated grade 3 skin toxicities. A docetaxel dose reduction of 25% was permitted in the case of docetaxel-related adverse events (AEs), intercurrent illnesses, or toxicities. However, stable or responding patients who developed intolerable neurotoxicity or nephrotoxicity were able to continue docetaxel therapy at a further reduced dose. Patients that discontinued docetaxel due to AEs were allowed to continue cetuximab therapy. Patients continued treatment until disease progression, protocol noncompliance, intolerable toxicity, or an intercurrent illness that mandated interruption of cetuximab therapy for more than 2 consecutive infusions.
Efficacy
Responses were defined by Response Evaluation Criteria in Solid Tumors (RECIST).20 Imaging studies were obtained at baseline and at 6-week intervals. Disease control was defined as the best tumor response of complete response (CR), partial response (PR), or stable disease (SD) that was confirmed and sustained for 4 weeks or longer. Duration of response was defined as the time from the initial response during combination therapy to progression of disease or death. Progression-free survival and median OS were calculated using the Kaplan-Meier method.
Safety and Tolerability
All patients receiving at least 1 dose of cetuximab were evaluated for safety analysis. Systemic and local treatment-emergent AEs were graded using the established National Cancer Institute-Common Toxicity Criteria (NCI-CTC), Version 2.0. Routine clinical and laboratory assessments were performed.
Pharmacokinetic Studies
For the determination of docetaxel concentration, 5-mL blood samples were collected from peripheral IV site into heparin-containing Vacutainer at the following time points: Predose, 55 minutes, 1.25, 1.5, 2.5, 6.5, and 24 hours after start of docetaxel infusion on cycle 1, Day 1 (docetaxel alone; cetuximab started on Day 2 for patients undergoing PK studies) and cycle 2 (docetaxel in combination with cetuximab). After collection, samples were centrifuged at 2500 rpm for 10 minutes at 5°C. Plasma was placed into a labeled cryovial and frozen at −70°C until analysis. Docetaxel concentrations were quantified using a validated reverse phase high-pressure liquid chromatography (HPLC) assay.21 The dynamic range for the assay was from 50 to 10,000 ng/mL with an intra-and inter-day standard deviation of <15%. Analytical system comprised Alliance HPLC system with a Waters 2487 tunable dual channel UV/Vis absorbance detector (Waters Corp, Mil-ford, Mass). A derived channel at 230 nm was extracted to create chromatograms for peak analysis. The docetaxel peak was positively identified from other peaks using ultra-violet (UV) absorbance spectrum and retention time. PK parameters for docetaxel were estimated using standard noncompartmental method (WinNonlin, version 3.1; Pharsight Corporation, Mountain View, Calif). To assess if cetuximab had an effect on the PK of docetaxel, with each patient serving as their own control, the parameters between cycle 1 (docetaxel alone) and cycle 2 (docetaxel in combination with cetuximab) were compared using the Student t test for paired data, with a priori level of significance of P = .05. Cetuximab concentration in serum was measured using a validated Biacore-based assay.22
Statistical Considerations
Simon's optimal 2-stage design,23 which allowed for early stopping for ineffectiveness, was implemented. It was expected the new regimen would have a targeted response rate of 25%. With a probability of 0.1 of accepting a response rate of ≤10% (ineffective regimen) or rejecting a response rate ≥25% (effective regimen), 3 patients were required to have a response among the first 21 patients treated so as to allow accrual to continue until the target total sample size of 50 patients was reached. At the end of the study, the new regimen would be rejected if the response rate was ≤14% (7 of 50) and would be accepted otherwise.
Results
Patients
Fifty-five patients were enrolled: o47 were evaluable for efficacy and 55 were evaluable for safety. All 55 patients were included in the denominator for the intent-to-treat analysis of response. Of the 8 patients who were not evaluable for tumor response, 1 patient withdrew due to disease progression, 3 patients died (2 due to disease complications and 1 due to an intercurrent illness), and 4 patients discontinued the study due to AEs before the first tumor assessment. Baseline characteristics of all enrolled patients are summarized in Table 1.
Table 1. Patient Characteristics (N = 55).
| Demographic and Baseline Characteristics | No. (%) |
|---|---|
| Age, y | |
| Median | 60 |
| Range | 31-76 |
| Sex | |
| Men | 26 (47.3) |
| Women | 29 (52.7) |
| KPS score | |
| 60 | 2 (3.6) |
| 70 | 2 (3.6) |
| 80 | 37 (67.3) |
| 90 | 8 (14.5) |
| 100 | 6 (10.9) |
| EGFR status* | |
| 11 | 2 (3.6) |
| 21 | 6 (10.9) |
| 31 | 47 (85.5) |
| Histology | |
| Adenocarcinoma | 36 (65.5) |
| Squamous cell carcinoma | 12 (22) |
| Other/unknown | 7 (13) |
| Prior therapy for lung cancer | |
| Chemotherapy | 55 (100.0) |
| Immunotherapy | 2 (3.6) |
| Radiotherapy | 26 (47.3) |
KPS indicates Karnofsky performance status; EGFR, epidermal growth factor receptor.
EGFR staining intensity guidelines: 1+, a faint/barely perceptible membrane staining is detected in more than 10% of the tumor cells; 2+, a weak to moderate membrane staining is present, completely surrounding the cells in more than 10% of the tumor cells; 3+, a strong complete membrane staining is observed in more than 10% of the tumor cells.
Exposure
Overall, 55 patients received a median of 11 (range 1 to 178) doses of cetuximab and 4 (range 1 to 23) doses of docetaxel (Table 2). None of the patients required a cetuximab dose reduction, whereas 4 patients required docetaxel dose reductions (3 patients to 60 mg/m2 and 1 patient to 56 mg/m2). Treatment delays of cetuximab or docetaxel occurred in 37 (67%) and 18 patients (33%), respectively. Reasons for treatment discontinuation were disease progression (41 patients, 75%), AEs (8 patients, 15%), death (3 patients, 6%), other (2 patients, 4%), and withdrawal of consent (1 patient, 2%).
Table 2. Extent of Exposure to Study Drug (N = 55).
| Extent of Exposure | Cetuximab | Docetaxel |
|---|---|---|
| Duration of treatment, wk | ||
| Median | 11.7 | 11.7 |
| Range | 1-248 | 3-80 |
| No. of doses | ||
| Median | 11.0 | 4 |
| Range | 1-178 | 1-23 |
| Cumulative dose, mg/m2 | ||
| Median | 2826 | 244 |
| Range | 400-44579 | 74-1484 |
| Dose intensity, mg/m2, wk | ||
| Median | 240.7 | 24 |
| Range | 142-259 | 19-26 |
| Relative dose intensity, % | ||
| Median | 96 | 97.7 |
| Range | 57-104 | 74.2-104.4 |
Efficacy
The investigator assessments of the best overall tumor responses in the evaluable population (N = 55) are shown in Table 3. The response rate (complete response [CR] + partial response [PR]) was 20% and 20 additional patients had stable disease producing a disease control rate of 56.4%. For the patients who responded (those with an objective response of CR or PR), the median duration of response was 225 days (95% confidence interval [CI], 66, 1684 + days). Data are also presented in Table 3 based on 47 patients excluding the 8 nonevaluable patients. In this analysis, the objective response rate was 23.4% and the disease control rate was 66%.
Table 3. Response Rates in the Evaluable Population.
| Tumor Response | Evaluable No. (%) n=47 | ITT No. (%) n=55 |
|---|---|---|
| Best response to treatment | ||
| CR | 1 (2.1) | 1 (1.8) |
| PR | 10 (21.3) | 10 (18.2) |
| SD | 20 (42.6) | 20 (36.4) |
| PD | 16 (34.0) | 16 (29.1) |
| Not evaluable | – | 8 (14.5) |
| Objective response (CR+PR) | 11 (23.4) | 11 (20.0) |
| 95% CI | (11.3-36.0) | (10.4-33.0) |
| Disease control (CR+PR+SD) | 31 (66.0) | 31 (56.4) |
| 95% CI | (52.4-79.5) | (42.3-69.7) |
ITT indicates intent to treat; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; CI, confidence interval.
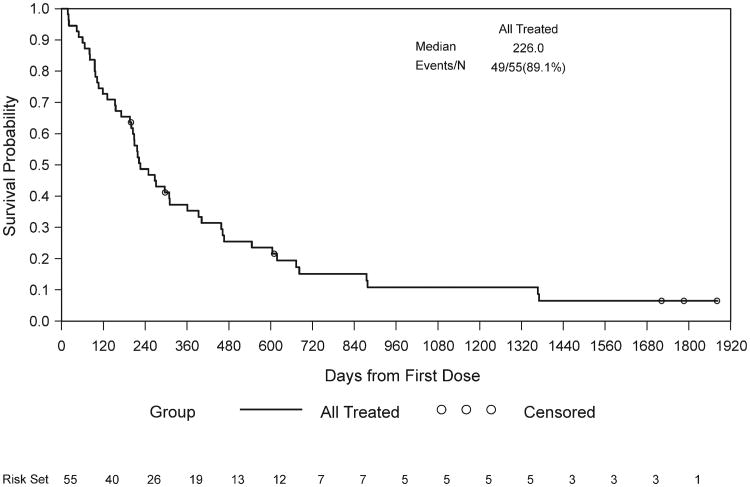
The median OS was 7.5 months (95% CI, 6.7 to 12 months) with a 1-year survival of 35% (Fig. 1). The median progression-free survival was 2.7 months (95% CI, 1.8 to 4.4 months). A subset analysis that correlates grade of rash and survival was performed. Patients with grade 3 rash had a median survival of 10.33 months, whereas the median survival of patients with grade 1-2 rash and no rash was 7.26 and 2.0 months, respectively. The Cox regression model was used to test whether patients with rash had a longer OS compared with patients without a rash. Patients with a grade 3 rash experienced a significantly longer OS compared with patients without rash (P = .0066). No correlation was observed between survival and grade of immunohistochemistry (IHC) staining.
Figure 1.
Kaplan-Meier curve of overall survival (OS) population: all treated patients.
Safety and Tolerability
Most AEs observed in this trial were mild, and there was no evidence of additional toxicity resulting from the combination of cetuximab and docetaxel. Forty-3 (78.2%) patients reported a grade 3 or 4 AE. The most frequent grade 3 and 4 AEs included leukopenia (23.6%), acne (21.8%), asthenia (20.0%), dyspnea (18.2%), and pneumonia (12.7%) (Table 4).
Table 4. Incidence of Hematologic and Selected Nonhematologic Adverse Events (Highest Grade per Patient; N = 55).
| Any Grade No. (%) | Grade 3 No. (%) | Grade 4 No. (%) | |
|---|---|---|---|
| Nonhematologic toxicities | |||
| Acne-like rash | 50 (90.9) | 12 (21.8) | – |
| Fatigue/malaise | 31 (56.4) | 11 (20.0) | – |
| Myalgia/arthralgia | 31 (56.4) | 5 (9.1) | – |
| Diarrhea | 28 (50.9) | – | 1 (1.8) |
| Mucositis/stomatitis | 26 (47.3) | – | – |
| Nausea/vomiting | 25 (45.5) | – | – |
| Fever/chills | 21 (38.2) | 5 (9.1) | 1 (1.8) |
| Dyspnea | 20 (36.4) | 6 (10.9) | 4 (7.3) |
| Neuropathy | 15 (27.3) | 2 (3.6) | – |
| Hypomagnesemia | 12 (21.8) | – | – |
| Hypersensitivity reaction | 6 (10.9) | 3 (5.5) | 1 (1.8) |
| Pneumonia | 11 (20) | 5 (9.1) | 2 (3.6) |
| Sepsis | 3 (5.5) | 2 (3.6) | 1 (1.8) |
| Pulmonary embolus | 3 (5.5) | 1 (1.8) | 2 (3.6) |
| Atrial fibrillation | 3 (5.5) | – | 3 (5.5) |
| Hematologic toxicities | |||
| Anemia | 8 (14.5) | 3 (5.5) | 1 (1.8) |
| Thrombocytopenia | 2 (3.6) | 1 (1.8) | 1 (1.8) |
| Leukopenia | 15 (27.3) | 6 (10.9) | 7 (12.7) |
Pharmacokinetics
From the 17 patients who consented to the optional blood collection for PK analysis, a summary of the peak and trough concentrations of cetuximab are depicted in Figure 2. After the first cycle of cetuximab, the mean trough concentration ranged from 67 + 32 mg/mL to 165 + 84 mg/mL and the 1-hour peak cetuximab concentrations ranged from 243 + 62 mg/mL to 385 + 176 mg/mL. For the determination of PK of docetaxel, 8 patients had adequate blood sampling for PK modeling in both cycles 1 (docetaxel alone) and 2 (docetaxel and cetuximab). The summary PK parameters are presented in Table 5. There were no differences in the PK parameters of docetaxel either alone or in combination with cetuximab (P > .05); in addition, these values are within ranges reported by other investigators using docetaxel as a single-agent dosing regimen.24-26
Figure 2.
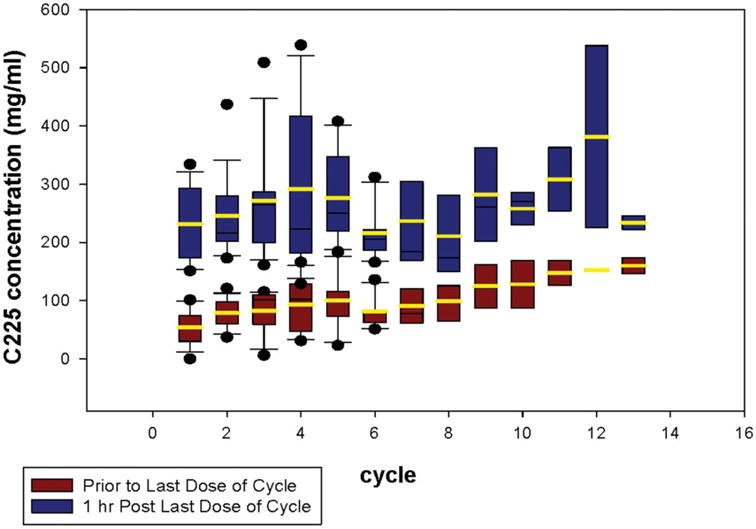
Peak (1-hour postdose) and trough concentrations of cetuximab (mean and standard of deviation).
Table 5. Summary of Docetaxel Pharmacokinetic Parameters.
| Docetaxel Alone (Cycle 1) (n=8) | Docetaxel+ Cetuximab (Cycle 2) (n=8) | P* | |
|---|---|---|---|
| AUC, μg/mL/h | 6.93±3.94 | 6.07±3.07 | .42 |
| Clearance, L/h/M2 | 14.40±8.64 | 14.9±6.72 | .52 |
| T½ beta, h | 23.09±8.46 | 21.9±9.75 | .31 |
AUC indicates area under the concentration-time curve; T½, terminal half-life.
The P value was derived from the Student t test.
Discussion
The landscape in NSCLC salvage therapy continues to be an evolving field. Current FDA-approved agents include docetaxel, pemetrexed, and erlotinib with reported response rates of less than 10% and median survivals of 5 to 8 months.4,6,7 This multicenter phase 2 study of combination cetuximab and docetaxel demonstrates activity in patients with chemotherapy refractory advanced NSCLC. The primary endpoint of response rate (20%) and disease control rate (56%) in the evaluable population compares favorably with historical controls in the second-line setting. However, this study targeted a chemotherapy refractory or resistant population, defined as patients who had disease progression while on chemotherapy or within 3 months of completing prior therapy, which may portend to a poorer overall prognosis. Previous randomized phase 3 studies of salvage therapy containing a single-agent docetaxel arm enrolled roughly only between 49% and 57% of chemorefractory/chemoresistant patients,6,27 and in at least 2 of these studies, the response rate to docetaxel in this subgroup was lower when compared with chemosensitive patients.5,6 Therefore, the response rates observed in the present trial are intriguing and raise the question whether cetuximab is capable of enhancing the activity of cytotoxic chemotherapy in patients with NSCLC. Enhanced activity of chemotherapy when combined with cetuximab has been observed in other tumors such as advanced colorectal cancer, which led to the approval of cetuximab in this disease: the EGFR-targeted antibody combined with irinotecan demonstrated by an increased response rate of the cetuximab-irinotecan arm as compared with the cetuximab alone arm in irinotecan refractory patients.19
In a previous phase 2 study, cetuximab single-agent elicited objective responses in 4.5% of previously treated NSCLC patients.28 Although the response rate was somewhat lower than what is usually observed with pemetrexed, docetaxel, and erlotinib monotherapy in this setting, the trial by Hanna et al enrolled a heavily pre-treated population (58% with ≥2 prior regimens) and achieved a median time to progression (2.3 months) and OS (8.9 months).28 These results added to the pool of data indicating that the EGFR is a valid target in treating NSCLC, either with the use of an antibody or a tyrosinekinase inhibitor as demonstrated by Shepherd et al with erlotinib.7
The strategy of combining chemotherapy with an EGFR-targeted drug is controversial in NSCLC, at least with the use of tyrosine-kinase inhibitors in the chemonaïve setting. Four randomized trials failed to demonstrate an improved activity of chemotherapy plus gefitinib or erlotinib as compared with chemotherapy alone in the frontline setting.29-32 In contrast, the addition of an EGFR-targeted antibody (ie, cetuximab) to chemotherapeutic regimens in the frontline setting has demonstrated prolonged progression-free survival in advanced colorectal cancer33 and an increase in OS and progression-free survival in advanced head and neck cancer.34 Furthermore, a recent phase 3 study (FLEX) reported the addition of cetuximab to vinorelbine/cisplatin statistically improved OS when compared with vinorelbine/cisplatin alone.35
However, these efficacy improvements have not been observed with regards to progression-free survival with cetuximab and chemotherapy in either the FLEX study or BMS-099, a randomized phase 3 study of carboplatin/taxane with or without cetuximab.36 The role of combining biologic and cytotoxic agents in the salvage treatment of NSCLC has never been proven and warrants further evaluation in randomized prospective studies.
There have been several reports that treatment efficacy may be improved based on tumor biomarker presence or degree of rash experienced while receiving an anti-EGFR agent. During a phase 2 trial by Hirsch et al of paclitaxel plus carboplatin given with or before cetuximab, patients with EGFR FISH-positive results had longer median survival times (15 vs 7 months; HR = .58; P = .046), 1-year survival rates (58% vs 32%), and a significantly longer median progression-free survival time (6 months vs 3 months; HR = .45; P = .0011) than patients with EGFR FISH-negative results, respectively.37 EGFR IHC was not predictive for efficacy in our study as most patients had high IHC expression. Our study also suggests that increasing grade of acneiform rash may be a predictive/prognostic factor in patients treated with cetuximab. This observation has also been described in studies with EGFR inhibitors in colorectal, lung, pancreatic, and head and neck cancer.33,38-40 Preliminary studies examining whether patients should be treated with higher drug levels until experiencing a clinically significant rash have been reported in the context of color-ectal cancer, and suggest improved efficacy with the use of the “dose-to-rash” strategy.41 However, this remains to be established in larger Phase 3 studies and in other tumor types.
In conclusion, future studies in the salvage treatment of NSCLC continue to build on the paradigm of combination therapy established in the first-line setting. Bevacizumab was the first biologic agent to demonstrate increased survival in combination with carboplatin and paclitaxel in selected patients based on safety.42 Ongoing studies in second-line therapy will assess the efficacy of doublet versus single-agent therapy. These combinations include chemotherapy + biologic therapy or biologic + biologic treatments. The results presented herein, although not definitive, further justify the need to investigate the possible role of EGFR-targeted agents (either antibodies or tyrosine kinase inhibitors) in this setting, especially in patients with a poor response to initial chemotherapy. In this regard, the SELECT trial, an ongoing phase 3 study that randomizes patients to either docetaxel or pemetrexed with or without cetuximab after failure of platinum-based frontline therapy, will help answer this question in salvage lung cancer treatment.
Acknowledgments
Funded by ImClone Systems Incorporated.
The following was disclosed: P. Windt is an employee of ImClone Systems and is an ImClone stockholder. E. Vokes has served as a consultant and has received research support and honoraria from sanofi-aventis, Bristol-Myers Squibb, and Imclone Systems. R. Herbst states a financial interest with ImClone Systems and Bristol-Myers Squibb. E. Kim has served as a consultant and has received research support and honoraria from sanofi-aventis, Bristol-Myers Squibb, and Imclone Systems.
Footnotes
Conflict of Interest Disclosures: Authors were asked to disclose relationships, including potential financial conflicts of interest.
References
- 1.Non-small Cell Lung Cancer Collaborative Group. Chemo-therapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 2.Schiller JH, Harrington D, Belani CP, et al. Comparison of 4 chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 3.Breathnach OS, Freidlin B, Conley B, et al. Twenty-two years of phase III trials for patients with advanced non-small-cell lung cancer: sobering results. J Clin Oncol. 2001;19:1734–1742. doi: 10.1200/JCO.2001.19.6.1734. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 5.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 6.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 8.Wikstrand CJ, Bigner DD. Prognostic applications of the epidermal growth factor receptor and its ligand, transforming growth factor-alpha. J Natl Cancer Inst. 1998;90:799–801. doi: 10.1093/jnci/90.11.799. [DOI] [PubMed] [Google Scholar]
- 9.Veale D, Kerr N, Gibson GJ, et al. The relationship of quantitative epidermal growth factor receptor expression in non-small cell lung cancer to long term survival. Br J Cancer. 1993;68:162–165. doi: 10.1038/bjc.1993.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowinsky EK. The erbB family: targets for therapeutic development against cancer and therapeutic strategies using monoclonal antibodies and tyrosine kinase inhibitors. Annu Rev Med. 2004;55:433–457. doi: 10.1146/annurev.med.55.091902.104433. [DOI] [PubMed] [Google Scholar]
- 11.Pavelic K, Banjac Z, Pavelic J, et al. Evidence for a role of EGF receptor in the progression of human lung carcinoma. Anticancer Res. 1993;13:1133–1137. [PubMed] [Google Scholar]
- 12.Goldstein NI, Prewett M, Zuklys K, et al. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311–1318. [PubMed] [Google Scholar]
- 13.Ciardiello F, Damiano V, Bianco R, et al. Antitumor activity of combined blockade of epidermal growth factor receptor and protein kinase A. J Natl Cancer Inst. 1996;88:1770–1776. doi: 10.1093/jnci/88.23.1770. [DOI] [PubMed] [Google Scholar]
- 14.Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:1935–1940. [PubMed] [Google Scholar]
- 15.Prewett M, Rothman M, Waksal H, et al. Mouse-human chimeric anti-epidermal growth factor receptor antibody C225 inhibits the growth of human renal cell carcinoma xenografts in nude mice. Clin Cancer Res. 1998;4:2957–2966. [PubMed] [Google Scholar]
- 16.Kurai J, Chikumi H, Hashimoto K, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res. 2007;13:1552–1561. doi: 10.1158/1078-0432.CCR-06-1726. [DOI] [PubMed] [Google Scholar]
- 17.Ciardiello F, Bianco R, Damiano V, et al. Antitumor activity of sequential treatment with topotecan and anti-epidermal growth factor receptor monoclonal antibody C225. Clin Cancer Res. 1999;5:909–916. [PubMed] [Google Scholar]
- 18.Prewett MC, Hooper AT, Bassi R, et al. Enhanced antitumor activity of anti-epidermal growth factor receptor monoclonal antibody IMC-C225 in combination with irinotecan (CPT-11) against human colorectal tumor xeno-grafts. Clin Cancer Res. 2002;8:994–1003. [PubMed] [Google Scholar]
- 19.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer. 2006;42:1031–1039. doi: 10.1016/j.ejca.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Vergniol JC, Bruno R, Montay G, Frydman A. Determination of Taxotere in human plasma by a semi-automated high-performance liquid chromatographic method. J Chromatogr. 1992;582:273–278. doi: 10.1016/0378-4347(92)80333-l. [DOI] [PubMed] [Google Scholar]
- 22.Robert F, Ezekiel MP, Spencer SA, et al. Phase I Study of anti-epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol. 2001;19:3234–3243. doi: 10.1200/JCO.2001.19.13.3234. [DOI] [PubMed] [Google Scholar]
- 23.Simon R. Optimal 2-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 24.Aapro M, Bruno R. Early clinical studies with docetaxel. Eur J Cancer. 1995;31:S7–S10. doi: 10.1016/0959-8049(95)00360-u. [DOI] [PubMed] [Google Scholar]
- 25.Extra JM, Rousseau F, Bruno R, et al. Phase I and pharmaco-kinetic study of Taxotere (RP 56976; NSC 628503) given as a short intravenous infusion. Cancer Res. 1993;53:1037–1042. [PubMed] [Google Scholar]
- 26.Bruno R, Hille D, Riva A, et al. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol. 1998;16:187–196. doi: 10.1200/JCO.1998.16.1.187. [DOI] [PubMed] [Google Scholar]
- 27.Ramlau R, Gervais R, Krzakowski M, et al. Phase III study comparing oral topotecan to intravenous docetaxel in patients with pretreated advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:2800–2807. doi: 10.1200/JCO.2005.03.6491. [DOI] [PubMed] [Google Scholar]
- 28.Hanna N, Lilenbaum R, Ansari R, et al. Phase II trial of cetuximab in patients with previously treated non-small-cell lung cancer. J Clin Oncol. 2006;24:5253–5258. doi: 10.1200/JCO.2006.08.2263. [DOI] [PubMed] [Google Scholar]
- 29.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 31.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 32.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 33.Van Cutsem E, Nowacki M, Lang I, et al. Randomized phase III study of irinotecan and 5-FU/FA with or without cetuximab in the first-line treatment of patients with meta-static colorectal cancer (mCRC): The CRYSTAL trial [abstract] J Clin Oncol. 2007;25(suppl) Abstract 4000. [Google Scholar]
- 34.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 35.Pirker R, Szczesna A, von Pawel J. FLEX: A randomized, multicenter, phase III study of cetuximab in combination with cisplatin/vinorelbine (CV) versus CV alone in the first-line treatment of patients with advanced non-small cell lung cancer (NSCLC) [abstract] J Clin Oncol. 2008;26(suppl 3) Abstract 3. [Google Scholar]
- 36.Lynch TJ, Dreisbach T, McCleod L, et al. A randomized multicenter phase III study of cetuximab (Erbitux) in combination with taxane/carboplatin versus taxane/carboplatin alone as first-line treatment for patients with advanced/metastatic non-small cell lung cancer (NSCLC) [abstract] J Thor Oncol. 2007;2:S340. Abstract B3-03. [Google Scholar]
- 37.Hirsch FR, Herbst RS, Olsen C, et al. Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non–small-cell lung cancer patients treated with cetuximab and chemotherapy. J Clin Oncol. 2008;26:3351–3357. doi: 10.1200/JCO.2007.14.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wacker B, Nagrani T, Weinberg J, et al. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in 2 large phase III studies. Clin Cancer Res. 2007;13:3913–3921. doi: 10.1158/1078-0432.CCR-06-2610. [DOI] [PubMed] [Google Scholar]
- 39.Saltz L, Kies M, Abbruzzese JL, et al. The presence and intensity of the cetuximab-induced acne-like rash predicts increased survival in studies across multiple malignancies [abstract] Proc Am Soc Clin Oncol. 2003;22(suppl) Abstract 817. [Google Scholar]
- 40.Cohen EE, Rosen F, Stadler WM, et al. Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2003;21:1980–1987. doi: 10.1200/JCO.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 41.Tejpar S, Peeters M, Humblet Y, et al. Phase I/II study of cetuximab dose-escalation in patients with metastatic color-ectal cancer (mCRC) with no or slight skin reactions on cetuximab standard dose treatment (EVEREST): Pharmaco-kinetic (PK), Pharmacodynamic (PD) and efficacy data [abstract] J Clin Oncol. 2007;25(suppl) Abstract 4037. [Google Scholar]
- 42.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]