Abstract
Background:
The Clavien–Dindo (CD) classification is used to evaluate the severity of surgical complications. However, its usefulness in esophageal reconstruction has not been reported. To address this, this case series study used the CD classification to evaluate the complications after cervical esophageal reconstruction with free jejunum transfer or supercharged pedicled intestinal transfer.
Methods:
All consecutive patients who underwent esophageal cancer surgery with larynx-preserving free jejunum or pedicled ileocolic transfer in June 2012–December 2015 were identified. The postoperative complications were classified using the CD classification.
Results:
In total, 22 patients (20 men and 2 women; mean age, 63.3 years) underwent esophageal cancer reconstruction with larynx-preserving free jejunum transfer (n = 9) and supercharged pedicled intestinal transfer (n = 13). Seven patients underwent prophylactic tracheotomy. Four patients underwent emergent tracheotomy 1 or 5 days after surgery. The most frequent complication was recurrent nerve paralysis (RNP) (n = 8). Of these 8 RNP cases, 6 and 2 were classified as CD I and III complications, respectively. Pneumonia was the next most common complication (n = 7). Of these 7 pneumonia cases, 5 and 2 were classified as CD II and III, respectively. There were 2 cases of intestinal anastomosis leakage (CD II and III). On average, patients were able to start oral alimentation 15.1 (9–35) days after surgery.
Conclusions:
Our analysis with the CD classification suggested that vascularized free jejunum transfer or supercharge-drainage pedicled ileocolic transfer prevents postoperative intestinal anastomosis leakage and that prophylactic tracheotomy is especially indicated in cases with significant surgical damage in the cervical region.
Before larynx-preserving esophageal reconstruction is performed, it is essential that the plastic and digestive surgeons reach a medical consensus on the complications that could arise. This will enable the choice of the most suitable surgical strategy. The Clavien–Dindo (CD) classification, which classifies the surgical complications according to their severity, was first introduced in 1992 and then revised in 2004. It has since become a standard practice to classify general surgical complications with this system.1–3 One study has assessed whether the CD classification is suitable for classifying complications after head and neck reconstruction, whereas another has identified the factors that lead to major (CD grade III–V) complications.4,5 However, little has been published about the efficacy of the CD classification in larynx-preserving esophageal reconstruction.
In esophageal cancer surgery, many patients undergo gastric tube interposition or pedicled colon transfer.6 However, partial necrosis at the oral side of the intestinal tube, fistula formation, or stricture sometimes occur, chiefly because of insufficient blood supply or excessive tension at the gastric anastomosis.7,8 When these complications are anticipated in our institution, we consider performing free jejunum or pedicled ileocolic transfer with supercharging to avoid the risks. Kadota et al9 and Miyata et al10 have reported that free jejunum transfer after larynx-preserving esophagectomy helps to prevent complications.
In this case series study, we reviewed our experiences with larynx-preserving esophagectomy with free jejunal transfer or supercharging pedicled intestinal transfer. The peri- and postoperative complications associated with either method were assessed using the CD classification. In light of the results, the indications and limitations of these methods were discussed.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of Nippon Medical School Hospital (approval number, 26-6-380). The need for informed consent was waived due to the retrospective nature of the study. The study was conducted according to the principles of the Declaration of Helsinki and its revisions.
Patients
All consecutive patients who underwent esophageal cancer surgery with microsurgical reconstruction in our tertiary care institution between June 2012 and December 2015 were identified by retrospective chart review. The patients who underwent larynx-preserving surgery with supercharge-drainage pedicled ileocolic transfer or free jejunum transfer were then selected for further analysis. The patients were diagnosed with esophageal cancer after undergoing computed tomography, fluoroscopic examination, and esophagogastroduodenoscopy. The diagnosis was then proven using biopsy.
Larynx-preserving Esophageal Cancer Surgery
Some but not all patients underwent chemotherapy before or after surgery or radiotherapy before surgery. Larynx-preserving surgery consisted of cervicothoracic esophageal resection with a gastric pull-up technique and free jejunal transfer. The gastric pull-up technique was used in the patients who received an insufficiently long free jejunal segment between the pharynx and the gastric tube. Alternatively, larynx-preserving surgery consisted of cervicothoracic esophageal resection with gastric resection and intestinal transfer with a microvascular technique for poor intestinal blood flow. Some patients underwent intestinal transfer and cervicothoracic esophageal resection without gastric resection because of a past total gastric resection. During surgery, the blood circulation of the gastric tube and the intestines was determined by subjectively assessing the intestinal color and by performing the indocyanine green (ICG) test. Some patients also underwent prophylactic tracheotomy.
Intestinal Blood Flow
Subjective evaluations of the intestinal color and the ICG test were used to assess the intestinal blood circulation. When the patient was considered to be at risk of gastric tube necrosis or fistula, free jejunal transfer or microvascular anastomosis transfer was employed. Free jejunal transfer is particularly required when the gastric tube is of insufficient length, whereas the supercharge-drainage technique is required when there is poor intestinal blood flow (Fig. 1).
Fig. 1.
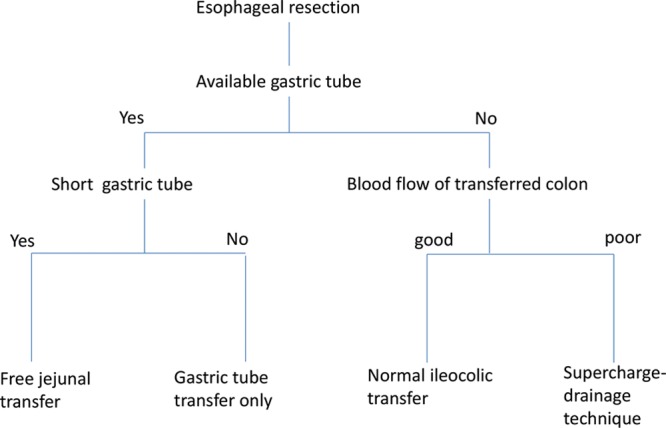
The algorithm of larynx-preserving esophageal reconstruction. Subjective evaluations of intestinal color and the indocyanine green test were used to determine intestinal blood circulation.
Tracheotomy
Figure 2 shows our institutional policy regarding the need for a tracheotomy during esophageal resection. Prophylactic tracheotomy is recommended when bilateral recurrent nerve damage is suspected as an outcome or when an esophageal reconstruction may result in severe edema.
Fig. 2.
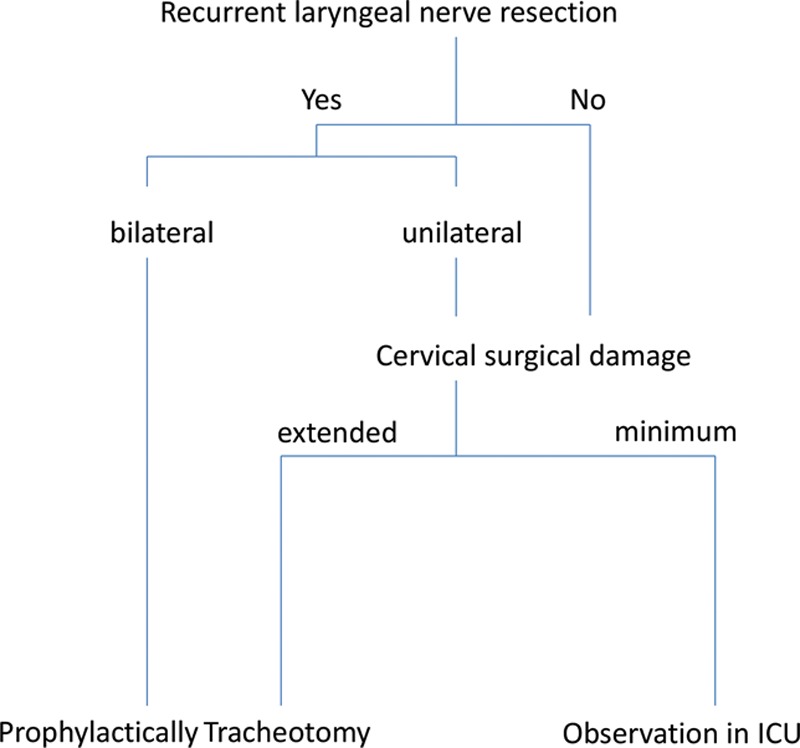
Institutional policy regarding the need for tracheotomy during larynx-preserving esophageal reconstruction surgery. Prophylactic tracheotomy is recommended in patients who probably seem to experience bilateral recurrent laryngeal nerve damage and extended cervical surgical damage.
Postoperative Care
All patients were taken to the intensive care unit and received respirator support. The supercharged transferred intestinal blood flow was observed by Doppler blood flow imaging. In all patients except the prophylactic tracheotomy cases, extubation was attempted 1 day after surgery under the aegis of an anesthesiologist. The patients were then moved to a general ward. They were allowed to leave hospital when they were capable of oral ingestion. They were then followed up by scheduled visits at 1 month and then every 2 months thereafter. Complications—including intestinal anastomosis leakage, recurrent nerve paralysis (RNP), and pneumonia—were recorded.
Data Extraction and Analysis
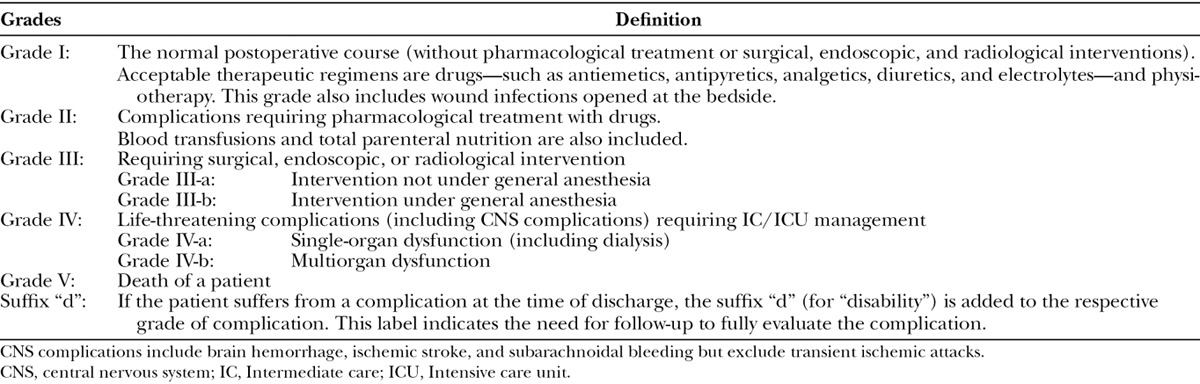
The preoperative demographic and clinical characteristics of the patients and the surgical factors and postoperative results were extracted from the medical records. The postoperative leakage ratio of intestinal anastomosis, the relationship between tumor invasion of the recurrent nerve and tracheotomy, the postoperative swallowing function, the vocal function, and the outcome of the patients at the last follow-up were assessed. The peri- and postoperative (up to 30 days after surgery) complications were categorized according to the CD classification (Table 1).
Table 1.
The Clavien–Dindo Classification of Surgical Complications
RESULTS
In total, 126 patients underwent esophageal cancer surgery during the study period. Of these, 28 patients underwent esophageal resection with microsurgical reconstruction. There were 23 men and 5 women, and their mean age was 63.6 years.
Of these 28 patients, 22 underwent larynx-preserving surgery with free jejunum or supercharge-drainage pedicled ileocolic transfer. There were 20 men and 2 women, and their mean age was 63.3 (range, 38–73) years (Table 2).
Table 2.
Preoperative Characteristics of the Patients
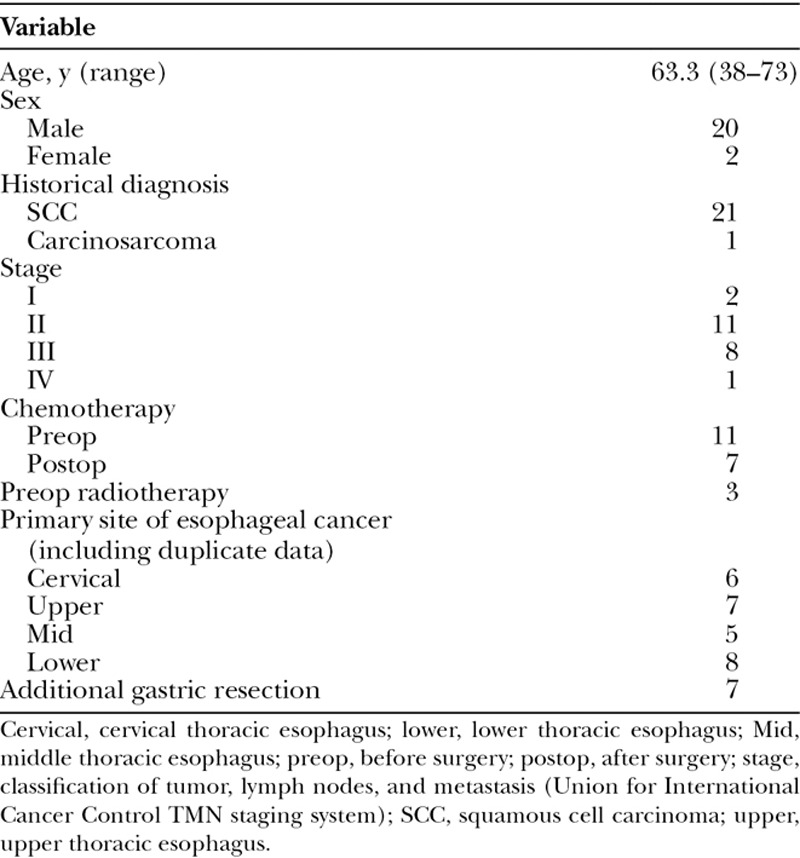
Squamous cell carcinoma was the most common histological diagnosis (n = 21). There was no particular tendency in terms of the distribution of primary esophageal cancer sites (defined as cervical, upper thoracic, middle thoracic, and/or lower thoracic esophagus) (Table 2).
Of the 22 patients, 9 underwent cervicothoracic esophageal resection with the gastric pull-up technique and free jejunal transfer. Another 7 patients underwent cervicothoracic esophageal resection with gastric resection and intestinal transfer with the microvascular technique for poor intestinal blood flow. Six patients underwent intestinal transfer and cervicothoracic esophageal resection without gastric resection because of a past total gastric resection. In 11, 6, and 5 patients, the presternal, retrosternal, and mediastinal route was selected, respectively. In 8 patients, the tumor invaded the recurrent laryngeal nerve, and thus it was excised together with the recurrent laryngeal nerve. In these patients, vagal-recurrent laryngeal nerve anastomosis was performed. Seven patients also underwent preoperative prophylactic tracheotomy because of edema of the larynx and pneumonia. One patient died during surgery (Table 3).
Table 3.
Reconstruction Method, Complications, Use of Tracheotomy, Start of Oral Ingestion, and Follow-up Duration
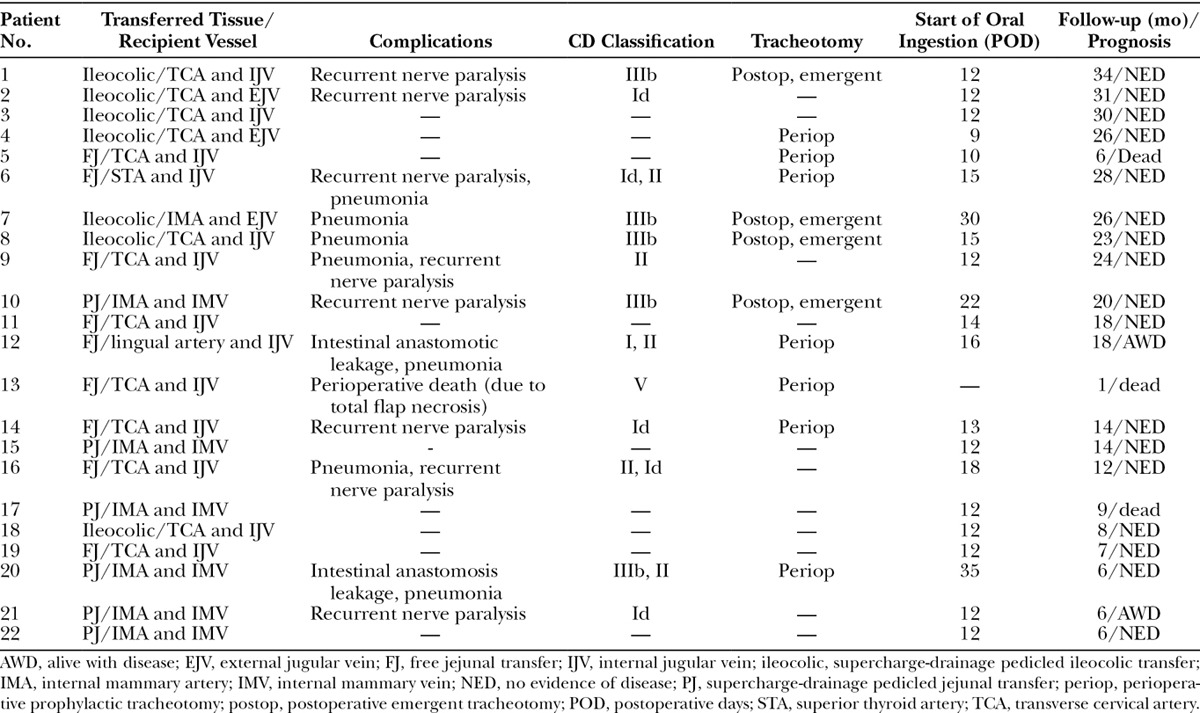
The average survival rate of the transferred tissue was 96.4%. None of the patients received intraoperative or postoperative antithrombotic medication. The blood flow of the transferred jejunal flap was observed by monitoring the jejunal flap that was resected 5 days after surgery.
In terms of postoperative complications, the most frequent was pneumonia (n = 7). Recurrent laryngeal nerve resection caused comorbid and recurrent nerve paralysis (RNP) in 8 patients. Four patients underwent emergent tracheotomy 1 or 5 days after surgery because of edema of the larynx and pneumonia. Two cases exhibited intestinal anastomosis leakage in the neck (Table 3). Of the 18 postoperative complications, 12 (67%) were classified as CD I or II. However, 2 cases of RNP, 2 cases of pneumonia, and 1 case of intestinal anastomosis leakage were classified as CD III (Fig. 3).
Fig. 3.
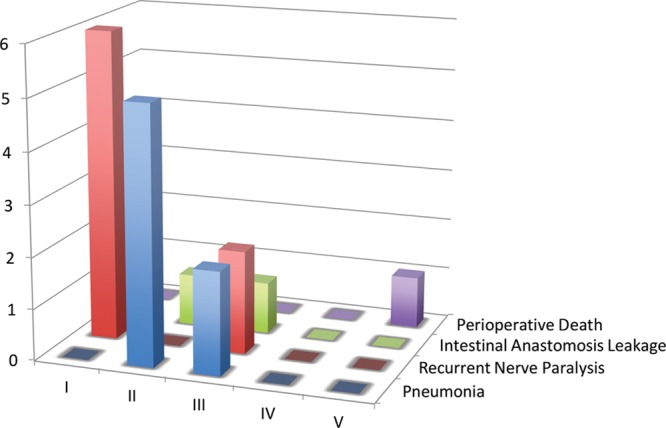
Frequency of specific complications of larynx-preserving esophageal reconstruction after applying the Clavien–Dindo classification. Recurrent nerve paralysis was the most frequent complication. Grade III–V complications were considered to be major complications.
On average, oral ingestion started 15.1 (range, 9–35) days after surgery. Most (n = 20) could start oral ingestion by day 22. The remaining patients (Patients 7 and 20) only started oral ingestion 30 and 35 days after surgery, respectively, because of pneumonia and anastomotic insufficiency in the cervical region.
Although 5 patients complained of postoperative trachyphonia, they had no trouble with daily life. As of May 10, 2016, after a mean follow-up duration of 16.6 (range, 1–34) months, 3 patients had died of systematic metastasis (n = 2) or surgical complications (n = 1). Of the remaining 19 patients, 17 were alive with no evidence of disease and 2 patients with disease. The weak point of this manuscript was the small number of patients; thus, we would like to plan a multicenter study using the CD classification in the future.
DISCUSSION
The CD classification is useful because it indicates the severity of surgical complications. It also helps plastic and digestive surgeons to realize how serious certain complications are. This CD classification-based analysis of the complications after esophageal reconstruction revealed the importance of prophylactic tracheotomy and reconstructive microsurgery in this setting, as follows.
In our case series, 15 patients did not undergo prophylactic preoperative tracheotomy. However, in 4 of these cases (27%), the postoperative complications of pneumonia and edema of the larynx led to the need for an emergent tracheotomy. Of these 4 patients, 2 had CD III pneumonia. In contrast, the patients who underwent prophylactic preoperative tracheotomy generally had CD I or II complications only; the exception was patient 13, who died during surgery. These observations suggest that prophylactic tracheotomy should perhaps be applied more liberally. In fact, the main reasons for the emergent tracheotomy are edema of the larynx and severe pneumonia, which suggest that these factors are more important when deciding to undergo prophylactic tracheotomy than the likelihood of unilateral RNP. In addition, several studies have not found a correlation between respiration-induced morbidity and the occurrence of recurrent laryngeal nerve paralysis after esophageal resection.11–13 This study shows that there is no strong relationship between unilateral recurrent laryngeal nerve resection and postoperative emergent tracheotomy. However, this may reflect the small numbers of patients.
In our series, 2 patients (9%) developed cervical intestinal anastomosis leakage. In the first patient (Patient 12), it was classified as CD I and was treated conservatively. However, in the second patient (Patient 20), the intestinal anastomosis leakage was treated by re-anastomosis surgery. In another case (Patient 13), the total flap necrosis led to mediastinitis and the perioperative death of the patient (CD V).
The blood and oxygen supply to the gastric tube is a major risk factor for anastomotic leakage and gastric tube ischemia/necrosis. With regard to ischemia/necrosis after esophageal reconstruction, Wormuth and Heitmiller14 reported that the average rates of ischemia after gastric tube, colon, and jejunum interposition are 3.2%, 5.1%, and 4.2%, respectively. Similarly, a retrospective analysis of 419 cases of esophageal cancer surgery at a single institute showed that 5.3% (n = 22) of the cases developed ischemic complications of the esophageal conduit; these included spontaneous closer necessitating longer than 1 month and additional surgery.15 In addition, the incidence of gastric tube ischemia/necrosis has been reported to range from 0.5% to 10.4% globally.14–16 However, in 2006, Shirakawa et al17 reported that the supercharge technique for colon interposition reduces the perioperative complications and improves the patient quality of life. Moreover, in 2013, Saeki et al18 reported the importance of superdrainage in esophageal cancer surgery with colon interposition. In our institute, superdrainage is performed with supercharging to reduce blood circulation complications in patients who undergo pedicled intestinal transfer. Both techniques completely prevent congestion or ischemia of the transferred pedicled intestinal tissue. In addition, the tension of intestinal anastomosis (which was caused by sclerotic tissue) and the inflammation in the stomach (such as was observed in Patient 1) are recognized as risk factors for gastric tube necrosis; in such cases, reconstruction with the microvascular technique should be considered.
Of the 22 patients, 21 (95.4%) were able to start oral alimentation on average 15.1 (9–35) days after surgery. Similarly, Matsumoto et al19 reported that after esophageal reconstruction by ileocolic interposition, patients generally started receiving an elemental diet from the supplemental feeding tube within 2 weeks of surgery. Thus, the results in our patient series were comparable to those observed in other institutions. In our series, 2 patients exhibited a particularly slow return to oral alimentation. Patient 7 could only start oral alimentation 30 days after surgery because of CD III pneumonia, whereas Patient 20 could only begin 35 days after surgery because of CD II pneumonia and CD III cervical intestinal leakage. The latter CD III complication of this patient was treated by pectoralis major musculocutaneous flap transfer.
Patients with cervical esophageal carcinoma have been reported to have a poor prognosis, even when they are treated with both laryngectomy and esophagectomy.20,21 However, patients who undergo cervical esophagectomy with or without laryngectomy have similar survival rates22 and Kadota et al9 concluded that preservation of the larynx does not decrease survival but does improve postoperative quality of life. In our series, the complications and functional outcomes were acceptable; moreover, the oncological outcomes were comparable to those in other institutions.9
CONCLUSIONS
The CD classification allowed us to better understand the indications and limitations of free jejunum transfer and supercharge-drainage pedicled intestinal transfer. In addition, our study of a series of larynx-preserving esophageal cancer surgery cases suggests that prophylactic tracheotomy is particularly necessary in patients who are likely to have bilateral RNP or who are likely to develop edema of the larynx or severe pneumonia.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111:518–526. [PubMed] [Google Scholar]
- 2.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clavien PA, Burkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 4.Monteiro E, Sklar MC, Eskander A, et al. Assessment of the Clavien-Dindo classification system for complications in head and neck surgery. Laryngoscope. 2014;124:2726–2731. doi: 10.1002/lary.24817. [DOI] [PubMed] [Google Scholar]
- 5.Peters TT, Post SF, van Dijk BA, et al. Free flap reconstruction for head and neck cancer can be safely performed in both young and elderly patients after careful patient selection. Eur Arch Otorhinolaryngol. 2015;272:2999–3005. doi: 10.1007/s00405-014-3268-z. [DOI] [PubMed] [Google Scholar]
- 6.Jamieson GG, Mathew G, Ludemann R, et al. Postoperative mortality following oesophagectomy and problems in reporting its rate. Br J Surg. 2004;91:943–947. doi: 10.1002/bjs.4596. [DOI] [PubMed] [Google Scholar]
- 7.Hagen JA, DeMeester SR, Peters JH, et al. Curative resection for esophageal adenocarcinoma: analysis of 100 en bloc esophagectomies. Ann Surg. 2001;234:520–530. doi: 10.1097/00000658-200110000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doki Y, Okada K, Miyata H, et al. Long-term and short-term evaluation of esophageal reconstruction using the colon or the jejunum in esophageal cancer patients after gastrectomy. Dis Esophagus. 2008;21:132–138. doi: 10.1111/j.1442-2050.2007.00738.x. [DOI] [PubMed] [Google Scholar]
- 9.Kadota H, Sakuraba M, Kimata Y, et al. Larynx-preserving esophagectomy and jejunal transfer for cervical esophageal carcinoma. Laryngoscope. 2009;119:1274–1280. doi: 10.1002/lary.20493. [DOI] [PubMed] [Google Scholar]
- 10.Miyata H, Yamasaki M, Takahashi T, et al. Larynx-preserving limited resection and free jejunal graft for carcinoma of the cervical esophagus. World J Surg. 2013;37:551–557. doi: 10.1007/s00268-012-1875-7. [DOI] [PubMed] [Google Scholar]
- 11.Hulcher JBF, van Sandrick JW, Devriese PP, et al. Vocal cord paralysis after subtotal oesophagectomy. Br J Surg. 1999;86:1583–1587. doi: 10.1046/j.1365-2168.1999.01333.x. [DOI] [PubMed] [Google Scholar]
- 12.Johnson PR, Kanegoanker GS, Bates T. Indirect laryngoscopic evaluation of vocal cord function in patients undergoing transhiatal esophagectomy. J Am Coll Surg. 1994;178:605–608. [PubMed] [Google Scholar]
- 13.Finley FJ, Lamy A, Clifton J, et al. Gastrointestinal function following esophagectomy for malignancy. Am J Surg. 1995;169:471–475. doi: 10.1016/s0002-9610(99)80197-4. [DOI] [PubMed] [Google Scholar]
- 14.Wormuth JK, Heitmiller RF. Esophageal conduit necrosis. Thorac Surg Clin. 2006;16:11–22. doi: 10.1016/j.thorsurg.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Matsubara T, Ueda M, Nakajima T, et al. Elongated stomach roll with vascular microanastomosis for reconstruction of the esophagus after pharyngolaryngoesophagectomy. J Am Coll Surg. 1995;180:613–615. [PubMed] [Google Scholar]
- 16.Ando N, Ozawa S, Kitagawa Y, et al. Improvement in the results of surgical treatment of advanced squamous esophageal carcinoma during 15 consecutive years. Ann Surg. 2000;232:225–232. doi: 10.1097/00000658-200008000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shirakawa Y, Naomoto Y, Noma K, et al. Colonic interposition and supercharge for esophageal reconstruction. Langenbecks Arch Surg. 2006;391:19–23. doi: 10.1007/s00423-005-0010-8. [DOI] [PubMed] [Google Scholar]
- 18.Saeki H, Morita M, Harada N, et al. Esophageal replacement by colon interposition with microvascular surgery for patients with thoracic esophageal cancer: the utility of superdrainage. Dis Esophagus. 2013;26:50–56. doi: 10.1111/j.1442-2050.2012.01327.x. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto H, Hirai T, Kubota H, et al. Safe esophageal reconstruction by ileocolic interposition. Dis Esophagus. 2012;25:195–200. doi: 10.1111/j.1442-2050.2011.01232.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang HW, Chu PY, Kuo KT, et al. A reappraisal of surgical management for squamous cell carcinoma in the pharyngoesophageal junction. J Surg Oncol. 2006;93:468–476. doi: 10.1002/jso.20472. [DOI] [PubMed] [Google Scholar]
- 21.Daiko H, Hayashi R, Saikawa M, et al. Surgical management of carcinoma of the cervical esophagus. J Surg Oncol. 2007;96:166–172. doi: 10.1002/jso.20795. [DOI] [PubMed] [Google Scholar]
- 22.Marmuse JP, Koka VN, Guedon C, et al. Surgical treatment of carcinoma of the proximal esophagus. Am J Surg. 1995;169:386–390. doi: 10.1016/s0002-9610(99)80182-2. [DOI] [PubMed] [Google Scholar]


