Supplemental Digital Content is available in the text.
Abstract
Background:
Recent research has identified mesenchymal stem cells (MSCs) within Dupuytren’s disease (DD) tissue and they have been proposed to give rise to the myofibroblasts, implicated in the progression of this condition. The aim of this study was to identify and characterize the primitive population that might be upstream of the MSC population, within DD.
Methods:
Formalin-fixed paraffin-embedded 4-µm-thick sections of DD cords and nodules obtained from 6 patients underwent 3,3-diaminobenzidine and immunofluorescent immunohistochemical staining for embryonic stem cell (ESC) markers OCT4, NANOG, SOX2, pSTAT3, and SALL4 and endothelial markers CD34 and ERG. NanoString gene expression analysis was performed to determine the transcriptional activation of these markers.
Results:
Immunohistochemical staining demonstrated the expression of ESC markers OCT4, NANOG, SOX2, pSTAT3, and SALL4 on the endothelium of the microvessels expressing CD34 and ERG, particularly those surrounding the DD nodules. NanoString analysis confirmed the transcriptional activation of OCT4, NANOG, STAT3, and SALL4, but not SOX2.
Conclusion:
This article demonstrates the novel finding of an ESC-like population expressing ESC markers OCT4, NANOG, SOX2, pSTAT3, and SALL4, localized to the endothelium of the microvessels within DD tissue, suggesting a potential therapeutic target for this condition.
Dupuytren’s disease (DD) is a chronic, irreversible, fibroproliferative condition1,2 involving the palmar fascia of the hand,1,2 with a male-to-female ratio of 5.9:1.3 The incidence rises with age, particularly in those of Northern European descent.1,2
An autosomal dominant trait has been observed in patients with DD.4,5 Although chromosomal abnormalities have been noted in DD,6 it may not be a simple monogenic disorder but a complex, multifactorial disease involving the dysfunction of multiple pathways.4,5 Overexertion of the hands, injury, diabetes mellitus,6 alcoholism,7 and liver diseases7 have been attributed to the onset of DD, although this remains speculative.4
Ledderhose disease (plantar fascia fibromatosis), Peyronie’s disease (penile fibromatosis), and Garrod’s nodules (affecting the dorsum of proximal interphalangeal joints) represent more generalized DD with extrapalmar manifestations.6
Fasciectomy—the most common treatment for DD, involving surgical removal of the cords and nodules to release the flexion contracture2,8—is associated with a 66% recurrence rate.1,8 Dermatofasciotomy, with additional excision of the overlying skin and surrounding tissues and full-thickness skin grafting,9,10 reduces the recurrence rate to 11.6%.1,2,8 This suggests that the overlying skin and tissues surrounding the diseased palmar fascia are crucial in the development of DD,9,10 potentially acting as the reservoir of progenitor cells which may give rise to myofibroblasts that characterize DD.1,2 This concept has been applied to fibrotic conditions affecting other organs such as the liver, in which hepatic stellate cells have been demonstrated to differentiate into myofibroblasts.2
Steroids have been used for the treatment of DD,11 by direct intralesional injections into the nodules to prevent progression of the disease, or onto the surgical sites postoperatively to prevent recurrence.11,12 A 66% success rate has been reported in patients without prior steroid injections or surgery for DD, receiving up to 3 cycles of injections.13 Complications associated with steroid injections for DD include infection, nerve injury, tendon laceration, and chronic pain.13
More recently, injection of collagenase Clostridium histolyticum (CCH)—an enzyme causing collagen lysis, leading to rupture of the DD cords—has been used as a nonoperative treatment.6,14 However, the long-term safety of CCH administration is unknown and repeated use may be associated with coagulation and neuromuscular disorders.5,15
While the origin of DD remains enigmatic,4 myofibroblasts—cells possessing features of both smooth muscle cells and fibroblasts1,4—have been attributed to its development.4,16 However, what remains unknown is the origin of these cells.1,2 DD manifests as a nodule through aberrant, uncontrolled proliferation of myofibroblasts2,16 which progresses to form thick collagenous cords along lines of tension, resulting in fixed flexion contracture of the affected digits with functional loss.1,2
Earlier studies suggest smooth muscle cells being the origin of myofibroblasts in DD,17 whereas more recent research demonstrates mesenchymal stem cells (MSCs) giving rise to the phenotypic myofibroblasts.18
We hypothesized the presence of an embryonic stem cell (ESC)–like population within DD, which may give rise to the MSC population that produces the aberrant myofibroblasts, leading to the development of DD. In this study, we analyzed the transcriptional and translational expression of the following ESC markers: NANOG, involved in the maintenance of pluripotency and self-renewal of ESCs19; SOX2, involved in ESC gene expression20; pSTAT3, critical for cellular regulation pathways21; SALL4, crucial for ESC pluripotency and regulation of OCT421,22; and OCT4, an essential factor for regulation of pluripotency and self-renewal,19,21 using immunohistochemical staining and NanoString gene expression analysis.
MATERIALS AND METHODS
Tissue Samples
DD tissue samples from 6 male patients aged 66–78 (mean, 69.3) years and a normal palmar fascia were sourced from the Gillies McIndoe Research Institute Tissue Bank for this study, which is approved by the Central Regional Health and Disability Ethics Committee (reference number, 13NTB155). Samples were divided by the operating surgeon (JRA) into cords and nodules, which were separately snap-frozen, formalin-fixed, and paraffin-embedded.
Histochemical and Immunohistochemical Staining
Hematoxylin and eosin (H&E) staining of 4-µm-thick formalin-fixed paraffin-embedded sections of the normal palmar fascia and DD tissues from 6 patients was used to confirm the presence of DD cords and nodules. 3,3-Diaminobenzidine (DAB) and immunofluorescent immunohistochemical staining were then performed using the Leica Bond Rx autostainer (Leica, Sydney, Australia) with the primary antibodies: CD34 (ready-to-use; cat# PA0212, Leica, Newcastle, United Kingdom), OCT4 (1:200; cat# NBPI-47923, Novus Biologicus, Littleton, Colo.), NANOG (1:1,000; cat# NBPI-04320, Novus Biologicus), SOX2 (1:500, cat# PA1-094, Thermo Fisher Scientific, Rockford, Calif.), pSTAT3 (1:100; cat# D3A7, Cell Signaling, Danvers, Mass.), and SALL4 (1:30; cat #6E3, Cell Marque, Rocklin, Calif.). All antibodies were diluted in Bond primary diluent (Leica).
A combination of Vectafluor Excel anti-rabbit 594 (ready-to-use, cat# VEDK-1594, Vector Laboratories, Burlingame, Calif.) and Alexa Fluor anti-mouse 488 (1:500, cat# A21202, Life Technologies) was used to detect combinations that included NANOG, SOX2, and pSTAT3; whereas that of Vectafluor Excel anti-mouse (ready-to-use, cat# VEDK2488, Vector Laboratories) and Alexa Fluor anti-rabbit 594 (1:500, cat# A21207, Life Technologies) to detect combinations that included OCT4 or SALL4.
DAB immunohistochemical–stained slides were mounted in Surgipath Micromount mounting medium (cat# 3801732, Leica) and immunofluorescent immunohistochemical–stained slides were mounted in Vectashield HardSet antifade mounting medium and counter-stained with 4′6-diamino-2-phenylindole (cat# H-1500, Vector Laboratories).
Positive human control samples were tonsil for pSTAT3,23 infantile hemangioma for SALL424 and NANOG,24 skin for SOX2,25 and myometrium for OCT4.26 Negative controls included sections of DD tissue by omitting the primary antibodies to determine the specificity of the amplification cascade.
Image Analysis.
DAB immunohistochemical–stained slides were viewed and imaged using the Olympus BX53 microscope fitted with an Olympus DP21 digital camera (Olympus, Tokyo, Japan). Immunofluorescent immunohistochemical–stained slides were viewed and imaged using the Olympus FV1200 biological confocal laser-scanning microscope and processed with cellSens Dimension 1.11 software using 2D deconvolution algorithm (Olympus).
Nanostring Gene Expression Analysis
RNA was extracted separately from snap-frozen DD cords and nodules from the original cohort of 6 patients used for DAB immunohistochemical staining, and was used for NanoString nCounter Gene Expression Assay (Nanostring Technologies, Seattle, Wash.). Total RNA was extracted using the MagJET RNA kit (Thermo Fisher Scientific) with the protocol adapted for tissue, and run on a KingFisher Duo machine (Thermo Fisher Scientific). RNA samples were then quantitated on a Qubit 2.0 fluorometer (Life Technologies) and were subjected to RNA integrity analysis using the 2100 Bioanalyzer Instrument (Agilent Technologies, Santa Clara, Calif.). Samples then underwent NanoString nCounter gene expression assay performed by New Zealand Genomics (Dunedin, New Zealand) according to the manufacturer’s protocol. Probes for the genes encoding NANOG (NM_024865.2), SALL4 (NM_020436.3), SOX2 (NM_003106.2), OCT4 (NM_002701.4), and STAT3 (NM_139276.2) and the housekeeping gene GUSB (NM_000181.1) were designed and manufactured by NanoString Technologies. Raw data was analyzed using nSolver software (NanoString Technologies) using standard settings and normalized against the housekeeping gene.
RESULTS
Histochemical and 3,3-Diaminobenzidine Immunohistochemical Staining
The cords (Fig. 1A) and nodules (Fig. 1B) including the microvessels within DD tissues were identified and confirmed on H&E-stained slides.
Fig. 1.
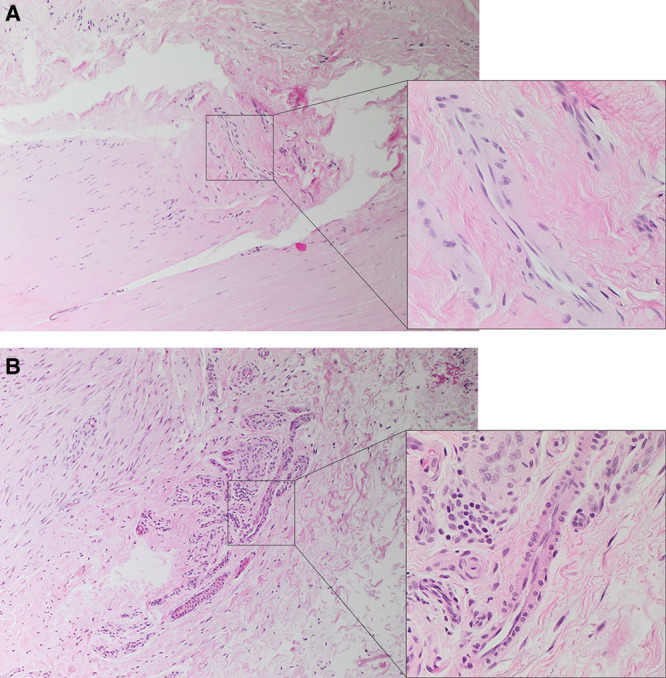
Representative H&E-stained slides of DD cords (A) and nodules (B) showing the microvessels within the nodules and cords. Original magnification: ×100 (inset showing the microvessels at ×400).
OCT4 was expressed, primarily localized to the endothelium of the microvessels within both DD cords and nodules (Figs. 2A, B, brown). Cytoplasmic staining of NANOG was also demonstrated on the endothelium of the microvessels within DD cords and nodules (Figs. 2C, D, brown). The presence of an ESC-like population within the endothelium of the microvessels associated with DD cords and nodules was further supported by nuclear and cytoplasmic staining of SOX2 (Figs. 2E, F, brown). SALL4 was present within the pericyte layer of the microvessels of the samples studied (Figs. 2G, H, brown). The ESC-like population on the endothelium of the microvessels within DD was confirmed by its intense nuclear staining for pSTAT3 (Figs. 2I, J, brown).
Fig. 2.
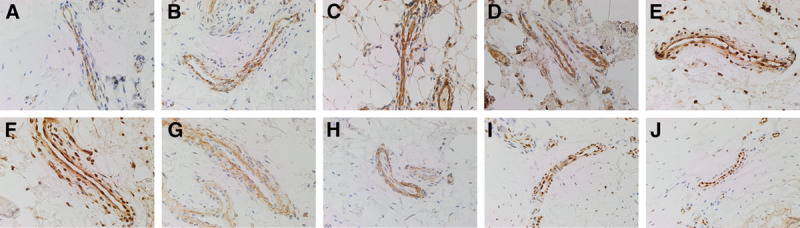
Representative DAB immunohistochemical–stained slides of DD cords (A, C, E, G, and I) and nodules (B, D, F, H, and J) showing the expression of OCT4 (A and B; brown), NANOG (C and D; brown), SOX2 (E and F; brown), SALL4 (G and H; brown), and pSTAT3 (I and J; brown) on the microvessels within DD tissues. The expression of OCT4 (E and F), NANOG (C and D), SOX2 (E and F), and pSTAT3 (I and J) was localized to the endothelium of the microvessels, whereas SALL4 (G and H) was primarily localized to the pericyte layer of the microvessels. Original magnification: ×400.
Similarly, DAB immunohistochemical staining for the normal palmar fascia revealed absence of staining for OCT4, NANOG, SOX2, SALL4, and pSTAT3 (See PDF, Supplemental Digital Content 1, which displays DAB immunohistochemical–stained images of normal palmer fascia for OCT4 (A, brown), NANOG (B, brown), SOX2 (C, brown), SALL4 (D, brown), pSTAT3 (E, brown), http://links.lww.com/PRSGO/A269).
Positive staining was demonstrated in myometrial blood vessels for OCT4 (A, brown); infantile hemangioma for NANOG (B, brown), SOX2 (C, brown), and SALL4 (D, brown); and tonsil for pSTAT3 (E, brown). Negative control DD tissue samples (F) demonstrated minimal staining (See PDF, Supplemental Digital Content 2, which displays DAB immunohistochemical–stained images of positive control human samples, http://links.lww.com/PRSGO/A270).
Immunofluorescent Immunohistochemical Staining
To confirm localization of the ESC markers to the endothelium, we performed immunofluorescent immunohistochemical costaining on two representative DD cord samples of these markers with the endothelial markers ERG27 (Figs. 3A–C, red) and CD3428 (Figs. 3A, D–F, green). This confirmed the expression of ERG (Fig. 3A, red) on the CD34+ endothelium (Fig. 3A, green). OCT4 (Fig. 3B, green) was expressed on the ERG+ endothelium of the microvessels (Figs. 3B, C, green), whereas SALL4 was expressed in the pericytes (Fig. 3C, green). SOX2 (Fig. 3D, red) was localized to the nuclei of the CD34+ endothelial cells (Fig. 3D, green). pSTAT3 (Fig. 3E, red) and NANOG (Fig. 3F, red) were localized to the CD34+ endothelium of the microvessels (Figs. 3E, F, green).
Fig. 3.
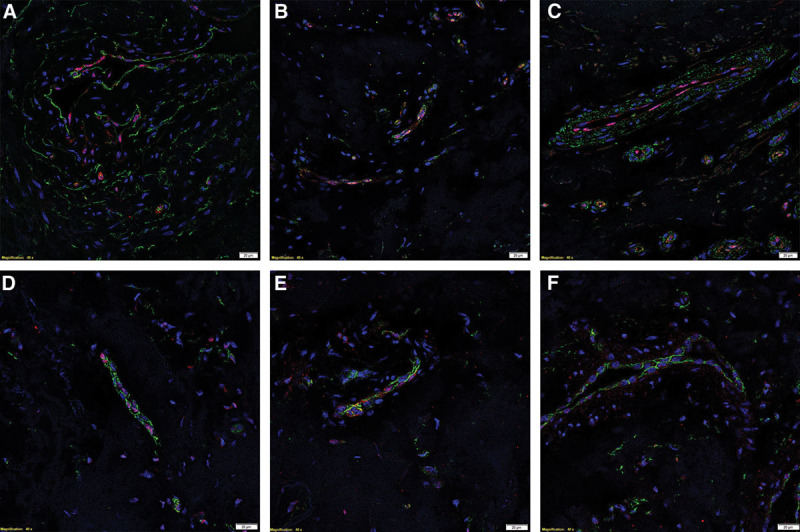
Representative immunofluorescent immunohistochemical–stained sections of DD cords, demonstrating coexpression of the endothelial markers ERG (A, red) on the CD34+ endothelium (A, green). OCT4 (B, green) and SALL4 (C, green) were localized to the ERG+ endothelium of the microvessels (B and C; red). SOX2 (D, red), pSTAT3 (E, red), and NANOG (F, red) were expressed on the CD34+ endothelium (D–F, green). The nuclei were counterstained with 4′6-diamino-2-phenylindole (A–F, blue). Scale bar: 20 µm.
Individual immunofluorescent immunohistochemical staining for each of the aforementioned proteins are presented in Supplemental Digital Content 3. (See PDF, Supplemental Digital Content 3, which displays split immunofluorescent immunohistochemical–stained images of DD cord presented in Figure 3 which shows ERG (A, red) and OCT4 (B, green) on the same tissue section; ERG (C, red) and SALL4 (D, green) on the same tissue section; and CD34 (E, G&I, green) on the sections probed for SOX2 (F, red), pSTAT3 (H, red) and NANOG (J, red) http://links.lww.com/PRSGO/A271.)
Nanostring Gene Expression Analysis
NanoString analysis confirmed the presence of the mRNA transcripts for SALL4, NANOG, OCT4, and STAT3 in the DD cords and nodules of all 6 patients (Fig. 4), whereas SOX2 was below the detectable levels in all the samples.
Fig. 4.
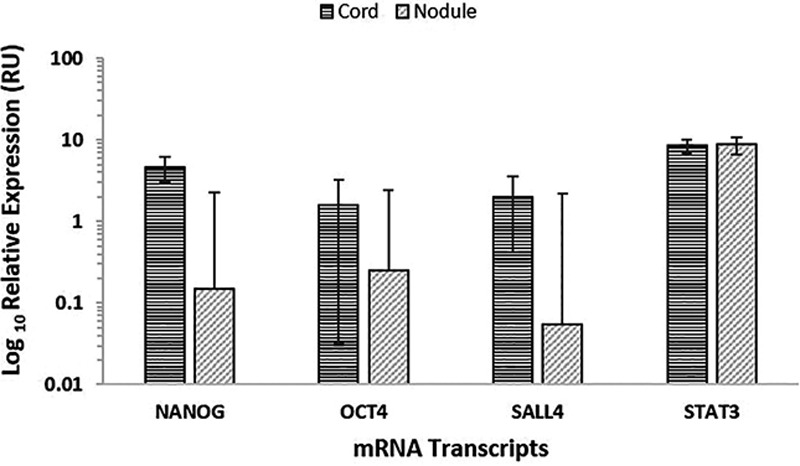
mRNA transcripts of NANOG, OCT4, SALL4, and STAT3 but not SOX2 were expressed in DD nodules and cords from 6 patients. Their expression was normalized over GUSB housekeeper and presented as relative units (RU).
DISCUSSION
Once labeled as the “Viking disease,” epidemiological studies on DD have implied a genetic basis to this condition with increased prevalence among individuals of Northern European descent, affecting nearly 30% of the Norwegian population aged over 60 years.3 However, the development of DD is not solely attributable to genetic predisposition and should be considered a complex disease with multiple intrinsic and extrinsic factors.
Studies of DD over the past 50 years have focused on the potential role of myofibroblasts. However, recent research has shifted the attention toward defining the progenitor cell population giving rise to myofibroblasts.1,2
Hindocha et al1 demonstrated increased expression of the common MSC markers CD13 and CD29 by cells within DD nodule, cord, perinodular fat, and skin, compared with control tissue from patients undergoing carpal tunnel surgery. Increased expression of these MSC markers, especially within DD nodules, has led to the postulation that MSCs provide a reservoir sustaining the myofibroblast population in DD.1 These findings implicate fat and skin tissues surrounding the diseased palmar fascia, in the formation of myofibroblasts.2
Our study identified and characterized an ESC-like population localized to the microvessels within the tissues surrounding the DD nodules and cords. These novel findings, along with previous studies, support the concept that the microvessels within DD contain a primitive population, and it is intriguing that the recently reported MSC population are also present in the similar area.1 It is therefore exciting to speculate that the ESC-like population reported here give rise to the downstream MSC population, potentially through an endothelial to mesenchymal transition (EndoMT), similarly reported in keloid scar (KS),29 although this requires further investigation.
The identification of an ESC-like population exhibiting increased multipotent and self-renewal capabilities within KS by us30 and others31 implies the presence of a similarly primitive population of stem cells within DD. Zhang et al31 proposed a “pathological niche” governing the deregulation of multiple pathways, especially the IL-17/IL-6 inflammatory axis, leading to the aberrant self-renewal and increased proliferation of cells within KS. The microenvironment of DD tissues may thus play a pivotal role in the deregulation of the homeostatic pathways maintaining the stem cell populations within DD.
The expression of ESC markers NANOG, pSTAT3, SALL4, and OCT4—both transcriptionally and translationally—defines an ESC-like population primarily localized to the endothelium of the microvessels in the cord and nodule of DD tissue. Interestingly, SOX2 was only detected by immunohistochemical staining but not NanoString analysis. This may be due to the transcriptional production of SOX2 being no longer active within the DD tissues used in our study, or the “hang-over” protein being present within the tissue. It is intriguing that SALL4 was predominantly expressed on the pericyte layer of the microvessels. We speculate that the SALL4+ cells are potentially derived from, and are therefore downstream of, the more primitive endothelium in a similar way that pericytes have been proposed to arise from the hemogenic endothelium,32 although this is beyond the scope of this study. Furthermore, the absence of these aforementioned ESC markers at the immunohistochemical level in normal palmar fascia is intriguing, and we infer a potential role for these phenotypic primitive cells in the ethiopathogenesis of DD.
It is also exciting to speculate that the endothelium of the microvessels within DD may undergo an EndoMT,33 by which the endothelial cells acquire a primitive stem cell phenotype and subsequent transition to a myofibroblastic phenotype,29,33 leading to the formation of nodules and cords in DD.1,2 This EndoMT process can be induced by a variety of growth factors, such as TGF-β, observed in many fibroproliferative disorders including KS.29,34 We hypothesize that EndoMT plays a fundamental role in DD and fibrotic conditions affecting other organs such as the heart, kidney, and liver.29 Alternatively, the microvessels within DD may act as a reservoir for ESCs that give rise to MSCs, the progenitor of myofibroblasts.35
To the best of our knowledge, this is the first report demonstrating the presence of an ESC-like population within the microvessels in DD nodules and cords, suggesting a potential cellular origin for the myofibroblasts in DD. The identification of this primitive population within DD underscores the need for further work to characterize these cells by identifying their regulatory pathway as potential novel therapeutic target for this condition.
ACKNOWLEDGMENT
We thank Ms. Liz Jones for her assistance in immunohistochemical staining.
Supplementary Material
Footnotes
Drs. Itinteang, Davis, and Tan are inventors of a patent application (number, 62/260,953): Treatment of Fibrotic Conditions. S.P. Koh and N. On were supported by summer scholarships from the Deane Endowment Trust. Neither of the other authors has any financial disclosures. The Article Processing Charge was paid for by the Gillies McIndoe Research Institute.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Hindocha S, Iqbal SA, Farhatullah S, et al. Characterization of stem cells in Dupuytren’s disease. Br J Surg. 2011;98:308–315. doi: 10.1002/bjs.7307. [DOI] [PubMed] [Google Scholar]
- 2.Iqbal SA, Manning C, Syed F, et al. Identification of mesenchymal stem cells in perinodular fat and skin in Dupuytren’s disease: a potential source of myofibroblasts with implications for pathogenesis and therapy. Stem Cells Dev. 2012;21:609–622. doi: 10.1089/scd.2011.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hindocha S, McGrouther DA, Bayat A. Epidemiological evaluation of Dupuytren’s disease incidence and prevalence rates in relation to etiology. Hand (N Y) 2009;4:256–269. doi: 10.1007/s11552-008-9160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musumeci M, Vadalà G, Russo F, et al. Dupuytren’s disease therapy: targeting the vicious cycle of myofibroblasts? Exp Opin Ther Targets. 2015;19:1677–1687. doi: 10.1517/14728222.2015.1068758. [DOI] [PubMed] [Google Scholar]
- 5.Shih B, Bayat A. Scientific understanding and clinical management of Dupuytren disease. Nat Rev Rheumatol. 2010;6:715–726. doi: 10.1038/nrrheum.2010.180. [DOI] [PubMed] [Google Scholar]
- 6.Bayat A, McGrouther DA. Management of Dupuytren’s disease–clear advice for an elusive condition. Ann R Coll Surg Engl. 2006;88:3–8. doi: 10.1308/003588406X83104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noble J, Arafa M, Royle S, et al. The association between alcohol, hepatic pathology and Dupuytren’s disease. J Hand Surg Br Eur. 1992;17:71–74. doi: 10.1016/0266-7681(92)90015-t. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong JR, Hurren JS, Logan AM. Dermofasciectomy in the management of Dupuytren’s disease. J Bone Joint Surg Br. 2000;82:90–94. doi: 10.1302/0301-620x.82b1.9808. [DOI] [PubMed] [Google Scholar]
- 9.Hueston JT. Recurrent Dupuytren’s contracture. Plast Reconstr Surg. 1963;31:66–69. doi: 10.1097/00006534-196301000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Hueston J. The control of recurrent Dupuytren’s contracture by skin replacement. Br J Plast Surg. 1969;22:152–6. doi: 10.1016/s0007-1226(69)80057-3. [DOI] [PubMed] [Google Scholar]
- 11.Sweet S, Blackmore S. Surgical and therapy update on the management of Dupuytren’s disease. J Hand Ther. 2014;27:77–83. doi: 10.1016/j.jht.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Meek R, McLellan S, Reilly J, et al. The effect of steroids on Dupuytren’s disease: role of programmed cell death. J Hand Surg Br Eur. 2002;27:270–273. doi: 10.1054/jhsb.2001.0742. [DOI] [PubMed] [Google Scholar]
- 13.Dala-Ali BM, Nakhdjevani A, Lloyd MA, et al. The efficacy of steroid injection in the treatment of trigger finger. Clin Ortho Surg. 2012;4:263–268. doi: 10.4055/cios.2012.4.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase Clostridium histolyticum for Dupuytren’s contracture. New Engl J Med. 2009;361:968–979. doi: 10.1056/NEJMoa0810866. [DOI] [PubMed] [Google Scholar]
- 15.Gilpin D, Coleman S, Hall S, et al. Injectable collagenase Clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg. 2010;35:2027–2038. doi: 10.1016/j.jhsa.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Viil J, Maasalu K, Maemets-Allas K, et al. Laminin-rich blood vessels display activated growth factor signaling and act as the proliferation centers in Dupuytren’s contracture. Arthritis Res Ther. 2015;17:144. doi: 10.1186/s13075-015-0661-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thurston AJ. Dupuytren’s disease. J Bone Joint Surg Br. 2003;85:469–477. doi: 10.1302/0301-620x.85b4.14215. [DOI] [PubMed] [Google Scholar]
- 18.Quante M, Tu SP, Tomita H, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin X, Zhang BH, Zheng SS, et al. Coexpression of gene Oct4 and Nanog initiates stem cell characteristics in hepatocellular carcinoma and promotes epithelial-mesenchymal transition through activation of Stat3/Snail signaling. J Hematol Oncol. 2015;8:23. doi: 10.1186/s13045-015-0119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weina K, Utikal J. SOX2 and cancer: current research and its implications in the clinic. Clin Transl Med. 2014;3:19. doi: 10.1186/2001-1326-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao W, Ji X, Zhang F, et al. Embryonic stem cell markers. Molecules. 2012;17:6196–6236. doi: 10.3390/molecules17066196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuri S, Fujimura S, Nimura K, et al. Sall4 is essential for stabilization, but not for pluripotency, of embryonic stem cells by repressing aberrant trophectoderm gene expression. Stem Cells. 2009;27:796–805. doi: 10.1002/stem.14. [DOI] [PubMed] [Google Scholar]
- 23.Skinnider BF, Elia AJ, Gascoyne RD, et al. Signal transducer and activator of transcription 6 is frequently activated in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood. 2002;99:618–626. doi: 10.1182/blood.v99.2.618. [DOI] [PubMed] [Google Scholar]
- 24.Itinteang T, Withers AH, Davis PF, et al. Biology of infantile hemangioma. Front Surg. 2014;1:38. doi: 10.3389/fsurg.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laga AC, Lai C-Y, Zhan Q, et al. Expression of the embryonic stem cell transcription factor SOX2 in human skin: relevance to melanocyte and merkel cell biology. Am J Pathol. 2010;176:903–913. doi: 10.2353/ajpath.2010.090495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ono M, Kajitani T, Uchida H, et al. OCT4 expression in human uterine myometrial stem/progenitor cells. Hum Reprod. 2010;25:2059–2067. doi: 10.1093/humrep/deq163. [DOI] [PubMed] [Google Scholar]
- 27.Miettinen M, Wang Z-F, Paetau A, et al. ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. Am J Surg Pathol. 2011;35:432–441. doi: 10.1097/PAS.0b013e318206b67b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fina L, Molgaard HV, Robertson D, et al. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75:2417–2426. [PubMed] [Google Scholar]
- 29.Lee WJ, Park JH, Shin JU, et al. Endothelial-to-mesenchymal transition induced by Wnt 3a in keloid pathogenesis. Wound Repair Regen. 2015;23:435–442. doi: 10.1111/wrr.12300. [DOI] [PubMed] [Google Scholar]
- 30.Grant C, Chudakova DA, Itinteang T, et al. Expression of embryonic stem cell markers in keloid associated lymphoid tissue. J Clin Pathol. 2016;69:643–646. doi: 10.1136/jclinpath-2015-203483. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Q, Yamaza T, Kelly AP, et al. Tumor-like stem cells derived from human keloid are governed by the inflammatory niche driven by IL-17/IL-6 axis. PLoS One. 2009;4:e7798. doi: 10.1371/journal.pone.0007798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall AP. Review of the pericyte during angiogenesis and its role in cancer and diabetic retinopathy. Toxicol Pathol. 2006;34:763–775. doi: 10.1080/01926230600936290. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez-Duffhues G, Orlova V, Ten Dijke P. In brief: endothelial-to-mesenchymal transition. J Pathol. 2016;238:378–380. doi: 10.1002/path.4653. [DOI] [PubMed] [Google Scholar]
- 34.Krieg T, Abraham D, Lafyatis R. Fibrosis in connective tissue disease: the role of the myofibroblast and fibroblast-epithelial cell interactions. Arthritis Res Ther. 2007;9(Suppl 2):S4. doi: 10.1186/ar2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witkowska-Zimny M, Walenko K. Stem cells from adipose tissue. Cell Mol Biol Lett. 2011;16:236–257. doi: 10.2478/s11658-011-0005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


