Abstract
The purpose of this study was to examine the hypothesis that excess maternal glucocorticoids in response to maternal undernutrition programs the expression of extracellular matrix (ECM) components potentially by miR-29c. We measured the expression of mRNA (qRT-PCR) and protein (Western blot) for collagen 3A1, collagen 4A5 and matrix metalloproteinase 2 (MMP2) in offspring carotid arteries from three groups of dams: 50% food-restricted in latter half of gestation [maternal undernutrition (MUN)], MUN dams who received metyrapone (MET) (500 mg/ml ) in drinking water from day 10 of gestation to term, and control dams fed an ad libitum diet. The expression of miR-29c was significantly decreased at 3 weeks, 3 months and 9 months in MUN carotid arteries, and these decreases in expression were partially blocked by treatment of dams with MET. The expression pattern of ECM genes that are targets of miR-29c correlated with miR-29c expression. Expression of mRNA was increased for elastin (ELN) and MMP2 mRNA in 3-week MUN carotids; in 9-month carotids there were also significant increases in expression of Col3A1 and Col4A5. These changes in mRNA expression of ECM genes at 3 weeks and 9 months were blocked by MET treatment. Similarly, the expression of ELN and MMP2 proteins at 3 weeks were increased in MUN carotids, and by 9 months there were also increases in expression of Col3A1 and Col4A5, which were blocked by MET in MUN carotids. Overall, the results demonstrate a close correlation between expression of miR-29c and the ECM proteins that are its targets thus supporting our central hypothesis.
Keywords: carotid remodeling, extracellular matrix, fetal programming, miR-29c
Introduction
The concept of fetal programming of adult diseases is now well accepted and numerous studies have established that many metabolic and cardiovascular disorders that manifest in adult life have their origins before birth.1 For example, fetal programming of adult onset hypertension has been demonstrated in multiple animal species, and can be induced pharmacologically by maternal glucocorticoid (GC) administration or physiologically by maternal undernutrition (MUN).2 Although the mechanisms whereby MUN influences long-term cardiovascular development and function remain unclear, exposure to excess GCs of maternal origin in response to an adverse uterine environment play a prominent role in this process.3,4 Correspondingly, GC administration to the mother is a widely used model of in utero programming.5 The early work of Langley-Evans and other investigators provided strong evidence that in the protein restriction model of fetal programming, GC of maternal origin plays a pivotal role as a programming agent for hypertension.6 In these studies, the administration of metyrapone (MET), which inhibits the synthesis of corticosterone through inhibition of 11β-hydroxylase in the pregnant rat and their fetuses, prevented development of hypertension in the offspring of mothers fed a low protein diet.7
Our previous studies have focused on changes in vascular composition and function in response to MUN. The extra-cellular matrix (ECM) composition of the vessel walls in these offspring exhibited significant remodeling that was associated with changes in the expression of matrix metalloproteinase 2 (MMP2) and matrix metalloproteinase 9 (MMP9).8 MUN induced increases in elastin (ELN) and glycosaminoglycans (GAG) in 1-day-old (P1) neonatal offspring, with parallel increases in collagen deposition and reduction in GAG content, consistent with stiff vessels by 2 months of life. Similar changes were found in mesenteric arterioles.8 We also reported changes in vascular smooth muscle content and phenotype in response to MUN. In aortae from P1 and P60 MUN offspring, we observed a significant increase in the smooth muscle content secondary to cellular hypertrophy.9 Through co-localization studies of smooth muscle α-actin with non-muscle and smooth muscle myosin heavy chain isoforms in middle cerebral arteries, we further demonstrated that MUN smooth muscle cells were altered toward a non-contractile phenotype.10 Together, these changes increased middle cerebral artery stiffness.10 In other studies, hypoxia during pregnancy also has been shown to increase offspring mesenteric artery stiffness.11 Other studies have demonstrated decreased vasodilator responses in mesenteric but not conduit arteries of offspring of maternal protein restricted dams,12 and reduced constriction to phenylephrine in carotid arteries of offspring of food restricted dams.13
Our own studies have also revealed that decreased expression of vascular endothelial growth factor (VEGF) in conductance and resistance MUN vessels can lead to vascular rarefaction and reduced microvessel density in the renal medulla, attenuated mesenteric branching, and reduced angiogenic potential of endothelial cells isolated from MUN vessels.14 Based on these findings we hypothesized that reduced angiogenesis and angiogenic potential of endothelial cells secondary to inhibition of VEGF expression is a potential mechanism for programmed hypertension,14 and this programming effect was secondary to excess maternal GCs in response to nutritional stress.14
The mechanism by which maternal GCs program the expression of angiogenic genes and ECM composition remains uncertain. A potential mechanism we have proposed involves miRNAs, which are short non-coding RNA molecules that regulate the expression of genes through translational repression and in some cases by enhanced mRNA degradation.15 Through use of a microarray, we identified several miRNAs whose expression was altered in MUN aortas, including miR-29c whose expression was inhibited.16 Interestingly, miR-29c regulates the expression of genes that are key components of ECM including both collagen and ELN,16 and therefore MUN through its effect on miR-29c could alter vascular contractility. Based on these findings, we have hypothesized that maternal GC may promote programming of ECM genes through their effect on miR-29c expression.16 To address this hypothesis we conducted the present study in which we determined the parallel effects of MUN on offspring carotid artery expression of ECM genes and miR-29c expression and the effect of blockade of GC synthesis in MUN dams through administration of MET on expression of miR-29c and ECM genes.
Materials and methods
Animals
The study was approved by the Animal Use and Care Committee at Los Angeles Biomedical Research Institute at the Harbor-UCLA Medical Center. We used a well-characterized animal model of fetal programming developed by our group.17 In this model, first-time-pregnant Sprague–Dawley rats (Charles River Laboratories Inc., Hollister, CA, USA) were housed in a facility with constant temperature and humidity with a 12 h light-12 h dark cycle. At 10 days of gestation, rats were provided either an ad libitum diet of standard laboratory chow (Lab Diet 5001, Brentwood, MO, USA: protein 23%, fat 4.5%, metabolizable energy 3030 kcal/kg) (Control group), or 50% food restricted diet determined by quantification of normal intake in the ad libitum fed rats (MUN group). The third group (MET) had 50% food restriction at 10 days of gestation onward and on days 11–22 of gestation were also given water bottles with MET (Sigma) (0.5 mg/ml). Control animals treated with MET were not used because the hypothalamic–pituitary–adrenal axis is intact in these animals and compensatory increased adrenocorticotropic hormone would be expected to normalize plasma corticosterone levels as previously reported by Warnes et al. in sheep.18 Maternal body weights, food and water intake were recorded daily. There were no differences in water consumption among all groups. Offspring were weaned on day 21 of life into individual cages. To avoid the effect of estrous cycles on the parameters studied, only male offspring were used. These offspring were sacrificed by decapitation at 3 weeks, 3 months and 9 months after birth.
RT-PCR
Total RNA was extracted using Trizol (Invitrogen, Grand Island, NY, USA). The quantity and quality of the isolated RNA was determined spectrophotometrically (ND-1000, NanoDrop Technologies, Wilmington, DE, USA). RNA samples of 10 ng were reverse transcribed using a specific stem-loop primer for miR-29c supplied by Applied Biosystems. RNA samples of 2 μg were reverse transcribed using random primers for collagen 3A1 (Col3A1), collagen 4A5 (Col4A5), MMP2 and ELN. Quantitative RT-PCR was carried out using TaqMan or SYBR gene expression master mixes, and TaqMan miRNA or TaqMan gene expression assays (Applied Biosystems). Reactions were incubated for 10 min at 95°C followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. Levels of mRNA and miRNA were quantified using the Invitrogen StepOne System and normalized to 18 S and RNU6B, respectively. All reactions were run in triplicate and relative expression was determined using the comparative cycle threshold method (2−ΔΔCT), as recommended by the supplier (Applied Biosystems). Abundance values were expressed as fold changes compared to the corresponding control group. The primer sequences used in the SYBR system for amplification for Col3A1 were sense 5′-TTGGGATGCAACTACCTTGGT-3′ and antisense 5′-CCCGAGTCGCAGACACATATT-3′; for Col4A5 were sense 5′-ATTACTATGCCAATTCTTACAGCTTTTG-3′ and antisense 5′-TGTCCTCAAGTCGCCTGCTT-3′; for MMP2 were sense 5′-GCACCGTCGCCCATCA-3′ and anti-sense 5′-GCACTGCCAACTCTTTGTCTGTT-3′; for ELN were sense 5′-AAAACCCCCGAAGCCCTATG-3′ and antisense 5′-TCACTTTCTCTTCCGGCCAC-3′; and for 18 S were sense 5′-GACGGACCAGAGCGAAAGC-3′ and antisense 5′-CCTCCGACTTTCGTTCTTGATT-3′.
Western blot analysis
Tissues were sonicated in a protein lysis buffer and protein concentrations determined by the BCA Protein Assay kit (Pierce, Rockford, IL, USA). For each sample, 40 μg of protein was separated on a 7.5% polyacrylamide gel. The separated proteins were transferred electrophoretically to Immobilon-P membranes (Millipore Corp., New Bedford, MA, USA). Membranes were blocked for 1 h in a 5% milk buffer before overnight incubation with the antibodies against ELN (15257-1-AP), Col4A5 (19797-1-AP), Col3A1 (13548-1-AP) (Proteintech Group, Chicago, IL, USA) and MMP2 (sc-10736) (Santa Cruz Labs, Santa Cruz, CA, USA) all of which are targets of miR-29c at a dilution recommended by the manufacturer. The blots were subjected to enhanced chemiluminescence (ECL Western Blotting Detection System, Amersham Corp, Arlington Heights, IL, USA) with enzyme conjugates of anti-rabbit IgG horseradish peroxidase as a secondary antibody. Blots were then exposed to autoradiography film. The resulting bands were then compared by scanning densitometry. To ensure equal loading, protein blots were stripped and re-probed for Glyceryl-aldehyde 3-phosphate dehydrogenase (Santa Cruz Biotechnology), which served as a loading control. Band densities were quantified using Image J software (http://imagej.nih.gov/ij/).
Statistics
All data are presented as mean ± S.E.M. The data were first analyzed for normality by the Kolmogrove–Smirnoff test, and comparisons were made by one-way ANOVA. Post hoc testing was done by Tuckey’s test. Statistical significance was established at P < 0.05.
Results
The blood pressure, body weight and plasma corticosterone of dams and offspring have been previously published.19 MUN offspring at birth weighed significantly less than control offspring, and MET-treated dam offspring had similar body weight as controls offspring. By 9 months of age male MUN offspring were heavier than controls whereas there were no differences in body weights between males in the MET and control groups. Blood pressures determined by tail plethsymography in the MUN males were higher compared with controls whereas there were no differences in MET offspring as compared with controls. Corticosterone levels in E20 dams were significantly higher in MUN group compared with controls whereas the MUN + ET group had similar corticosterone levels as the controls.19
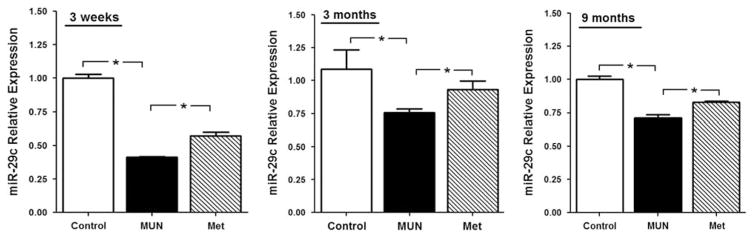
The levels of miR-29c mRNA expressed in carotid arteries are shown in Fig. 1. At 3 weeks of age, the levels of miR-29c were significantly less in MUN than in the Control arteries, and levels in the MET group were significantly greater than in the MUN group (Fig. 1, left panel). This pattern of expression was also evident in the 3-month old (Fig. 1, middle panel) and 9-month old (Fig. 1, right panel) offspring. This pattern generally paralleled that found in the aortae from these animals.16
Fig. 1.
The level of miR-29c in 3-week (left panel, control n =5, MUN n =5, MET n =6), 3-month (middle panel, n =6 per group) and 9-month (right panel, control n =5, MUN n =8, MET n =8)-old offspring of maternal undernutrition (MUN) and metyrapone (MET)-treated dams by qRT-PCR. *P <0.05.
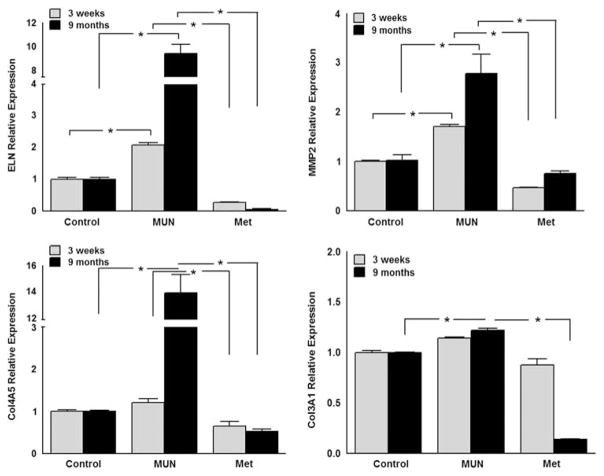
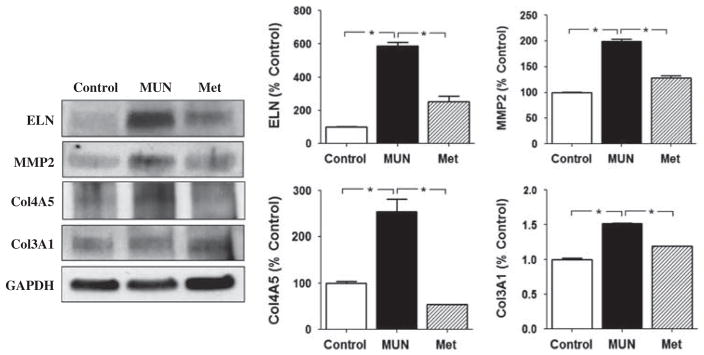
The effects of MUN and MET on both the mRNA and protein levels of molecules targeted by miR-29c are summarized in Figs 2–4. At 3 weeks of age, the abundance of mRNA for ELN and MMP2, but not Col3A1 and Col 4A5, were significantly increased in MUN compared with Control carotid arteries (Fig. 2). Treatment of MUN dams with MET reduced the abundance of ELN, MMP2 and Col4A5 mRNA compared with the MUN group in carotids from 3-week-olds (Fig. 2). Protein abundance for ELN, and MMP2 were significantly greater in 3-week-old carotid arteries from the MUN group compared with controls (Fig. 3); at this age the abundance for Col3A1 and Col4A5 were similar in MUN and Control arteries. The protein abundance for ELN, MMP2, Col3A1 and Col4A5 were all significantly less in carotids from the MET group than from the MUN group at 3 weeks of age.
Fig. 2.
The mRNA levels of elastin (ELN) (upper left panel), matrix metalloproteinase 2 (MMP2) (upper right panel), collagen 4A5 (Col4A5) (lower left panel) and collagen 3A1 (Col3A1) (lower right panel) in 3-week and 9-month-old offspring of maternal undernutrition (MUN) and metyrapone (MET)-treated dams by qRT-PCR. *P < 0.05.
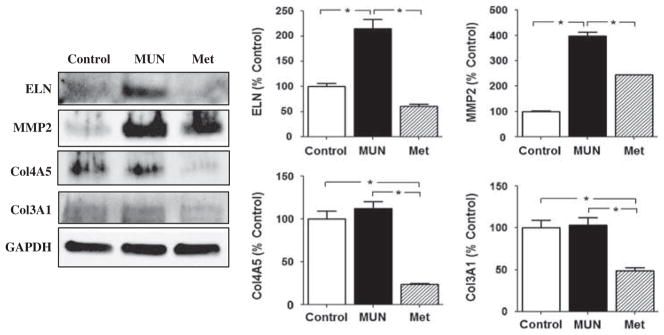
Fig. 4.
The protein expression of elastin (ELN), matrix metalloproteinase 2 (MMP2), collagen 4A5 (Col4A5) and collagen 3A1 (Col3A1) in 9-month-old offspring of maternal undernutrition (MUN) and metyrapone (MET)-treated dams by Western blotting . The band density of ELN (upper left), MMP2 (upper right), Col4A5 (lower left) and Col3A1 (lower right) was shown, respectively. *P < 0.05.
Fig. 3.
The protein expression of elastin (ELN), matrix metalloproteinase 2 (MMP2), collagen 4A5 (Col4A5) and collagen 3A1 (Col3A1) in 3-week-old offspring of maternal undernutrition (MUN) and metyrapone (MET)-treated dams by Western blotting (upper left). The band density of ELN (upper left), MMP2 (upper right), Col4A5 (lower left) and Col3A1 (lower right) was shown, respectively. *P < 0.05.
By 9 months of age, the abundance of mRNA for ELN, MMP2, Col4A5, and Col3A1 were all significantly increased relative to control in MUN carotid arteries (Fig. 2). In 9-month-olds, treatment with MET reduced the abundance of ELN, MMP2, Col4A5 and Col3A1 mRNA compared with the MUN group (Fig. 2). In carotid arteries from 9-month-olds, the protein abundance for ELN, MMP2, Col3A1 and Col4A5 were all significantly greater in the MUN group compared with the Control group (Fig. 4). In addition, at 9 months the abundance of ELN, MMP2, Col3A1 and Col4A5 protein were all significantly less in carotids from the MET group than from the MUN group (Fig. 4).
Discussion
The results of this study support the hypothesis that MUN programs the expression of a number of structural protein components of the ECM of blood vessels, and contributes to remodeling by promoting changes in abundances of ELN, Col4A5, Col3A1 and MMP2. The mRNAs for these proteins are targets of miR-29c, which is suppressed by MUN as is evident at postnatal day 1 and throughout adult life both in aorta16 and as shown here, in carotid arteries. The ability of MUN to increase abundances of ELN, collagen and MMP2 in carotid arteries are consistent with our previously published findings in the aorta8 and indicate that MUN-induced programming of ECM protein expression affects multiple vessel types. Importantly, our current data extend our previous findings and reinforce the hypothesis that excess maternal GC secretion in response to nutritional stress contributes directly to programming the expression of these key ECM components. Given that increased expression of these proteins was blocked by treatment of MUN dams with MET, a blocker of 11β-hydroxylase enzyme and thereby GC synthesis, our find-ings strongly implicate GC synthesis in this process. Because miR-29c targets ELN, Col4A5, Col3A120 and MMP2,21 and MET treatment of MUN dams partially blocked the inhibition of miR-29c in offspring carotid arteries (Fig. 1) we speculate that excess maternal GC induces feedback inhibition of offspring GCs and miR-29c which in turn should relieve inhibition of miR-29c targets, including ELN, Col4A5, Col3A1 and MMP2.
ECM composition is a key determinant of arterial compliance and blood pressure.22,23 Composed primarily of ELN, collagen, GAG, the vascular ECM serves as a scaffold for smooth muscle cells, and also as a reservoir for growth factors and cytokines.23 In blood vessels ELN is organized with other proteins in microfibrils that maintain elastic function and enable long range deformability and passive recoil.23 Conditions such as aging, hypertension, and atherosclerosis are associated with decreased vascular ELN content and compliance.24 In turn, observations that MUN offspring typically exhibit hypertension19,14 and reduced vascular compliance10 suggest that MUN blood vessels may also exhibit decreased ELN content. In contrast, our findings that ELN content was increased in MUN offspring carotid arteries (Figs 3 and 4), and also in the aorta8 demonstrates that MUN-induced reductions in compliance cannot be attributed to reduced ELN, and instead must be due to changes either in ELN organization, cross-linking and/or association with other myofibrillar proteins. Consistent with this interpretation are our previous observations of a marked disruption of the architecture of elastic fibers with partial loss of the typical wavy patterns and reduced inter-elastic laminae area in MUN aortas.8 These findings raise the possibility that MUN-induced increases in ELN content may in some way impair vascular function due perhaps to altered structural organization of the laminae needed to provide elastic recoil. Interestingly, the increase in vascular ELN found in MUN vessels was not found in MET-treated dams, indicating that excess maternal GC in MUN dams are closely associated with upregulation of ELN expression, perhaps through fetal programming of the ELN gene. GCs can stimulate ELN synthesis in rabbit lung,25 in fetal human lung cells,26 and in chick aortic tissue in an age-dependent manner.27 This stimulatory effect of GC on ELN synthesis is most probably transcriptional, as glucoco-corticoid response elements have been identified in the human ELN promoter.28 Additional mechanisms by which maternal GC could program overexpression of offspring vascular ELN include direct epigenetic modification of the ELN gene (methylation/histone acetylation) or indirect enhancement of ELN mRNA translation mediated by GC-induced inhibition of miR-29c,16 which targets the ELN gene.29
A second major component of the vascular ECM is collagen, which provides tensile strength and rigidity to the vessel wall. Of the more than two dozen forms of collagens identified,30 the major types in rodent vessels include collagens I, III, IV, V and VI.23 Of these, collagen III is the main fibril-forming collagen and together with collagen type 1 is most important for imparting tensile strength.23 Because collagens 3A1 and 4A5 are targets of miR-29c,20 the present study focused on the correlations between expression of these collagens and expression of miR-29c. In our earlier published work we reported increased expression of collagen in both aorta and mesenteric arterioles of adult MUN offspring and postulated that this increase in collagen was the structural basis for blood vessel stiffening.8 In this report we extend these findings to carotid arteries and furthermore, demonstrate that blockade of maternal excess GC in MUN dams also inhibits overexpression of collagen in MUN vessels and prevents the vascular stiffening observed in the middle cerebral artery10 and other vessel types31,32 following MUN. Whereas the changes in vascular structure induced by MET could be caused indirectly by normalization of blood pressure, as we and others have reported in various animal models of fetal programming,19 it is even more likely that direct effects of GC on collagen synthesis are involved. In many different blood vessels, GC can stimulate collagen synthesis.33,34 Although the roles of epigenetic mechanisms in these effects of GC have not been explored, their involvement seems likely given that: (1) both the Col3A1 and Col4A5 genes are targets of miR-29c).20 (2) MUN attenuates miRNA29c abundance; and (3) inhibition of GC synthesis with MET reverses the effects of MUN on miR-29c as well as Col3A1, Col4A5, and ELN.
Vascular stiffness and compliance are governed not only by the expression levels of ELN and collagen but also by the cross-linking, organization and selective degradation of these proteins, which is strongly governed by the activity and abundance of MMPs,35 and MMP2 and MMP9,36 in particular. From this perspective, the increased MMP2 expression observed in MUN offspring is important. How this gelatinase could have contributed to increased arterial stiffness is unknown, because limited tissue availability prohibited measurements of multiple types of MMP, tissue inhibitors of metalloproteinases and MMP enzyme activity. The involvement of other collagenases such as MMP1, MMP9 and MMP13,37 and tissue inhibitors of metalloproteinases may also have contributed significantly. For MMP2, the factors that enhanced its expression are also unknown, but it is unlikely that these involved a direct effect of GC on MMPs because GC generally inhibits MMP expression, at least in tissues such as sarcomas,38 and nasal polyps.39 Undoubtedly, many other factors are involved in this complex interaction between MUN, elevated maternal GC, and upregulated ECM proteins. Prime candidates for other molecular participants include vasoactive peptides such as Angiotensin ll, endothelin-140,41 and growth factors.
Increased collagen and ELN deposition can contribute to stenosis and increase vulnerability for ischemic stroke, 20% of which are due to extra cranial atherosclerotic carotid stenosis.42 Increased ELN content can promote formation of carotid plaques that can enhance symptoms of cerebral ischemia.43 Equally important, the ameliorative effect of treatment of MUN dams with MET is not unique to the ECM of blood vessels but also apply to lung alveoli.44,45
In summary, the present study examines the parallel effects of MUN on the expression of collagen, ELN, MMP2, and miR-20c in offspring carotid arteries. The effects of MUN on ECM composition reported here in offspring carotid arteries corroborate and extend our previous findings in offspring aortae.8 The ECM proteins analyzed here are all determinants of vascular compliance, and all are also targets of miR-29c, which in our previous microarray study of offspring aortae16 identified as the miRNA with the greatest response to MUN. Because treatment of MUN dams with a GC synthesis inhibitor, partially relieved inhibition of miR-29c expression but attenuated enhanced expression of multiple ECM proteins these data demonstrate a tight inverse coupling between the expression of miR-29c and ECM proteins. Together, these findings support the hypothesis that excess maternal GC secretion in response to nutritional stress programs the expression of ECM proteins through a miR-29c-mediated pathway.
Acknowledgments
The authors wish to thank David Enriquez and Antonia Enriquez for their assistance with manuscript preparation.
Financial Support
American Heart Association Western Affiliates (4290098), and NIH RO3 (HD054920-01) (OK) and USPHS Grants HD31266, HL64867, and NS076945 (WJP).
Footnotes
Conflict of Interests
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and use of laboratory animals (American Association for Accreditation of Laboratory Care and National Institutes of Health guidelines) and has been approved by Labiomed Animal Care Committee.
References
- 1.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. Br Med J. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 3.Langley-Evans SC, McMullen S. Developmental origins of adult disease. Med Princ Pract. 2010;19:87–98. doi: 10.1159/000273066. [DOI] [PubMed] [Google Scholar]
- 4.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Seckl JR. Prenatal glucocorticoids and long-term programming. Eur J Endocrinol. 2004;151(Suppl 3):U49–U62. doi: 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- 6.Gardner DS, Jackson AA, Langley-Evans SC. Maintenance of maternal diet-induced hypertension in the rat is dependent on glucocorticoids. Hypertension. 1997;30:1525–1530. doi: 10.1161/01.hyp.30.6.1525. [DOI] [PubMed] [Google Scholar]
- 7.Langley-Evans SC. Hypertension induced by foetal exposure to a maternal low-protein diet, in the rat, is prevented by pharmacological blockade of maternal glucocorticoid synthesis. J Hypertens. 1997;15:537–544. doi: 10.1097/00004872-199715050-00010. [DOI] [PubMed] [Google Scholar]
- 8.Khorram O, Momeni M, Desai M, Ross MG. Nutrient restriction in utero induces remodeling of the vascular extracellular matrix in rat offspring. Reprod Sci. 2007;14:73–80. doi: 10.1177/1933719106298215. [DOI] [PubMed] [Google Scholar]
- 9.Khorram O, Momeni M, Ferrini M, Desai M, Ross MG. In utero undernutrition in rats induces increased vascular smooth muscle content in the offspring. Am J Obstet Gynecol. 2007;196:486–488. doi: 10.1016/j.ajog.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Durrant LM, Khorram O, Buchholz JN, Pearce WJ. Maternal food restriction modulates cerebrovascular structure and contractility in adult rat offspring: effects of metyrapone. Am J Physiol Regul Integr Comp Physiol. 2014;306:R401–R410. doi: 10.1152/ajpregu.00436.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemmings DG, Williams SJ, Davidge ST. Increased myogenic tone in 7-month-old adult male but not female offspring from rat dams exposed to hypoxia during pregnancy. Am J Physiol Heart Circ Physiol. 2005;289:H674–H682. doi: 10.1152/ajpheart.00191.2005. [DOI] [PubMed] [Google Scholar]
- 12.Torrens C, Brawley L, Barker AC, et al. Maternal protein restriction in the rat impairs resistance artery but not conduit artery function in pregnant offspring. J Physiol. 2003;547:77–84. doi: 10.1113/jphysiol.2002.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams SJ, Campbell ME, McMillen IC, Davidge ST. Differential effects of maternal hypoxia or nutrient restriction on carotid and femoral vascular function in neonatal rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R360–R367. doi: 10.1152/ajpregu.00178.2004. [DOI] [PubMed] [Google Scholar]
- 14.Khorram O, Khorram N, Momeni M, et al. Maternal undernutrition inhibits angiogenesis in the offspring: a potential mechanism of programmed hypertension. Am J Physiol Regul Integr Comp Physiol. 2007;293:R745–R753. doi: 10.1152/ajpregu.00131.2007. [DOI] [PubMed] [Google Scholar]
- 15.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 16.Khorram O, Han G, Bagherpour R, et al. Effect of maternal undernutrition on vascular expression of micro and messenger RNA in newborn and aging offspring. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1366–R1374. doi: 10.1152/ajpregu.00704.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai M, Gayle D, Babu J, Ross MG. Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol. 2005;288:R91–R96. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]
- 18.Warnes KE, McMillen IC, Robinson JS, Coulter CL. Metyrapone infusion stimulates adrenal growth without activating the cell cycle or the IGF system in the late gestation fetal sheep. Endocr Res. 2004;30:535–539. doi: 10.1081/erc-200043619. [DOI] [PubMed] [Google Scholar]
- 19.Khorram O, Ghazi R, Chuang TD, et al. Excess maternal glucocorticoids in response to in utero undernutrition inhibit offspring angiogenesis. Reprod Sci. 2014;21:601–611. doi: 10.1177/1933719113508819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Taylor NE, Lu L, et al. Renal medullary microRNAs in Dahl salt-sensitive rats: miR-29b regulates several collagens and related genes. Hypertension. 2010;55:974–982. doi: 10.1161/HYPERTENSIONAHA.109.144428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Zhu Y, Zhao M, et al. miRNA-29c suppresses lung cancer cell adhesion to extracellular matrix and metastasis by targeting integrin beta1 and matrix metalloproteinase2 (MMP2) PLoS One. 2013;8:e70192. doi: 10.1371/journal.pone.0070192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shadwick RE. Mechanical design in arteries. J Exp Biol. 1999;202:3305–3313. doi: 10.1242/jeb.202.23.3305. [DOI] [PubMed] [Google Scholar]
- 23.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89:957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karnik SK, Brooke BS, Bayes-Genis A, et al. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411–423. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 25.Anceschi MM, Palmerini CA, Codini M, Luzi P, Cosmi EV. Collagen and elastin in rabbit fetal lung: ontogeny and effects of steroids. J Dev Physiol. 1992;18:233–236. [PubMed] [Google Scholar]
- 26.Nakamura T, Liu M, Mourgeon E, Slutsky A, Post M. Mechanical strain and dexamethasone selectively increase surfactant protein C and tropoelastin gene expression. Am J Physiol Lung Cell Mol Physiol. 2000;278:L974–L980. doi: 10.1152/ajplung.2000.278.5.L974. [DOI] [PubMed] [Google Scholar]
- 27.Keeley FW, Johnson DJ. Age differences in the effect of hydrocortisone on the synthesis of insoluble elastin in aortic tissue of growing chicks. Connect Tissue Res. 1987;16:259–268. doi: 10.3109/03008208709006980. [DOI] [PubMed] [Google Scholar]
- 28.Del MM, Covello SP, Kennedy SH, et al. Identification of novel glucocorticoid-response elements in human elastin promoter and demonstration of nucleotide sequence specificity of the receptor binding. J Invest Dermatol. 1997;108:938–942. doi: 10.1111/1523-1747.ep12295241. [DOI] [PubMed] [Google Scholar]
- 29.van RE, Sutherland LB, Thatcher JE, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brawley L, Itoh S, Torrens C, et al. Dietary protein restriction in pregnancy induces hypertension and vascular defects in rat male offspring. Pediatr Res. 2003;54:83–90. doi: 10.1203/01.PDR.0000065731.00639.02. [DOI] [PubMed] [Google Scholar]
- 32.Sathishkumar K, Elkins R, Yallampalli U, Yallampalli C. Protein restriction during pregnancy induces hypertension and impairs endothelium-dependent vascular function in adult female offspring. J Vasc Res. 2009;46:229–239. doi: 10.1159/000166390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leitman DC, Benson SC, Johnson LK. Glucocorticoids stimulate collagen and noncollagen protein synthesis in cultured vascular smooth muscle cells. J Cell Biol. 1984;98:541–549. doi: 10.1083/jcb.98.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poiani GJ, Tozzi CA, Thakker-Varia S, Choe JK, Riley DJ. Effect of glucocorticoids on collagen accumulation in pulmonary vascular remodeling in the rat. Am J Respir Crit Care Med. 1994;149:994–999. doi: 10.1164/ajrccm.149.4.8143066. [DOI] [PubMed] [Google Scholar]
- 35.Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother. 2003;57:195–202. doi: 10.1016/s0753-3322(03)00065-9. [DOI] [PubMed] [Google Scholar]
- 36.Yasmin Mc, Eniery CM, Wallace S, et al. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:372. doi: 10.1161/01.ATV.0000151373.33830.41. [DOI] [PubMed] [Google Scholar]
- 37.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 38.Roomi MW, Kalinovsky T, Monterrey J, Rath M, Niedzwiecki A. In vitro modulation of MMP-2 and MMP-9 in adult human sarcoma cell lines by cytokines, inducers and inhibitors. Int J Oncol. 2013;43:1787–1798. doi: 10.3892/ijo.2013.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yigit O, Acioglu E, Gelisgen R, et al. The effect of corticosteroid on metalloproteinase levels of nasal polyposis. Laryngoscope. 2011;121:667–673. doi: 10.1002/lary.21462. [DOI] [PubMed] [Google Scholar]
- 40.Touyz RM. Intracellular mechanisms involved in vascular remodelling of resistance arteries in hypertension: role of angiotensin II. Exp Physiol. 2005;90:449–455. doi: 10.1113/expphysiol.2005.030080. [DOI] [PubMed] [Google Scholar]
- 41.De Mey JG, Schiffers PM, Hilgers RH, Sanders MM. Toward functional genomics of flow-induced outward remodeling of resistance arteries. Am J Physiol Heart Circ Physiol. 2005;288:H1022–H1027. doi: 10.1152/ajpheart.00800.2004. [DOI] [PubMed] [Google Scholar]
- 42.Pelisek J, Eckstein HH, Zernecke A. Pathophysiological mechanisms of carotid plaque vulnerability: impact on ischemic stroke. Arch Immunol Ther Exp (Warsz ) 2012;60:431–442. doi: 10.1007/s00005-012-0192-z. [DOI] [PubMed] [Google Scholar]
- 43.Goncalves I, Moses J, Dias N, et al. Changes related to age and cerebrovascular symptoms in the extracellular matrix of human carotid plaques. Stroke. 2003;34:616–622. doi: 10.1161/01.STR.0000058157.69113.F6. [DOI] [PubMed] [Google Scholar]
- 44.Paek DS, Sakurai R, Saraswat A, et al. Metyrapone alleviates deleterious effects of maternal food restriction on lung development and growth of rat offspring. Reprod Sci. 2015;22:207–222. doi: 10.1177/1933719114537712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rehan VK, Li Y, Corral J, et al. Metyrapone blocks maternal food restriction-induced changes in female rat offspring lung development. Reprod Sci. 2014;21:517–525. doi: 10.1177/1933719113503404. [DOI] [PMC free article] [PubMed] [Google Scholar]