Abstract
17β-Estradiol (E2) exerts protective effects on right ventricular (RV) function in pulmonary arterial hypertension (PAH). Since acute exercise-induced increases in afterload may lead to RV dysfunction in PAH, we sought to determine whether E2 allows for superior RV adaptation after an acute exercise challenge. We studied echocardiographic, hemodynamic, structural, and biochemical markers of RV function in male and female rats with sugen/hypoxia (SuHx)-induced pulmonary hypertension, as well as in ovariectomized (OVX) SuHx females, with or without concomitant E2 repletion (75 μg·kg−1·day−1) immediately after 45 min of treadmill running at 75% of individually determined maximal aerobic capacity (75% aerobic capacity reserve). Compared with males, intact female rats exhibited higher stroke volume and cardiac indexes, a strong trend for better RV compliance, and less pronounced increases in indexed total pulmonary resistance. OVX abrogated favorable RV adaptations, whereas E2 repletion after OVX markedly improved RV function. E2's effects on pulmonary vascular remodeling were complex and less robust than its RV effects. Postexercise hemodynamics in females with endogenous or exogenous E2 were similar to hemodynamics in nonexercised controls, whereas OVX rats exhibited more severely altered postexercise hemodynamics. E2 mediated inhibitory effects on RV fibrosis and attenuated increases in RV collagen I/III ratio. Proapoptotic signaling, endothelial nitric oxide synthase phosphorylation, and autophagic flux markers were affected by E2 depletion and/or repletion. Markers of impaired autophagic flux correlated with endpoints of RV structure and function. Endogenous and exogenous E2 exerts protective effects on RV function measured immediately after an acute exercise challenge. Harnessing E2's mechanisms may lead to novel RV-directed therapies.
Keywords: sugen/hypoxia, fibrosis, apoptosis, endothelial nitric oxide synthase, autophagy
pulmonary arterial hypertension (PAH) is a sexually dimorphic disease with a female-to-male ratio of up to 4:1 (3). Despite availability of 14 Food and Drug Administration-approved medications, PAH remains a devastating, progressive, and incurable disease, evidenced by the disappointing 3-yr survival rate of only 55% (22). Even though both female sex and right ventricular (RV) function are major determinants of survival in PAH (5, 22, 23), no RV- or sex steroid-directed therapies exist. This is of importance, since women, despite being more prone to PAH development, exhibit better survival than male patients, a phenomenon attributed, at least in part, to better RV function in women (22, 23, 26, 34, 62).
We and others demonstrated that the female sex hormone 17β-estradiol (E2) exerts protective effects on RV function in experimental PAH (11, 36, 37, 60), findings that support observations made in healthy postmenopausal hormone replacement therapy users, where circulating E2 levels correlate with better RV ejection fraction (61). Given the superior RV function of female PAH patients (23, 26), the exquisite sensitivity of the RV to increases in afterload (19), and the recent observation that exercise and physical activity may worsen RV afterload and RV wall stress and induce RV contractile dysfunction (56), we sought to determine whether E2 favorably affects the RV response to an acute afterload increase in experimental pulmonary hypertension (PH). Specifically, it was our goal to study whether endogenous or exogenous E2 allows for better RV responses after an episode of acute exercise, thus mimicking the clinically prevalent scenario where a PAH patient's RV is stressed by acute exertion. A better understanding of E2's effects in this context could help with the development of individualized activity levels and optimized exercise regimens for PAH patients. In addition, given the known sex differences in responses to PAH therapies (12, 48), a better understanding of the underlying mechanisms of E2-mediated changes in RV responses to exercise may facilitate the development of sex- and RV-specific therapies.
Given the paucity of mechanistic studies of sex hormone signaling employing both sexes in robust animal models of human PAH (31, 34, 57), we performed our studies in male and female rats with sugen/hypoxia (SuHx)-induced PH (SuHx-PH). We hypothesized that female rats with severe angio-proliferative PH exhibit better RV function after a bout of acute strenuous exercise than males, and that the superior function of female RVs is mediated by E2. Employing male, as well as intact, sex hormone-depleted or E2-replete female SuHx rats, we measured markers of RV function immediately after a bout of acute, strenuous exercise. We complemented these studies with measurements of pathogenetically relevant biochemical and molecular modifiers of RV function. The cardiopulmonary phenotype of these animals at rest was previously published (11). We now report that female SuHx rats tolerate an acute exercise challenge better than their male counterparts, and that E2 mediates beneficial effects on RV function and remodeling, as well as RV proapoptotic signaling, endothelial nitric oxide synthase (eNOS) activation, and autophagic flux.
MATERIALS AND METHODS
Animal care.
Studies were performed in age-matched adult male and female Sprague-Dawley rats (175–200 and 150–175 g, respectively; Charles River, Wilmington, MA). Animals were allowed ad libitum access to food and water for the duration of the experimentation. All animals received care in compliance with the Guide for the Care and Use of Laboratory Animals and followed the Declaration of Helsinki conventions for the use and care of animals. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine.
SuHx exposure and experimental groups.
A time line of the experimental protocol is provided in Fig. 1. Su5416 (20 mg/kg subcutaneously; dissolved in DMSO; Sigma Aldrich, St. Louis, MO) was administered immediately before hypoxia exposure. Hypoxia exposure occurred in a hypobaric chamber (atmospheric pressure = 362 mmHg; equivalent to 10% inspired O2 fraction), as described previously (32). After 3 wk of hypoxia, animals were returned to room air for another 4 wk. Experimental groups included intact male and female SuHx rats, ovariectomized (OVX) female SuHx rats, and OVX female SuHx rats replete with E2. Male and female animals not undergoing SuHx were employed as control groups. OVX was performed 2 wk before SuHx induction under isoflurane anesthesia (2%) using sterile technique, as described previously (11). E2 was administered via subcutaneous pellets at a dose of 75 μg·kg−1·day−1, as described previously (11). This E2 dose was previously demonstrated to result in E2 plasma levels within the physiological range (15–25 pg/ml) (11). E2 levels in the target range were confirmed using a previously described Calbiotech Mouse/Rat E2 ELISA (Calbiotech, Spring Valley, CO) (11) and are shown in Table 1. E2 pellets were implanted under isoflurane anesthesia (2%) using sterile technique at the time of OVX. E2 placebo pellets were tested in previous studies and found to not exert any relevant effects on cardiopulmonary parameters (11).
Fig. 1.
Experimental design and timeline. Experimental groups are shown at top of graph. SuHx-PH was generated in male or female age-matched Sprague-Dawley rats by administration of Su5416 (sugen) followed by 3 wk of hypobaric hypoxia (atmospheric pressure = 362 mmHg; equivalent to 10% inspired O2 fraction) and 4 wk of reexposure to room air. Subsets of female SuHx rats underwent ovariectomy (OVX) ± repletion of 17β-estradiol (E2). OVX was performed 2 wk before Su5416 administration. E2 pellets were implanted subcutaneously (sq) at the time of OVX. Male and female rats not exposed to Su5416 or hypoxia served as controls. Familiarization training was performed for 1 wk before exercise testing. Acute exercise challenge was performed via treadmill running at 75% of maximal aerobic capacity (V̇o2max) reserve, followed immediately by echocardiographic and hemodynamic assessment and subsequent death and organ harvest. d, Days.
Table 1.
E2 levels, body weights, and tibia lengths in experimental groups
| Endpoint | Male Normoxia Control | Female Normoxia Control | Male SuHx | Female SuHx | Female OVX SuHx | Female OVX+E2 SuHx |
|---|---|---|---|---|---|---|
| E2 level, pg/ml | 7.2 ± 0.6 | 13.1 ± 3.3† | 6.9 ± 0.5 | 12.8 ± 2.9 | 6.0 ± 0.3‡ | 14.9 ± 0.4§ |
| Body weight, g | 513 ± 18 | 309 ± 10† | 445 ± 7* | 278 ± 10† | 358 ± 12‡ | 244 ± 8*§ |
| Tibia length, mm | 45.3 ± 0.2 | 40.7 ± 0.4† | 44.0 ± 0.2 | 39.9 ± 0.4† | 41.8 ± 0.3‡ | 37.6 ± 0.5*§ |
Values are means ± SE. P < 0.05 vs.
same sex normoxia control,
male normoxia or SuHx control,
female SuHx, and
female OVX SuHx.
Exercise testing and acute exercise challenge.
A time line for these procedures is depicted in Fig. 1. Maximal aerobic capacity (V̇o2max, expressed relative to body mass) was determined before Su5416 administration and at the end of the 7-wk SuHx period via an indirect open-circuit calorimetric system, as described previously (7, 11, 16). V̇o2max was identified using standard criteria for rats, including three consecutive failures to return to running after contacting the shock stimulus at the rear of the treadmill and a respiratory exchange ratio > 1.015 (4). Animals were familiarized with the exercise system by treadmill running for ∼5 min/day for 1 wk before each V̇o2max test (7, 11, 16). We then waited for 48 h after post-SuHx V̇o2max testing until we performed the acute exercise challenge to allow for any potential confounding effects of V̇o2max testing to resolve (7, 11, 16). Animals then performed a single bout of acute, moderately intense exercise that consisted of treadmill running for 45 min. The workload for the exercise challenge was set relative to each rat's individual post-SuHx V̇o2max value and was determined using the Karvonen formula to calculate 75% V̇o2 reserve (V̇o2R) (7, 25). The intensity of 75% V̇o2R was chosen as it corresponds to the upper end of the exercise intensity range recommended by the American College of Sports Medicine for exercise prescription in cardiopulmonary patients (59). Each rat was permitted to warm up for 5 min at 6–10 m/min and 0% incline, then ran the duration of the 45 min at the speed corresponding to their 75% V̇o2R, with an incline of 10%.
To determine how acute exercise affects cardiopulmonary hemodynamics in intact, OVX, and OVX+E2 SuHx rats, we compared acute exercise responses in these animals to control rats not undergoing an acute exercise challenge. Control animals were placed in the running chamber for 45 min with the treadmill turned off. Other procedures, including hormone manipulation, SuHx induction, and V̇o2max testing, were identical to those performed in acutely exercised rats.
Echocardiography, hemodynamics, and organ harvest.
Immediately after the conclusion of the acute exercise bout, animals underwent echocardiography, followed by immediate hemodynamic assessments via transjugular approach for RV pressures and via carotid artery catheterization for systemic pressure, and subsequent death and organ harvest [all performed under light isoflurane anesthesia (2%), as described previously (7, 11, 16, 32)]. Echocardiography endpoints were obtained within 15 min after conclusion of exercise; hemodynamic endpoints [RV systolic and diastolic pressure (RVSP and RVDP)] were assessed within 40 min. As done previously (11), due to significant differences in weights between male and female animals, we indexed stroke volume (SV) and cardiac output (CO) and their derived parameters to body weight. Indexed total pulmonary resistance (TPRi) was calculated as RVSP/cardiac index (CI), with the latter being determined by echocardiography. RV compliance was determined as measured RV SV index (SVI, measured by echocardiography) divided by RV pulse pressure (calculated as RVSP − RVDP, measured by right heart catheterization). Tissue harvest occurred within 60 min after conclusion of exercise.
RV hypertrophy.
RV hypertrophy was assessed by measuring the Fulton index [weight of RV divided by weight of the left ventricle (LV) plus septum; RV/(LV+S)], as described previously (32). Immediately after determination of RV and LV+S weights, sections of the RV were snap-frozen for further biochemical analyses or immersed in 10% buffered formalin for immunohistochemistry studies.
Tibia length measurements.
The left tibia was excised from each rat, cleaned of soft tissue, and stored in saline soaked gauze at −20°C. Bone length (mm) from the medial condyle to medial malleous was measured in duplicate using digital calipers (65).
Pulmonary vascular remodeling.
Pulmonary vascular remodeling was assessed by immunofluorescence staining for α-smooth muscle actin (α-SMA; Sigma Aldrich, St. Louis, MO) and von Willebrand factor (vWF; Dako, Carpinteria, CA). Primary antibodies were used at a dilution of 1:400 (α-SMA) and 1:50 (vWF). Anti-rabbit-Rhodamine Red (Jackson Immuno Research) and anti-mouse-fluorescein (Vector Labs, Burlingame, CA) conjugated secondary antibodies were used at a dilution of 1:300 and 1:200, respectively. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI) (prolong antifade with DAPI; Life Technologies, Carlsbad, CA). Nuclei were stained with DAPI. Lungs were flushed, inflated, harvested, paraffin-embedded, and sectioned, as described previously (11, 32). Pulmonary arteries (PAs) were identified by vWF positivity and classified in a blinded fashion as nonmuscularized (α-SMA staining <25% of inner vessel circumference), partially muscularized (α-SMA staining 25–75% of inner vessel circumference), or fully muscularized (α-SMA staining >75% of inner vessel circumference), as described previously (32). Since PAs associated with terminal bronchioles are usually fully muscularized, only small PAs (<200 μm) associated with alveolar ducts were included in the analysis. The percentages of nonmuscularized and partially or fully muscularized vessels were calculated by dividing the number of vessels in each category by the total number of blood vessels counted per animal. At least 20 PAs per animal were assessed in each analysis. Images were obtained using a Nikon Eclipse 80i microscope with camera and NIS-Elements 4.0 software (Nikon Instruments, Melville, NY).
Assessment of RV fibrosis.
RV tissues were fixed in 10% buffered formalin and embedded in paraffin. Tissue sections of 4 μm were stained with picrosirius red to measure collagen abundance. Tissue sections were visualized using an inverted microscope (TE-2000; Nikon Instruments, Melville, NY) equipped with a filter to provide polarized illumination. Tissue images were captured at ×10 or ×20 using a Spot camera and analyzed using Metavue image analysis software (Optical Analysis, Nashua, NH). Interstitial or perivascular collagen deposition was characterized using collagen area fraction, defined as a ratio of the area positive for collagen to the total tissue area (36). Interstitial collagen type I and III were identified as different interference colors under polarized light, with collagen type I defined as areas of yellow, orange, or red color, and collagen type III defined as areas of green color (24, 50). Absolute and relative (ratio of collagen type I to type III) values from three to four regions of interest per slide were averaged to obtain a single measurement for each animal.
Western blotting.
Western blotting was performed using the following antibodies: mouse monoclonal anti-vinculin (clone cp74, CalBiochem/EMD Millipore, Billerica, MA), anti-eNOS (BD Biosciences, San Jose, CA), and anti-phospho-eNOSThr495 (BD Biosciences), as well as rabbit polyclonal anti-phospho-eNOSSer1177 (Cell Signaling, Danvers, MA), anti-Bax (Cell Signaling), anti-Bcl-2 (Cell Signaling), anti-LC3B (Sigma), and anti-p62 (Cell Signaling). Secondary antibodies were anti-mouse horseradish peroxidase (KPL, Gaithersburg, MD) and anti-rabbit-horseradish peroxidase (Cell Signaling). Tissue was homogenized in ice-cold RIPA buffer (ThermoFisher Scientific, Waltham, MA) containing proteinase inhibitor cocktail (Sigma) and PhosSTOP phosphatase inhibitor cocktail (Roche, Indianapolis, IN). Protein concentration was measured using the BCA protein assay (ThermoFisher Scientific). Western blots were performed as previously described (11, 32) in homogenates from the RV outflow tract. All primary antibodies were used at a dilution of 1:1,000 in 5% BSA in Tris-buffered saline-Tween 20 (25 mM Tris, 1 M NaCl, 1% Tween 20), except for anti-LC3B, which was used at a dilution of 1:3,000. Secondary antibodies were diluted 1:5,000 in 5% BSA in Tris-buffered saline-Tween 20.
Data and statistical analysis.
Results are expressed as means ± SE. Experimental groups were compared by one-way ANOVA, with post hoc Tukey's or Dunnett's multiple comparisons test (GraphPad Prism version 6.0b, La Jolla, CA). Where appropriate, two-way ANOVA was performed. Kruskal-Wallis ANOVA by ranks was performed on nonparametric data. Correlation analyses were performed via linear regression analyses. Differences at an α-level of 0.05 (P < 0.05) were considered statistically significant. Investigators performing analyses were blinded to treatment assignments.
RESULTS
E2 exerts beneficial effects on exercise capacity.
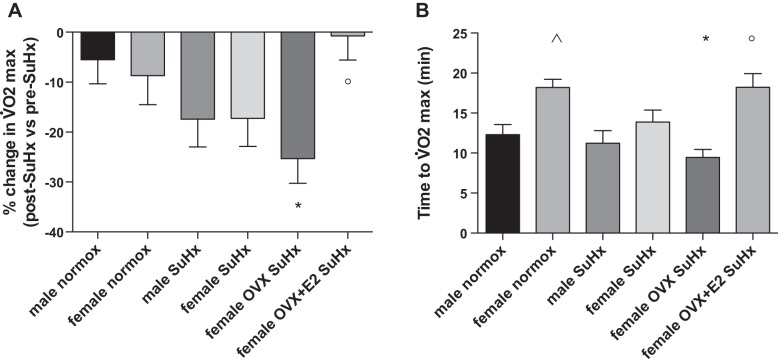
Since V̇o2max was required to establish a relative intensity for the acute exercise bout, we first evaluated E2's effects on this parameter. While no sex differences existed in SuHx-induced decreases in V̇o2max (measured as change in baseline vs. post-SuHx V̇o2max; Fig. 2A), OVX females exhibited the most pronounced decrease in this parameter. On the other hand, E2 repletion in OVX animals prevented the SuHx-induced decrease in V̇o2max. A similar pattern was observed when we evaluated time to V̇o2max (Fig. 2B). Taken together, these data suggest that E2 increases exercise capacity in SuHx-PH.
Fig. 2.
17β-Estradiol (E2) exerts beneficial effects on exercise capacity in SuHx rats. Effects are shown of sex, OVX, and E2 repletion on change in post-SuHx vs. pre-SuHx (baseline) V̇o2max (A) and time to V̇o2max in SuHx rats (B). Note pronounced decrease in V̇o2max and time to V̇o2max in OVX SuHx females, whereas E2-replete OVX SuHx females exhibited attenuation of these changes. SuHx exposure, OVX, and E2 repletion were as outlined in Fig. 1. Normox, untreated normoxia control; SuHx, untreated SuHx groups; female OVX SuHx, ovariectomized SuHx rats; female OVX+E2 SuHx, ovariectomized female SuHx rats with concomitant E2 repletion. Values are means ± SE; N = 8–12/group. P < 0.05 vs. *same sex normoxia control, °female OVX SuHx, and ^male normoxia control (one-way ANOVA with post hoc Tukey's test).
E2 exerts beneficial effects on postexercise RV function.
Immediately after conclusion of the 45-min acute exercise challenge, we evaluated RV function by echocardiography. We noted that intact female SuHx rats exhibited a significantly higher SVI and CI compared with male SuHx rats (Fig. 3, A and B). Hormone depletion (OVX) eliminated this difference, decreasing the values of females SuHx rats to the levels of males. E2 repletion, on the other hand, restored both parameters to values seen in intact female SuHx animals. Of note, this increase in SVI and CI in E2-treated rats occurred despite a marked decrease in RV hypertrophy (Fig. 3C). To account for possible confounding effects of differences in body weight between groups (Table 1), we also evaluated parameters that are not normalized for this parameter, such as SV and CO, as well as velocity time integral (VTI) and pulmonary artery acceleration time. While changes in SV and CO with and without E2 were not as pronounced as those in SVI and CI, we noted that male, but not female, rats exhibited decreased SV and CO after SuHx induction (Fig. 3, D and E). When evaluating VTI, we noted that SuHx females had higher VTI values than SuHx males, and that OVX eliminated this difference (Fig. 3F). Pulmonary artery acceleration time was not different between SuHx males and females, but was decreased by OVX and increased after E2 repletion (Fig. 3G). To further evaluate E2 effects on weight-independent parameters, we normalized SV and CO for tibia length (65) (tibia lengths shown in Table 1). We found patterns that, while not as robust as those for SVI or CI, followed changes in SVI or CI, with E2 replete OVX rats exhibiting an increase in SV/tibia length, and with male SuHx rats demonstrating a decrease in CO/tibia length (compared with normoxia controls) that was not seen in female SuHx rats (Fig. 3, H and I). Taken together, these data suggest that, even though subtle differences exist between the various endpoints measured, female rats tend to exhibit more stable parameters of RV function after acute exercise, that OVX tends to worsen RV function, and that E2 repletion after OVX improves RV performance.
Fig. 3.
E2 exerts beneficial effects on RV function and RV compliance in acutely exercised SuHx rats. Effects are shown of sex, OVX, and E2 repletion on stroke volume index (SVI; A), cardiac index (CI; B), RV hypertrophy [expressed as Fulton index; RV/(LV+S); C], stroke volume (SV; D), cardiac output (CO; E), velocity time integral (VTI; F), pulmonary artery acceleration time (PAAT; G), SV normalized for tibia length (H), CO normalized for tibia length (I), and RV compliance [expressed as SVI divided by pulse pressure (RVSP − RVDP); J]. Note overall pattern of improved RV function and structure in animals with endogenous and/or exogenous E2. K: representative images of Doppler signal in the RV outflow tract. Note notching of Doppler signaling in male SuHx and female OVX groups (arrows), indicating RV dysfunction. SuHx exposure, OVX, and E2 repletion were as outlined in Fig. 1. Values are means ± SE; N = 5–8/group. P < 0.05 vs. *same sex normoxia control, #female SuHx, °female OVX SuHx, and ^male SuHx (one-way ANOVA with post hoc Tukey's test).
Echocardiography was followed by invasive hemodynamic assessment. We measured RVSP and RVDP and calculated RV pulse pressure; this allowed us to determine RV compliance (Fig. 3J). As expected, RV compliance decreased in SuHx rats. However, female SuHx rats exhibited more preserved RV compliance than males. OVX decreased compliance to levels found in males, whereas E2 repletion restored compliance to values found in healthy animals. Lastly, male and OVX female SuHx rats were the only groups that consistently exhibited notching of the Doppler signaling in the RV outflow tract, a sign of RV dysfunction (1) (Fig. 3K). In conglomerate, our echocardiographic data indicate salutary effects of E2 on postexercise RV function.
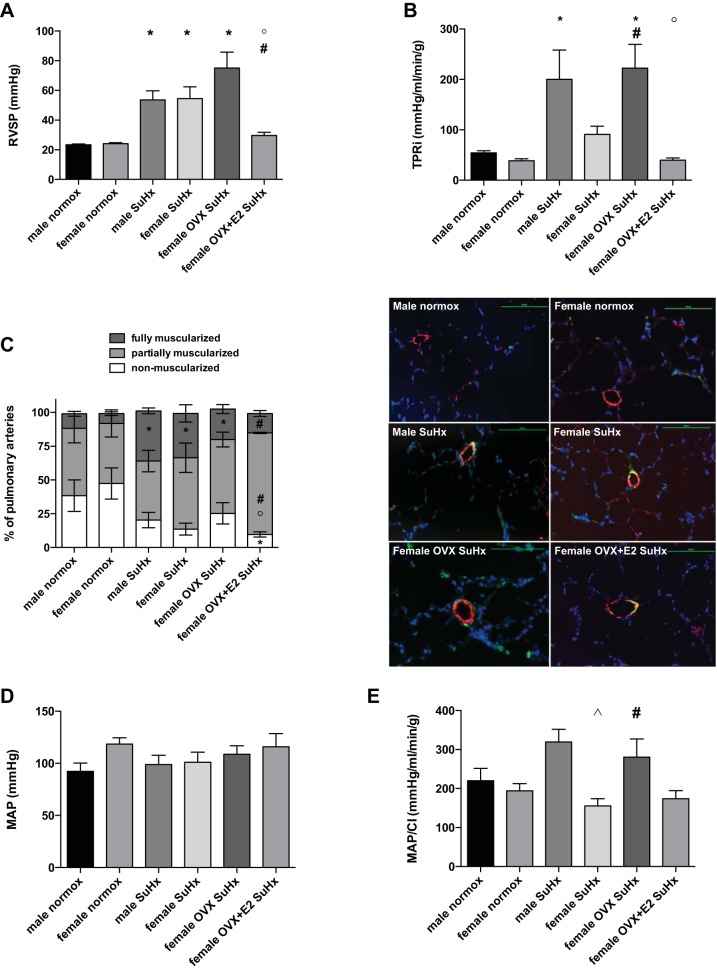
E2 exerts beneficial effects on postexercise RVSP and total pulmonary resistance.
We did not detect differences in postexercise RVSP between male and female SuHx rats (Fig. 4A). OVX, however, tended to further increase RVSP values. E2 repletion, on the other hand, resulted in a 60% decrease in RVSP, leading to values even lower than those observed in intact SuHx females. In a next step, we calculated postexercise TPRi as a surrogate for pulmonary vascular resistance (30) (Fig. 4B). We noted a significant tripling of postexercise TPRi in male SuHx rats. Postexercise TPRi in SuHx females, while twice as high as in healthy controls, was not statistically different from that group. OVX, however, resulted in a fourfold increase in postexercise TPRi compared with healthy controls and in doubling compared with intact SuHx females. E2 repletion dramatically decreased TPRi, resulting in values similar to those observed in healthy controls. Analysis of PA remodeling yielded complex results (Fig. 4C). We did not note any sex differences after SuHx induction, and OVX females did not differ statistically from intact females. E2 repletion after OVX decreased the percentage of nonmuscularized PAs, but also prevented the SuHx-induced increase in abundance of fully muscularized PA noted in the other groups (resulting in an overall increase in partially muscularized PAs compared with intact female or OVX).
Fig. 4.
E2 exerts beneficial effects on RVSP and total pulmonary resistance in acutely exercised SuHx rats, while having modest effects on pulmonary artery remodeling. Effects are shown of sex, OVX, and E2 repletion on RV systolic pressure (RVSP; A), total pulmonary resistance index (TPRi; calculated as RVSP/CI; B), pulmonary artery (PA) remodeling (expressed as percentage of non-, partially, or fully muscularized PAs; C), mean arterial pressure (MAP; D), and MAP/CI (used as a surrogate for systemic vascular resistance; E). Note worsening of RVSP and TPRi in female SuHx rats after OVX, with dramatic improvement after E2 repletion (A and B). C, left: analysis of PA muscularization (bar graph) reveals distinct distribution of non-, partially, or fully muscularized PAs after E2 repletion in OVX animals. C, right: representative images, depicting nonmuscularized PAs in male and female normoxia control groups and partially muscularized PAs in SuHx groups [von Willebrand factor staining in red, α-smooth muscle actin staining in green, nuclei staining in blue (DAPI)]. Size bars = 100 μm. D: MAP was not affected by SuHx, sex, or E2. SuHx exposure, OVX, and E2 repletion were as outlined in Fig. 1. Values are means ± SE; N = 5–8/group. P < 0.05 vs. *same sex normoxia control, #female SuHx, °female OVX SuHx, and ^male SuHx (one-way ANOVA with post hoc Tukey's test). Statistical symbols in C are included for each of the three muscularization categories.
To rule out confounding E2 effects on the systemic circulation, we measured postexercise mean arterial pressure (MAP; Fig. 4D). We did, however, not note any effects of sex or E2 on this parameter. We calculated the ratio of MAP and CI as a surrogate for systemic vascular resistance index. Since differences between groups were driven by CI rather than MAP, trends were similar to those noted for TPRi, but overall less pronounced (Fig. 4E). These data suggest beneficial effects of E2 on postexercise hemodynamics that are primarily targeted to the cardiopulmonary system.
E2 prevents worsening of cardiopulmonary hemodynamics after acute exercise.
Given E2's profound effects on cardiopulmonary hemodynamics, we next sought to determine whether acute exercise elicits differential RV responses in female SuHx rats with or without E2 (Fig. 5). We compared PH endpoints in acutely exercised rats to control groups of rats that did not undergo acute exercise. Interestingly, in animals with endogenous or exogenous E2, postexercise RVSP, SVI, and TPRi were similar to that in unexercised controls, whereas postexercise hemodynamics (in particular, RVSP and TPRi) were profoundly more altered in OVX animals. These data suggest that, unlike OVX SuHx females, intact SuHx females and E2-replete OVX SuHx females do not exhibit worsened hemodynamics after acute exercise.
Fig. 5.
E2 prevents worsening of cardiopulmonary hemodynamics after acute exercise. Effects are shown of OVX and E2 repletion on RV systolic pressure (RVSP; A), stroke volume index (SVI; B), and total pulmonary resistance index (TPRi; C) after acute exercise. Values are depicted as fold change vs. values in unexercised control rats. Values >1 represent increase compared with unexercised controls; values <1 represent decrease. Note that postexercise hemodynamics remained unchanged or improved in intact female or E2-replete OVX female SuHx rats, whereas postexercise RVSP and TPRi increased in OVX female SuHx rats. SuHx exposure, OVX, and E2 repletion were as outlined in Fig. 1. Values are means ± SE; N = 5–8/group. P < 0.05 vs. #female SuHx and °female OVX SuHx (one-way ANOVA with post hoc Tukey's or Dunnett's test). ex, Exercised.
E2 attenuates RV fibrosis.
Having established E2 as a mediator of superior RV adaptation to acute exercise, we sought to determine the underlying structural correlates of this effect. Given the prominent role of fibrosis in mediating RV contractile and diastolic dysfunction (51, 55), we evaluated E2's effects on RV collagen content (Fig. 6A). We noted a significant increase in RV interstitial collagen abundance in SuHx males, but not in SuHx females (note, however, that females exhibited a slightly higher baseline). OVX, on the other hand, resulted in a significant 40% increase in interstitial collagen compared with that in control females, whereas E2 repletion in OVX females attenuated collagen accumulation by 25%. Similar trends were noted for RV perivascular collagen accumulation (data not shown). We evaluated this further by specifying the ratio of type I to type III collagen, with a higher ratio indicating a stiffer RV (40, 45) (Fig. 6B). Interestingly, the collagen I-to-III ratio increased by 100% in SuHx males, whereas it remained largely unchanged in SuHx females. OVX, however, doubled this ratio (resulting in values similar to those in SuHx males), whereas E2 administration prevented the SuHx-induced increase in collagen I-to-III ratio. In summary, these results indicate that E2 attenuates SuHx-induced changes in RV collagen content and composition.
Fig. 6.
E2 attenuates SuHx-induced changes in RV interstitial collagen content and composition. Effects are shown of sex, OVX, and E2 repletion on RV interstitial collagen fraction (A) and RV collagen type I-to-type III ratio (B). Collagen was stained with picrosirius red; collagen fraction was calculated as ratio of the area positive for collagen (stained in pink; see arrows) to the total tissue area. Collagen types I and III were identified as different interference colors under polarized light, with collagen type I defined as areas of yellow, orange, or red color, and collagen type III defined as areas of green color. Note lack of increase in RV collagen content and lack of increase in collagen I-to-III ratio in intact and E2-replete OVX SuHx females. SuHx exposure, OVX, and E2 repletion were as outlined in Fig. 1. Values are means ± SE; N = 4–6/group. P < 0.05 vs. *same sex normoxia control and °female OVX SuHx (one-way ANOVA with post hoc Tukey's or Dunnett's test).
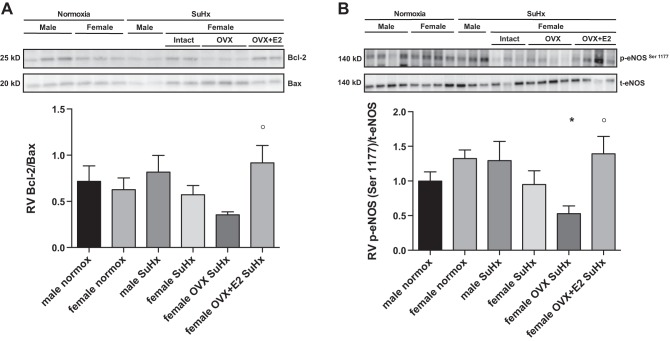
E2 alters proapoptotic signaling and enhances eNOS activation in the RV of acutely exercised rats.
Cardiomyocyte apoptosis has been linked to development of fibrosis in the RV (53). We, therefore, studied E2's effects on the apoptosis inhibitor Bcl-2 and the apoptosis mediator Bax, with a lower Bcl-2-to-Bax ratio (Bcl-2/Bax) indicating a shift toward proapoptotic signaling (8, 49) (Fig. 7A). While we found no sex differences in Bcl-2/Bax, OVX tended to decrease this ratio; however, the decrease was not statistically significant (P = 0.07 vs. female normoxia control). E2 administration, on the other hand, more than doubled the Bcl-2/Bax (vs. OVX), indicating less proapoptotic signaling after E2 repletion. Since eNOS is a critical regulator of cardiomyocyte and cardiac endothelial cell homeostasis and survival, as well as cardiomyocyte contraction and relaxation (29, 46, 47), we hypothesized that E2 would increase activation of this parameter [assessed as ratio of phospho-eNOSSer1177 to total eNOS (9); Fig. 7B]. While there were no sex differences in eNOS activation, OVX decreased phospho-eNOSSer1177/total eNOS by 50% (compared with female normoxia control). Conversely, E2 repletion more than doubled phospho-eNOSSer1177/total eNOS. Phosphorylation of eNOSThr495 was not affected by sex or E2 (data not shown). Taken together, our Bcl-2/Bax and eNOS data suggest that E2 favorably affects cardiomyocyte prosurvival signaling and homeostasis, suggesting a biochemical substrate for the favorable functional and structural changes observed in E2-treated animals.
Fig. 7.
E2 increases the Bcl-2-to-Bax ratio and enhances eNOS activation in the RV of acutely exercised rats. Effects are shown of sex, OVX, and E2 repletion on RV Bcl-2-to-Bax ratio (A) and RV eNOS activation [measured as ratio of phospho-eNOSSer1177 (p-eNOSSer1177) to total eNOS (t-eNOS); B]. Note trend for decrease in Bcl-2/Bax and significant decrease in eNOS activation in female SuHx rats after OVX, with dramatic improvement in both parameters after E2 repletion. SuHx exposure, OVX, and E2 repletion were as outlined in Fig. 1. Values are means ± SE; N = 5–8/group. P < 0.05 vs. *female normoxia control and °female OVX SuHx (one-way ANOVA with post hoc Tukey's test). P = 0.07 for female OVX SuHx vs. female normoxia control in A.
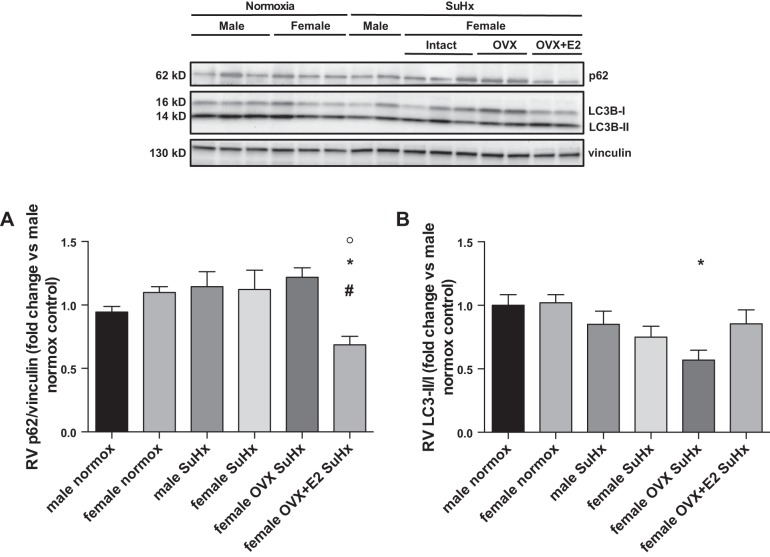
E2 enhances autophagic flux in the RV of acutely exercised rats.
Autophagy is a major signaling pathway involved in regulating cellular stress responses (35). Since impaired autophagy has been linked to cardiomyocyte death and contractile dysfunction in the LV (41, 66), and given the known role of autophagy in promoting degradation of collagen type I (27), we hypothesized that E2 would favorably affect autophagic flux in the RV. We evaluated the effects of sex and E2 on two major markers of autophagic flux, p62 and LC3B (Fig. 8, A and B). Accumulation of p62 and decreased conversion of LC3B-I to LC3B-II [indicated by a lower LC3B-II-to-LC3B-I ratio (LC3B-II/I)] suggest impaired autophagic flux (28). While neither sex nor OVX affected p62 in SuHx rats, E2 repletion in OVX animals resulted in a 40% decrease in p62 abundance (compared with OVX), consistent with improved autophagic flux. The LC3B-II/I tended to be decreased in SuHx animals, but values were not statistically different from normoxia controls. OVX, however, lead to a statistically significant 40% reduction in LC3B-II/I, whereas no such a decrease was noted after E2 repletion. Taken together, the constellation of E2's effects on p62 and LC3B is consistent with a stimulatory effect on autophagic flux (28).
Fig. 8.
E2 enhances autophagic flux in the RV of acutely exercised rats. Effects are shown of sex, OVX, and E2 repletion on RV p62 accumulation (A) and RV LC3II-to-LC3I ratio (B). Note trend for increased p62 accumulation, as well as significant decrease in LC3II-to-LC3I ratio in female SuHx rats after OVX, with reversal of OVX effects on both parameters after E2 repletion. SuHx exposure, OVX, and E2 repletion were as outlined in Fig. 1. Values are means ± SE; N = 5–6/group. P < 0.01 vs. *female normoxia control, #female SuHx, and °female OVX SuHx (one-way ANOVA with post hoc Tukey's test). P = 0.06 for female OVX+E2 SuHx vs. female OVX SuHx in B.
Expression of autophagy proteins correlates with functional alterations in acutely exercised female SuHx rats.
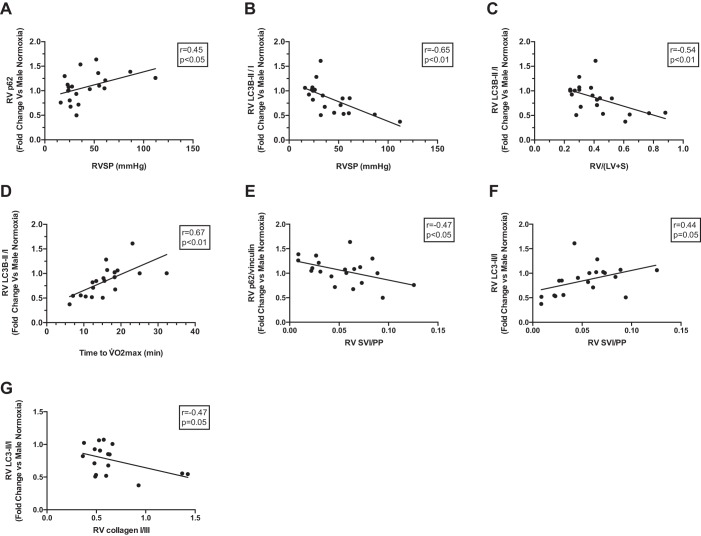
Given the links between impaired autophagy and cardiac dysfunction and fibrosis (27, 41), we correlated p62 expression and LC3B-II/I with RV function and fibrosis endpoints. We noted a positive correlation between p62 and RVSP (Fig. 9A), as well as negative correlations between LC3B-II/I and RVSP or RV/(LV+S) (Fig. 9, B and C). LC3B-II/I correlated positively with time to V̇o2max (Fig. 9D). These correlations suggest that preserved autophagic flux is associated with better RV function and less RV hypertrophy. Both p62 expression and the LC3B-II/I correlated with RV compliance (with indicators of more preserved autophagic flux being associated with higher RV compliance; Fig. 9, E and F). Lastly, increases in conversion of LC3B-I to LC3B-II correlated negatively with increases in collagen I-to-collagen III ratio (collagen I/III) (Fig. 9G), suggesting that intact autophagic flux is associated with a more preserved collagen I/III. In summary, these correlations suggest a potential link between impaired autophagic flux and worsening RV hypertrophy, fibrosis, and function.
Fig. 9.
Indicators of impaired autophagic flux are associated with worsening RV function and remodeling. Correlations are shown between RV expression of p62 or LC3B-II/I with RVSP (A and B), RV hypertrophy [measured as RV/(LV+S); C], exercise capacity (expressed as time to V̇o2max; D), RV compliance (measured as RV SVI/PP; E and F), and RV collagen type I-to-III ratio (G). Correlations were determined by linear regression analysis. Higher p62 and lower LC3B-II/I indicate impaired autophagy flux. Note that indicators of impaired autophagic flux correlated with indicators of impaired RV function or increased RV remodeling. p62 was normalized for RV vinculin abundance.
DISCUSSION
Our data in male and female SuHx rats undergoing a challenge of acute strenuous exercise reveal several novel findings. We demonstrate that E2 exerts beneficial effects on RV function and compliance (Fig. 3), as well as RVSP and TPRi (Fig. 4). Animals with endogenous or exogenous E2 exhibit more preserved hemodynamics after an acute exercise challenge than their E2-depleted counterparts (Fig. 5). Structural and biochemical correlates of these observations include beneficial effects of E2 on RV collagen abundance and collagen I/III (Fig. 6). Proapoptotic signaling, eNOS phosphorylation, and markers of autophagic flux, while not robustly affected by SuHx, were altered by E2 depletion and/or repletion (Figs. 7 and 8), suggesting regulation of these processes by E2. Lastly, we demonstrate that markers of impaired autophagic flux robustly correlate with endpoints of RV structure and function (Fig. 9), suggesting a potential link between impaired autophagic flux and worsening RV hypertrophy, fibrosis, and function. In addition, our studies expand upon our laboratory's prior findings (11) of stimulatory effects of E2 on exercise capacity in SuHx rats (Fig. 2). Taken together, these data indicate that endogenous or exogenous E2 mediates favorable effects on the cardiopulmonary axis that allow for greater exercise capacity and superior adaptation of RV function after an acute exercise challenge.
Our laboratory previously demonstrated that E2 mediates protective effects on RV function in male and female SuHx rats at rest (11). These effects included beneficial effects on SuHx-induced alterations in proapoptotic and proinflammatory signaling, oxidative stress, and mitochondrial dysfunction. We now expand these findings by demonstrating that E2 also mediates RV-protective effects after the clinically highly relevant stimulus of acute exercise. This is important for two reasons. First, while many PAH patients exhibit stable symptoms at rest, they develop significant cardiopulmonary insufficiency during or after strenuous exercise. In fact, exercise, especially if too intense, may promote RV injury and mortality in PAH animal models and PAH patients (18, 20, 56). Cardiopulmonary compromise may occur during or after exercise (18, 20, 56). A better understanding of the roles of sex and sex hormones in this context may reveal novel therapeutic strategies to derive benefit from exercise training while avoiding exercise-induced RV damage. Second, in light of recent recommendations to incorporate cardiopulmonary exercise training into the care of PAH patients (13), and in light of recent data demonstrating sex-specific responses to PAH treatments (12, 48), it is important to decipher how sex and sex hormones affect cardiopulmonary responses during and immediately after exercise, as this may allow for individualizing exercise prescriptions and for maximizing the effects of exercise training in both sexes. While we know from prior study that baseline differences exist between sexes and E2-depleted and -replete animals in RV adaptation to chronic pressure overload from PAH, the present study demonstrates that E2's favorable effects extend to a scenario of acute-on-chronic pressure overload. Specifically, comparisons between exercised and nonexercised rats demonstrate that E2-depleted females (who exhibit the most compromised RV function at rest) exhibit cardiopulmonary hemodynamics after exercise that are even more altered than in their nonexercised counterparts, while animals with endogenous or exogenous E2 maintained their hemodynamics at a range similar to (or even better than in) nonexercised animals. This indicates that E2-mediated differences, while present at baseline, are potentiated after acute exercise. Extrapolated to the clinical context, the results of our postexercise phenotyping study would indicate that female PAH patients with preserved E2 levels might tolerate acute exercise challenges better than those with lower levels (e.g., men or postmenopausal women).
Since echocardiography in exercising rodents is not technically feasible, we assessed RV function immediately after cessation of the exercise stimulus, thereby measuring the consequences of acute exercise on hemodynamics rather than the real-time impact of exercise. Even though some of the hemodynamic effects of acute exercise may have resolved at the time of assessment, we believe this is a clinically relevant time period, since PAH patients frequently develop RV pump failure after rather than during exercise (18, 20).
Strengths of our study include the comprehensive exercise testing with assessment of V̇o2max, the individualized exercise stimulus, the assessment of multiple clinically relevant endpoints, and the robust model of angio-proliferative PAH. The latter point is of particular importance, since most previous studies of sex and sex differences in PAH were performed in models that do not closely recapitulate the human phenotype (15, 57). Given that many of these studies revealed conflicting results, it is imperative to perform studies in animal models that are relevant to the human pathology (31, 34). This aspect, together with our assessment of clinically relevant endpoints, supports a recent initiative by the National Institutes of Health to enhance rigor and reproducibility (NIH notice NOT-OD-16-011).
While we previously demonstrated that proapoptotic signaling is attenuated by E2 in resting SuHx rats, we now extend these findings by identifying RV collagen abundance and collagen I/III, eNOS activation, and autophagic flux as novel targets of E2 in the RV. E2-mediated decreases in collagen abundance and increases in eNOS activation have previously been reported in the pulmonary vasculature; we now show that these endpoints also are regulated by E2 in the RV (21, 36, 37). To the best of our knowledge, this has not been reported before. Similarly, decreases in RV collagen I/III, which would be expected to lessen RV stiffness (40, 45), are a novel finding and may be the correlate of the increased RV compliance we noted with E2 treatment. Lastly, we identify autophagy as a novel target of E2 in the RV; this finding is of particular interest, since E2 effects on autophagy are not well described, and since currently available studies are limited to the oncology field (10).
Even though we demonstrate evidence of improved RV function, structure, and biochemical processes in animals with endogenous or exogenous E2, it remains unknown whether these effects are mediated directly by E2 exerting effects on the RV, or more indirectly by E2 exerting effects on the pulmonary vasculature. Since both compartments are targets of E2 in SuHx-PH (11, 36), and since E2 decreased TPRi both by decreasing RVSP and increasing CI, this question currently is difficult to answer. Clearly, an anti-remodeling effect of E2 in the PA would be expected to affect RV function by decreasing RV afterload. While our laboratory, as previously reported (11), did not identify sex or OVX as modifiers of PA remodeling in SuHx rats, we noted a small decrease in abundance of fully muscularized PAs in E2-treated OVX females. Given this observation, and given the decrease in RVSP with E2 in the present study, it is conceivable that pulmonary vascular effects indeed may play a role in the superior RV adaptation to acute exercise. However, compared with the magnitude of changes in RV parameters, E2's effects on PA remodeling were relatively modest. In addition, while decreasing the percentage of fully muscularized PAs, E2 also decreased the percentage of nonmuscularized PAs and increased abundance of partially muscularized PAs, suggesting that E2's effects on PA remodeling are complex and likely not the primary mode of action of how E2 improves RV function in SuHx-PH. Other potential protective pulmonary vascular effects of E2 could include increased proximal PA compliance (36, 37), PA vasodilation (33), or the opening of intrapulmonary shunts during exercise, a phenomenon previously identified as an important mediator of decreased PVR during exercise (30, 39). Future PV loop measurements will help dissect these effects.
On the other hand, in light of previous reports of direct effects of E2 on LV and RV function (23, 36, 54), one would expect direct RV-protective effects of E2 that are independent of its effects on the pulmonary vasculature. We, therefore, speculate that E2's protective effects on RV fibrosis, proapoptotic signaling, eNOS activity, and autophagic flux are mediated, at least in part, by direct effects on RV cardiomyocytes. Future studies using pressure volume loops, PA banding models, and isolated cardiomyocytes should be able to address this hypothesis. Lastly, E2 may also affect peripheral muscle cells, ventilatory parameters, and/or systemic metabolic processes that may modify acute exercise responses. Beneficial effects of lower body weight in E2-treated animals may play a role as well. This is currently under study in our laboratory.
One interesting observation is that OVX SuHx animals replete with E2 exhibited more favorable functional, structural, and biochemical adaptations than their intact female SuHx counterparts. We made similar observations in our prior studies (11). Since E2 levels in E2-replete OVX rats were similar to those in intact females, this observation cannot be explained by higher E2 levels after exogenous administration. One potential explanation is that continuous administration of exogenous E2 is more cardioprotective than endogenous, cyclically released E2. Continuous E2 administration may avoid an estrogen withdrawal phenomenon, which has been implicated in the worsening of other vascular diseases during menstruation (17, 38, 42). Furthermore, OVX may eliminate potentially detrimental sex hormones or estrogen metabolites that may counteract beneficial effects of endogenous E2 in intact females; E2 repletion after OVX may thus allow for unopposed E2 signaling. While we did not detect effects of OVX on RVSP or RV hypertrophy in prior experiments in normoxia controls (data not shown), studies of E2 repletion after OVX in absence of PH may shed further light on this issue.
The role of autophagy in the RV has only been minimally explored. Studies from LV injury models suggest that impaired autophagic flux is associated with apoptosis and LV functional impairment (41). In addition, autophagy is important for collagen degradation (27). Since both proapoptotic signaling and fibrosis were favorably affected by E2, and since E2 regulates autophagy (10), we studied E2's effect on the autophagy mediators p62 and LC3B. Our data suggest beneficial effects of E2 on autophagic flux. Since our correlation analyses linked impaired autophagic flux to worsening RV function and remodeling, these data suggest that improved autophagic flux may, at least in part, be a mechanism of how E2 mediates RV-protective effects after acute exercise. Along these lines, stimulatory effects on autophagy have recently been suggested as a mediator of RV-protective and anti-fibrotic effects of the prostacyclin ilopprost (14). Taken together, these data suggest that autophagy may be a potentially important modifier of RV function in PAH.
The results of the present study, viewed in context of other recent studies by others and us (11, 23, 36, 60), further contribute to a paradigm of RV-protective effects of E2. Such a paradigm would explain, at least in part, why female PAH patients exhibit better RV function and survival. It would, however, not explain why women are more prone to PAH development. One potential theory to reconcile these discrepancies would be that the same prosurvival and anti-apoptotic properties of E2 that are beneficial in the pressure-overloaded RV may contribute to increased pulmonary vascular wall cell proliferation and remodeling. While we did not note worsening PA remodeling with E2 in our laboratory's prior studies of SuHx or chronically hypoxic rats (rather, we noted improvement) (11, 32), E2 may worsen PA remodeling in the context of genetic alterations in BMPR2 or serotonin signaling (2, 43, 44, 63, 64). E2 may be “good for the RV” but, at least in certain contexts, “bad for the pulmonary vasculature” (31, 34).
Our study has limitations. While we focused on RV responses after exercise, real-time measurement of RV function in exercising rats would have provided additional insights into cardiopulmonary adaptations during exercise. We have established this expensive and time-consuming technique in our group (7) and hope to include it in future studies. PV loop measurement during acute exercise as a means of assessment of load-independent RV function parameter would be the gold standard for assessment of RV responses during exercise. Since this procedure is technically extremely challenging to perform in rodents, PV loops after sex hormone depletion and repletion should be assessed in future studies using dobutamine infusion to mimic cardiopulmonary effects of exercise (58). Second, while we demonstrate favorable effects of endogenous or exogenous E2 on proapoptotic signaling, eNOS phosphorylation, and autophagic flux, these parameters were not significantly affected by SuHx or sex. While this may suggest that these pathways do not play a role in RV failure, this is unlikely in light of previously published data that demonstrate pathophysiological relevance (6, 14, 29, 46, 47, 52). A more likely explanation is that the present studies were underpowered to detect more profound changes in these endpoints. Alternatively, potential differences at rest may have been masked by exercise-induced changes. Along those lines, it should be noted that our control and SuHx animals, due to the 9-wk experimental time frame, were about 5 mo old at the time of death. Increases in weight and age may have confounded some of the results in the control animals. We also consider this an explanation for the slight, albeit nonsignificant, decrease in V̇o2max that we noted in our controls, which may have arisen as a consequence of metabolic changes from aging, weight gain, and/or sedentary life style. SuHx rats (especially E2-repleted females), on the other hand, tend to exhibit a less pronounced weight increase; this may have favorably affected endpoints.
In conclusion, we demonstrate that E2 mediates favorable effects on cardiopulmonary adaptations in SuHx rats after an acute exercise challenge. E2's effects target both the pulmonary vasculature and RV, but appear to be more pronounced in the latter compartment. These results have implications for individualizing exercise prescriptions and for maximizing the effects of exercise training in PAH. Furthermore, they provide a potential explanation for sexually dimorphic survival rates in PAH, and they may facilitate the development of novel treatment strategies for PAH patients of either sex.
GRANTS
This work was supported by the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative (T. Lahm), Gilead Sciences Research Scholars Program in PAH (T. Lahm), VA Merit 1I01BX002042-01A2 (T. Lahm), National Institutes of Health (NIH) 5TL1TR001107-02 (A. L. Frump), NIH R01HL086939 (N. C. Chesler), and American Heart Association Midwest Affiliates Scientist Development Grant 13SDG17140066 (M. B. Brown).
DISCLOSURES
T. Lahm received funding for investigator-initiated research from Gilead and Pfizer (paid to institution), serves on the speaker bureau for Bayer, and received reagents from Eli Lilly.
AUTHOR CONTRIBUTIONS
T.L., A.L.F., and M.B.B. conception and design of research; T.L., A.L.F., T.G.C., T.J.J., B.Y., J.W., R.K.F., A.L., N.C.C., and M.B.B. analyzed data; T.L., A.L.F., T.J.J., A.L., N.C.C., and M.B.B. interpreted results of experiments; T.L. and A.L. prepared figures; T.L. drafted manuscript; T.L., A.L.F., A.L., N.C.C., and M.B.B. edited and revised manuscript; T.L., A.L.F., M.A., A.J.F., T.G.C., T.J.J., B.Y., J.W., R.K.F., A.L., N.C.C., and M.B.B. approved final version of manuscript; A.L.F., M.A., A.J.F., T.G.C., B.Y., J.W., R.K.F., and M.B.B. performed experiments.
ACKNOWLEDGMENT
The authors acknowledge the expert technical assistance of Alexandra Vayl and Dr. Robert G. Presson, Jr (Indiana University).
REFERENCES
- 1.Arkles JS, Opotowsky AR, Ojeda J, Rogers F, Liu T, Prassana V, Marzec L, Palevsky HI, Ferrari VA, Forfia PR. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am J Respir Crit Care Med 183: 268–276, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Austin ED, Cogan JD, West JD, Hedges LK, Hamid R, Dawson EP, Wheeler LA, Parl FF, Loyd JE, Phillips JA 3rd. Alterations in oestrogen metabolism: implications for higher penetrance of familial pulmonary arterial hypertension in females. Eur Respir J 34: 1093–1099, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 137: 376–387, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Bedford TG, Tipton CM, Wilson NC, Oppliger RA, Gisolfi CV. Maximum oxygen consumption of rats and its changes with various experimental procedures. J Appl Physiol 47: 1278–1283, 1979. [DOI] [PubMed] [Google Scholar]
- 5.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 122: 164–172, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation 120: 1951–1960, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Brown MB, Chingombe TJ, Zinn AB, Reddy JG, Novack RA, Cooney SA, Fisher AJ, Presson RG, Lahm T, Petrache I. Novel assessment of hemodynamic kinetics with acute exercise in a rat model of pulmonary arterial hypertension. Exp Physiol 100: 742–754, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Condorelli G, Morisco C, Stassi G, Notte A, Farina F, Sgaramella G, de Rienzo A, Roncarati R, Trimarco B, Lembo G. Increased cardiomyocyte apoptosis and changes in proapoptotic and antiapoptotic genes bax and bcl-2 during left ventricular adaptations to chronic pressure overload in the rat. Circulation 99: 3071–3078, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Fan D, Liu SY, van Hasselt CA, Vlantis AC, Ng EK, Zhang H, Dong Y, Ng SK, Chu R, Chan AB, Du J, Wei W, Liu X, Liu Z, Xing M, Chen GG. Estrogen receptor alpha induces prosurvival autophagy in papillary thyroid cancer via stimulating reactive oxygen species and extracellular signal regulated kinases. J Clin Endocrinol Metab 100: E561–E571, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Frump AL, Goss KN, Vayl A, Albrecht M, Fisher A, Tursunova R, Fierst J, Whitson J, Cucci AR, Brown MB, Lahm T. Estradiol improves right ventricular function in rats with severe angioproliferative pulmonary hypertension: effects of endogenous and exogenous sex hormones. Am J Physiol Lung Cell Mol Physiol 308: L873–L890, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabler NB, French B, Strom BL, Liu Z, Palevsky HI, Taichman DB, Kawut SM, Halpern SD. Race and sex differences in response to endothelin receptor antagonists for pulmonary arterial hypertension. Chest 141: 20–26, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galie N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, Klepetko W, McGoon MD, McLaughlin VV, Preston IR, Rubin LJ, Sandoval J, Seeger W, Keogh A. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol 62: D60–D72, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Arroyo J, Sakagami M, Syed AA, Farkas L, Van Tassell B, Kraskauskas D, Mizuno S, Abbate A, Bogaard HJ, Byron PR, Voelkel NF. Iloprost reverses established fibrosis in experimental right ventricular failure. Eur Respir J 45: 449–462, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Arroyo J, Saleem SJ, Mizuno S, Syed AA, Bogaard HJ, Abbate A, Taraseviciene-Stewart L, Sung Y, Kraskauskas D, Farkas D, Conrad DH, Nicolls MR, Voelkel NF. A brief overview of mouse models of pulmonary arterial hypertension: problems and prospects. Am J Physiol Lung Cell Mol Physiol 302: L977–L991, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goss KN, Cucci AR, Fisher AJ, Albrecht M, Frump A, Tursunova R, Gao Y, Brown MB, Petrache I, Tepper RS, Ahlfeld SK, Lahm T. Neonatal hyperoxic lung injury favorably alters adult right ventricular remodeling response to chronic hypoxia exposure. Am J Physiol Lung Cell Mol Physiol 308: L797–L806, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenstein D, Jeffcote N, Ilsley D, Kester RC. The menstrual cycle and Raynaud's phenomenon. Angiology 47: 427–436, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Grunig E, Lichtblau M, Ehlken N, Ghofrani HA, Reichenberger F, Staehler G, Halank M, Fischer C, Seyfarth HJ, Klose H, Meyer A, Sorichter S, Wilkens H, Rosenkranz S, Opitz C, Leuchte H, Karger G, Speich R, Nagel C. Safety and efficacy of exercise training in various forms of pulmonary hypertension. Eur Respir J 40: 84–92, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease. II. Pathophysiology, clinical importance, and management of right ventricular failure. Circulation 117: 1717–1731, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Handoko ML, de Man FS, Happe CM, Schalij I, Musters RJ, Westerhof N, Postmus PE, Paulus WJ, van der Laarse WJ, Vonk-Noordegraaf A. Opposite effects of training in rats with stable and progressive pulmonary hypertension. Circulation 120: 42–49, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Hisamoto K, Ohmichi M, Kurachi H, Hayakawa J, Kanda Y, Nishio Y, Adachi K, Tasaka K, Miyoshi E, Fujiwara N, Taniguchi N, Murata Y. Estrogen induces the Akt-dependent activation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem 276: 3459–3467, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Cottin V, Degano B, Jais X, Montani D, Souza R, Simonneau G. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 122: 156–163, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs W, van de Veerdonk MC, Trip P, de Man F, Heymans MW, Marcus JT, Kawut SM, Bogaard HJ, Boonstra A, Vonk Noordegraaf A. The right ventricle explains sex differences in survival in idiopathic pulmonary arterial hypertension. Chest 145: 1230–1236, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Junqueira LC, Cossermelli W, Brentani R. Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch Histol Jpn 41: 267–274, 1978. [DOI] [PubMed] [Google Scholar]
- 25.Karvonen J, Vuorimaa T. Heart rate and exercise intensity during sports activities. Practical application. Sports Med 5: 303–311, 1988. [DOI] [PubMed] [Google Scholar]
- 26.Kawut SM, Al-Naamani N, Agerstrand C, Rosenzweig EB, Rowan C, Barst RJ, Bergmann S, Horn EM. Determinants of right ventricular ejection fraction in pulmonary arterial hypertension. Chest 135: 752–759, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SI, Na HJ, Ding Y, Wang Z, Lee SJ, Choi ME. Autophagy promotes intracellular degradation of type I collagen induced by transforming growth factor (TGF)-beta1. J Biol Chem 287: 11677–11688, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12: 1–222, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kojda G, Kottenberg K. Regulation of basal myocardial function by NO. Cardiovasc Res 41: 514–523, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Kovacs G, Olschewski A, Berghold A, Olschewski H. Pulmonary vascular resistances during exercise in normal subjects: a systematic review. Eur Respir J 39: 319–328, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Lahm T. Sex differences in pulmonary hypertension: are we cleaning up the mess? Eur Respir J 47: 390–393, 2016. [DOI] [PubMed] [Google Scholar]
- 32.Lahm T, Albrecht M, Fisher AJ, Selej M, Patel NG, Brown JA, Justice MJ, Brown MB, Van Demark M, Trulock KM, Dieudonne D, Reddy JG, Presson RG, Petrache I. 17beta-Estradiol attenuates hypoxic pulmonary hypertension via estrogen receptor-mediated effects. Am J Respir Crit Care Med 185: 965–980, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lahm T, Crisostomo PR, Markel TA, Wang M, Wang Y, Tan J, Meldrum DR. Selective estrogen receptor-α and estrogen receptor-β agonists rapidly decrease pulmonary artery vasoconstriction by a nitric oxide-dependent mechanism. Am J Physiol Regul Integr Comp Physiol 295: R1486–R1493, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lahm T, Tuder RM, Petrache I. Progress in solving the sex hormone paradox in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 307: L7–L26, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 132: 27–42, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu A, Schreier D, Tian L, Eickhoff JC, Wang Z, Hacker TA, Chesler NC. Direct and indirect protection of right ventricular function by estrogen in an experimental model of pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 307: H273–H283, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu A, Tian L, Golob M, Eickhoff JC, Boston M, Chesler NC. 17beta-Estradiol attenuates conduit pulmonary artery mechanical property changes with pulmonary arterial hypertension. Hypertension 66: 1082–1088, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lloyd GW, Patel NR, McGing E, Cooper AF, Brennand-Roper D, Jackson G. Does angina vary with the menstrual cycle in women with premenopausal coronary artery disease? Heart 84: 189–192, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lovering AT, Stickland MK, Amann M, Murphy JC, O'Brien MJ, Hokanson JS, Eldridge MW. Hyperoxia prevents exercise-induced intrapulmonary arteriovenous shunt in healthy humans. J Physiol 586: 4559–4565, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Low RB, Stirewalt WS, Hultgren P, Low ES, Starcher B. Changes in collagen and elastin in rabbit right-ventricular pressure overload. Biochem J 263: 709–713, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Diwan A. Autophagy is impaired in cardiac ischemia-reperfusion injury. Autophagy 8: 1394–1396, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A. Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology 67: 2154–2158, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Mair KM, Wright AF, Duggan N, Rowlands DJ, Hussey MJ, Roberts S, Fullerton J, Nilsen M, Loughlin L, Thomas M, MacLean MR. Sex-dependent influence of endogenous estrogen in pulmonary hypertension. Am J Respir Crit Care Med 190: 456–467, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mair KM, Yang XD, Long L, White K, Wallace E, Ewart MA, Docherty CK, Morrell NW, MacLean MR. Sex affects bone morphogenetic protein type II receptor signaling in pulmonary artery smooth muscle cells. Am J Respir Crit Care Med 191: 693–703, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marijianowski MM, van der Loos CM, Mohrschladt MF, Becker AE. The neonatal heart has a relatively high content of total collagen and type I collagen, a condition that may explain the less compliant state. J Am Coll Cardiol 23: 1204–1208, 1994. [DOI] [PubMed] [Google Scholar]
- 46.Massion PB, Balligand JL. M odulation of cardiac contraction, relaxation and rate by the endothelial nitric oxide synthase (eNOS): lessons from genetically modified mice. J Physiol 546: 63–75, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: ten years after, and continuing. Circ Res 93: 388–398, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Mathai SC, Hassoun PM, Puhan MA, Zhou Y, Wise RA. Sex differences in response to tadalafil in pulmonary arterial hypertension. Chest 147: 188–197, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Misao J, Hayakawa Y, Ohno M, Kato S, Fujiwara T, Fujiwara H. Expression of bcl-2 protein, an inhibitor of apoptosis, and Bax, an accelerator of apoptosis, in ventricular myocytes of human hearts with myocardial infarction. Circulation 94: 1506–1512, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Montes GS, Junqueira LC. The use of the Picrosirius-polarization method for the study of the biopathology of collagen. Mem Inst Oswaldo Cruz 86, Suppl 3: 1–11, 1991. [DOI] [PubMed] [Google Scholar]
- 51.Rain S, Handoko ML, Trip P, Gan CT, Westerhof N, Stienen GJ, Paulus WJ, Ottenheijm CA, Marcus JT, Dorfmuller P, Guignabert C, Humbert M, Macdonald P, Dos Remedios C, Postmus PE, Saripalli C, Hidalgo CG, Granzier HL, Vonk-Noordegraaf A, van der Velden J, de Man FS. Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation 128: 2016–2025, 2013. [DOI] [PubMed] [Google Scholar]
- 52.Rawat DK, Alzoubi A, Gupte R, Chettimada S, Watanabe M, Kahn AG, Okada T, McMurtry IF, Gupte SA. Increased reactive oxygen species, metabolic maladaptation, and autophagy contribute to pulmonary arterial hypertension-induced ventricular hypertrophy and diastolic heart failure. Hypertension 64: 1266–1274, 2014. [DOI] [PubMed] [Google Scholar]
- 53.Ryan JJ, Archer SL. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res 115: 176–188, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sasaki H, Nagayama T, Blanton RM, Seo K, Zhang M, Zhu G, Lee DI, Bedja D, Hsu S, Tsukamoto O, Takashima S, Kitakaze M, Mendelsohn ME, Karas RH, Kass DA, Takimoto E. PDE5 inhibitor efficacy is estrogen dependent in female heart disease. J Clin Invest 124: 2464–2471, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shehata ML, Lossnitzer D, Skrok J, Boyce D, Lechtzin N, Mathai SC, Girgis RE, Osman N, Lima JA, Bluemke DA, Hassoun PM, Vogel-Claussen J. Myocardial delayed enhancement in pulmonary hypertension: pulmonary hemodynamics, right ventricular function, and remodeling. AJR Am J Roentgenol 196: 87–94, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spruijt OA, de Man FS, Groepenhoff H, Oosterveer F, Westerhof N, Vonk-Noordegraaf A, Bogaard HJ. The effects of exercise on right ventricular contractility and right ventricular-arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med 191: 1050–1057, 2015. [DOI] [PubMed] [Google Scholar]
- 57.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297: L1013–L1032, 2009. [DOI] [PubMed] [Google Scholar]
- 58.Tabima DM, Hacker TA, Chesler NC. Measuring right ventricular function in the normal and hypertensive mouse hearts using admittance-derived pressure-volume loops. Am J Physiol Heart Circ Physiol 299: H2069–H2075, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson WR. (Editor). ACSM's Guidelines for Exercise Testing and Prescription. Baltimore, MD: Lippincott Williams and Wilkins, 2010. [Google Scholar]
- 60.Umar S, Iorga A, Matori H, Nadadur RD, Li J, Maltese F, van der Laarse A, Eghbali M. Estrogen rescues preexisting severe pulmonary hypertension in rats. Am J Respir Crit Care Med 184: 715–723, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ventetuolo CE, Ouyang P, Bluemke DA, Tandri H, Barr RG, Bagiella E, Cappola AR, Bristow MR, Johnson C, Kronmal RA, Kizer JR, Lima JA, Kawut SM. Sex hormones are associated with right ventricular structure and function: the MESA-right ventricle study. Am J Respir Crit Care Med 183: 659–667, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ventetuolo CE, Praestgaard A, Palevsky HI, Klinger JR, Halpern SD, Kawut SM. Sex and hemodynamics in pulmonary arterial hypertension. Eur Respir J 43: 523–30, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wallace E, Morrell NW, Yang XD, Long L, Stevens H, Nilsen M, Loughlin L, Mair KM, Baker AH, MacLean MR. A sex-specific microRNA-96/5-hydroxytryptamine 1B axis influences development of pulmonary hypertension. Am J Respir Crit Care Med 191: 1432–1442, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.White K, Dempsie Y, Nilsen M, Wright AF, Loughlin L, MacLean MR. The serotonin transporter, gender, and 17beta oestradiol in the development of pulmonary arterial hypertension. Cardiovasc Res 90: 373–382, 2011. [DOI] [PubMed] [Google Scholar]
- 65.Yin FC, Spurgeon HA, Rakusan K, Weisfeldt ML, Lakatta EG. Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am J Physiol Heart Circ Physiol 243: H941–H947, 1982. [DOI] [PubMed] [Google Scholar]
- 66.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest 117: 1782–1793, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]