Abstract
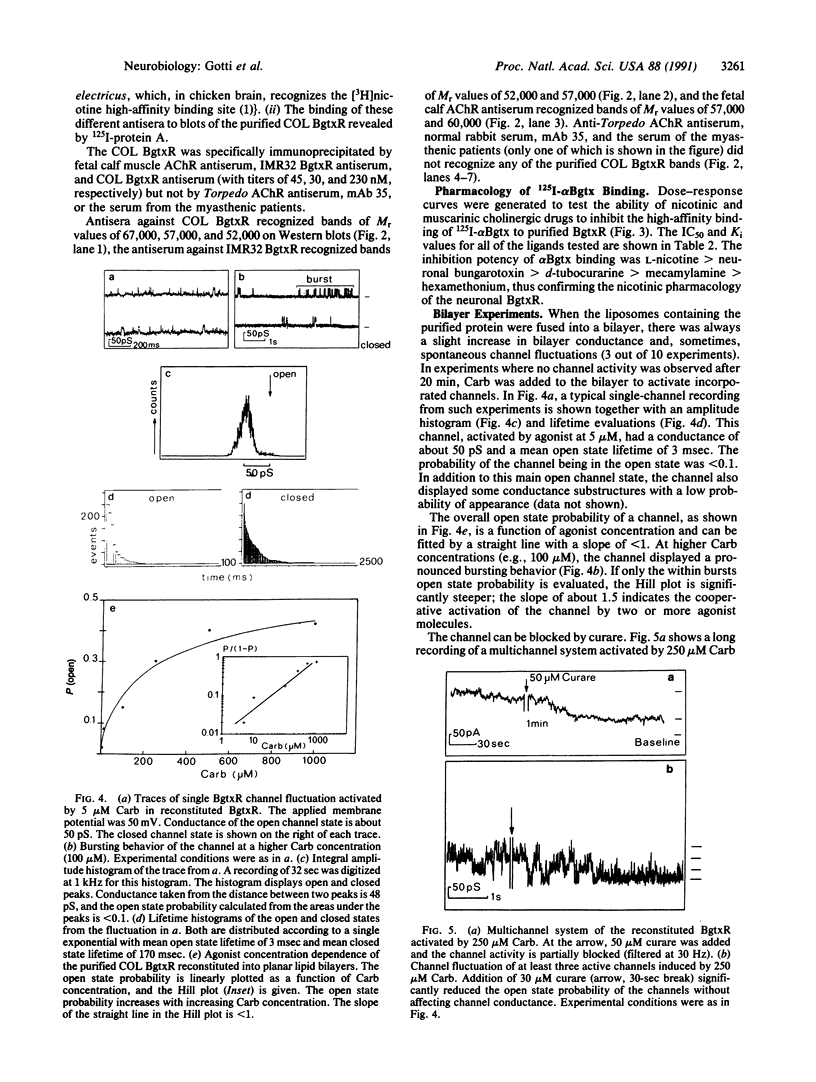
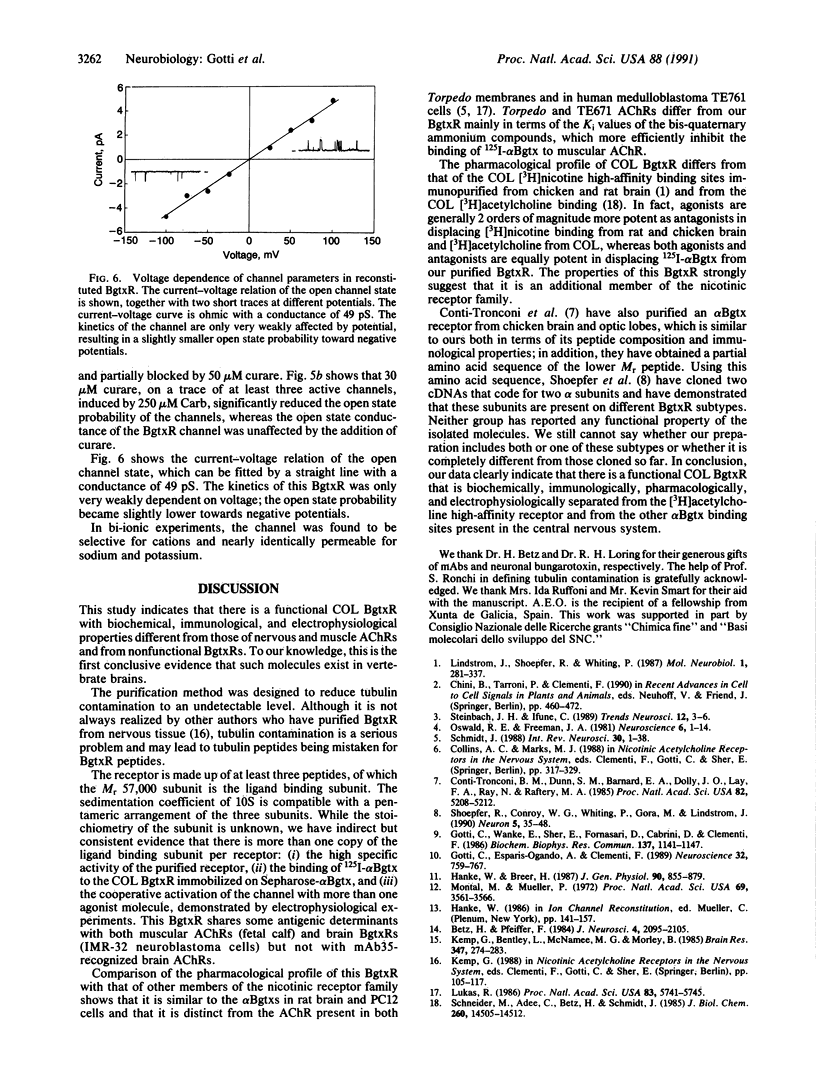
Neither the structure nor the function of alpha-bungarotoxin (alpha Bgtx) binding molecules in the nervous system have yet been completely defined, although it is known that some of these molecules are related to cation channels and some are not. Using an improved method of affinity chromatography, we have isolated a toxin binding molecule from chicken optic lobe that contains at least three subunits with apparent Mr values of 52,000, 57,000, and 67,000. The Mr 57,000 subunit binds alpha Bgtx and seems to be present in two copies per receptor. The receptor is recognized by antibodies raised against the alpha Bgtx receptors of human neuroblastoma cells, fetal calf muscle, and chicken optic lobe but not by antibodies raised against Torpedo acetylcholine receptor, the serum of myasthenic patients, or monoclonal antibody, 35. 125I-labeled alpha Bgtx binding to the isolated receptor is blocked, with the same potency, by nicotinic agonists and antagonists, such as nicotine, neuronal bungarotoxin and, d-tubocurarine. When reconstituted in a planar lipid bilayer, the purified alpha Bgtx receptor forms cationic channels with a conductance of 50 pS. These channels are activated in a dose-dependent manner by carbamylcholine and blocked by d-tubocurarine.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz H., Pfeiffer F. Monoclonal antibodies against the alpha-bungarotoxin-binding protein of chick optic lobe. J Neurosci. 1984 Aug;4(8):2095–2105. doi: 10.1523/JNEUROSCI.04-08-02095.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Dunn S. M., Barnard E. A., Dolly J. O., Lai F. A., Ray N., Raftery M. A. Brain and muscle nicotinic acetylcholine receptors are different but homologous proteins. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5208–5212. doi: 10.1073/pnas.82.15.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C., Ogando A. E., Clementi F. The alpha-bungarotoxin receptor purified from a human neuroblastoma cell line: biochemical and immunological characterization. Neuroscience. 1989;32(3):759–767. doi: 10.1016/0306-4522(89)90296-0. [DOI] [PubMed] [Google Scholar]
- Gotti C., Wanke E., Sher E., Fornasari D., Cabrini D., Clementi F. Acetylcholine operated ion channel and alpha-bungarotoxin binding site in a human neuroblastoma cell line reside on different molecules. Biochem Biophys Res Commun. 1986 Jun 30;137(3):1141–1147. doi: 10.1016/0006-291x(86)90344-x. [DOI] [PubMed] [Google Scholar]
- Hanke W., Breer H. Characterization of the channel properties of a neuronal acetylcholine receptor reconstituted into planar lipid bilayers. J Gen Physiol. 1987 Dec;90(6):855–879. doi: 10.1085/jgp.90.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp G., Bentley L., McNamee M. G., Morley B. J. Purification and characterization of the alpha-bungarotoxin binding protein from rat brain. Brain Res. 1985 Nov 18;347(2):274–283. doi: 10.1016/0006-8993(85)90187-8. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Schoepfer R., Whiting P. Molecular studies of the neuronal nicotinic acetylcholine receptor family. Mol Neurobiol. 1987 Winter;1(4):281–337. doi: 10.1007/BF02935740. [DOI] [PubMed] [Google Scholar]
- Lukas R. J. Immunochemical and pharmacological distinctions between curaremimetic neurotoxin binding sites of central, autonomic, and peripheral origin. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5741–5745. doi: 10.1073/pnas.83.15.5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald R. E., Freeman J. A. Alpha-bungarotoxin binding and central nervous system nicotinic acetylcholine receptors. Neuroscience. 1981;6(1):1–14. doi: 10.1016/0306-4522(81)90239-6. [DOI] [PubMed] [Google Scholar]
- Schneider M., Adee C., Betz H., Schmidt J. Biochemical characterization of two nicotinic receptors from the optic lobe of the chick. J Biol Chem. 1985 Nov 25;260(27):14505–14512. [PubMed] [Google Scholar]
- Schoepfer R., Conroy W. G., Whiting P., Gore M., Lindstrom J. Brain alpha-bungarotoxin binding protein cDNAs and MAbs reveal subtypes of this branch of the ligand-gated ion channel gene superfamily. Neuron. 1990 Jul;5(1):35–48. doi: 10.1016/0896-6273(90)90031-a. [DOI] [PubMed] [Google Scholar]
- Steinbach J. H., Ifune C. How many kinds of nicotinic acetylcholine receptor are there? Trends Neurosci. 1989 Jan;12(1):3–6. doi: 10.1016/0166-2236(89)90145-8. [DOI] [PubMed] [Google Scholar]