Abstract
Background:
The objective of the present investigation was to compare the effect of neoadjuvant irradiation on the microvascular anastomosis in cervical bundle using an experimental model in rats.
Methods:
One hundred forty male Sprague–Dawley rats were allocated into 4 groups: group I, control, arterial microanastomosis; group II, control, venous microanastomosis; group III, arterial microanastomosis with previous irradiation (20 Gy); and group IV, venous microanastomosis with previous irradiation (20 Gy). Clinical parameters, technical values of anastomosis, patency, and histopathological parameters were evaluated.
Results:
Irradiated groups (III and IV) and vein anastomosis groups (II and IV) showed significantly increased technical difficulties. Group IV showed significantly reduced patency rates (7/35) when compared with the control group (0/35). Radiotherapy significantly decreased the patency rates of the vein (7/35) when compared with the artery (1/35). Groups III and IV showed significantly reduced number of endothelial cells and also showed the presence of intimal thickening and adventitial fibrosis as compared with the control group.
Conclusion:
Neoadjuvant radiotherapy reduces the viability of the venous anastomosis in a preclinical rat model with a significant increase in the incidence of vein thrombosis.
The widespread success of microvascular free tissue transfer has enabled more aggressive tumor resection while regaining form and function necessary for acceptable quality of life. Malignant tumor treatments are based on a multidisciplinary approach that generally requires the combination of wide surgical margins, radiotherapy, and chemotherapy.1–4
Radiation therapy is used against many types of cancer. About 60% of cancer cases require radiation therapy.5 The usual radiation protocols are preoperative or postoperative external beam treatment, or brachytherapy.4,6–9 The advantages of neoadjuvant radiotherapy include smaller field sizes,7,10 lower doses,11,12 and radiation-induced tumor regression, which may reduce the extent of surgical resections and spare critical structures.4,6–8,13,14
Despite potential advantages of neoadjuvant radiotherapy, it is not universally practiced, especially because several studies have identified preoperative radiotherapy as an independent risk factor for complications after microvascular reconstruction,1,7,12 such as wound infection, flap loss, delayed wound healing, and prolonged hospitalization.15–25 Success rates of microsurgery procedures in nonirradiated tissues range from 90% to 99% depending on the procedure.19,26–29 Whether these results are the same in previously irradiated tissues remains controversial.1–3,17,19,28,29 Microvascular surgical techniques in a previously irradiated field are technically demanding, with increased incidence of postoperative complications. Currently it has been debated whether radiation has an effect on free flap survival.1–3 The existing clinical data on free flap reconstruction after radiotherapy focus primarily on wound-related complications and average flap survival without reporting the rate of thrombosis of the vascular microanastomosis.4,6,7,30,31 It is widely agreed that the majority of microsurgical failures are due to vascular phenomena such as arterial thrombosis, venous thrombosis, or both.17,27,32–37 Thrombosis is defined as the formation of a clot inside a blood vessel. It has been described that microvascular compromise occurs due to venous thrombosis leading to flap congestion and necrosis rather than to arterial thrombosis.38–40 In addition to technical difficulties in the execution of the microanastomosis because of the thin wall of the veins, the slow blood flow due to low blood pressure in the venous system contributes to thrombus formation.
To date, there is a paucity of reliable studies that have specifically addressed the effect of prior radiation therapy on the rate and specific type of microvascular alterations after vascular anastomosis. There is also lack of consensus in previous experimental studies.32,41–44 Moreover, these studies are not recent and are bereft of statistical evidence.42 Thus, the underlying vascular pathology remains unclear.6,7,14 This experimental study was performed to define the thrombotic effect of preoperative irradiation on vascular anastomoses.
MATERIALS AND METHODS
The ethics committees of Vall d'Hebron Research Institute and Hospital Universitari Vall d'Hebron (Universitat Autònoma de Barcelona) approved the experimental protocol. All animals received care in compliance with the principles of laboratory animal care formulated by the National Society for Medical Research and the Guide for Care and Use of Laboratory Animals prepared by the National Institutes of Health (publication number, 80-23, revised 1985) and the Spanish law of protection of experimental animals (Real Decreto 223, 1988).
One hundred forty adult male Sprague–Dawley rats, weighing on average 300 g (range, 250–400 g), were obtained from Janvier Labs (Roubaix, France). The animals were divided into 4 groups of 35 rats each: group I, control, anastomosis in the common carotid artery without previous irradiation; group II, control, anastomosis in the external jugular vein without previous irradiation; group III, anastomosis in the common carotid artery with previous irradiation; and group IV, anastomosis in the external jugular vein with previous irradiation (Fig. 1). All animals were followed up after surgery for 6 weeks, when the end point was established according to previous animal studies31,32,41,42,45,46 to be able to recognize acute and subacute thrombosis.
Fig. 1.
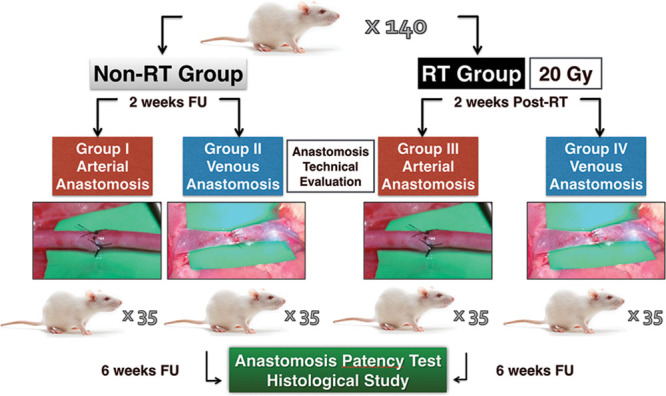
Flowchart of the experimental study. Time lapse between the radiotherapy (RT) and the day of the surgery and time tracking are detailed. FU, follow-up.
Irradiation Technique
Computed tomographic images were used as a basis for dose planning before the irradiation (Fig. 2). Radiotherapy was given 14 days before the operation in groups III and IV. The time lapse between the radiotherapy and the day of the surgery was established following previous animal models,31,32,41,42,45,46 which demonstrated that surgery carried out between the second and tenth days is still dangerous because of hypervascularity stage of the tissues, and therefore surgery should be done between 2 and 6 weeks after irradiation in the hypovascular stage of tissue. The rats in the irradiated groups were exposed in subgroups of 4 rats simultaneously to a single fraction of 20 Gy, delivered at a mean dose rate of 1.0 Gy per minute, at an 200-mm source-to-skin distance. A central lead plate allowed simultaneous irradiation of 4 fields. The radiation was applied to the left hemicervical region after anesthesia with ketamine hydrochloride (50 mg/kg; Ketalar, Parke Davis, Eczacibaşi, Istanbul, Turkey) and xylazine (5 mg/kg; Rompun, Bayer AG, Leverkusen, Germany). Irradiation procedures were performed using a Cobalt-60 megavoltage radiotherapy machine. Irradiation field size to the left hemicervical region was 2 × 2 cm (Fig. 3). Animals were allowed to breathe spontaneously during the irradiation. The rats were given 5 ml of Ringer’s lactate solution subcutaneously to prevent postirradiation dehydration. Buprenorphine was administered subcutaneously as an analgesic with a dose of 0.01 mg/kg twice daily after the procedure to all animal subjects.
Fig. 2.
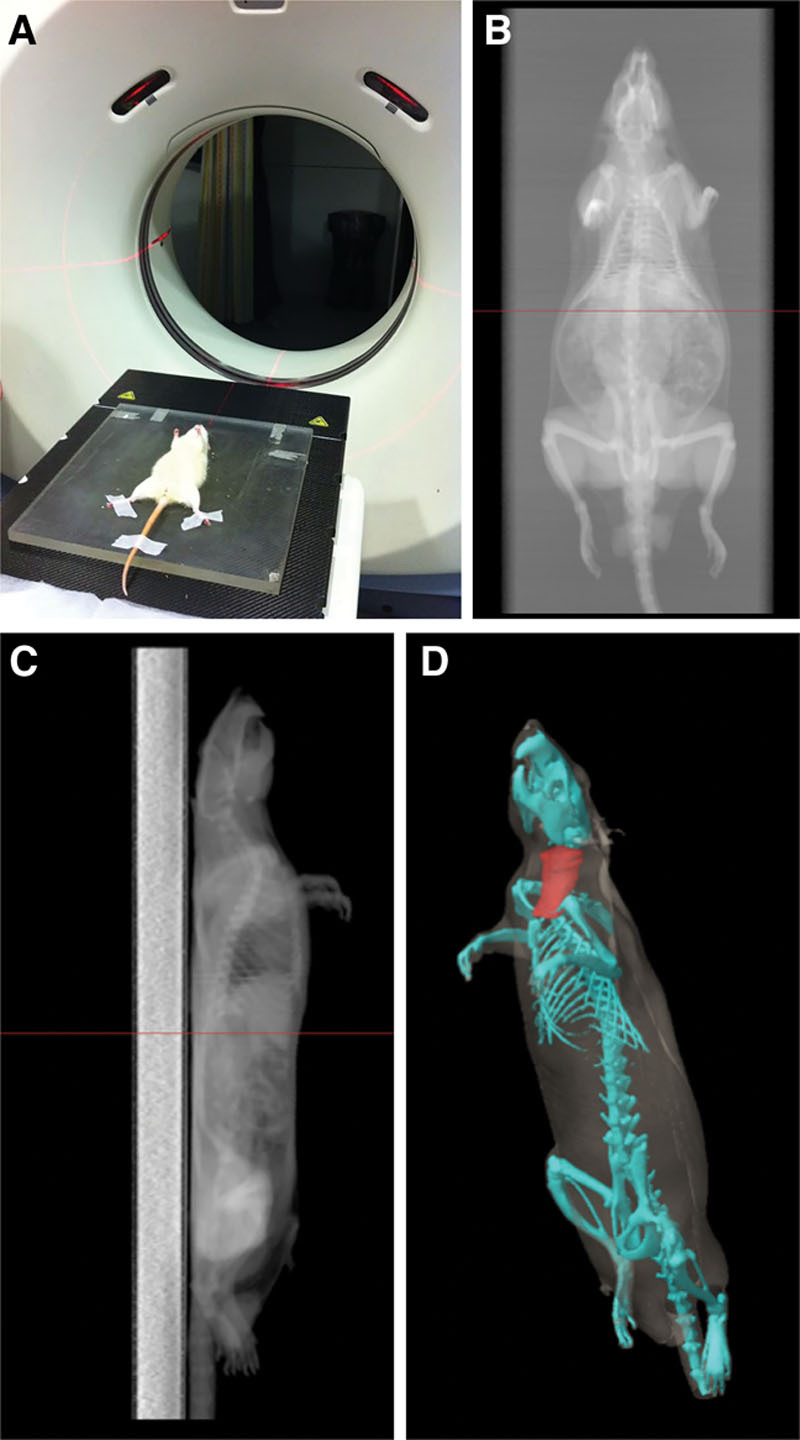
Tests carried out for planning the radiation field. A, Cervical topographical study by computed tomography (CT). B, C, CT images obtained showing depth, characteristics, and localization of the cervical neurovascular bundle; coronal (B) and sagittal (C) sections. D, Tridimensional reconstruction.
Fig. 3.
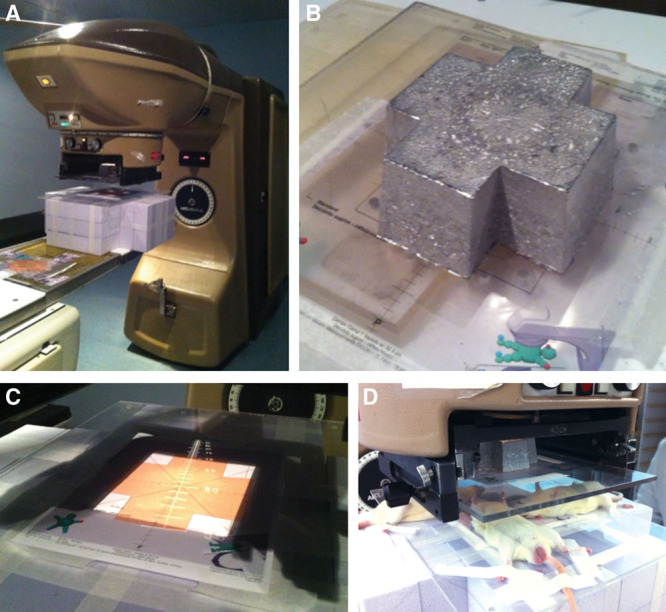
Infrastructure of the radiotherapy unit. A, Cobalt-60 treatment unit Theraton used in the experimental study; it magnifies the cross-shaped Cerrobend plate placed in the middle of the radiation beam. B, Four simultaneous 2 × 2 cm field irradiations, which allow us 4 rats at the same time. C, 4-cm2 frontal field over the left hemicervical region. D, Irradiation of 4 rats simultaneously.
Surgical Procedures
All the anastomoses and permeability tests were carried out by 1 experienced surgeon (S.B.-O.). Surgical procedure was established according to previous animal studies.38,41 After an overnight fast, all rats were anesthetized by an intraperitoneal injection of ketamine hydrochloride (50 mg/kg) and xylazine (5 mg/kg). A midline cervical approach was performed and the carotid artery or jugular vein was dissected and isolated with the aid of a microscope at 10× magnification. We measured the cervical vessel diameters at this point with a standardized background measuring grid before extensive dissection/stripping. The vessels were occluded with clamps (P-2, S&T, Neuhausen, Switzerland) and sectioned. Standardized end-to-end microvascular anastomosis was performed at 20× magnification with interrupted stitches of 9.0 nylon suture with a 50-μm needle (9.0 monofilament polypropylene, Prolene, Ethicon, Weymouth, United Kingdom). We measured the time needed to perform the microvascular anastomosis (minutes), the number of stitches for every anastomosis, and the number of attempts for a successful anastomosis. Subsequently, the clamps were removed to allow recovery of the blood flow, and at this moment we measured the time to achieve the hemostasis of the anastomotic site (seconds). Patency of the microvascular anastomosis was assessed by visual observation and a patency test at 1, 3, and 5 minutes after the procedure. The empty and refill patency test should be performed gently. Two pairs of smooth forceps are used to occlude the vessel distal to the anastomosis. The more “downstream” forceps is then moved gently approximately 1 cm down the vessel to create an empty segment between the 2 forceps. The proximal compression is then released, and rapid filling of the empty segment indicates patency of the anastomosis. This test is useful for either arteries or veins and for any size of vessel. The approach was closed with interrupted 5.0 nylon suture (monofilament polypropylene, Prolene, Ethicon, Weymouth, United Kingdom). Buprenorphine was administered subcutaneously as an analgesic with a dose of 0.01 mg/kg twice daily after the surgical procedure to all animal subjects.
All animals were surgically explored 6 weeks after the microsurgery under the same anesthesia protocol used in the first surgery. Patency of both common carotid arteries (left and right) and both external jugular veins (left and right) at the time of sacrifice was assessed by visual observation and patency test. This variable was used to define thrombosis macroscopically. Direct inspection of both arterial and venous anastomoses under the microscope may reveal signs of patency. Arterial patency is performed by nicely dilating vessels showing pulsatile elongation (“wriggling”) or expansile pulsation. Gently lifting the vessel distal to the anastomosis by placing forceps underneath will demonstrate the “flicker” of blood flowing across this area, but it is easily visible only in thin-walled vessels.
Specimens, including the anastomosed site, were taken for histopathological study. All biopsies were preserved in formalin solution, and then stained with hematoxylin–eosin (H&E) and Masson’s trichrome and evaluated with light microscopy by the same pathologist.
Histological Analysis
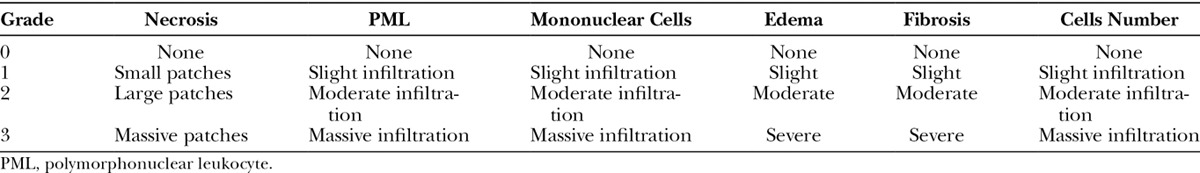
A conventional binocular Zeiss (Carl Zeiss Microscopy, Jena, Germany) light microscope was used with an ocular magnification of 10× and objectives 25/0.45 and 40/0.65 for examination of the H&E-stained sections. All observations were performed by 1 examiner (C.R.), who was unaware of the origin of the slides. A semiquantitative scale adapted from Verhofstad et al47 and containing various parameters for analysis of the wound healing sequence was used (Table 1). The amount of necrosis was expressed as none (0 point), only small patches (1 point), some patches (2 points), or massive (3 points). In the anastomotic area, accumulation of polymorphonuclear cells, macrophages, and lymphocytes was assessed in terms of none or normal number (0 point), slight increase (1 point), marked infiltration (2 points), or massive infiltration (3 points). Edema, expressed as the ratio of maximum thickness of the wall at the anastomosis to the thickness of the normal vascular wall as present at the end of the section, was established in terms of none (0 point), some (1–1.5× normal thickness; 1 point), marked (1.5–2× normal thickness; 2 points), or severe (>2× normal thickness; 3 points). Healing of the anastomotic site was expressed as normal, that is, endothelial layer with restored simple endothelial cells (0 point), small patches without endothelial cells (1 point), large patches without endothelial cells (2 points), or endothelial completely devoid of endothelial cell coverage (3 points). Intimal layer and muscular repair was assessed in terms of good (0 point), average (1 point), poor (2 points), or no (3 points) fibroblast stretching and bridging the anastomotic wound.
Table 1.
Parameters of Histological Study
Statistical Analysis
Statistical analysis was performed using SPSS Statistics, Windows version 18.0 (SPSS Inc., Chicago, Ill.). Data were expressed as mean ± SD if normally distributed or as median and range. For all tests, P < 0.05 was considered significant. Results were analyzed through Kruskal–Wallis variance analysis. Mann–Whitney test was used to evaluate the differences between the mean of both groups. Chi-square analysis was used with categorical variables and the t test was used with continuous variables to assess for univariate predictors of thrombosis after a microanastomosis.
RESULTS
Survival Rate
Groups I and II: All rats survived the 6-week study period 100%. Group III: 97% survival rate, with 1 rat lost during the acute period post-radiotherapy. Group IV: 97% survival rate, with 1 rat was lost during the subacute period post-radiotherapy (2–4 weeks). Statistically significant differences were not observed between groups (P > 0.05).
Weight
Results are depicted in Figure 4. There was a progressive increase in body weight in control groups (I and II) and the irradiated groups (III and IV) until the conclusion of the study. No significant differences were observed between groups (P > 0.05).
Fig. 4.
Data represent mean values of body weight on the day of the surgery and at 2, 4, and 6 weeks postoperative. Numbers represent P values of the significant differences observed between groups.
Vascular Patency and Macroscopic Appearance
Patency was 100% in the control groups (groups I and II), whereas it was 97.1% in group III (only 1 patency test was negative) and 80% in group IV (7 patency tests were negative; (Fig. 5). The patency rates of the arterial radiotherapy group (group III) were not statistically different from those of the control arteries (group I; χ2 test, P > 0.05). The patency rates of the venous radiotherapy group (group IV) were statistically significantly different from those of the control veins (group II; χ2 test, P < 0.05). The patency rates of control arteries (group I) were not statistically different from those of the control veins (group II; χ2 test, P > 0.05). The patency rates of radiotherapy arteries (group III) were statistically significantly different from radiotherapy veins (group IV; χ2 test, P < 0.05).
Fig. 5.
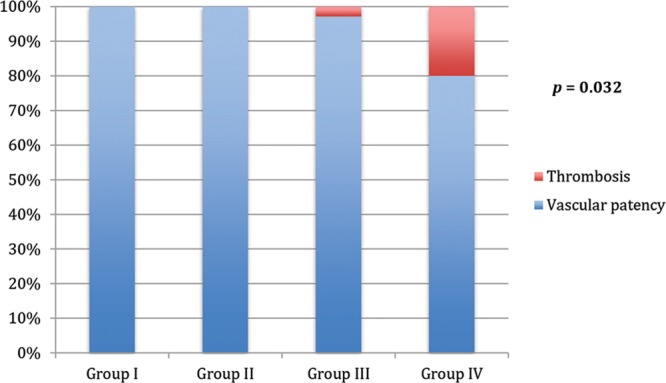
Vascular patency vs thrombosis. Data represent the percentage of thrombosis rates in the 4 groups. Numbers represent P values of the significant differences observed between groups.
Macroscopically, both irradiated and nonirradiated arteries and veins did not show surface or endovascular alterations visible through the surgical microscope.
Anastomosis Technical Evaluation
A correlation between the vascular patency and the technical difficulties in the execution of the anastomosis was performed. Results of the univariate analysis are summarized in Figure 6. This revealed 4 statistically significant variables that increased vascular thrombosis: time of anastomosis, number of stitches, number of attempts for a successful anastomosis, and time to achieve hemostasis. All the thrombosed vessels (8/140) needed more than 1 (2–3, range) anastomosis attempt to achieve an initial vascular patency success.
Fig. 6.
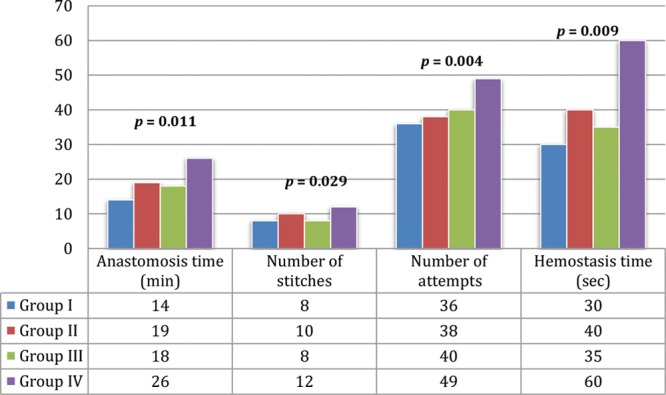
Anastomosis technical evaluation. Data represent the mean result of the different parameters evaluated in the 4 groups. Numbers represent P values of the significant differences observed between groups.
Histological Study
Statistically significant differences were not observed between groups I versus II (non-radiotherapy groups) and III versus IV (radiotherapy groups; P > 0.05).
In the radiotherapy groups, we found significant changes compared with the non-radiotherapy groups (Fig. 7); with moderate decrease in the number of endothelial cells, the intimal layer was thicker with moderate fibroblast proliferation (Fig. 8); the number of muscle cells in the media was slightly decreased and vacuolation was observed (Fig. 9).
Fig. 7.
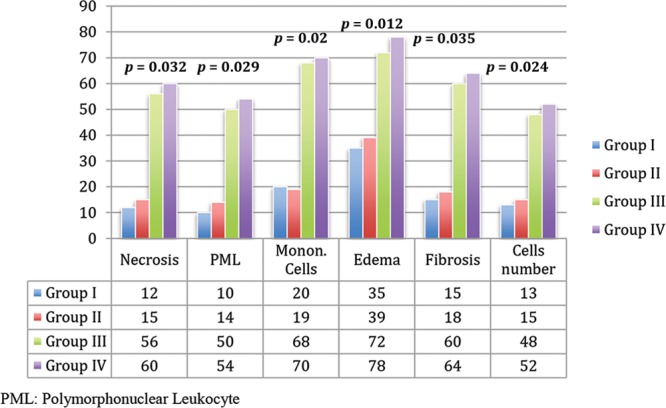
Histological evaluation. Data represent the mean result of the different parameters evaluated in the 4 groups. Numbers represent P values of the significant differences observed between groups.
Fig. 8.
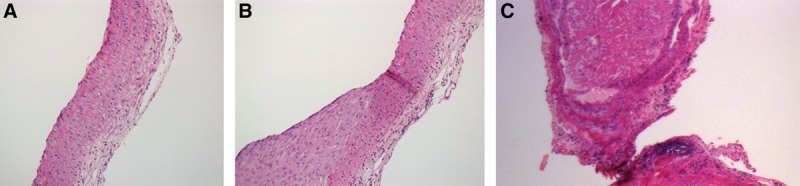
Arterial histological section. H&E staining. A, Longitudinal section, ×10; group I: any histological alterations in the vascular structure are observed. B, Longitudinal section, ×10; group III: tunica intima hyperplasia. C, Coronal section, ×10; group III: organized thromboembolism on the anastomosis site.
Fig. 9.
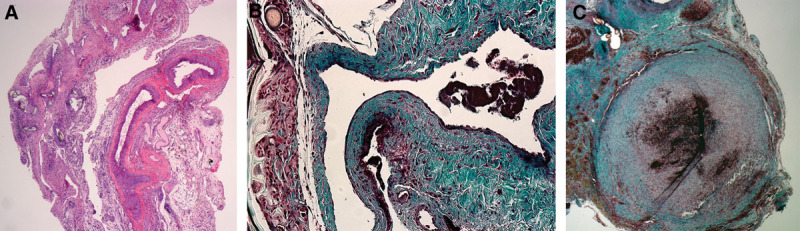
Venous histological section. A, H&E staining; longitudinal section, ×10; group II: any histological alterations in the vascular structure are observed. B, Masson’s trichrome staining; longitudinal section, ×10; group IV: endothelial discontinuity, muscular layer disorganization, and adventitial fibrosis. C, Coronal section, ×10; group IV: organized thrombus on the anastomosis site.
Some histological changes were observed in both groups (non-radiotherapy vs radiotherapy groups) without significant differences between groups (P > 0.05), as the external elastic lamina was altered with fibrosis, microcalcifications, and necrotic fields around suture holes. There was also edema in the adventitia, and there was a slight infiltration of polymorphonuclear leucocysts in the medial layers and adventitia. These changes were considered due to surgical procedure.
The histological study confirmed the presence of a thrombus on the anastomotic site in all the cases where thrombosis was observed macroscopically or through a negative patency test.
DISCUSSION
Preoperative radiotherapy provoked histological changes in both arterial and venous microanastomoses but only resulted in an increased rate of venous thrombosis in our rat model.
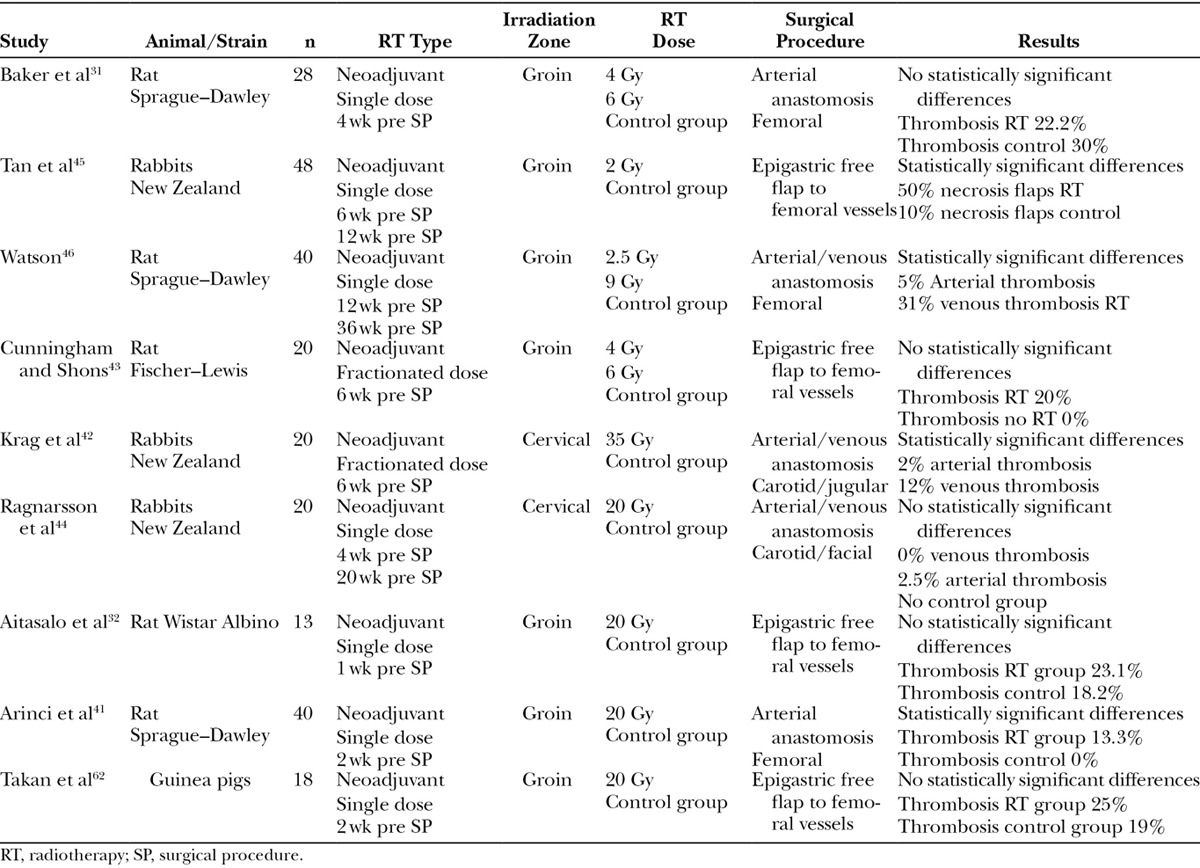
Regarding the experimental literature, some previous models have attempted to examine radiation-induced changes on microvascular anastomoses (Table 2).31,32,41–43,45,46 Results obtained regarding patency have shown high variability. Furthermore, this patency variability increases with its association with radiotherapy.7,12,15,19–25 Some of these experimental studies concluded that acute irradiation may predispose to free flap failures.31,42,45,46 On the contrary, other studies suggest that radiation may not alter the patency of arteries32,41,43,44 but have detrimental effects only on veins.44 These studies are inconclusive due to uncontrolled biases or lack of statistical power. Many series were too small with an insufficient number of observations to provide a minimal risk of type II error.31,32,41–46 In some cases, their experimental series were extremely small.6,34,42,44 To overcome this methodological mistake, we used the statistically recommended number of animals in each group providing an adequate statistical power. Previous experimental studies also show differences in the applied surgical technique,31,32,43,45 radiotherapy dose, and animal models42,44 that impede a direct and objective comparison.31,32,41–46 There is only 1 previous study in rats evaluating the patency rates of anastomosis in artery and vein46 but it had significant methodological failures such as the small series used, insufficient radiotherapy dosage (2.5–9 Gy),41 and the long interval between the neoadjuvant radiotherapy (3–6 months before the microanastomosis) and the moment of the surgery, as compared with the current clinical protocols.7,10–12,14,30
Table 2.
Previous Experimental Models of Microanastomosis on Irradiated Population
In our experimental study, apart from providing enough statistical power, we tried to decrease confounding factors to isolate the relations between anastomotic patency, which is believed to be the most important factor in microvascular surgery,31,42,45,46,48,49 and radiotherapy. Thus, we did not opt for an irradiation model over a flap’s pedicle but a model of 2 independent vessels in the same surgical field approached by a single incision. After a flap necrosis due to a vascular alteration, it is not possible to discriminate the origin of the thrombosis in either artery or vein.31,32,43,45 In addition, we decreased other causes of flap failure such as infection and wound dehiscence, more frequent in free flaps.
The dosage used in our experimental model is greater than those administrated in other previous models, which used doses from 2.5 to 15 Gy, even using fractioned doses.27,28,41,42,45 We have selected this radiotherapy method with a radiotherapy of 20 Gy unit dose to reproduce with maximum fidelity all the adverse clinical conditions and to favor the appearance of postoperative radiotherapy local side effects. Post-radiotherapy arterial and venous histological changes, such as a reduced number of endothelial cells, reduction in the number of smooth muscle nuclei in the media, intimal proliferation, edema, and fibrosis of the adventitial tunica, were similar to the changes observed in tissue samples from clinical studies.27,28,36,41 Significant histological changes when comparing endothelial cells, intimal layer, and muscular layer between radiotherapy and non-radiotherapy groups were considered to be due to radiotherapy. Changes in external elastic lamina, medial layer, and adventitia were considered to be due to surgical procedure.
Although macroscopically normal-looking through the microscope, the irradiated venous showed a high rate of thrombosis (20%) at the end-to-end anastomotic site, compared with irradiated arterial (3%) and nonirradiated recipient (0%) vessels. Furthermore, higher surgical difficulties as reflected in the higher number of attempts and thus an increased surgical time and number of stitches have been found in the irradiated group. Microsurgical anastomoses are largely technically dependent; however, there exists a finite rate of failure, with often devastating consequences, such as venous thrombosis, the most frequent complication.38–40,50–58 We could not confirm that there were not mechanical traumas associated with the repeated number of attempts applied to the irradiated vessels. Repetitive attempts of vascular anastomosis are traumatic and should be performed as gently and infrequently as possible.59 According to our thrombosis results on non-radiotherapy groups (0/70), we could obviate the technical error as the cause of vein thrombosis.
Both arteries and veins showed histological changes related to radiation. However, only veins showed a significant increased ratio of thrombosis. Tissular changes related to radiotherapy, a thin-walled vessel32,41 (ie, vein), the technical difficulties in the execution of the microanastomosis, and the slower blood flow in the venous system might be the main factors contributing to thrombus formation.60 All the thrombosed vessels needed more than 1 attempt to obtain a permeable anastomosis. Thus, trauma related to repetitive attempts of anastomosis on a pathologic and thin-walled vessel could explain the increased rate of vessel thrombosis. We would support those authors recommending to perform a dual vein anastomosis whenever possible2,15,16,49 and the use of pharmacologic agents to reduce the rate of thrombosis after free tissue transfer.61
The current study, performed in an animal model, presents inherent limitations in the clinical translation of the information obtained. We chose an anastomosis model instead of a free flap model, which is very different from a clinical scenario, but it allows us to reduce confounding factors that might influence the viability of the anastomosis. Similar to other preclinical studies, other limitations of the model are the radiotherapy-induced changes at both ends of the anastomosis, different from patients where the tumor bed vessels are irradiated but the free flaps vessels are not irradiated.31,32,43,45 We believe that this research provides valuable contribution to current knowledge as our results demonstrate that irradiated vein anastomoses have higher failure rates than irradiated arterial and nonirradiated recipient vessels. However, further studies are still needed to determine the role of each theoretical factor contributing to the development of vein thrombosis so it will allow surgeons to design the strategies to prevent it.
CONCLUSION
Preoperative radiotherapy influences viability of the vein anastomosis in a rat model with a significant increase in the incidence of vein thrombosis.
ACKNOWLEDGMENTS
We would like to thank Vall d'Hebron Research Institute, A. Rojo, and M. Rosal for their precious help in the care of the animals. We would also like to thank the Radiotherapy and Pathology departments of Hospital Universitari Vall d'Hebron for their practical suggestions and encouragement.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The authors have received a grant from Sociedad Española Cirugía Ortopédica y Traumatología (grant number: 0012-2012) and from Societat Catala Cirurgia Ortopèdica i Traumatologia (grant number: 0347-2012). The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Bengtson BP, Schusterman MA, Baldwin BJ, et al. Influence of prior radiotherapy on the development of postoperative complications and success of free tissue transfers in head and neck cancer reconstruction. Am J Surg. 1993;166:326–330. doi: 10.1016/s0002-9610(05)80325-3. [DOI] [PubMed] [Google Scholar]
- 2.Choi S, Schwartz DL, Farwell DG, et al. Radiation therapy does not impact local complication rates after free flap reconstruction for head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130:1308–1312. doi: 10.1001/archotol.130.11.1308. [DOI] [PubMed] [Google Scholar]
- 3.Nahabedian MY, Singh N, Deune EG, et al. Recipient vessel analysis for microvascular reconstruction of the head and neck. Ann Plast Surg. 2004;52:148–155. doi: 10.1097/01.sap.0000095409.32437.d4. discussion 156. [DOI] [PubMed] [Google Scholar]
- 4.O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 5.Wang CC, editor. Clinical Radiation Oncology: Indications, Techniques, and Results. 2nd ed. New York, NY: Wiley-Liss, Inc.; 2000. [Google Scholar]
- 6.O’Sullivan B, Gullane P, Irish J, et al. Preoperative radiotherapy for adult head and neck soft tissue sarcoma: assessment of wound complication rates and cancer outcome in a prospective series. World J Surg. 2003;27:875–883. doi: 10.1007/s00268-003-7115-4. [DOI] [PubMed] [Google Scholar]
- 7.Cheng EY, Dusenbery KE, Winters MR, et al. Soft tissue sarcomas: preoperative versus postoperative radiotherapy. J Surg Oncol. 1996;61:90–99. doi: 10.1002/(SICI)1096-9098(199602)61:2<90::AID-JSO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 8.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 9.Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859–868. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen OS, Cummings B, O’Sullivan B, et al. Preoperative and postoperative irradiation of soft tissue sarcomas: effect of radiation field size. Int J Radiat Oncol Biol Phys. 1991;21:1595–1599. doi: 10.1016/0360-3016(91)90337-4. [DOI] [PubMed] [Google Scholar]
- 11.Sadoski C, Suit HD, Rosenberg A, et al. Preoperative radiation, surgical margins, and local control of extremity sarcomas of soft tissues. J Surg Oncol. 1993;52:223–230. doi: 10.1002/jso.2930520405. [DOI] [PubMed] [Google Scholar]
- 12.Wilson AN, Davis A, Bell RS, et al. Local control of soft tissue sarcoma of the extremity: the experience of a multidisciplinary sarcoma group with definitive surgery and radiotherapy. Eur J Cancer. 1994;30A:746–751. doi: 10.1016/0959-8049(94)90286-0. [DOI] [PubMed] [Google Scholar]
- 13.Karasek K, Constine LS, Rosier R. Sarcoma therapy: functional outcome and relationship to treatment parameters. Int J Radiat Oncol Biol Phys. 1992;24:651–656. doi: 10.1016/0360-3016(92)90710-y. [DOI] [PubMed] [Google Scholar]
- 14.Wunder JS, Healey JH, Davis AM, et al. A comparison of staging systems for localized extremity soft tissue sarcoma. Cancer. 2000;88:2721–2730. doi: 10.1002/1097-0142(20000615)88:12<2721::aid-cncr10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 15.Carlson GW, Page AL, Peters K, et al. Effects of radiation therapy on pedicled transverse rectus abdominis myocutaneous flap breast reconstruction. Ann Plast Surg. 2008;60:568–572. doi: 10.1097/SAP.0b013e31815b6ced. [DOI] [PubMed] [Google Scholar]
- 16.Williams JK, Carlson GW, Bostwick J, 3rd, et al. The effects of radiation treatment after TRAM flap breast reconstruction. Plast Reconstr Surg. 1997;100:1153–1160. doi: 10.1097/00006534-199710000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Kroll SS, Robb GL, Reece GP, et al. Does prior irradiation increase the risk of total or partial free-flap loss? J Reconstr Microsurg. 1998;14:263–268. doi: 10.1055/s-2007-1000179. [DOI] [PubMed] [Google Scholar]
- 18.Kunisada T, Ngan SY, Powell G, et al. Wound complications following pre-operative radiotherapy for soft tissue sarcoma. Eur J Surg Oncol. 2002;28:75–79. doi: 10.1053/ejso.2001.1213. [DOI] [PubMed] [Google Scholar]
- 19.Fosnot J, Fischer JP, Smartt JM, Jr, et al. Does previous chest wall irradiation increase vascular complications in free autologous breast reconstruction? Plast Reconstr Surg. 2011;127:496–504. doi: 10.1097/PRS.0b013e3181fed560. [DOI] [PubMed] [Google Scholar]
- 20.Chmell MJ, Schwartz HS. Analysis of variables affecting wound healing after musculoskeletal sarcoma resections. J Surg Oncol. 1996;61:185–189. doi: 10.1002/(SICI)1096-9098(199603)61:3<185::AID-JSO4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Prosnitz LR, Maguire P, Anderson JM, et al. The treatment of high-grade soft tissue sarcomas with preoperative thermoradiotherapy. Int J Radiat Oncol Biol Phys. 1999;45:941–949. doi: 10.1016/s0360-3016(99)00272-2. [DOI] [PubMed] [Google Scholar]
- 22.Bujko K, Suit HD, Springfield DS, et al. Wound healing after preoperative radiation for sarcoma of soft tissues. Surg Gynecol Obstet. 1993;176:124–134. [PubMed] [Google Scholar]
- 23.Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg. 1994;93:980–987. doi: 10.1097/00006534-199404001-00012. [DOI] [PubMed] [Google Scholar]
- 24.Deutsch M, Kroll SS, Ainsle N, et al. Influence of radiation on late complications in patients with free fibular flaps for mandibular reconstruction. Ann Plast Surg. 1999;42:662–664. doi: 10.1097/00000637-199906000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Singh B, Cordeiro PG, Santamaria E, et al. Factors associated with complications in microvascular reconstruction of head and neck defects. Plast Reconstr Surg. 1999;103:403–411. doi: 10.1097/00006534-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Halle M, Ekström M, Farnebo F, et al. Endothelial activation with prothrombotic response in irradiated microvascular recipient veins. J Plast Reconstr Aesthet Surg. 2010;63:1910–1916. doi: 10.1016/j.bjps.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Virolainen P, Aitasalo K. Effect of postoperative irradiation on free skin flaps: an experimental study in rats. Scand J Plast Reconstr Surg Hand Surg. 2002;36:257–261. doi: 10.1080/028443102320791789. [DOI] [PubMed] [Google Scholar]
- 28.Sidorov VB, Minachenko VK, Rekhter MD, et al. The influence of radiotherapy and chemotherapy on regeneration at arterial microanastomoses: an experimental and clinical study. Ann Plast Surg. 1994;32:45–51. doi: 10.1097/00000637-199401000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Nasir S, Aydin MA, Karahan N, et al. New microvenous anastomosis model for microsurgical training: external jugular vein. J Reconstr Microsurg. 2006;22:625–630. doi: 10.1055/s-2006-956236. [DOI] [PubMed] [Google Scholar]
- 30.Cannon CP, Ballo MT, Zagars GK, et al. Complications of combined modality treatment of primary lower extremity soft-tissue sarcomas. Cancer. 2006;107:2455–2461. doi: 10.1002/cncr.22298. [DOI] [PubMed] [Google Scholar]
- 31.Baker SR, Krause CJ, Panje WR. Radiation effects on microvascular anastomosis. Arch Otolaryngol. 1978;104:103–107. doi: 10.1001/archotol.1978.00790020045010. [DOI] [PubMed] [Google Scholar]
- 32.Aitasalo K, Aro HT, Virolainen P, et al. Healing of microvascular free skin flaps in irradiated recipient tissue beds. Am J Surg. 1992;164:662–666. doi: 10.1016/s0002-9610(05)80730-5. [DOI] [PubMed] [Google Scholar]
- 33.Dormand E-L, Banwell PE, Goodacre TE. Radiotherapy and wound healing. Int Wound J. 2005;2:112–127. doi: 10.1111/j.1742-4801.2005.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muramatsu K, Ihara K, Miyoshi T, et al. Transfer of latissimus dorsi muscle for the functional reconstruction of quadriceps femoris muscle following oncological resection of sarcoma in the thigh. J Plast Reconstr Aesthet Surg. 2011;64:1068–1074. doi: 10.1016/j.bjps.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Innocenti M, Abed YY, Beltrami G, et al. Quadriceps muscle reconstruction with free functioning latissimus dorsi muscle flap after oncological resection. Microsurgery. 2009;29:189–198. doi: 10.1002/micr.20607. [DOI] [PubMed] [Google Scholar]
- 36.Schultze-Mosgau S, Keilholz L, Rödel F, et al. Experimental model for transplantation of a modified free myocutaneous gracilis flap to an irradiated neck region in rats. Int J Oral Maxillofac Surg. 2001;30:63–69. doi: 10.1054/ijom.2000.0015. [DOI] [PubMed] [Google Scholar]
- 37.Cooper JS, Fu K, Marks J, et al. Late effects of radiation therapy in the head and neck region. Int J Radiat Oncol Biol Phys. 1995;31:1141–1164. doi: 10.1016/0360-3016(94)00421-G. [DOI] [PubMed] [Google Scholar]
- 38.Acland R. Thrombus formation in microvascular surgery: an experimental study of the effects of surgical trauma. Surgery. 1973;73:766–771. [PubMed] [Google Scholar]
- 39.Hupkens P, Cooley BC. Comparison of arterial and venous patency in a rat model of subendothelium-stimulated thrombosis. Microsurgery. 1996;17:226–229. doi: 10.1002/(SICI)1098-2752(1996)17:4<226::AID-MICR10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 40.Kroll SS, Schusterman MA, Reece GP, et al. Timing of pedicle thrombosis and flap loss after free-tissue transfer. Plast Reconstr Surg. 1996;98:1230–1233. doi: 10.1097/00006534-199612000-00017. [DOI] [PubMed] [Google Scholar]
- 41.Arinci A, Topalan M, Aydin I, et al. Effects of early pre- and postoperative irradiation on the healing of microvascular anastomoses. J Reconstr Microsurg. 2000;16:573–576. doi: 10.1055/s-2000-8398. [DOI] [PubMed] [Google Scholar]
- 42.Krag C, De Rose G, Lyczakowski T, et al. Free flaps and irradiated recipient vessels: an experimental study in rabbits. Br J Plast Surg. 1982;35:328–336. doi: 10.1016/s0007-1226(82)90122-9. [DOI] [PubMed] [Google Scholar]
- 43.Cunningham BL, Shons AR. Free flap transfers in rats using an irradiated recipient site. Br J Plast Surg. 1979;32:137–140. doi: 10.1016/0007-1226(79)90016-x. [DOI] [PubMed] [Google Scholar]
- 44.Ragnarsson R, Berggren A, Klintenberg C, et al. Microvascular anastomoses in irradiated vessels: a comparison between the Unilink system and sutures. Plast Reconstr Surg. 1990;85:412–418. [PubMed] [Google Scholar]
- 45.Tan E, O’Brien BM, Brennen M. Free flap transfer in rabbits using irradiated recipient vessels. Br J Plast Surg. 1978;31:121–123. doi: 10.1016/s0007-1226(78)90059-0. [DOI] [PubMed] [Google Scholar]
- 46.Watson JS. Experimental microvascular anastomoses in radiated vessels: a study of the patency rate and the histopathology of healing. Plast Reconstr Surg. 1979;63:525–533. doi: 10.1097/00006534-197904000-00014. [DOI] [PubMed] [Google Scholar]
- 47.Verhofstad MH, Lange WP, van der Laak JA, et al. Microscopic analysis of anastomotic healing in the intestine of normal and diabetic rats. Dis Colon Rectum. 2001;44:423–431. doi: 10.1007/BF02234744. [DOI] [PubMed] [Google Scholar]
- 48.Pignatti M, Benati D, Cavadas PC. Effect of the two-wall-stitch mistake upon patency of rat femoral vein anastomosis: preliminary observations. Microsurgery. 2004;24:339–344. doi: 10.1002/micr.20026. [DOI] [PubMed] [Google Scholar]
- 49.Halle M, Bodin I, Tornvall P, et al. Timing of radiotherapy in head and neck free flap reconstruction—a study of postoperative complications. J Plast Reconstr Aesthet Surg. 2009;62:889–895. doi: 10.1016/j.bjps.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Ezzat WH, Dahl JP, Luginbuhl A, et al. Recombinant human tissue factor pathway inhibitor prevents thrombosis in a venous tuck model. Laryngoscope. 2010;120:2172–2176. doi: 10.1002/lary.20898. [DOI] [PubMed] [Google Scholar]
- 51.Simşek T, Eroglu L, Engin MS, et al. End-to-end microvascular anastomosis in the rat carotid artery using continuous horizontal mattress sutures. J Reconstr Microsurg. 2006;22:631–640. doi: 10.1055/s-2006-956237. [DOI] [PubMed] [Google Scholar]
- 52.Nagler RM, Nagler A. Effects of ionizing irradiation and beta-adrenergic stimulation on gene expression pattern in rat submandibular glands. Anticancer Res. 1996;16:2749–2756. [PubMed] [Google Scholar]
- 53.Nagler RM, Baum BJ, Fox PC. Acute effects of X irradiation on the function of rat salivary glands. Radiat Res. 1993;136:42–47. [PubMed] [Google Scholar]
- 54.Nagler RM. Extended-term effects of head and neck irradiation in a rodent. Eur J Cancer. 2001;37:1938–1945. doi: 10.1016/s0959-8049(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 55.Stiubea-Cohen R, David R, Neumann Y, et al. Effect of irradiation on cell transcriptome and proteome of rat submandibular salivary glands. PLoS One. 2012;7:e40636. doi: 10.1371/journal.pone.0040636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kubo T, Yano K, Hosokawa K. Management of flaps with compromised venous outflow in head and neck microsurgical reconstruction. Microsurgery. 2002;22:391–395. doi: 10.1002/micr.10059. [DOI] [PubMed] [Google Scholar]
- 57.Blackwell KE. Unsurpassed reliability of free flaps for head and neck reconstruction. Arch Otolaryngol Head Neck Surg. 1999;125:295–299. doi: 10.1001/archotol.125.3.295. [DOI] [PubMed] [Google Scholar]
- 58.Farina JA, Jr, Piccinato CE, Campos AD, et al. Comparative study of isovolemic hemodilution with 3% albumin, dextran-40, and prophylactic enoxaparin (LMWH) on thrombus formation at venous microanastomosis in rats. Microsurgery. 2006;26:456–464. doi: 10.1002/micr.20270. [DOI] [PubMed] [Google Scholar]
- 59.Pederson WC. Bone and soft tissue reconstruction: principles of microvascular surgery. In: Wolfe SW, Hotchkiss RN, Pederson WC, et al., editors. In: Green’s Operative Hand Surgery. 6th ed. Vol. 1. New York, NY: Churchill-Livingstone; 2011. pp. 1553–1584. [Google Scholar]
- 60.Farina JA, Jr, Piccinato CE, Rossi MA, et al. Effect of isovolemic hemodilution with 3% albumin on thrombus formation at venous microanastomosis in rats. Microsurgery. 2002;22:152–157. doi: 10.1002/micr.21743. [DOI] [PubMed] [Google Scholar]
- 61.Moore MG, Deschler DG. Clopidogrel (Plavix) reduces the rate of thrombosis in the rat tuck model for microvenous anastomosis. Otolaryngol Head Neck Surg. 2007;136:573–576. doi: 10.1016/j.otohns.2006.06.1276. [DOI] [PubMed] [Google Scholar]
- 62.Dvali LT, Dagum AB, Pang CY, et al. Effect of radiation on skin expansion and skin flap viability in pigs. Plast Reconstr Surg. 2000;106:624–629. doi: 10.1097/00006534-200009030-00015. [DOI] [PubMed] [Google Scholar]


