Abstract
Background:
Human acellular dermal matrix (HADM) is commonly used to provide coverage and support for breast reconstruction. The primary purpose of this study was to evaluate the complication rates associated with breast reconstruction procedures when performed in conjunction with multiple types of HADM in a consecutive series.
Methods:
After receiving institutional review board approval, medical records from a single surgeon were retrospectively reviewed for 126 consecutive patients (170 breasts and 211 procedures) who received a breast reconstruction or revision with implantation of HADM between 2012 and 2014. Patient demographics, surgical technique, and the complication profile of 4 major types of HADM were evaluated by procedure. Complication data were primarily evaluated for infection, seroma formation, necrosis, and other complications requiring additional surgery.
Results:
The total complication rate was 19.4%. The complication rates were not statistically different between all 4 types of HADM: Alloderm (n = 143); Alloderm RTU (n = 19); FlexHD (n = 18); hMatrix (n = 32) (P > 0.05). Smokers and large-breasted women (≥500 g) had a significantly higher complication rate than the rest of the population (P < 0.01 and P < 0.03, respectively). The complication rates associated with all other patient cohorts analyzed (age, body mass index, comorbid conditions, cancer diagnosis, prepectoral technique) showed no influence on complication rates (P > 0.05).
Conclusions:
In characteristically similar cohorts, there was no statistically significant difference in complication rates based on type of HADM; however, certain risk factors and anatomy should be considered before HADM-assisted breast reconstruction.
The use of acellular dermal matrix (ADM) as an adjunct for implant-based breast reconstruction (IBBR) has been steadily on the rise since first applied for this purpose in 2001.1,2 The use of ADM for IBBR is now widely accepted among reconstructive breast surgeons (utilization rates reported between 50% and 80%) owing to a number of advantages shown to be associated with its use.3–7 The use of ADM is typically used in reconstructions involving thin, well-vascularized flaps. When elected, ADM is used during breast reconstruction for soft-tissue reinforcement and to provide coverage and support, supplement muscle coverage, and shape the breast and inframammary fold (IMF).1,8 Additionally, because ADMs may reduce tension on the mastectomy flap, they allow for higher initial fill volumes and reduced tissue-expansion time and, in some cases, eliminate the need for a tissue-expansion phase altogether, thus requiring fewer surgeries.9,10
In the United States, human acellular dermal matrices (HADMs) are used in IBBR with much higher frequency than their xenogenic or synthetic counterparts.11 HADMs are processed from cadaveric donor-derived skin. Generally, the epidermal layer of skin is removed, followed by decellularization of the remaining tissue to minimize the risk of immunogenic response upon transplant. The resulting decellularized product is a biocompatible collagen scaffold rich in elastin and proteoglycans. This remaining structural lattice is ideal for cellular ingrowth and remodeling, as the natural architecture and vascular channels of the native tissue have been retained. All HADMs are aseptically processed; however, some offer further assurance of sterility through terminal sterilization methods. There have been several new HADMs introduced to the market over the last 5 years, most recently hMatrix (Bacterin International; Bozeman, Mont.); however, because of the preponderance of conflicting data, it is still unclear how differences in the processing, packaging, and sterilization of HADMs correlate with postoperative outcomes. Table 1 summarizes the key processing parameters of several HADM manufacturers.
Table 1.
Currently Commercially Available HADMs
Recent literature has cautioned against the adoption of ADM use in IBBR as the standard of care because of reports of increased postoperative complications and the relative paucity of prospective clinical efficacy and safety data.10,12 Indeed, careful patient selection to mitigate demonstrated risk factors is important. Patient comorbidities, adjuvant therapies, body mass index (BMI), and breast weight/implant size are a few factors that have been shown to be associated with higher postoperative complication rates.12–18 It has also been demonstrated that total complication rates decrease as surgeons become more experienced with ADM-assisted breast procedures, indicating that there is a certain “learning curve” to the technique.15,19
Despite the correlation of ADM use in IBBR to postoperative complication rates in some patient populations, some studies have shown that its use improves cosmetic outcomes and reduces the rate of capsular contracture.3,6,11 ADM-assisted breast reconstruction can also allow for a prepectoral placement of the breast implant especially with advanced mastectomy techniques such as nipple sparing (NS). Before these advances, the expanders were traditionally placed in the submuscular pocket because of concerns with thin and poorly vascularized flaps.20 Prepectoral placement may improve cosmetic outcomes, reduce pain, and eliminate the animation defect often associated with a traditional subpectoral implant placement.21,22
The primary purpose of this study was to establish the equivalency of a newly introduced HADM, to those already on the market and to better define optimal patient populations for its use. Although HADM use in patients presenting with certain risk factors should be carefully considered, HADMs are a safe and effective adjunct to breast reconstruction surgery.
PATIENTS AND METHODS
After institutional review board approval, comprehensive case histories of a consecutive series of 140 patients having undergone breast reconstruction or revision with implantation of a dermal matrix by a single surgeon between 2012 and 2014 were retrospectively reviewed. Follow-up data were recorded throughout the tissue-expansion process where applicable and in all cases at least 3 months beyond surgery date. A total of 126 patients met the inclusion criteria of the study, which consisted of women with a current or past cancer diagnosis (including BRCA + prophylactic procedures) who elected IBBR after mastectomy and who underwent a primary reconstruction or revision procedure utilizing HADM between 2012 and 2014. The surgeon’s decision to choose a certain HADM brand was primarily determined by insurance formulary inclusion/exclusion. Revision cases were defined as reconstructions performed after an initially failed tissue expander (TE) or a permanent implant procedure whereby the pocket had already been created. Procedures that were strictly cosmetic in nature (those breasts without mastectomy history) were excluded, in addition to those utilizing xenogenic or synthetic ADM, and also any fenestrated HADM. Of the 140 patients reviewed, 211 procedures representing 170 unique breasts and 126 patients were included in the data analysis.
Patient demographic data were collected to include age, BMI, comorbidities, cancer diagnosis, adjuvant cancer therapy (when available), and history of smoking. Procedural factors including mastectomy type, number of operative breasts (unilateral vs bilateral), dermal graft material used, breast weight, TE and implant volumes, operative technique, and type of reconstruction were also recorded.
Infection, seroma, and tissue necrosis were recorded as applicable for each procedure reviewed. Infections requiring intravenous antibiotics or additional surgery were defined as “major,” whereas those treated with oral antibiotics were considered “minor.” Only those seromas occurring clearly independent of infection were categorized as such. Any other complication requiring additional surgery (eg, implant exposure or loss, delayed wound healing) was included in the overall complication rate. Red breast syndrome was excluded due to its unknown etiology. Because of the relatively short follow-up for some patients, it was inaccurate to report on cosmetic outcomes and pain.
Statistical analysis of the incidence of complications was performed using Fisher’s exact tests and Chi-squared analyses. Linear regression analyses and Pearson correlations were also used, when appropriate, to determine the relationship between continuous variables and incidence of complications. Analyses of variance and Chi-squared analyses of demographic data to include age, gender, BMI, reason for surgery, and risk factors/comorbidities were used to confirm homogeneity between groups. SPSS v. 21 (SPSS, Inc.; Chicago, Ill.) was used for all analyses, and significance was set at P < 0.05.
Surgical Reconstruction Technique
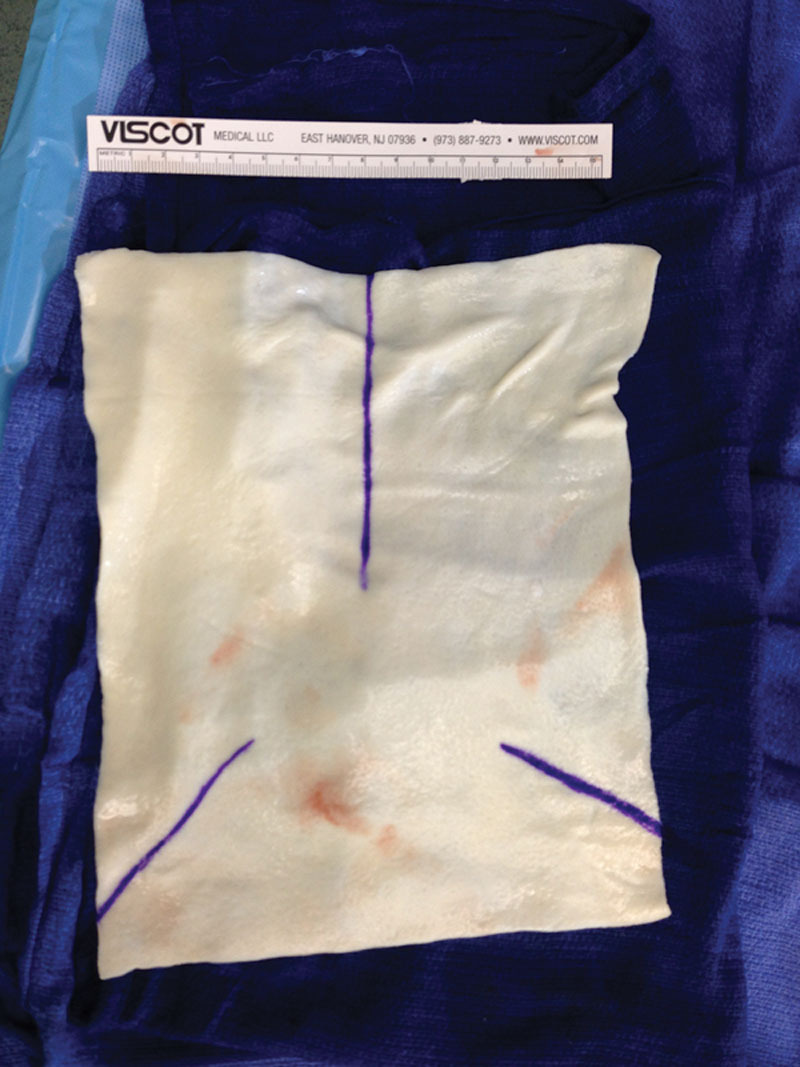
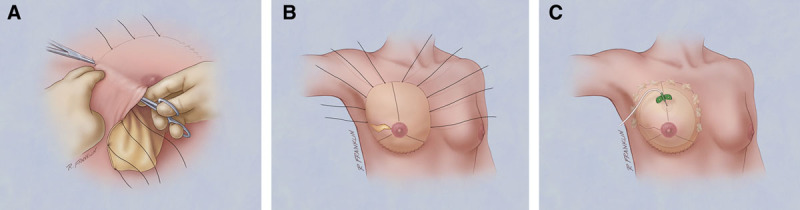
The majority of the procedures were prepectoral placement of the implant with the HADM secured anteriorly. The HADM, prepared according to the manufacturer’s instructions, was trimmed and contoured to fit the pocket dimensions typically using size 16 × 16 or 16 × 20. In most cases, because the same general surgeon was used for the mastectomies incorporating a NS technique, a moderate thickness (0.8–1.3 mm) was most often selected, which was dependent on patient anatomy and surgeon preference. If flap thickness was of concern, perfusion imaging was used at the beginning and at the end of the case. The appropriate orientation was determined (long vs short axis) along the IMF, dermal or epidermal side toward/away from mastectomy flap (not applicable for hMatrix as this product has a sans basement membrane option). Orientation marks were placed at the 12-, 4-, and 8-o’clock positions with the 12-o’clock position made longer for clarity (Fig. 1). The pocket was rinsed with dilute betadine before the HADM was inserted. Using a 5/8 circle taper cut 3-0 monocryl “urology” suture (Ethicon; New Brunswick, N.J.), the HADM was anchored into the IMF beginning at the medial border (4-o’clock position for the right breast and 8-o’clock position for the left breast). The suture was attached to the “marked” HADM at the medial position before insertion and the tail left long to tie to after reversal. In a running fashion (continuously or intermittently locked), the HADM was anchored into the IMF with each bite securely engaging the chest wall fascia/muscle and also the HADM. Each bite advanced 1/2 to 1 cm. After reaching the lateral margin of the IMF (8-o’clock position for the right breast and 4-o’clock position for the left breast), the suture was reversed and passed medially along the IMF securely engaging the chest wall fascia/muscle and HADM a second time. After reaching the medial border (starting point), the suture was tied to the tail left long previously. The 3-0 chromic sutures on an FS or straight needle (Ethicon; New Brunswick, N.J.) were then attached along the remaining free medial, superior, and lateral margins. Four to six sutures were initially placed along the medial “hemi” margin, passed from inside the pocket to the exterior at the pocket margin (Fig. 2A), cut in half and secured with 1 or 2 hemostats, with the remaining half used to complete the lateral “hemi” margin draw marionette sutures (Fig. 2B). The HADM can then be manipulated in the pocket for drain and TE/implant placement with the marionette sutures used to reposition the HADM over the TE/implant (also utilizing digital manipulation) and also securely anchoring the HADM into final position by drawing on the chromic sutures and anchoring them to the skin with steristrips after applying skin adhesive (Fig. 2C). In the author’s experience, any absorbable suture may be utilized, and permanent sutures are not personally desired. The prosthesis (TE or implant) was then inserted prepectorally under the HADM (Fig. 3). All implants were bathed in a 5000 units per 10 ml of bacitracin. If utilized, the prosthesis anchor tab was attached to the muscle, and remote ports were created in the flank. The HADM was then draped over the implant using the draw sutures (Fig. 4) and digital manipulation. Final adjustments for fill and positioning of the prosthesis were performed. The incisions were closed in multiple layers with absorbable sutures. Perfusion imaging was performed if indicated and volume adjustments made. Suture lines were dressed with Aquacel Silver strips (Convatec; Bridgewater, N.J.) held by 2-inch Hypafix tape (Smith & Nephew; Andover, Mass.). Biopatches (Ethicon; New Brunswick, N.J.) held by Hypafix tape were placed around the drain sites. Absorbent pads and front fastening sports bra were applied after closure and wound dressing.
Fig. 1.
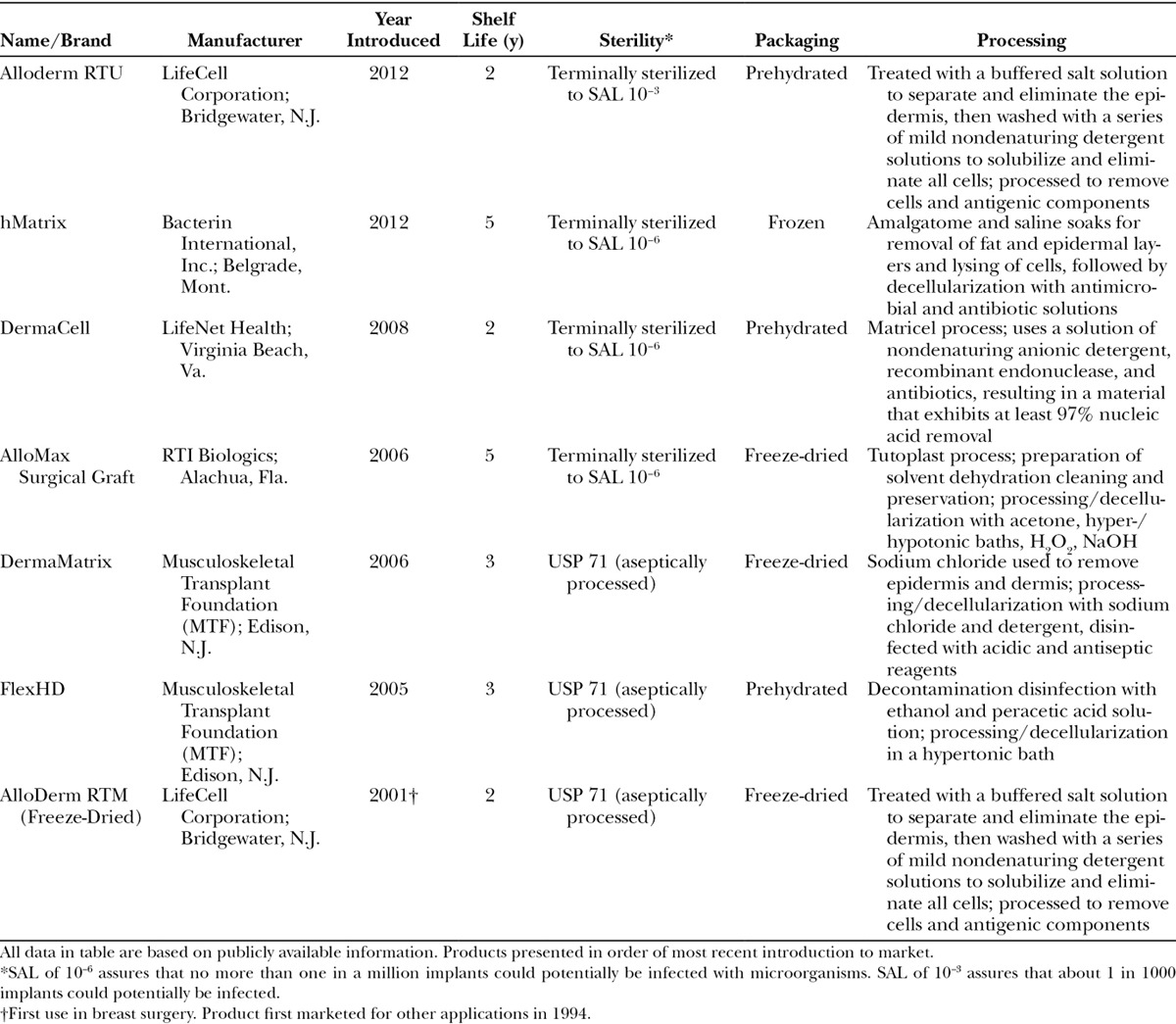
HADM contoured and marked correctly oriented over the breast.
Fig. 2.
A, Passing the marionette sutures through the skin. B, Marionette sutures after completion of skin passage. C, Marionette sutures collected and secured to the skin.
Fig. 3.
Prosthesis placed prepectorally.
Fig. 4.
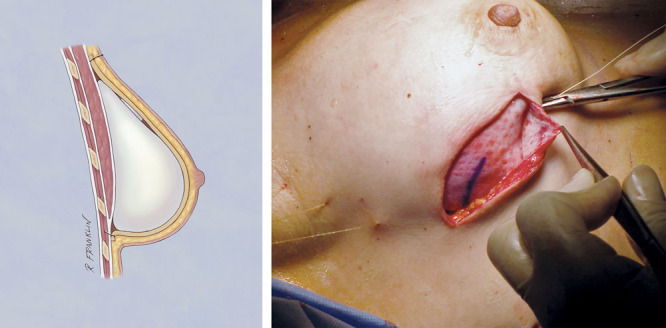
HADM pulled up by marionette sutures.
RESULTS
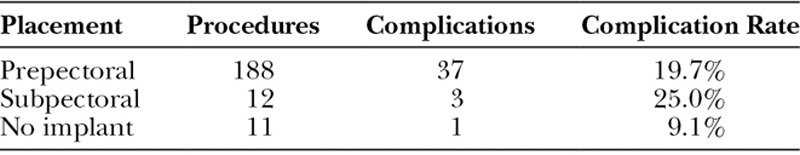
From 2012 to 2014, 211 HADM-assisted breast reconstructions after mastectomy procedures were performed by a single surgeon on 126 patients comprising 170 unique breasts. The average age was 54.0 years (range: 29–76) and the average BMI was 27.3 (range: 17.8–51.2). Thirty-two patients were obese (BMI ≥30) and 18 had a smoking history (Table 2). Of the data available for breast weight (N = 165), 55 procedures were performed on large breasts (breast weight ≥500 g). Of the 211 HADM-assisted procedures within the time frame of 2012 and 2014, 53 were immediate reconstructions after mastectomy (14 bilateral and 25 unilateral). Only one of these primary reconstructions was a single-stage, direct-to-implant procedure, whereas the other 52 were TE-based. There were 101 procedures related to a breast carcinoma diagnosis, 39 prophylactic procedures, and 71 revisions. One hundred and eighty-eight implants were prepectorally placed, of which 36 of those procedures were conversions from a subpectoral implant to a prepectoral placement (Table 3).
Table 2.
Patient Demographics, 2012 to 2014
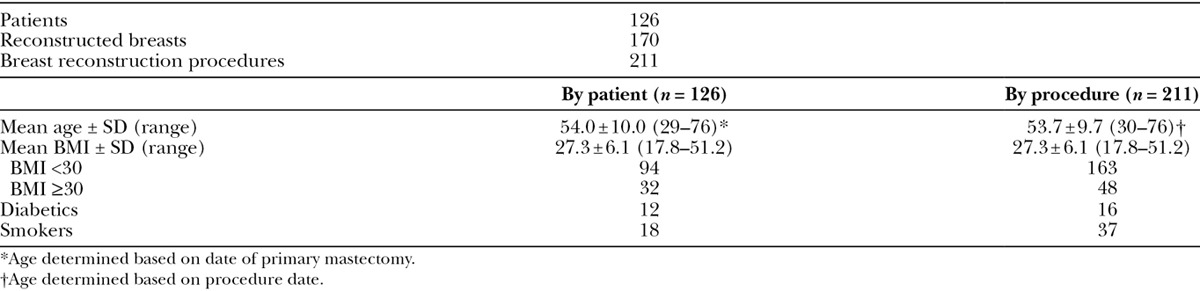
Table 3.
HADM Procedural Data, 2012 to 2014
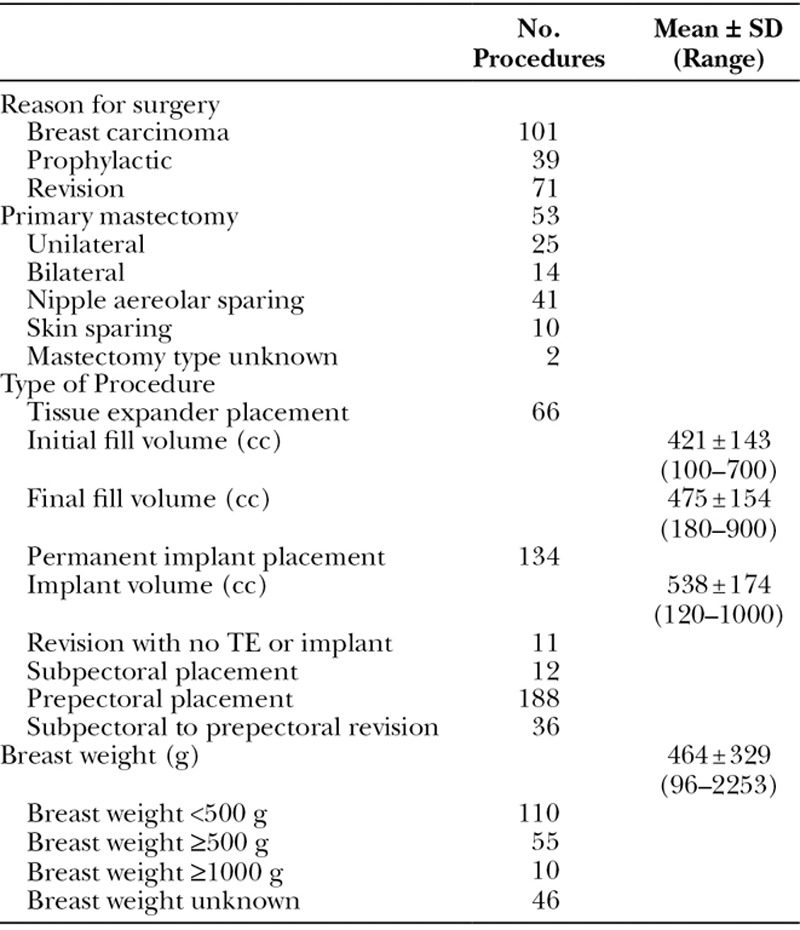
The total complication rate for the 211 procedures was 19.4% (N = 41/211) and is broken down according to HADM type implanted (Table 4, Table 5). There was no statistically significant difference (P > 0.05) when comparing the complication rates of the 4 types of HADM utilized. Also, no statistically significant difference was found between the HADMs when comparing the complication rates in each of the 4 categories analyzed (major and minor infection, seroma, tissue necrosis, and other). Additionally, there was no significant difference in complication rate between the cohort of prepectorally placed implants (19.7% in 188 procedures) and those with subpectoral placement (25.0% in 12 procedures), P > 0.05 (Table 6).
Table 4.
Per Procedure Complications by Type of HADM
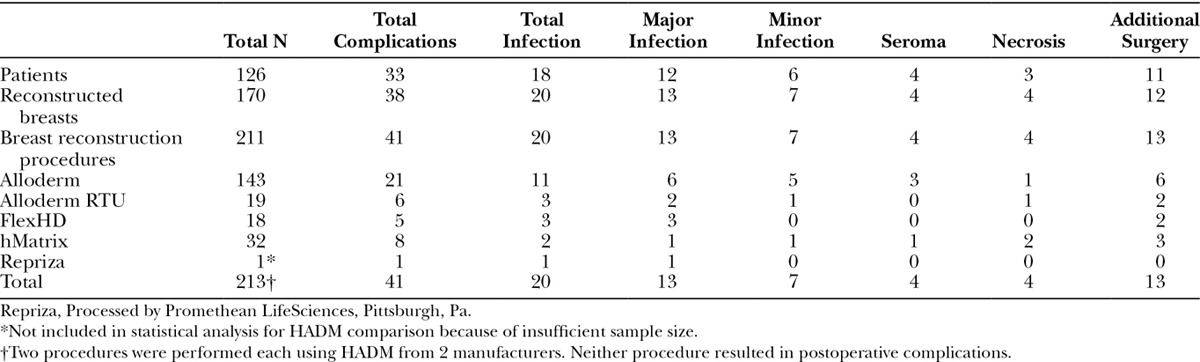
Table 5.
Per Procedure Complication Rate by Type of HADM
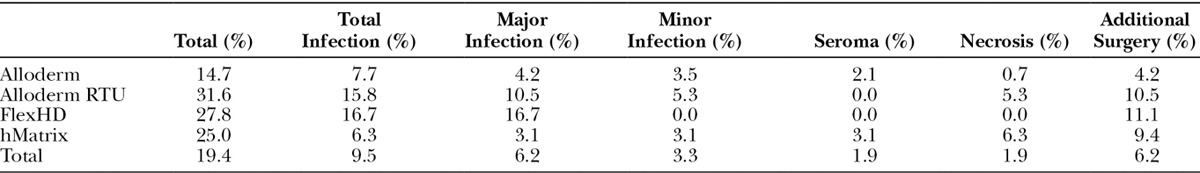
Table 6.
Prepectoral Versus Subpectoral Complication Rates
Smoking history and breast weight were found to significantly impact the total complication rate. Of procedures performed on patients with a smoking history, 38% had complications (N = 14/37) compared with a 16% complication rate associated with nonsmokers (N = 27/174, P < 0.01). Additionally, the complication rate associated with procedures performed on large breasts (breast weight ≥500 g) was significantly increased over those without large breasts (33% vs 16%, P = 0.027). The complication rate by breast weight is shown in Figure 5. The average breast weight was 464 g (range: 96–2253 g).
Fig. 5.
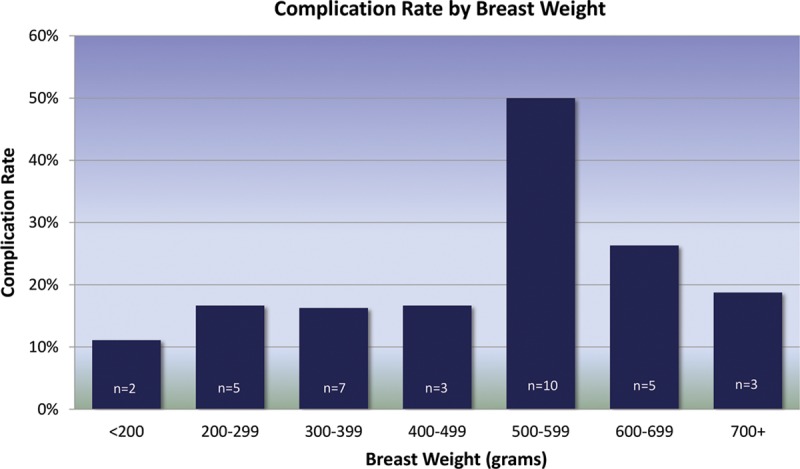
Total complication rate by breast weight. n represents the total number of complications in each category. Breast weights ≥500 g were associated with a significantly higher complication rate than those <500 g (P = 0.027).
None of the other factors analyzed significantly affected the total complication rate (obesity, diabetes, cancer diagnosis, fill volume; P > 0.05). Consistent data were not available regarding radiation and chemotherapy.
DISCUSSION
The use of ADM for breast reconstruction remains a frequent topic of interest, research, and discussion, largely with regard to complication rates. As of the date of this article, a PubMed database search using the keywords “acellular dermal matrix AND breast reconstruction” contains over 300 publications, with nearly 40% published over the last 2 years. The majority of these publications have been met with mixed reviews, bias, and an unclear answer as to which, if any, of the ADMs are better than the other. For HADMs, the published data show a variance in total complications as high as 48.7% and as low as 3.2%.23 A meta-analysis by Kim et al23 reports the total HADM complication rate at 15.4%, which is not significantly different than the rate in this study (19.4%, P > 0.05).
When further analyzing the total complication rate by HADM manufacturers, there are some clinically relevant differences. For example, although some authors have argued over the effect of graft sterility on infection rates,14,24 it is noteworthy that the lowest infection rate of the 4 HADMs included in this study was associated with the only terminally, device-level (sterile assurance level 10−6) sterile product (hMatrix), and the highest infection rate was associated with a nonsterile, aseptically processed product (FlexHD, Musculoskeletal Transplant Foundation (MTF); Edison, N.J.). Furthermore, Alloderm RTU (LifeCell Corporation; Bridgewater, N.J.), although provided sterile (SAL 10−3), had the highest total complication rate (31.6%) of the 4 HADMs studied, which, in part, is hypothesized to be a result of immunogenicity issues with the preservatives in the product packing solution.
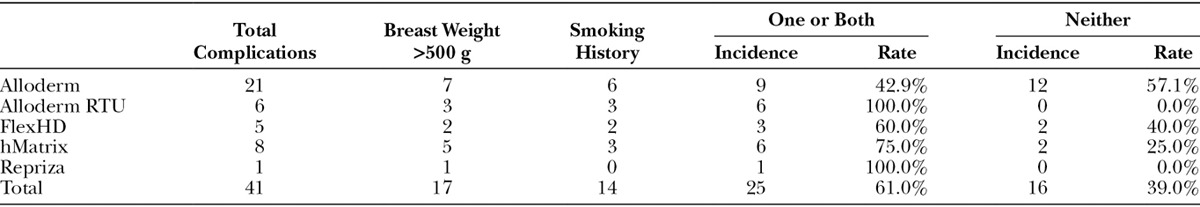
Despite the lack of statistically significant differences in outcomes between the 4 HADM manufacturers analyzed in this study, another story emerges when looking specifically at those patients with and without risk factors. The data show that smoking history and breast weight significantly influenced the complication rate. Compared with an overall complication rate for the entire population of 19.4%, those procedures performed on smokers had a 37.8% total complication rate (P < 0.01), and those performed on large breasts (≥500 g) had a 32.7% total complication rate (P = 0.027) (Table 7). Seventy-five percent of the cases of postoperative seroma formation or tissue necrosis and 70% of postoperative infections occurred in patients with one or both of these risk factors. Conversely, in the subset of patients with neither significant risk factor (nonsmokers and breast weights <500 g), the incidence of complications differs considerably between the manufacturers (57% Alloderm [LifeCell Corporation; Bridgewater, N.J.], 40% FlexHD, 25% hMatrix, 0% Alloderm RTU), with the highest being associated with Alloderm (Table 8). Although not statistically significant due to sample size (n = 41 complications in the entire patient population), it is of note that hMatrix and Alloderm RTU had such relatively low complication rates among those patients not otherwise considered at increased risk.
Table 7.
Complications among At-risk Population*
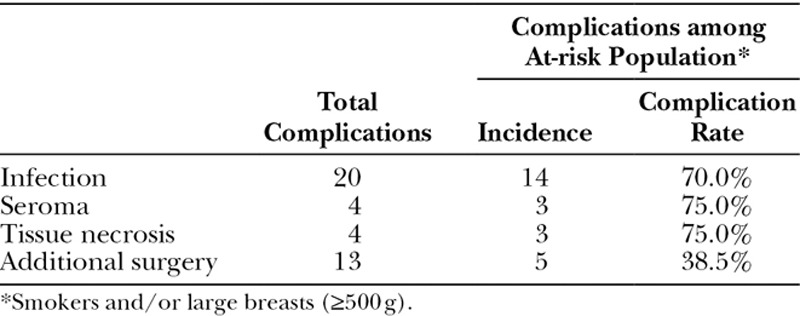
Table 8.
Complications in At-risk and Not–at-risk Populations by HADM Manufacturer
With these data in mind, careful attention should be made to high-risk patients when electing the use of an HADM to include patient morphology, flap thickness, fill rates, ADM and mastectomy type, and cancer treatment. Specifically in smokers, the success of direct-to-implant reconstruction and more radical mastectomies is limited by their vasoconstriction and compromised vascularity. Similarly, ADM incorporation requires adequate perfusion and is more difficult to achieve in vasocompromised patients. Pre- and postoperative pharmacologic intervention may be additionally considered to counteract the effects of nicotine. Large-breasted patients are also less perfused, which reduces treatment options in favor of a more conservative approach. To improve patient outcomes, this surgeon counsels large-breasted women to envelope reducing procedures.
Given the surgeon’s preference toward prepectoral implant placement, a secondary evaluation of this technique was of interest. Prepectoral implant placement is also an emerging option for improved breast reconstruction outcomes resulting in fewer operations, faster healing, and reduced need for fat grafting,20–22 all of which ultimately result in lower overall cost of treatment and higher patient satisfaction.9 Additional advantages to this technique are improved aesthetic outcomes associated with reduced incidence of animation defect. Consequently, because less aggressive mastectomies and NS techniques have come into favor, performing a prepectoral technique affords potentially less damage to the chest wall while improving cosmesis, which can be ideal for young, active patients with low BMI and thin mastectomy flaps. Analysis of this study’s prepectoral subset shows reduced complication rates when compared with the subpectoral population, although the difference is not statistically significant (19.7% prepectoral vs 25.0% subpectoral, P > 0.05) (Table 6).
There are limitations to this study, to include the relative lack of sample size for 3 of the 4 HADM manufacturers analyzed and also the large variety of reconstructive procedures. Alloderm has the largest sample size primarily because of its longevity and ease of reimbursement by the insurance companies compared with newer products where utilization has been limited by the absence of reimbursement formularies. The large variety of procedures included in this consecutive series introduced a number of variables that could be confounding to the conclusions presented. Larger sample sizes and further stratification and analysis of the individual procedures by type of HADM may increase the analytical integrity.
CONCLUSIONS
In conclusion, when deciding to use an HADM for breast reconstruction, the complication rates for Alloderm, Alloderm RTU, FlexHD, and hMatrix are statistically similar. Careful patient selection determines not only the procedure type but also when/if HADM should be implanted. In general, this surgeon prefers a formulary-approved, terminally sterile HADM in conjunction with prepectoral implantation as a good option for indicated patients. Finally, a prospective analysis including the effect of radiation, chemotherapy, and other adjuvant cancer treatments should be considered to better determine complication rates.
ACKNOWLEDGMENTS
The authors thank M. Russell Giveans, PhD, with Elite Research Institute in Minneapolis, Minn. for performing the statistical analysis contained in this article. The authors additionally thank Mark Jennings, MD, and Robert R. McCormack for their assistance with data collection.
Footnotes
Disclosure: RH Schnarrs has consulted for and spoken on behalf of LifeCell, Novadaq, Allergan, Mentor, Sientra, Bacterin, and LifeNet. He received no financial compensation in support of the study presented in this article. SA Chase and KA Rossmeier are employees of Bacterin International, Inc. Neither of the other authors has any financial disclosures. The article processing charge was paid for by Bacterin International, Inc.
REFERENCES
- 1.Salzburg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg. 2006;57:1–5. doi: 10.1097/01.sap.0000214873.13102.9f. [DOI] [PubMed] [Google Scholar]
- 2.Salzberg CA, Dunavant C, Nocera N. Immediate breast reconstruction using porcine acellular dermal matrix (Strattice™): long-term outcomes and complications. J Plast Reconstr Aesthet Surg. 2013;66:323–328. doi: 10.1016/j.bjps.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim AM, Koolen PG, Ganor O, et al. Does acellular dermal matrix really improve aesthetic outcome in tissue expander/implant-based breast reconstruction? Aesthetic Plast Surg. 2015;39:359–368. doi: 10.1007/s00266-015-0484-x. [DOI] [PubMed] [Google Scholar]
- 4.Lanier ST, Wang ED, Chen JJ, et al. The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Ann Plast Surg. 2010;64:674–678. doi: 10.1097/SAP.0b013e3181dba892. [DOI] [PubMed] [Google Scholar]
- 5.Gurunluoglu R, Gurunluoglu A, Williams SA, et al. Current trends in breast reconstruction: survey of American Society of Plastic Surgeons 2010. Ann Plast Surg. 2013;70:103–110. doi: 10.1097/SAP.0b013e31822ed5ce. [DOI] [PubMed] [Google Scholar]
- 6.JoAnna Nguyen T, Carey JN, Wong AK. Use of human acellular dermal matrix in implant- based breast reconstruction: evaluating the evidence. J Plast Reconstr Aesthet Surg. 2011;64:1553–1561. doi: 10.1016/j.bjps.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Leong M, Basu CB, Hicks MJ. Further evidence that human acellular dermal matrix decreases inflammatory markers of capsule formation in implant-based breast reconstruction. Aesthet Surg J. 2015;35:40–47. doi: 10.1093/asj/sju014. [DOI] [PubMed] [Google Scholar]
- 8.Bullocks JM. DermACELL: a novel and biocompatible acellular dermal matrix in tissue expander and implant-based breast reconstruction. Eur J Plast Surg. 2014;37:529–538. doi: 10.1007/s00238-014-0995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez-Feliz J, Codner MA. Embrace the change: incorporating single-stage implant breast reconstruction into your practice. Plast Reconstr Surg. 2015;136:221–231. doi: 10.1097/PRS.0000000000001448. [DOI] [PubMed] [Google Scholar]
- 10.Potter S, Chambers A, Govindajulu S, et al. Early complications and implant loss in implant-based breast reconstruction with and without acellular dermal matrix (Tecnoss Protexa®): a comparative study. Eur J Surg Oncol. 2015;41:113–119. doi: 10.1016/j.ejso.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim AM, Koolen PG, Ashraf AA, et al. Acellular dermal matrix in reconstructive breast surgery: survey of current practice among plastic surgeons. Plast Reconstr Surg Glob Open. 2015;3:e381. doi: 10.1097/GOX.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avraham T, Weichman KE, Wilson S, et al. Postoperative expansion is not a primary cause of infection in immediate breast reconstruction with tissue expanders. Breast J. 2015;21:501–507. doi: 10.1111/tbj.12448. [DOI] [PubMed] [Google Scholar]
- 13.Selber JC, Wren JH, Garvey PB, et al. Critical evaluation of risk factors and early complications in 564 consecutive two-stage implant-based breast reconstructions using acellular dermal matrix at a single center. Plast Reconstr Surg. 2015;136:10–20. doi: 10.1097/PRS.0000000000001327. [DOI] [PubMed] [Google Scholar]
- 14.Lewis P, Jewell J, Mattison G, et al. Reducing postoperative infections and red breast syndrome in patients with acellular dermal matrix-based breast reconstruction: the relative roles of product sterility and lower body mass index. Ann Plast Surg. 2015;74:S30–S32. doi: 10.1097/SAP.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 15.Lardi AM, Ho-Asjoe M, Mohanna PN, et al. Immediate breast reconstruction with acellular dermal matrix: factors affecting outcome. J Plast Reconstr Aesthet Surg. 2014;67:1098–1105. doi: 10.1016/j.bjps.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Fischer JP, Nelson JA, Serletti JM, et al. Peri-operative risk factors associated with early tissue expander (TE) loss following immediate breast reconstruction (IBR): a review of 9305 patients from the 2005-2010 ACS-NSQIP datasets. J Plast Reconstr Aesthet Surg. 2013;66:1504–1512. doi: 10.1016/j.bjps.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 17.Barber MD, Williams L, Anderson ED, et al. Outcome of the use of acellular-dermal matrix to assist implant-based breast reconstruction in a single centre. Eur J Surg Oncol. 2015;41:100–105. doi: 10.1016/j.ejso.2014.08.475. [DOI] [PubMed] [Google Scholar]
- 18.Martin L, O’Donoghue JM, Horgan K, et al. Association of Breast Surgery and the British Association of Plastic, Reconstructive and Aesthetic Surgeons. Acellular dermal matrix (ADM) assisted breast reconstruction procedures: joint guidelines from the Association of Breast Surgery and the British Association of Plastic, Reconstructive and Aesthetic Surgeons. Eur J Surg Oncol. 2013;39:425–429. doi: 10.1016/j.ejso.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Colwell AS, Damjanovic B, Zahedi B, et al. Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: indications, complications, trends, and costs. Plast Reconstr Surg. 2011;128:1170–1178. doi: 10.1097/PRS.0b013e318230c2f6. [DOI] [PubMed] [Google Scholar]
- 20.Becker H, Lind JG, III, Hopkins EG. Immediate implant-based prepectoral breast reconstruction using a vertical incision. Plast Reconstr Surg Glob Open. 2015;3:e412. doi: 10.1097/GOX.0000000000000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: a new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg. 2015;68:162–167. doi: 10.1016/j.bjps.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Hammond DC, Schmitt WP, O’Connor EA. Treatment of breast animation deformity in implant-based reconstruction with pocket change to the subcutaneous position. Plast Reconstr Surg. 2015;135:1540–1544. doi: 10.1097/PRS.0000000000001277. [DOI] [PubMed] [Google Scholar]
- 23.Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129:28–41. doi: 10.1097/PRS.0b013e3182361fd6. [DOI] [PubMed] [Google Scholar]
- 24.Yuen JC, Yue CJ, Erickson SW, et al. Comparison between Freeze-dried and Ready-to-use AlloDerm in alloplastic breast reconstruction. Plast Reconstr Surg Glob Open. 2014;2:e119. doi: 10.1097/GOX.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]


