Supplemental Digital Content is available in the text.
Abstract
Background:
Rhinoplasty has traditionally been preferred for correction of nasal defects. Long-term clinical experience with hyaluronic acid (HA) injection as an alternative or complement to rhinoplasty is presented.
Methods:
A retrospective review of the author’s clinical experience with HA gel for nasal reshaping from 1997 to 2012 was conducted, with treatments performed during 1998, 2005, and 2012 selected for detailed review.
Results:
More than 250 patients were treated for nasal reshaping with HA since 1997. In addition to being a complement to surgery, HA injection successfully addressed nasal defects that would have been difficult to correct surgically. The effect persisted for >1 year in most patients (>5 y in some patients), with individual variations. No serious complications occurred. When comparing the 3 years reviewed in detail, new indications for nasal reshaping with HA gel became evident over time, which was also reflected by the increase in number of patients treated (1998: n = 2; 2005: n = 22; 2012: n = 51). Of these patients, 55 (73%) received HA injection instead of rhinoplasty, 20 (27%) received HA injection after rhinoplasty, and 5 (7%) underwent rhinoplasty after HA injection. The mean injection volume was 0.4 mL HA gel/treatment. All patients were satisfied with the primary outcome of treatment. Retreatment was performed in 32 patients (43%).
Conclusions:
Injection of HA gel is a valuable tool for nasal reshaping. It can also be used for correction of minor postrhinoplasty defects in appropriate patients.
Rhinoplasty was the fifth most common surgical procedure in 2013.1 In the author’s experience, and despite most patients requesting a nasal reduction, rhinoplasty often involves reshaping rather than reduction. Because nasal skin has a limited ability to contract, especially in the tip and if the skin is thick, nasal reshaping/augmentation may be preferred from an aesthetic point of view. In fact, enhancement of parts of the nose can lead to it being perceived as smaller. Despite the challenge to explain to patients who seek a nasal reduction that they would benefit from enhancement of parts of the nose, plastic surgeons should be aware of this effect.
Nasal reshaping may be achieved with injectables such as calcium hydroxylapatite2–5 and stabilized hyaluronic acid (HA).6–15 Use of injectables is appealing because patients generally prefer nonsurgical minimally invasive procedures over invasive surgical procedures. In fact, nonsurgical procedures accounted for >80% of all cosmetic procedures in the United States in 2013.1 In the author’s experience, HA injection is a valuable tool for minimally invasive nasal reshaping and has the advantage of being reversible with hyaluronidase. However, retreatment may be needed to maintain the desired aesthetic correction because HA degrades over time. The efficacy and safety of HA have been established for facial aesthetic indications,16–23 but information on nasal treatments is limited; clinical experience7,9–12,14,15 and data from 2 small studies8,13 and 1 retrospective case series24 have been described. Here, the author presents his clinical experience of nasal treatments with HA during 15 years, with case descriptions to illustrate the use of HA as an alternative or complement to primary and secondary rhinoplasty.
MATERIALS AND METHODS
The recommendations presented here are based on the author’s clinical experience as a plastic surgeon for 33 years and experience of nasal reshaping with HA fillers since 1996. This article focuses on HA injections performed between 1998 and 2012 with those executed during 1998, 2005, and 2012 selected for detailed review (Table 1). All treatments were performed at the author’s clinic, in keeping with routine clinical practice.
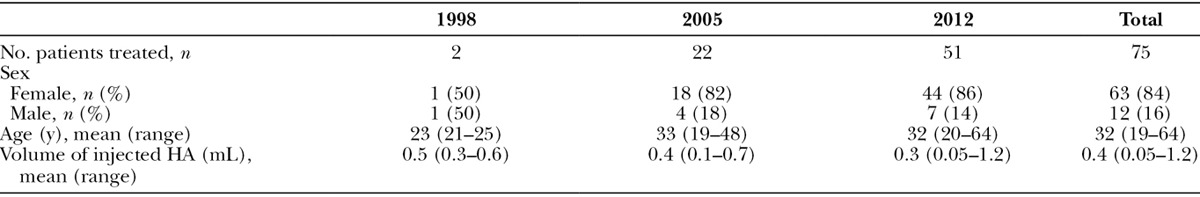
Table 1.
Demographics and Injected Volumes
Pretreatment Analysis
It is important to evaluate the nasal balance to judge if a small augmentation of certain areas can produce a more balanced and/or smaller-looking nose. If the patient has thick nasal skin (Fig. 1), it may be difficult to reduce the nose as thick skin has a limited ability to contract, but an increased projection may achieve balance and give the illusion of a smaller nose. If the patient has thin nasal skin, HA gel can be used to mask protruding cartilage.
Fig. 1.
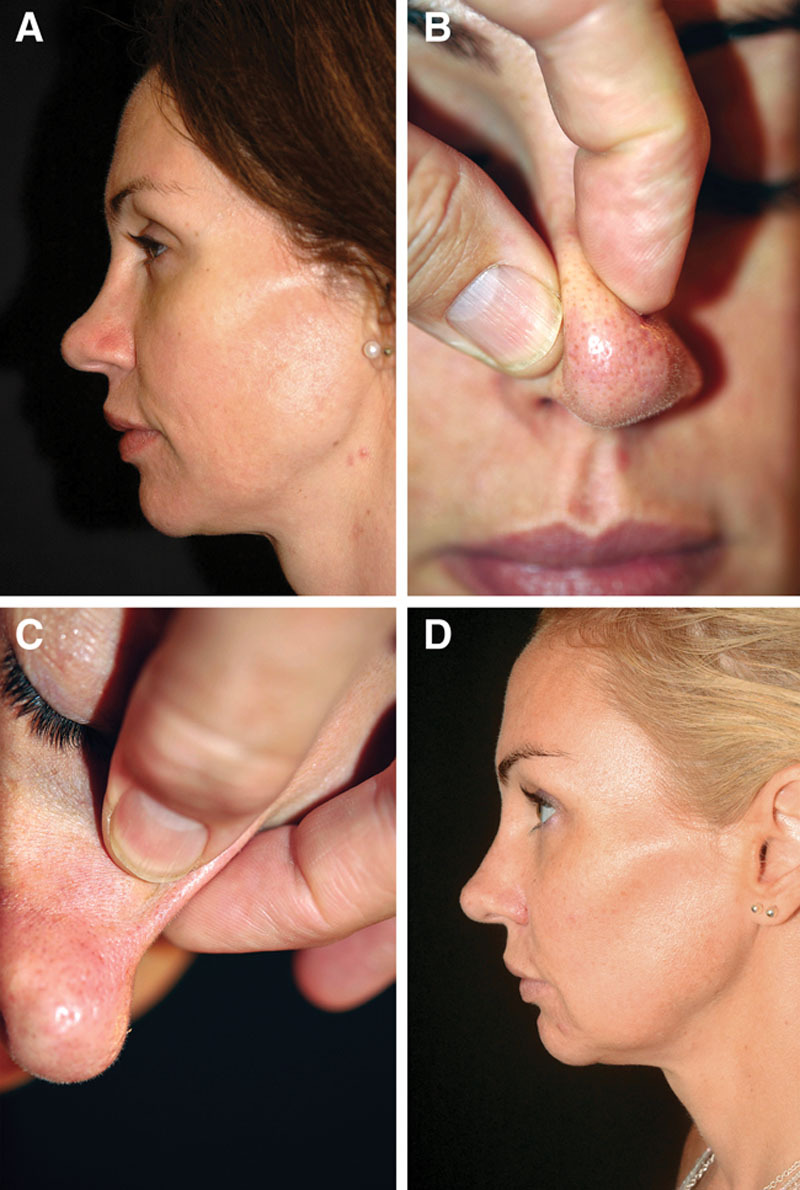
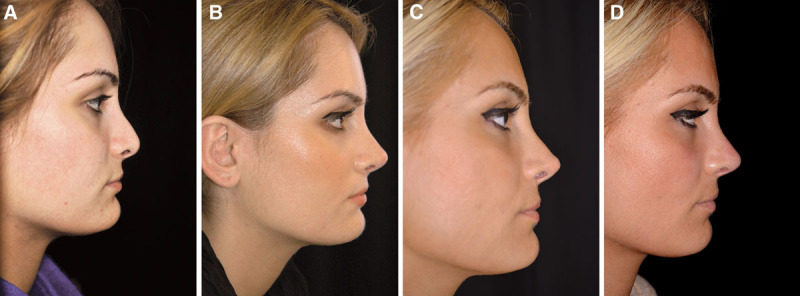
Case 1—to elevate low dorsum and increase tip definition. A, Pretreatment profile view of a healthy 38-year-old woman requesting a reduction of her bulbous tip. She rejected augmentation surgery because she considered that her nose was already too big and had no previous nasal trauma or surgery. B and C, There was a considerable difference in skin thickness when comparing the tip (B) and dorsum (C). Reduction surgery was thus considered to be contraindicated, and the patient agreed to try HA injection instead. Immediately after injection of 0.8 mL Restylane Perlane in the tip (0.15 mL intradermally with a sharp 29-G needle) and dorsum (0.25 mL intradermally with a sharp 29-G needle; 0.4 mL subcutaneously with a blunt 25-G cannula), the patient was positively surprised to find that her nose looked smaller as a result of the injection. D, Two years after the last of 3 injections (2.1 mL in total), the patient said that she had been satisfied with the effect but now considered that most had disappeared. However, after reviewing the before and after pictures, she was impressed to see that the effect actually still persisted and that the difference in appearance at 2 years after the last injection was relatively small compared with immediately after the injection.
A full facial documentation with photographs in 6 positions (frontal, “worms-eye,” oblique, and profile views [left and right]) is recommended. Pretreatment analysis may also involve a 2-dimensional or 3-dimensional picture morphing to illustrate the estimated injection outcome.
Injection Procedure
Injection into areas with signs of infections and acne should be avoided. Treatment area should be cleaned with 70% alcohol and aseptic conditions maintained during the injection. Topical local anesthesia (eg, EMLA cream [AstraZeneca AB; Södertälje, Sweden] for 30–60 min) may be offered for increased treatment comfort. The safety and efficacy profiles of lidocaine-containing HA gels are similar to those of corresponding products without lidocaine.16,23,25 The author used Restylane, Restylane Lidocaine, Restylane Perlane, or Restylane Perlane Lidocaine (Q-Med AB; Uppsala, Sweden) for the majority of nasal treatments, and more recently Emervel Classic, Emervel Deep, or Emervel Lips (Q-Med AB; Uppsala, Sverige, Sweden) was used. Nasal tip injections were generally done using the copacked sharp needle; a blunt 25-G Pix’L cannula (Thiebaud SAS; Paris, France) was favored for the nasal dorsum. HA gel placement is illustrated in Figure 2 and Video 1. (See video, Supplemental Digital Content 1, which shows nasal reshaping with 0.25 mL Restylane Perlane Lidocaine, injected intradermally into the nasal tip [0.15 mL] and intradermally and supraperiostally in the dorsum [0.1 mL] with a sharp 29-G needle. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A283.)
Fig. 2.
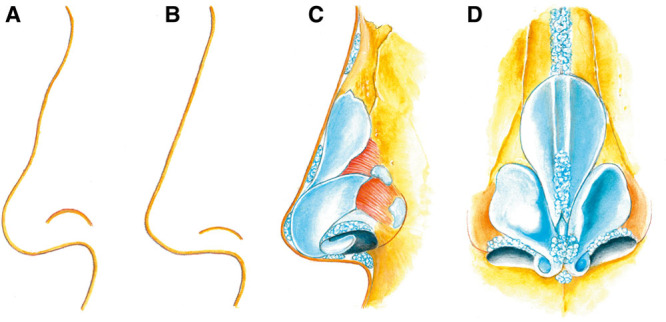
Illustration of HA gel placement for nasal reshaping. A, Example of nasal profile. Common treatment requests include lowering of hump, shortening of the nose, lowering of the alar rim, and widening of the nasolabial angle. B, Example of desired nasal profile, which can be achieved by adding volume rather than reduction of tissue. C, Profile view of HA gel placement to achieve the desired nasal shape. D, Frontal view of HA gel placement to achieve the desired nasal shape. The illustration is published with permission from the copyright holder, Jenny Hanzon.
Video Graphic 1.

Nasal reshaping; this video, Supplemental Digital Content 1, shows nasal reshaping with 0.25 mL Restylane Perlane Lidocaine, injected intradermally into the nasal tip (0.15 mL) and intradermally and supraperiostally in the dorsum (0.1 mL) with a sharp 29-G needle. This video is available in the “Related Videos” section of the full-text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A283.
Minute amounts of gel should be carefully injected, while gradually evaluating the change in shape and with bolus doses avoided to avert intravascular injection. Small volumes of HA are usually sufficient for a pronounced effect, especially in the nasal tip. Overcorrection should be avoided because this may produce a widened and less refined tip. In the nasal tip, the benefit of a superficial (intradermal/hypodermal) injection with a thin needle is the achievement of a better tip definition. Deep injection (directly on the cartilages) usually requires larger volumes of product and may widen the lower lateral cartilages.
The most important difference between injections in alar rim versus alar sill is the risk for intravascular injections. Along the rim, injection is done superficially in the intradermal plane; in the sill, more subcutaneous placement of the gel is needed for widening of the sill.
Important anatomical considerations include the superficial vascular network and the potential risk of intravascular injections. Serious complications, eg, blindness and facial skin necrosis, have been reported after HA injection in the nasal and periorbital region.26–29 To minimize this risk, the author uses blunt cannulas (25-G or wider) in the subcutis and sharp needles only supraperiostally and intradermally. Constant movement of the needle and injection of minimal volumes reduce the risk further. Knowledge of vascular anatomy is of importance for avoiding such complications,30 especially in patients who have had previous nasal surgeries.
The injected product should be smoothed out, not massaged. A full facial photographic documentation is recommended immediately after the treatment.
Posttreatment Assessments
Patients should avoid pressure and massage on the injected area for the first 1 to 2 weeks after treatment. A follow-up visit should be scheduled 6 to 8 weeks after, during which a full facial photographic documentation should be done and, if applicable, touch-up injection. Before and after photographs are useful when discussing treatment results with patients.
RESULTS
This review covers nasal reshaping with HA performed by the author in >250 patients during the period 1997 to 2012. The frequency of nasal HA gel treatments changed from a few treatments per year to several treatments every week. Indications for nasal reshaping with HA gel broadened over time, which was also reflected by the increase in number of patients treated (Table 1). In particular, the frequency of secondary surgical rhinoplasties markedly reduced although the total number of rhinoplasties did not decrease.
Indications for nasal treatments with HA are broader than for conventional rhinoplasty (Fig. 3). HA injection can be used instead of surgical rhinoplasty in patients unwilling to go through general anesthesia or when it is unsuitable for medical reasons. It is also an option for patients with small deformities, for patients who are hesitant toward surgical rhinoplasty, and for whom HA gel injection can give an idea of what could be achieved with surgical rhinoplasty (ie, HA injection becomes a “door opener” for rhinoplasty). In some cases, HA gel injection can also be used as a complement to surgery or for correction of minor postsurgical defects, for instance, in those patients who are not candidates for secondary rhinoplasty and even those fairly satisfied with results of primary rhinoplasty. HA gel can also be used in patients for whom nasal reshaping is required but for whom surgical rhinoplasty is unsuitable (eg, psychological reasons, extent of previous surgeries). Secondary rhinoplasty can be more challenging than primary rhinoplasty and may be technically demanding. In these instances, HA gel injections may also be useful as a simple alternative to surgery to evaluate how masking of the defect may affect its appearance. However, special attention must be given to any alterations in nasal vasculature resulting from previous surgeries. Most patients are unaware of how HA gel injection can change the facial balance; nasal reshaping often gives a greater “wow” effect than other HA injection indications. Patients who undergo HA gel injections for other reasons may also have nasal reshaping as a “bonus” with remaining product after being informed about its versatility.
Fig. 3.
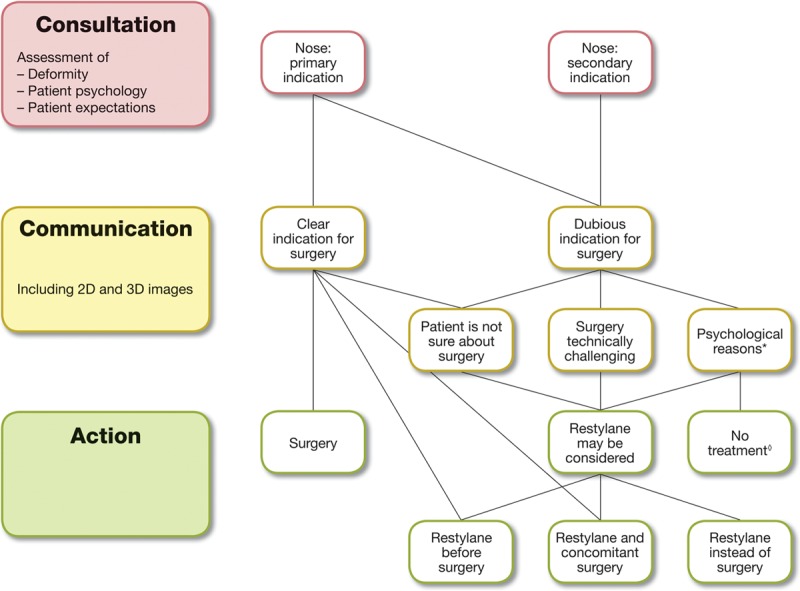
Flow chart illustrating that HA gel injections can be considered for most patients requesting nasal reshaping. *, Psychological reasons may be body dysmorphic disorder; high or unrealistic expectations; fixation with details; dissatisfaction with previous treatment; previous surgery elsewhere; inability to understand treatment limitations; inability to understand information provided and/or difficulty to communicate. ◊, Eg, if the patient has body dysmorphic disorder.
Contraindications include vascular rosacea, unrealistic expectations of treatment results, patients assessed as unsuitable due to psychological reasons, pronounced scarring in the treatment area, bleeding disorders/anticoagulant therapy, or allergy to lidocaine or HA.
Precautions include previous nasal surgery and any intervention that could affect the vasculature or increase risk of vascular compromise. In general, precautions should be considered before rhinoplasty after a filler procedure in the nose. This is especially true if another physician has administrated HA in an unknown manner. Careful examination including palpation of the nose is recommended. Large depositions of HA can be distinguished as “soft” areas and sometimes as a Tyndall effect where the transparency of the gel is seen through the skin. Thus, it is always important to avoid overfilling; only small amounts of HA should be used.
Procedures and Outcomes
Among the 75 patients treated during 1998, 2005, and 2012, the average age was 32 years (range: 19–64 y); 84% were women (Table 1). Mean injection volume was 0.4 mL/treatment. Restylane Perlane Lidocaine was most commonly used. In general, small volumes of HA gel were used (<0.1 mL in the tip and <0.8 mL in the dorsum). The most common corrections were increased tip definition and straightening of dorsal hump (Table 2, Figs. 1, 4–8, and Video 1). Fifty-five patients (73%) received HA injection instead of rhinoplasty; 20 (27%) received HA injection as a secondary correction after rhinoplasty, which had mainly been performed elsewhere (12/20). Five patients (7%) underwent rhinoplasty as a secondary procedure to HA fillers (Table 2); the time lapse between filler procedures and secondary rhinoplasty was >6 months. The reasons for secondary rhinoplasty include that duration of the filler was too short (2 patients), a more drastic effect was sought (1 patient), and the HA injection was a door opener for rhinoplasty (2 patients). Only 16% of patients had a touch-up treatment 6 to 8 weeks after initial injection; retreatment (usually after 1 y) was performed in 32 patients (43%). The ratio of patients receiving retreatment indicates durable cosmetic effect, supported by a high satisfaction rate after treatment and a long duration of effect in a substantial number of patients.
Table 2.
Treatment Indications
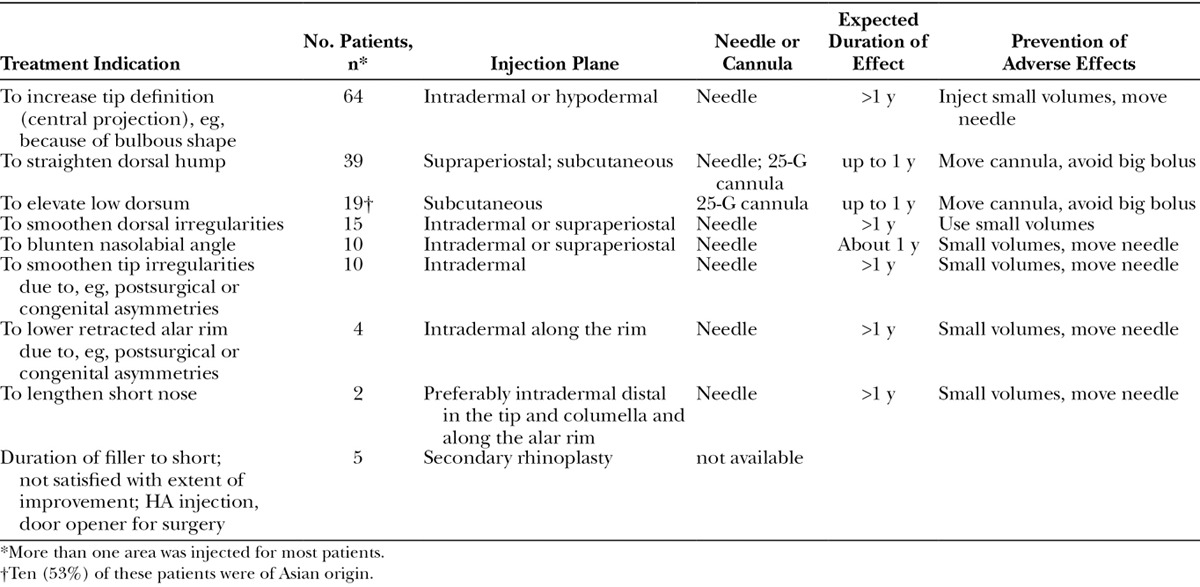
Fig. 4.
Case 2—to straighten dorsal hump and increase tip definition. A, Profile view of a healthy 34-year-old woman who had had an unknown permanent filler injected into the upper lip (in another country) and presented with an unfavorable result and a wish to address her profile view. She had no other relevant medical or surgical history. B, Two weeks after an endoscopic forehead lift and simultaneous lower lip augmentation with 0.8 mL Emervel Lips (using a 25-G blunt cannula) and nasal reshaping with the remaining Emervel Lips (0.2 mL in the tip and 0.3 mL along the dorsum using a sharp 30-G × ½-inch needle), the nose looked smaller (shorter and more balanced).
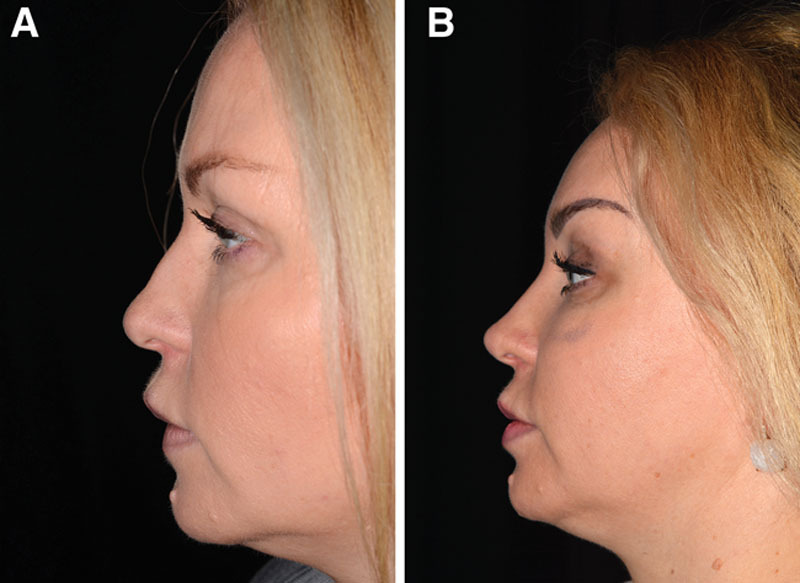
Fig. 8.
Case 6—to lengthen short nose. A, Profile view of a healthy 19-year-old woman who wished to lengthen the nose. As this was considered to be a difficult surgical procedure, the author decided to try to improve the length by HA injection. She had no relevant medical or surgical history. B, Improvement in nasal length after 2 years and 2 injections of Restylane Perlane (total volume injected: 1.5 mL). The HA was deposited intradermally with a sharp 27-G needle in the distal end of the tip in the columella (0.7 mL), along the alar rims (0.2 mL) and in the nasion (0.1 mL), and subcutaneously in the nasion along the dorsum with a 25-G cannula (0.5 mL). Even after 5 years, the author has noted that the length of the patient’s nose was still maintained (image not provided).
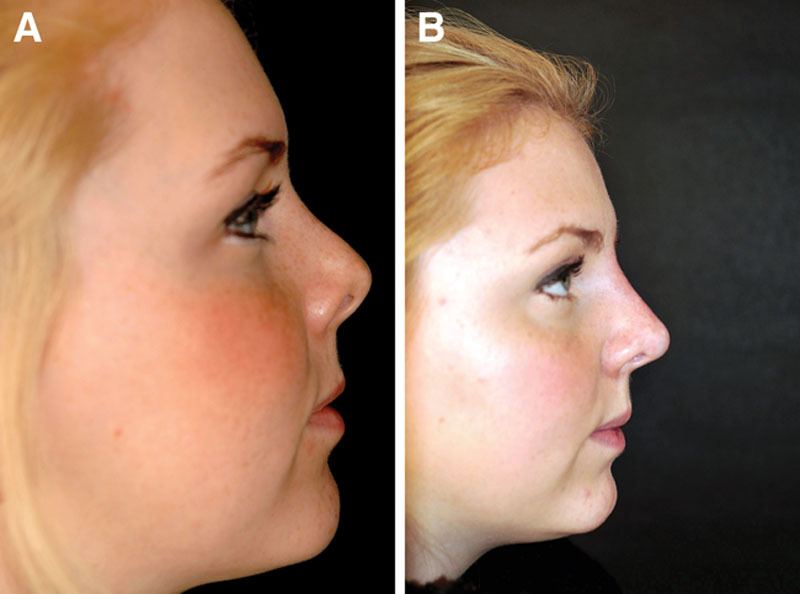
Fig. 5.
Case 3—to smoothen postsurgical tip irregularities. A, Profile view of a 25-year-old woman who presented with a poorly defined and underprojected tip after undergoing 2 previous rhinoplasties (performed elsewhere). She had no other relevant medical or surgical history. B, Appearance 10 months after a successful closed rhinoplasty with triple conchal tip grafts. C, Four years after the tertiary surgery, the patient presented with increasing visibility of the tip grafts. D, Appearance 2 years after intradermal injection of 0.1 mL Restylane Perlane using a sharp 29-G needle around the tip graft (mainly in the supratip area), which effectively masked the graft visibility.
Fig. 7.
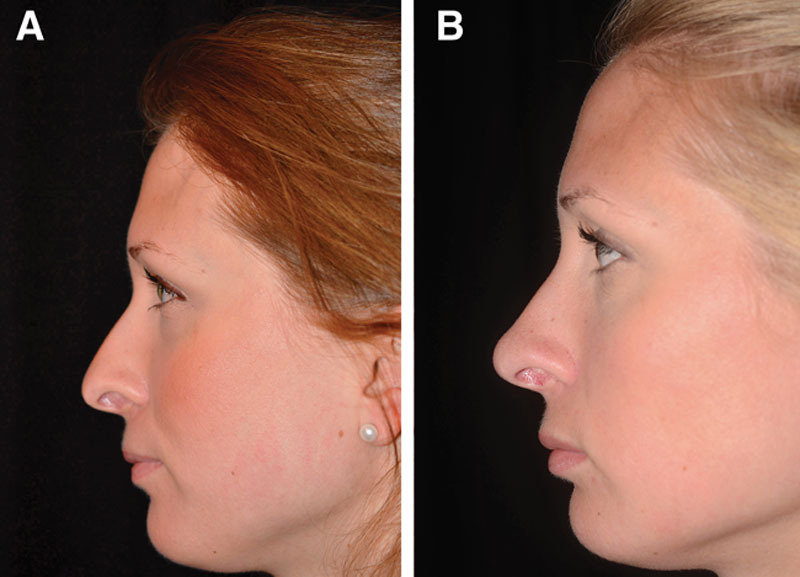
Case 5—to increase tip definition, elevate low dorsum, and lower retracted alar rim. A, Profile view of a healthy 27-year-old woman who presented with an underprojected tip and also wished to address the height of the dorsum and the retracted alar rim. She had no relevant medical or surgical history. B, Appearance 6 months after injection of 1 mL Restylane (0.1 mL/side along the alar rim, 0.2 mL in the tip, and 0.6 mL in the nasion) to correct the shape of the tip, alar rim, and nasion. The HA was deposited not only intradermally with a sharp 27-G needle in all treated regions but also supraperiostally in the nasion.
In addition to use for aesthetic purposes, the author has also injected HA gel to address functional nasal deficiencies. For example, 1 patient experienced improved nasal breathing after intranasal injection along the dorsal septal edge (under the upper lateral cartilage) to simulate the effect of a spreader graft, combined with injection in the base of the nostril to widen the alar rim (Video 2). (See video, Supplemental Digital Content 2 , which shows functional nasal reshaping with intranasal injection of 0.3 mL Restylane Perlane using a sharp 29-G needle along the dorsal septal edge [under the upper lateral cartilage] to simulate the effect of a spreader graft, combined with injection in the base of the nostril to widen the alar rim. This video is available in the “Related Videos” section of the full-text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A284.) Duration of effect was >1 year.
Video Graphic 2.
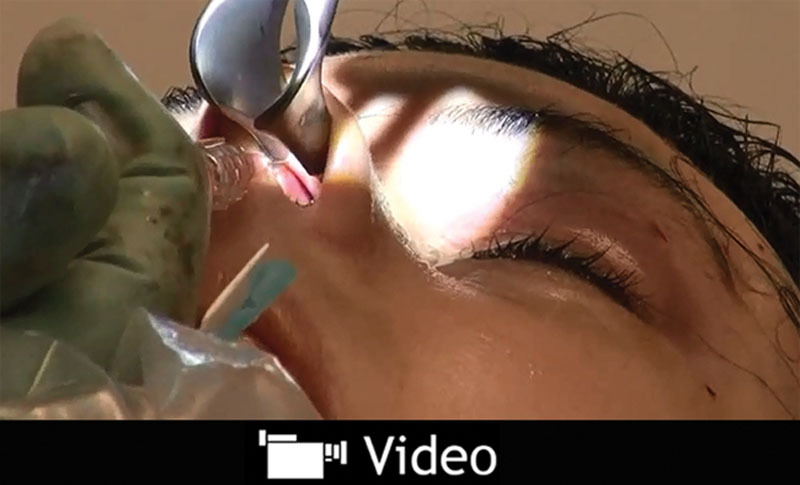
Functional nasal reshaping; this video, Supplemental Digital Content 2, shows functional nasal reshaping with intranasal injection of 0.3 mL Restylane Perlane using a sharp 29-G needle along the dorsal septal edge (under the upper lateral cartilage) to simulate the effect of a spreader graft, combined with injection in the base of the nostril to widen the alar rim. This video is available in the “Related Videos” section of the full-text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A284.
Assessment of Effect and Adverse Events
All patients treated during 1998, 2005, and 2012 were satisfied (35%) or very satisfied (65%) with the primary outcome as determined by a self-assessment scale with the following 5 points: “very dissatisfied,” “dissatisfied,” “neither dissatisfied nor satisfied,” “satisfied,” or “very satisfied.” The vast majority (93%) were still at least satisfied at the follow-up visit 6 to 8 weeks after the primary injection.
Long-term, less than one-third of all patients treated during 1997 to 2012 wished that the duration of effect would have been longer. In most patients, the duration of effect was >1 year after the treatment. In approximately 30% of patients, the duration was >2 years, and in some cases >8 years (Fig. 6).
Fig. 6.
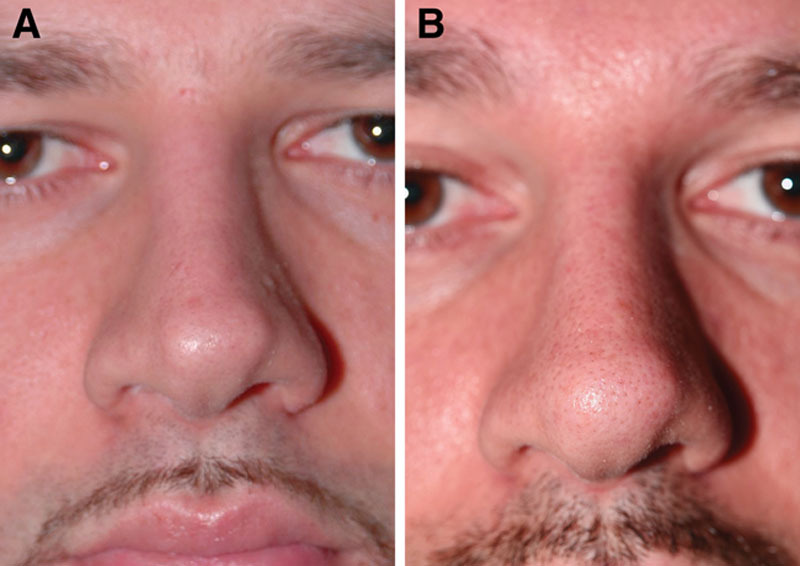
Case 4—to smoothen postsurgical tip irregularities. A, Two years after rhinoplasty in a 26-year-old man; the patient came back wishing to address an increasing tip graft visibility that he had first started to notice during the past year. He had no other relevant medical or surgical history. Secondary surgery would necessitate a difficult and relatively risky procedure for such a small correction because an open rhinoplasty would likely be needed, and there would also be a higher risk for supratip swelling and worsening of the defect. Therefore, HA injection was preferred for this patient. B, Ten years after intradermal injection of 0.1 mL Restylane with a sharp 29-G needle around the edges of the tip graft; the tip graft was still effectively masked, and the patient was satisfied (1 retreatment intradermal injection with 0.05 mL Restylane using a sharp 29-G needle was given 5½ y after the first injection). The photographs are published with permission from the patient.
Adverse effects occurred in a relatively small proportion of patients. Although >250 patients were treated with HA injections, no serious complications, eg, infections, skin necroses, or visual impairment, occurred, and hyaluronidase injection was not required in any case. These risks were prevented by ensuring treatment of proper patient population, use of sterile conditions, slow injections of minimal volumes of HA; moving the needle while injecting; and avoiding bolus injections. Three patients had an increase in telangiectatic vessels and erythema; however, review of their medical history and pretreatment pictures revealed signs of rosacea or nasal/perinasal telangiectatic vessels. Treatment with pulsed dye laser (V-beam Perfecta; Candela, Irvine, Calif.) resolved the problem.
Short duration (<6 mo) or insufficient effect was noted in approximately 10% of the patients. Among these, some patients had severely scarred nasal tips that were difficult to correct.
DISCUSSION
In the author’s experience, injection of HA gel is a valuable tool for minimally invasive nasal reshaping. Experienced plastic surgeons can use HA injection as an alternative/complement to many indications for rhinoplasty because of its versatility (Fig. 3). In the author’s opinion, this is infrequently considered by many surgeons.
Benefits with HA injection include a quick and noninvasive method to change nasal features without need for general anesthesia. The procedure is associated with no/minimal downtime and with lower cost per treatment compared with rhinoplasty.1 Minor and sometimes time-consuming and risky secondary surgical procedures can sometimes be avoided with HA injection. In addition, HA gel injections are useful for preserving the height of the nose, which can be challenging with a surgical reshaping rhinoplasty. The nonpermanent nature of HA and reversibility with hyaluronidase are also favorable properties. Limitations include a relatively short duration of effect in some cases and thus need for retreatment.
Although use of HA in aesthetic facial treatments is well established for treatment of wrinkles and folds, most patients are unaware of nasal indications. As a nonsurgical minimally invasive alternative to rhinoplasty, it would likely appeal to many patients who wish to modify the appearance of their nose. HA treatment may also serve as a door opener to surgery for patients who are reluctant to undergo rhinoplasty.
A thorough pretreatment assessment and knowledge of the anatomy of the nose are of utmost importance for a successful treatment result. With a more generous attitude toward small refinements after rhinoplasty, a higher patient satisfaction can be achieved, and these small adjustments are often long lasting. It is not uncommon that patients forget how their nose used to look (eg, as in Figure 1) and complain about short duration of effect. Before and after pictures are therefore essential. When reviewing such pictures, most patients are surprised to note that the treatment effect persisted. In the author’s experience, the duration of effect persisted >1 year for most patients and in some cases >5 years. This range in duration may be a reflection of individual variability in gel degradation, which has been reported from clinical studies where HA was used for other indications.31,32 Persistence in nasolabial folds for at least 6 months,22 18 months (with 1 retreatment),18 and 36 months (with 2 retreatments)19 has been reported. Although the data presented here are not from a controlled clinical study, the duration of effect after 1 treatment seems to be longer in the nose than in nasolabial folds, consistent with the experience of other investigators.15 Possible explanations may be lower degradation rates in the nose, minimal muscular activity, and/or differences in metabolic activity compared with, eg, lips and nasolabial folds. Other investigators propose that injection of cross-linked HA stimulates collagen synthesis,33,34 which could be another possible explanation.
Use of permanent fillers in the nose should be avoided, because of association with granuloma formation35,36 and complications that are difficult to manage.37 Possible complications with HA fillers include vascular emboli and necrosis,38–42 ocular ischemia,27 vision loss,26,28,29 discoloration/Tyndall effect,43 and infection. None of these complications have occurred in the >250 patients treated by the author to date.
To avoid complications, a thorough patient consultation and pretreatment assessment are recommended. HA injections in the nose should only be done by experienced plastic surgeons with a profound knowledge of the nasal anatomy, with special attention to any alterations resulting from previous nasal surgeries. Only small volumes of HA gel should be injected, using a slow and gentle technique; overfilling must be avoided because of the risk of serious complications (eg, pressure necrosis or vascular embolus). If large volumes are needed, placement of autologous cartilage grafts may be advantageous to not risk tissue necrosis or implant visibility due to thin, transparent skin. Injection close to nasal vessels should be avoided, and also use of a sharp needle in the perivascular area. The facial artery’s nasal branches are especially important to consider. In addition, the dorsal nasal vessels sometimes anastomose over the midline,30 making the risk for vascular embolization lower but still possible in the midline. To minimize the risk of intravascular injection and potential skin necrosis or blindness, the following recommendations are suggested. Injection into the rhinion of the nose should be done directly on the periosteum. Risk of vessel penetration is also reduced with 25-G or wider cannulas in the subdermal layer. Risk for intra-arterial injection is minimized with cannulas especially with thin cannulas (28- to 30-G). If sharp needles are used, injections should be placed directly on the periosteum or in an intradermal or hypodermal position. When using a sharp needle, it is important to only inject minute volumes, to move the needle (eg, as shown in Video 1) and avoid bolus injections.
Although it is important to minimize the risk of complications, it is equally important to be prepared to manage complications that may arise. A quick recognition of vascular events and quick and aggressive treatment are necessary to avoid serious, potentially irreversible complications. The injecting physician should always be prepared to treat not only complications with hyaluronidase but also allergies toward hyaluronidase.
If vascular compromise is suspected/occurs, a similar protocol as suggested by not only DeLorenzi44 but also various others36,45,46 is recommended. Immediately discontinue injection if whitening of the skin distal to the cannula tip or intense pain occurs. Inject hyaluronidase, at least 100 U for every 1 mL of filler used. To induce vasodilation, apply gentle massage, warm compresses and nitroglycerin ointment to the affected area, and apply gentle massage to dissipate the mass with resolution. Administer anticoagulants (low–molecular-weight heparin/aspirin). If visual impairment occurs, immediately refer the patient to an ophthalmologist/oculoplastic surgeon for further treatment (eg, injection of hyaluronidase in a retrobulbar location or into the ophthalmic artery [possibly by radiology-assisted catheterization or periocular arterial catheterization] and reduction of intraocular pressure).47
Aspiration of HA may be attempted if Tyndall effect occurs, but the author prefers hyaluronidase injection for effective treatment. Suspected or confirmed infection should be treated with antibiotics commenced before use of hyaluronidase.
CONCLUSIONS
Injection of HA gel is a valuable tool for plastic surgeons to consider for nasal reshaping. Small corrective refinements offered to patients may help achieve higher patient satisfaction and have in many cases had a surprisingly long duration of effect. The clinical experience gained with HA gel injections for nasal treatments over 15 years has also shown that HA gel can be used for correction of minor postrhinoplasty defects in appropriate patients.
ACKNOWLEDGMENTS
Medical writing assistance was provided by Dr. Carolina Edwartz on behalf of Galderma. The author thanks Ms. Jenny Hanzon for the drawings illustrating HA gel placement.
PATIENT CONSENT
Patients provided written consent for the use of their images.
Supplementary Material
Footnotes
Presented in part at the 8th International Beauty through Science meeting, Waterfront Conference Center, Stockholm, Sweden, June 7–9, 2012.
Disclosure: Dr Hedén is a consultant for Galderma and has received payment for lectures and travel but has no other financial interest in the company or their products. Medical writing assistance was funded by Galderma. The Article Processing Charge was paid for by the author.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.The American Society for Aesthetic Plastic Surgery. Cosmetic surgery national data bank statistics. Available at: http://www.surgery.org/sites/default/files/Stats2013_3.pdf. Accessed April 03, 2014. [DOI] [PubMed]
- 2.Jacovella PF. Aesthetic nasal corrections with hydroxylapatite facial filler. Plast Reconstr Surg. 2008;121:338e–339e. doi: 10.1097/PRS.0b013e31816b112e. [DOI] [PubMed] [Google Scholar]
- 3.Rokhsar C, Ciocon DH. Nonsurgical rhinoplasty: an evaluation of injectable calcium hydroxylapatite filler for nasal contouring. Dermatol Surg. 2008;34:944–946. doi: 10.1111/j.1524-4725.2008.34182.x. [DOI] [PubMed] [Google Scholar]
- 4.Siclovan HR, Jomah JA. Injectable calcium hydroxylapatite for correction of nasal bridge deformities. Aesthetic Plast Surg. 2009;33:544–548. doi: 10.1007/s00266-008-9281-0. [DOI] [PubMed] [Google Scholar]
- 5.Stupak HD, Moulthrop TH, Wheatley P, et al. Calcium hydroxylapatite gel (Radiesse) injection for the correction of postrhinoplasty contour deficiencies and asymmetries. Arch Facial Plast Surg. 2007;9:130–136. doi: 10.1001/archfaci.9.2.130. [DOI] [PubMed] [Google Scholar]
- 6.Braccini F, Dohan Ehrenfest DM. [Medical rhinoplasty: rationale for atraumatic nasal modelling using botulinum toxin and fillers]. Rev Laryngol Otol Rhinol (Bord) 2008;129:233–238. [PubMed] [Google Scholar]
- 7.Bray D, Hopkins C, Roberts DN. Injection rhinoplasty: non-surgical nasal augmentation and correction of post-rhinoplasty contour asymmetries with hyaluronic acid: how we do it. Clin Otolaryngol. 2010;35:227–230. doi: 10.1111/j.1749-4486.2010.02125.x. [DOI] [PubMed] [Google Scholar]
- 8.Han SK, Shin SH, Kang HJ, et al. Augmentation rhinoplasty using injectable tissue-engineered soft tissue: a pilot study. Ann Plast Surg. 2006;56:251–255. doi: 10.1097/01.sap.0000198549.64341.17. [DOI] [PubMed] [Google Scholar]
- 9.Humphrey CD, Arkins JP, Dayan SH. Soft tissue fillers in the nose. Aesthet Surg J. 2009;29:477–484. doi: 10.1016/j.asj.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Redaelli A. Medical rhinoplasty with hyaluronic acid and botulinum toxin A: a very simple and quite effective technique. J Cosmet Dermatol. 2008;7:210–220. doi: 10.1111/j.1473-2165.2008.00391.x. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka Y, Matsuo K, Yuzuriha S. Westernization of the Asian nose by augmentation of the retropositioned anterior nasal spine with an injectable filler. Eplasty. 2011;11:e7. [PMC free article] [PubMed] [Google Scholar]
- 12.Xue K, Chiang CA, Liu K, et al. Multiplane hyaluronic acid rhinoplasty. Plast Reconstr Surg. 2012;129:371e–372e. doi: 10.1097/PRS.0b013e31823af0bd. [DOI] [PubMed] [Google Scholar]
- 13.Liapakis IE, Englander M, Vrentzos NP, et al. Secondary rhinoplasty fixations with hyaluronic acid. J Cosmet Dermatol. 2013;12:235–239. doi: 10.1111/jocd.12046. [DOI] [PubMed] [Google Scholar]
- 14.Wong GR, Chen WP. Phosphatidylcholine/deoxycholate lipolysis and hyaluronic acid augmentation to enhance nonsurgical lower facial contouring using botulinum toxin type A. J Cosmet Dermatol. 2011;10:159–162. doi: 10.1111/j.1473-2165.2011.00549.x. [DOI] [PubMed] [Google Scholar]
- 15.Kurkjian TJ, Ahmad J, Rohrich RJ. Soft-tissue fillers in rhinoplasty. Plast Reconstr Surg. 2014;133:121e–126e. doi: 10.1097/01.prs.0000437246.61294.33. [DOI] [PubMed] [Google Scholar]
- 16.Brandt F, Bank D, Cross SL, et al. A lidocaine-containing formulation of large-gel particle hyaluronic acid alleviates pain. Dermatol Surg. 2010;36:1876–1885. doi: 10.1111/j.1524-4725.2010.01777.x. [DOI] [PubMed] [Google Scholar]
- 17.Glogau RG, Bank D, Brandt F, et al. A randomized, evaluator-blinded, controlled study of the effectiveness and safety of small gel particle hyaluronic acid for lip augmentation. Dermatol Surg. 2012;38:1180–1192. doi: 10.1111/j.1524-4725.2012.02473.x. [DOI] [PubMed] [Google Scholar]
- 18.Narins RS, Dayan SH, Brandt FS, et al. Persistence and improvement of nasolabial fold correction with nonanimal-stabilized hyaluronic acid 100,000 gel particles/mL filler on two retreatment schedules: results up to 18 months on two retreatment schedules. Dermatol Surg. 2008;34:S2–S8. doi: 10.1111/j.1524-4725.2008.34236.x. [DOI] [PubMed] [Google Scholar]
- 19.Narins RS, Brandt FS, Dayan SH, et al. Persistence of nasolabial fold correction with a hyaluronic acid dermal filler with retreatment: results of an 18-month extension study. Dermatol Surg. 2011;37:644–650. doi: 10.1111/j.1524-4725.2010.01863.x. [DOI] [PubMed] [Google Scholar]
- 20.Rzany B, Cartier H, Kestemont P, et al. Full-face rejuvenation using a range of hyaluronic acid fillers: efficacy, safety, and patient satisfaction over 6 months. Dermatol Surg. 2012;38:1153–1161. doi: 10.1111/j.1524-4725.2012.02470.x. [DOI] [PubMed] [Google Scholar]
- 21.Taylor SC, Burgess CM, Callender VD. Safety of nonanimal stabilized hyaluronic acid dermal fillers in patients with skin of color: a randomized, evaluator-blinded comparative trial. Dermatol Surg. 2009;35:1653–1660. doi: 10.1111/j.1524-4725.2009.01344.x. [DOI] [PubMed] [Google Scholar]
- 22.Taylor SC, Burgess CM, Callender VD. Efficacy of variable-particle hyaluronic acid dermal fillers in patients with skin of color: a randomized, evaluator-blinded comparative trial. Dermatol Surg. 2010;36:741–749. doi: 10.1111/j.1524-4725.2009.01344.x. [DOI] [PubMed] [Google Scholar]
- 23.Weiss R, Bank D, Brandt F. Randomized, double-blind, split-face study of small-gel-particle hyaluronic acid with and without lidocaine during correction of nasolabial folds. Dermatol Surg. 2010;36:750–759. [Google Scholar]
- 24.Chen L, Li SR, Yu P, et al. Comparison of Artecoll, Restylane and silicone for augmentation rhinoplasty in 378 Chinese patients. Clin Invest Med. 2014;37:E203–E210. doi: 10.25011/cim.v37i4.21725. [DOI] [PubMed] [Google Scholar]
- 25.Hedén P, Fagrell D, Jernbeck J, et al. Injection of stabilized hyaluronic acid-based gel of non-animal origin for the correction of nasolabial folds: comparison with and without lidocaine. Dermatol Surg. 2010;36:775–781. [Google Scholar]
- 26.Kim SN, Byun DS, Park JH, et al. Panophthalmoplegia and vision loss after cosmetic nasal dorsum injection. J Clin Neurosci. 2014;21:678–680. doi: 10.1016/j.jocn.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Kim YJ, Kim SS, Song WK, et al. Ocular ischemia with hypotony after injection of hyaluronic acid gel. Ophthal Plast Reconstr Surg. 2011;27:e152–e155. doi: 10.1097/IOP.0b013e3182082f37. [DOI] [PubMed] [Google Scholar]
- 28.He MS, Sheu MM, Huang ZL, et al. Sudden bilateral vision loss and brain infarction following cosmetic hyaluronic acid injection. JAMA Ophthalmol. 2013;131:1234–1235. doi: 10.1001/jamaophthalmol.2013.1603. [DOI] [PubMed] [Google Scholar]
- 29.Kim EG, Eom TK, Kang SJ. Severe visual loss and cerebral infarction after injection of hyaluronic acid gel. J Craniofac Surg. 2014;25:684–686. doi: 10.1097/SCS.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 30.Lee HJ, Kang IW, Won SY, et al. Description of a novel anatomic venous structure in the nasoglabellar area. J Craniofac Surg. 2014;25:633–635. doi: 10.1097/SCS.0000000000000649. [DOI] [PubMed] [Google Scholar]
- 31.Camenisch CC, Tengvar M, Hedén P. Macrolane for volume restoration and contouring of the buttocks: magnetic resonance imaging study on localization and degradation. Plast Reconstr Surg. 2013;132:522e–529e. doi: 10.1097/PRS.0b013e31829fe47e. [DOI] [PubMed] [Google Scholar]
- 32.Hedén P, Olenius M, Tengvar M. Macrolane for breast enhancement: 12-month follow-up. Plast Reconstr Surg. 2011;127:850–860. doi: 10.1097/PRS.0b013e318200ae57. [DOI] [PubMed] [Google Scholar]
- 33.Quan T, Wang F, Shao Y, et al. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J Invest Dermatol. 2013;133:658–667. doi: 10.1038/jid.2012.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang F, Garza LA, Kang S, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143:155–163. doi: 10.1001/archderm.143.2.155. [DOI] [PubMed] [Google Scholar]
- 35.Carruthers J, Cohen SR, Joseph JH, et al. The science and art of dermal fillers for soft-tissue augmentation. J Drugs Dermatol. 2009;8:335–350. [PubMed] [Google Scholar]
- 36.Narins RS, Jewell M, Rubin M, et al. Clinical conference: management of rare events following dermal fillers–focal necrosis and angry red bumps. Dermatol Surg. 2006;32:426–434. doi: 10.1111/j.1524-4725.2006.32086.x. [DOI] [PubMed] [Google Scholar]
- 37.Andre P, Lowe NJ, Parc A, et al. Adverse reactions to dermal fillers: a review of European experiences. J Cosmet Laser Ther. 2005;7:171–176. doi: 10.1080/14764170500344393. [DOI] [PubMed] [Google Scholar]
- 38.Grunebaum LD, Bogdan Allemann I, Dayan S, et al. The risk of alar necrosis associated with dermal filler injection. Dermatol Surg. 2009;35:1635–1640. doi: 10.1111/j.1524-4725.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 39.Inoue K, Sato K, Matsumoto D, et al. Arterial embolization and skin necrosis of the nasal ala following injection of dermal fillers. Plast Reconstr Surg. 2008;121:127e–128e. doi: 10.1097/01.prs.0000300188.82515.7f. [DOI] [PubMed] [Google Scholar]
- 40.Kim SG, Kim YJ, Lee SI, et al. Salvage of nasal skin in a case of venous compromise after hyaluronic acid filler injection using prostaglandin E. Dermatol Surg. 2011;37:1817–1819. doi: 10.1111/j.1524-4725.2011.02188.x. [DOI] [PubMed] [Google Scholar]
- 41.Menick FJ. Aesthetic and reconstructive rhinoplasty: a continuum. J Plast Reconstr Aesthet Surg. 2012;65:1169–1174. doi: 10.1016/j.bjps.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Sung HM, Suh IS, Lee HB, et al. Case reports of adipose-derived stem cell therapy for nasal skin necrosis after filler injection. Arch Plast Surg. 2012;39:51–54. doi: 10.5999/aps.2012.39.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandez EM, Mackley CL. Soft tissue augmentation: a review. J Drugs Dermatol. 2006;5:630–641. [PubMed] [Google Scholar]
- 44.DeLorenzi C. Complications of injectable fillers, part 2: vascular complications. Aesthet Surg J. 2014;34:584–600. doi: 10.1177/1090820X14525035. [DOI] [PubMed] [Google Scholar]
- 45.Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295–316. doi: 10.2147/CCID.S50546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beleznay K, Humphrey S, Carruthers JD, et al. Vascular compromise from soft tissue augmentation: experience with 12 cases and recommendations for optimal outcomes. J Clin Aesthet Dermatol. 2014;7:37–43. [PMC free article] [PubMed] [Google Scholar]
- 47.Carruthers JD, Fagien S, Rohrich RJ, et al. Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg. 2014;134:1197–1201. doi: 10.1097/PRS.0000000000000754. [DOI] [PubMed] [Google Scholar]


