Supplemental Digital Content is available in the text.
Abstract
Background:
In abdominal wall reconstruction, the retrorectus plane offers an ideal location for mesh placement. Mesh fixation in this plane is often achieved using transfascial sutures, which risks entrapping intercostal nerves and causing significant pain, and takes time to place. A novel alternative is the use of sutureless self-adhering mesh. Although the use of this mesh in inguinal hernias has been well described, studies on its use in abdominal wall reconstruction are lacking.
Methods:
Consecutive patients who underwent ventral hernia repair with retrorectus mesh were reviewed. This included patients who received transfascially sutured mesh and those who received sutureless self-adhering mesh. All patients were followed up for at least 12 months. The amount of narcotics required by each patient postoperatively was calculated. Surgical-site occurrences (SSOs) and hernia recurrence and bulge were measured.
Results:
Twenty-six patients underwent abdominal wall reconstruction with retrorectus mesh. This included 12 patients with transfascially sutured mesh and 14 patients with self-adhering mesh. Mean follow-up was 600 days. Baseline characteristics were similar between the 2 groups. Patients receiving self-adhering mesh required significantly less narcotics than patients with transfascially sutured mesh. There were no significant differences in the rate of SSOs between the 2 groups. No hernia recurrences, bulges, or chronic pain occurred in either group.
Conclusions:
This is the first study to compare the outcomes of retrorectus self-adhering mesh and transfascially sutured mesh in abdominal wall reconstruction. Our results show low rates of SSO, recurrence, and bulge with both options, with significantly less acute pain with self-adhering mesh.
Incisional ventral hernias are a prevalent problem, affecting up to 10% of patients who have undergone a laparotomy.1 At the time of the initial hernia repair, it is important to achieve the most durable reconstruction as the risk of hernia recurrence increases with every subsequent reconstructive attempt.2
Multiple well-designed studies have shown that, to reduce hernia recurrence, most hernia repairs should be reinforced with mesh.3–7 In addition, primary fascial reapproximation should be obtained to reduce the risk of recurrence and bulge.8 Furthermore, to minimize the risks of surgical-site occurrences (SSOs) and hernia recurrence, most authors agree that the ideal plane for mesh placement is the retrorectus plane, with at least 4 cm of overlap between the mesh and the fascia on either side.9 This highly vascular plane is particularly attractive as it avoids contact between the mesh and the intraabdominal contents and protects the mesh from exposure in case of wound healing complication.
Fixation of mesh in the retrorectus plane can be performed using sutures between the mesh and the rectus sheath reflection at the semilunar line. Alternatively, percutaneous sutures can be placed through the anterior rectus sheath or through the obliques/transversus abdominis complex, which risks entrapping intercostal nerves in the sutures or even devascularizing segments of the muscle. Another alternative, which is the technique evaluated in this study, is the use of self-adhering mesh.
ProGrip (Covidien, Dublin, Ireland) is a semiresorbable self-adhering mesh, consisting of a permanent monofilament macroporous polyester mesh, with resorbable microgrips made of polylactic acid. The microgrips provide secure mesh fixation and distribute the tension along the entire surface area of the mesh, allowing strong purchase of the mesh against the tissue.10 The strength of the mesh purchase against tissues has been found to be superior to laparoscopic staples and fibrin glue at 5 days and 2 months, respectively.11 The mesh also has been found to achieve good tissue incorporation.10
Self-adhering mesh has been used extensively in inguinal hernia repair where its ability to be inset without sutures constitutes a particularly attractive feature12 as the use of sutures in inguinal hernia repairs has raised concern for nerve entrapment and chronic groin pain postoperatively.13,14 The use of self-adhering mesh in inguinal hernia repair has been found in some studies to result in lower levels of acute pain in the early postoperative period,15 leading to faster return to normal activity,16 although other studies failed to demonstrate a reduction in chronic groin pain.17–23 Self-adhering mesh has also been found to provide a durable repair in inguinal hernias, providing equivalent hernia recurrence rates to sutured polypropylene mesh. Birk et al24 performed inguinal hernia repairs using ProGrip via the laparoscopic transabdominal preperitoneal approach and found a 1.9% recurrence at 22.8 months. Other authors did not observe any recurrences.15 The equivalent durability between ProGrip and sutured polypropylene mesh in inguinal hernia repairs has been confirmed by multiple randomized controlled trials17,25–29 and meta-analyses.18–21
Although the use of self-adhering mesh in inguinal hernia repair has been very well described and studied, studies on its use in ventral hernia repair are limited. Verhelst et al30 described their outcomes with retrorectus placement of ProGrip mesh. They did not observe any hernia recurrences although their average follow-up duration was only 12 weeks. In addition, there was no comparison group, and postoperative pain was not evaluated.
Our purposes with this study were to describe our pilot experience with the use of self-adhering, sutureless mesh in ventral hernia repair and to compare our short- and long-term outcomes with the use of this mesh with our outcomes with the use of transfascially sutured mesh.
METHODS
After institutional review board approval, a retrospective review of 26 consecutive patients who underwent elective ventral hernia repair with mesh placement in the retrorectus plane with primary musculofascial reapproximation with at least 12-month follow-up was performed. This included patients who underwent placement of sutureless, self-adhering mesh and patients who underwent placement of synthetic or biologic mesh fixated using transfascial sutures. All patients underwent the procedure by the same surgeon (J.E.J.) at an academic medical center. Preoperative patient and hernia characteristics, intraoperative details, and postoperative complications were collected. The patients were risk stratified using the Kanters grade, a validated grading system that predicts the risk of SSOs (Table 1).31
Table 1.
Kanters Grading System31

The average daily amount of narcotic analgesics required postoperatively by each patient while in the hospital was calculated and converted to milligrams of oral morphine equivalents. SSOs were measured at 30 days postoperatively, consisting of hematoma, seroma, infection, dehiscence, mesh exposure/extrusion, and enterocutaneous fistula. Postoperative hernia recurrence and bulge were detected either clinically or using computer tomography at least 12 months postoperatively. Statistical analyses included t test analysis and Fisher’s exact test. Multivariate logistic regression was used to determine the predictors of lower narcotic requirement, using the following variables: use of self-adhering mesh, narrow hernia width, epidural catheter, multimodal analgesia, and no previous narcotic usage. Statistical analyses were performed using Minitab 17 (Minitab Inc., State College, Penn.).
Preoperative Patient Selection and Surgical Technique
In all patients, the operation began with an exploratory laparotomy including lysis of adhesions. The retrorectus plane was then developed: the rectus muscles on either side were palpated, noting that they were often quite lateralized. Manual palpation was used to identify the medial edge of each rectus muscle, and a small incision was made along the medial rectus reflection, which was continued superiorly and inferiorly. The rectus muscle and the retrorectus fat were then carefully dissected off the posterior rectus sheath, starting cranially and medially and heading caudally and laterally. Special caution was exercised caudal to the arcuate line, maintaining the continuity of the posterior sheath. In addition, caution was exercised not to injure the deep inferior epigastric artery or its paired venae comitantes, given their primary axial blood supply to the rectus muscles. The retrorectus plane was developed laterally to the semilunar line, preserving the segmental motor nerves to the rectus muscles. Once the retrorectus planes were developed on both sides, they were joined across the midline cranially and caudally to create a contiguous space to receive the mesh.
The ability to close the posterior rectus sheath was then assessed. Two Kocher or Allis clamps were placed on the medial edge of the posterior rectus sheath on each side, and an attempt was made to reapproximate the 2 sides in the midline. If there was undue tension at this point, unilateral or bilateral minimally invasive anterior component separation was performed, similar to description by Butler and Campbell,32 with minimal skin undermining. Alternatively, unilateral or bilateral posterior component separation was performed, similar to description by Novitsky et al,33 depending on the patient. The choice of whether to perform anterior or posterior component separation depended on several factors: in patients with very wide defects, in whom additional excursion of both the anterior and posterior sheaths is needed, and in those with preexisting skin undermining (caused by the hernia sac), in whom anterior component separation would not require significant additional undermining, anterior component separation was performed. In contrast, in patients with moderately sized defects in whom additional excursion is needed in the posterior rectus sheath, but not the anterior rectus sheath, and in those with no preexisting skin undermining, posterior components separation was performed. Anterior component separation and posterior component separation were never combined in the same patient. The posterior sheath was then primarily reapproximated using 1 unidirectional or 2 bidirectional running #0-looped polyglyconate sutures.
Before adopting self-adhering mesh, we used a variety of synthetic and biologic meshes in the retrorectus plane. Uncoated macroporous synthetic mesh was only used in patients in whom the posterior rectus sheath could be fully reapproximated. Retrorectus mesh was fixated via multiple transfascial sutures. In most cases, 8 transfascial sutures were used. Using a #1-polyglyconate suture, U-stitches were placed in the mesh about 1 cm from its edge at the 12, 3, 6, and 9 o’clock positions, and then 4 additional sutures were placed to bisect the quadrants. Those sutures were then passed through the abdominal wall lateral to the semilunar line using a laparoscopic suture passer. The anterior rectus sheath was then closed using a running #0-looped polyglyconate suture(s).
After the self-adhering mesh became available to us, we adopted its use in patients in whom the posterior rectus sheath could be closed fully. The steps involved in developing the retrorectus plane and inserting the self-adhering mesh are shown in Video 1 (See video, Supplemental Digital Content 1, which displays the development of the retrorectus plane and insertion of the self-adhering mesh. This video is available in the “related videos” section of the full-text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A302). The mesh was immersed for at least 5 minutes in triple antibiotic solution, consisting of 1 g of cefazolin, 80 mg of gentamicin, and 50,000 units of bacitracin in 500 mL of normal saline, as described by Adams et al.34 Although the mesh was immersed in antibiotic solution, hemostasis was ensured in the retrorectus plane. Povidone iodine was used to reprep the skin, and triple antibiotic irrigation was used. The surgeon then donned new talc-free gloves, and only 1 surgeon touched the mesh. This “no-touch” technique was used, whereas the contact of the mesh with any other objects was minimized.35 This was especially true of laparotomy sponges and towels as the microgrips on the mesh tend to adhere to fabric and cause cotton threads to be embedded in the mesh. Four Richardson retractors were used to retract the skin and rectus muscles laterally. The mesh was held above the abdomen, with the microgrips toward the closed posterior rectus sheath. It was folded in half along its long axis, and the fold was placed in the midline against the posterior rectus sheath repair. The mesh was then carefully unfolded laterally, avoiding and/or minimizing skin contact, and any adherence to the underside of the rectus muscle. This was especially important inferiorly where the microgrips should be not be allowed to adhere to the deep inferior epigastric pedicle. Any mesh edges extending beyond the retrorectus space were trimmed to ensure elimination of redundancy and to be sure that the mesh lay completely flat. A 15-French drain was then placed in the retrorectus space over the mesh.
Video Graphic 1.

See video, Supplemental Digital Content 1, which displays the development of the retrorectus plane and insertion of the self-adhering mesh. This video is available in the “related videos” section of the full-text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A302.
The anterior rectus sheath was then closed using running #0-looped polyglyconate suture(s). Any tenuous or undermined skin near the midline was excised. One or 2 19-French drains were placed in the subcutaneous space, and multiple progressive tension sutures were placed to advance the skin flaps toward the midline and obliterate any dead space.36 Meticulous layered closure of the skin was performed.
RESULTS
Twenty-six patients underwent elective abdominal wall reconstruction with synthetic retrorectus mesh with primary musculofascial midline reapproximation between December 2013 and December 2015. This included 14 patients who received self-adhering mesh and 12 patients who received transfascially sutured mesh. All patients had follow-up greater than 12 months, with an average follow-up of 600 days (612 days for self-adhering mesh and 587 days for transfascially sutured mesh). All other patients who did not receive retrorectus mesh with midline fascial reapproximation were excluded.
Table 2 shows the baseline characteristics of the patients in the 2 groups. In the 14 patients who received self-adhering mesh, 3 were classified as Kanters grade 1 and 11 as Kanters grade 2. The average hernia width was 7.5 cm (range, 2.1–14.9 cm). Two patients (14.3%) were on preoperative narcotics for chronic pain. Primary fascial reapproximation was achieved in all patients. Eight of the patients required minimally invasive anterior component separation. Postoperatively, 6 of these patients (44.4%) received multimodal analgesia as part of an enhanced recovery pathway, which included scheduled acetaminophen, celecoxib, and/or gabapentin.
Table 2.
Baseline Characteristics of Patients Who Underwent Retrorectus Mesh Placement

In the 12 patients who received mesh with transfascial suture fixation, 4 were classified as Kanters grade 1 and 8 as Kanters grade 2. One patient (8.3%; P = 0.6 compared with self-adhering mesh) was on preoperative narcotics for chronic pain. Eight patients received uncoated midweight polypropylene mesh, and 4 received noncrosslinked porcine acellular dermal matrix. The average hernia width was 7.9 cm (range, 4.1–14.2 cm; P = 1.0 compared with self-adhering mesh). Primary fascial reapproximation was achieved in all patients. Five of the patients required minimally invasive anterior component separation, and 1 patient required posterior component separation. Postoperatively, 7 of these patients (58.3%; P = 0.7 compared with self-adhering mesh) received multimodal analgesia as part of an enhanced recovery pathway.
Table 3 shows the outcomes of the patients in the 2 groups. The rate of SSOs in patients who received self-adhering mesh was 14.3%, which was not statistically different from those who received transfascially sutured mesh (8.3%; P = 1.0). The 2 SSOs in the self-adhering mesh group were both episodes of cellulitis that resolved with oral antibiotics. One occurred on postoperative day 16 and required clindamycin for 7 days. The second one occurred on postoperative day 3 and required treatment with linezolid for 25 days. The only SSO in the conventional mesh group was delayed wound healing along the incision, which healed with local wound care.
Table 3.
Outcomes in Patients Who Underwent Retrorectus Mesh Placement
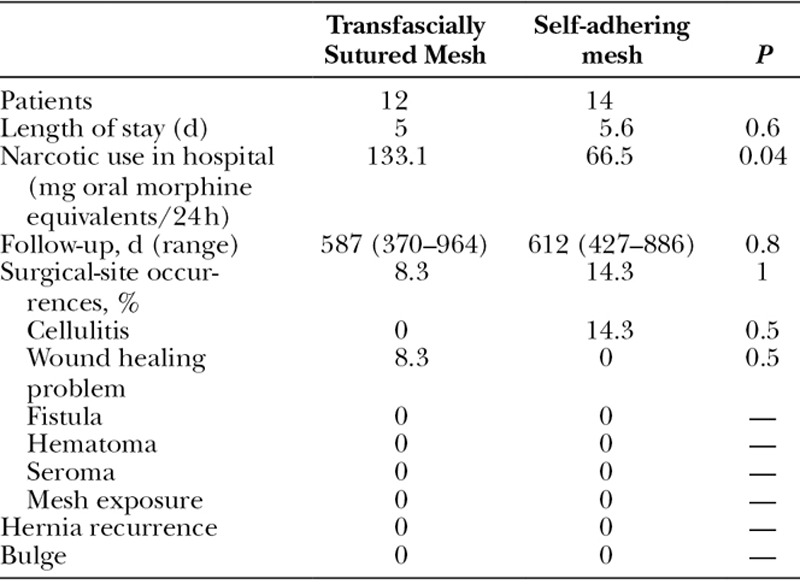
The average length of hospital stay was also similar between the 2 groups (5.6 vs 5 days; P = 0.6). Patients who received self-adhering mesh required 50% less narcotics in the hospital than patients who received transfascially sutured mesh (66.5 mg of oral morphine equivalents per day vs 133.1 mg of oral morphine equivalents per day; P = 0.04).
No hernia recurrences or bulges occurred in either group. No patients have developed chronic pain in their abdominal wall. No mesh required explantation in either group.
One potential confounder is the fact that more patients in the self-adhering mesh group received an epidural catheter (28.6% vs 8.3%; P = 0.3). To control for the differences in the proportion of patients who received an epidural catheter, 2 additional analyses were performed. In the first analysis, multivariate logistic regression of all potential predictors of narcotic requirement postoperatively showed that the only predictor of lower narcotic requirement was the use of self-adhering mesh (P = 0.04; Table 4). In the second analysis, univariate analysis was performed on all patients, except those who received an epidural catheter. The outcomes in these patients are shown in Table 5. Outcomes were similar between the 2 groups, except that patients who received self-adhering mesh required 46.4% less narcotics than patients who received transfascially sutured mesh (66.6 vs 124.3 mg; P = 0.05).
Table 4.
Multivariate Logistic Regression of the Predictors of Lower Narcotic Requirement

Table 5.
Outcomes Excluding Patients Who Received an Epidural Catheter

DISCUSSION
Multiple studies have shown that the highly vascular retrorectus plane offers significant advantages for mesh placement. However, there is little agreement regarding how much fixation is required for mesh in the retrorectus plane. Some authors perform minimal or no mesh fixation,37 relying instead on the apposition of the mesh against the posterior rectus sheath and the rectus muscle to prevent mesh migration. Other authors place numerous sutures to fixate the mesh to the fascia,38 using the mesh to offload tension off the fascia. It is unclear which approach is superior. Our main purpose in using some type of fixation is to obtain close apposition between the mesh and the fascia, avoiding wrinkles and folds in the mesh that may impair incorporation, and to provide support to the posterior rectus sheath postoperatively as intraabdominal pressure increases (which unsutured traditional mesh would not be able to do).
In the past, we have used mesh with transfascial suture fixation. Theoretical risks of transfascial sutures are intercostal nerve entrapment and acute and chronic pain. The self-adhering mesh offers advantages in that regard, namely the lack of need for suture mesh fixation. We found that patients who received sutureless self-adhering mesh required significantly lower doses of narcotics postoperatively than those who received transfascially sutured mesh. Patients in the 2 groups had similar rates of preoperative narcotics for chronic pain and postoperative multimodal analgesia. The difference in postoperative narcotic requirement persisted when controlling for the presence of an epidural catheter. Our findings mirror the body of literature on the use of self-adhering mesh in inguinal hernia repair where this mesh has been shown to result in less acute pain,15,16 without a clear effect on chronic pain.17–23 The reduction in acute pain is very important in ensuring patient satisfaction and limiting patient suffering.39 Pain management is also a tracked outcome on the Hospital Consumer Assessment of Healthcare Providers and Systems scores on which value-based purchasing is based,40 so there is not only patient benefit but also hospital system benefit from improving pain control. Furthermore, uncontrolled acute pain is believed to increase susceptibility to surgical-site infections,41 particularly troubling complications in patients undergoing abdominal wall reconstruction. Patients with more acute pain also tend to require higher doses of narcotics. Adverse effects of postoperative narcotics are dose dependent42 and include constipation, nausea, and confusion. To reduce pain and narcotic requirements in abdominal wall reconstruction, authors have recommended strategies such as instituting multimodal analgesia with nonopioid medications,43 performing a transversus abdominis plane block44 and considering a neuraxial catheter.45 We found that patients who received sutureless self-adhering mesh required significantly lower doses of opioids postoperatively compared with patients who received transfascially sutured mesh, with no deterioration in reconstructive outcomes.
Disadvantages of self-adhering polyester mesh include the fact that it can be applied only to a subset of patients, namely those without current abdominal wall infection or contamination,46 who have an intact posterior rectus sheath. Another disadvantage of self-adhering mesh is the fact that it is not placed under significant tension using sutures, but it is simply laid on top of the repaired posterior rectus sheath albeit with multiple points of adherence/fixation. The mesh, therefore, does not theoretically take as much tension off the healing fascial repair in the early postoperative period. It is unclear what role significant tension offloading with mesh plays in the early postoperative period. Does mesh need to be placed under significant tension to offload tension off the fascia and prevent early recurrence? Or is the function of mesh simply to incorporate and provide long-term reinforcement for the fascia? The answer is currently unclear. Self-adhering mesh has been shown to have superior gripping strength to both laparoscopic staples and fibrin glue over the short and medium terms.11 It has also been shown to incorporate well over the long term,10 thus acting as a durable reinforcement to the fascia.
Our study has several limitations. First, our study is retrospective in nature. Second, the patients were not randomized to 1 mesh type versus the other. Third, although we were able to demonstrate a reduction in narcotic analgesic requirements in the hospital with the use of self-adhering mesh, we did not evaluate patient perception of their acute pain using validated instruments. Because our electronic medical record system only records total surgical time, we could not measure the lengths of the general surgery and plastic surgery portions of the procedures separately. We were, thus, unable to determine whether the use of self-adhering mesh decreased operative time for the reconstructive portion. Lastly, we had a limited number of patients in each group in our pilot study although there was no dropout and all patients were followed up for over 12 months. Despite the small patient numbers, we were able to demonstrate a statistically significant reduction in narcotic requirements with the use of self-adhering mesh.
CONCLUSIONS
This is the first study evaluating the short-term and long-term outcomes of incisional ventral hernia repair using self-adhering mesh. Our results show favorable outcomes, with low rates of SSOs and hernia recurrence. We also demonstrated lower narcotic needs in patients who received self-adhering mesh compared with patients who received transfascially sutured mesh. No patients developed chronic abdominal wall pain after hernia repair in either group. Future studies will focus on elucidating the exact role that mesh plays when placed in the retrorectus plane: acute tension-offloading device or chronic reinforcement.
Supplementary Material
Footnotes
Presented as a podium presentation at the 17th Annual Hernia Repair meeting of the American Hernia Society, March 30 to April 2, 2016, Wash., and as a podium presentation at the 59th Annual Meeting of the Ohio Valley Society of Plastic Surgeons, June 3 to 5, 2016, Dayton, Ohio.
Disclosure: Dr. Janis is a consultant for LifeCell, has received previous honoraria from Pacira, KCI and Bard, and receives royalties from CRC Press. Dr. Khansa has no financial disclosures. The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Le Huu Nho R, Mege D, Ouaïssi M, et al. Incidence and prevention of ventral incisional hernia. J Visc Surg. 2012;149(5 Suppl):e3–e14. doi: 10.1016/j.jviscsurg.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Flum DR, Horvath K, Koepsell T. Have outcomes of incisional hernia repair improved with time? A population-based analysis. Ann Surg. 2003;237:129–135. doi: 10.1097/00000658-200301000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luijendijk RW, Hop WC, van den Tol MP, et al. A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med. 2000;343:392–398. doi: 10.1056/NEJM200008103430603. [DOI] [PubMed] [Google Scholar]
- 4.Yezhelyev MV, Deigni O, Losken A. Management of full-thickness abdominal wall defects following tumor resection. Ann Plast Surg. 2012;69:186–191. doi: 10.1097/SAP.0b013e31821d0715. [DOI] [PubMed] [Google Scholar]
- 5.Breuing K, Butler CE, Ferzoco S, et al. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148:544–558. doi: 10.1016/j.surg.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Burger JW, Luijendijk RW, Hop WC, et al. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004;240:578–583. doi: 10.1097/01.sla.0000141193.08524.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stabilini C, Stella M, Frascio M, et al. Mesh versus direct suture for the repair of umbilical and epigastric hernias. Ten-year experience. Ann Ital Chir. 2009;80:183–187. [PubMed] [Google Scholar]
- 8.Booth JH, Garvey PB, Baumann DP, et al. Primary fascial closure with mesh reinforcement is superior to bridged mesh repair for abdominal wall reconstruction. J Am Coll Surg. 2013;217:999–1009. doi: 10.1016/j.jamcollsurg.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Albino FP, Patel KM, Nahabedian MY, et al. Does mesh location matter in abdominal wall reconstruction? A systematic review of the literature and a summary of recommendations. Plast Reconstr Surg. 2013;132:1295–1304. doi: 10.1097/PRS.0b013e3182a4c393. [DOI] [PubMed] [Google Scholar]
- 10.Gruber-Blum S, Riepl N, Brand J, et al. A comparison of Progrip(®) and Adhesix (®) self-adhering hernia meshes in an onlay model in the rat. Hernia. 2014;18:761–769. doi: 10.1007/s10029-014-1258-0. [DOI] [PubMed] [Google Scholar]
- 11.Hollinsky C, Kolbe T, Walter I, et al. Comparison of a new self-gripping mesh with other fixation methods for laparoscopic hernia repair in a rat model. J Am Coll Surg. 2009;208:1107–1114. doi: 10.1016/j.jamcollsurg.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 12.García Ureña MÁ, Hidalgo M, Feliu X, et al. Multicentric observational study of pain after the use of a self-gripping lightweight mesh. Hernia. 2011;15:511–515. doi: 10.1007/s10029-011-0811-3. [DOI] [PubMed] [Google Scholar]
- 13.Birk D, Hess S, Garcia-Pardo C. Low recurrence rate and low chronic pain associated with inguinal hernia repair by laparoscopic placement of Parietex ProGrip™ mesh: clinical outcomes of 220 hernias with mean follow-up at 23 months. Hernia. 2013;17:313–320. doi: 10.1007/s10029-013-1053-3. [DOI] [PubMed] [Google Scholar]
- 14.Chastan P. Tension-free open hernia repair using an innovative self-gripping semi-resorbable mesh. Hernia. 2009;13:137–142. doi: 10.1007/s10029-008-0451-4. [DOI] [PubMed] [Google Scholar]
- 15.Bresnahan E, Bates A, Wu A, et al. The use of self-gripping (Progrip™) mesh during laparoscopic total extraperitoneal (TEP) inguinal hernia repair: a prospective feasibility and long-term outcomes study. Surg Endosc. 2015;29:2690–2696. doi: 10.1007/s00464-014-3991-y. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz A, Yener O, Kaynak B, et al. Self-gripping Covidien™ ProGrip™ mesh versus polypropylene mesh in open inguinal hernia repair: multicenter short term results. Prague Med Rep. 2013;114:231–238. doi: 10.14712/23362936.2014.12. [DOI] [PubMed] [Google Scholar]
- 17.Chatzimavroudis G, Papaziogas B, Koutelidakis I, et al. Lichtenstein technique for inguinal hernia repair using polypropylene mesh fixed with sutures vs. self-fixating polypropylene mesh: a prospective randomized comparative study. Hernia. 2014;18:193–198. doi: 10.1007/s10029-013-1211-7. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Ji Z, Li Y. The comparison of self-gripping mesh and sutured mesh in open inguinal hernia repair: the results of meta-analysis. Ann Surg. 2014;259:1080–1085. doi: 10.1097/SLA.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 19.Pandanaboyana S, Mittapalli D, Rao A, et al. Meta-analysis of self-gripping mesh (Progrip) versus sutured mesh in open inguinal hernia repair. Surgeon. 2014;12:87–93. doi: 10.1016/j.surge.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 20.Sajid MS, Farag S, Singh KK, et al. Systematic review and meta-analysis of published randomized controlled trials comparing the role of self-gripping mesh against suture mesh fixation in patients undergoing open inguinal hernia repair. Updates Surg. 2014;66:189–196. doi: 10.1007/s13304-013-0237-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C, Li F, Zhang H, et al. Self-gripping versus sutured mesh for inguinal hernia repair: a systematic review and meta-analysis of current literature. J Surg Res. 2013;185:653–660. doi: 10.1016/j.jss.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 22.Fang Z, Zhou J, Ren F, et al. Self-gripping mesh versus sutured mesh in open inguinal hernia repair: system review and meta-analysis. Am J Surg. 2014;207:773–781. doi: 10.1016/j.amjsurg.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 23.Pierides G, Scheinin T, Remes V, et al. Randomized comparison of self-fixating and sutured mesh in open inguinal hernia repair. Br J Surg. 2012;99:630–636. doi: 10.1002/bjs.8705. [DOI] [PubMed] [Google Scholar]
- 24.Birk D, Pardo CG. Self-gripping Parietene and Parietex Progrip mesh laparoscopic hernia repair: have we found the ideal implant? Surg Technol Int. 2012;22:93–100. [PubMed] [Google Scholar]
- 25.Jorgensen LN, Sommer T, Assaadzadeh S, et al. Danish Multicentre DANGRIP Study Group. Randomized clinical trial of self-gripping mesh versus sutured mesh for Lichtenstein hernia repair. Br J Surg. 2013;100:474–481. doi: 10.1002/bjs.9006. [DOI] [PubMed] [Google Scholar]
- 26.Kapischke M, Schulze H, Caliebe A. Self-fixating mesh for the Lichtenstein procedure–a prestudy. Langenbecks Arch Surg. 2010;395:317–322. doi: 10.1007/s00423-010-0597-2. [DOI] [PubMed] [Google Scholar]
- 27.Kingsnorth A, Gingell-Littlejohn M, Nienhuijs S, et al. Randomized controlled multicenter international clinical trial of self-gripping Parietex™ ProGrip™ polyester mesh versus lightweight polypropylene mesh in open inguinal hernia repair: interim results at 3 months. Hernia. 2012;16:287–294. doi: 10.1007/s10029-012-0900-y. [DOI] [PubMed] [Google Scholar]
- 28.Fumagalli Romario U, Puccetti F, Elmore U, et al. Self-gripping mesh versus staple fixation in laparoscopic inguinal hernia repair: a prospective comparison. Surg Endosc. 2013;27:1798–1802. doi: 10.1007/s00464-012-2683-8. [DOI] [PubMed] [Google Scholar]
- 29.Sanders DL, Nienhuijs S, Ziprin P, et al. Randomized clinical trial comparing self-gripping mesh with suture fixation of lightweight polypropylene mesh in open inguinal hernia repair. Br J Surg. 2014;101:1373–1382. doi: 10.1002/bjs.9598. [DOI] [PubMed] [Google Scholar]
- 30.Verhelst J, de Goede B, Kleinrensink GJ, et al. Open incisional hernia repair with a self-gripping retromuscular Parietex mesh: a retrospective cohort study. Int J Surg. 2015;13:184–188. doi: 10.1016/j.ijsu.2014.11.043. [DOI] [PubMed] [Google Scholar]
- 31.Kanters AE, Krpata DM, Blatnik JA, et al. Modified hernia grading scale to stratify surgical site occurrence after open ventral hernia repairs. J Am Coll Surg. 2012;215:787–793. doi: 10.1016/j.jamcollsurg.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Butler CE, Campbell KT. Minimally invasive component separation with inlay bioprosthetic mesh (MICSIB) for complex abdominal wall reconstruction. Plast Reconstr Surg. 2011;128:698–709. doi: 10.1097/PRS.0b013e318221dcce. [DOI] [PubMed] [Google Scholar]
- 33.Novitsky YW, Elliott HL, Orenstein SB, et al. Transversus abdominis muscle release: a novel approach to posterior component separation during complex abdominal wall reconstruction. Am J Surg. 2012;204:709–716. doi: 10.1016/j.amjsurg.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Adams WP, Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstr Surg. 2006;117:30–36. [PubMed] [Google Scholar]
- 35.Mladick RA. “No-touch” submuscular saline breast augmentation technique. Aesthetic Plast Surg. 1993;17:183–192. doi: 10.1007/BF00636260. [DOI] [PubMed] [Google Scholar]
- 36.Janis JE, Khansa I. Evidence-based abdominal wall reconstruction: the maxi-mini approach. Plast Reconstr Surg. 2015;136:1312–1323. doi: 10.1097/PRS.0000000000001831. [DOI] [PubMed] [Google Scholar]
- 37.Novitsky YW, Fayezizadeh M, Orenstein SB. Outcomes of posterior component separation with transversus abdominis muscle release and synthetic mesh sublay reinforcement. Ann Surg. 2016;264:226–232. doi: 10.1097/SLA.0000000000001673. [DOI] [PubMed] [Google Scholar]
- 38.Cheesborough JE, Dumanian GA. Simultaneous prosthetic mesh abdominal wall reconstruction with abdominoplasty for ventral hernia and severe rectus diastasis repairs. Plast Reconstr Surg. 2015;135:268–276. doi: 10.1097/PRS.0000000000000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janis JE, Joshi GP. Introduction to “current concepts in pain management in plastic surgery”. Plast Reconstr Surg. 2014;134(4 Suppl 2):6S–7S. doi: 10.1097/PRS.0000000000000683. [DOI] [PubMed] [Google Scholar]
- 40.Hospital Consumer Assessment of Healthcare Providers and Systems Survey. Available at: http://www.hcahpsonline.org/surveyinstrument.aspx. Accessed April 15, 2016. [Google Scholar]
- 41.Baratta JL, Schwenk ES, Viscusi ER. Clinical consequences of inadequate pain relief: barriers to optimal pain management. Plast Reconstr Surg. 2014;134(4 Suppl 2):15S–21S. doi: 10.1097/PRS.0000000000000681. [DOI] [PubMed] [Google Scholar]
- 42.Funk RD, Hilliard P, Ramachandran SK. Perioperative opioid usage: avoiding adverse effects. Plast Reconstr Surg. 2014;134(4 Suppl 2):32S–39S. doi: 10.1097/PRS.0000000000000680. [DOI] [PubMed] [Google Scholar]
- 43.Fayezizadeh M, Petro CC, Rosen MJ, et al. Enhanced recovery after surgery pathway for abdominal wall reconstruction: pilot study and preliminary outcomes. Plast Reconstr Surg. 2014;134(4 Suppl 2):151S–159S. doi: 10.1097/PRS.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 44.Petersen PL, Mathiesen O, Torup H, et al. The transversus abdominis plane block: a valuable option for postoperative analgesia? A topical review. Acta Anaesthesiol Scand. 2010;54:529–535. doi: 10.1111/j.1399-6576.2010.02215.x. [DOI] [PubMed] [Google Scholar]
- 45.Momoh AO, Hilliard PE, Chung KC. Regional and neuraxial analgesia for plastic surgery: surgeon’s and anesthesiologist’s perspectives. Plast Reconstr Surg. 2014;134(4 Suppl 2):58S–68S. doi: 10.1097/PRS.0000000000000702. [DOI] [PubMed] [Google Scholar]
- 46.Harth KC, Broome AM, Jacobs MR, et al. Bacterial clearance of biologic grafts used in hernia repair: an experimental study. Surg Endosc. 2011;25:2224–2229. doi: 10.1007/s00464-010-1534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


