Abstract
BACKGROUND
Hybrid coronary revascularization (HCR) combines minimally invasive surgical coronary artery bypass grafting of the left anterior descending artery with percutaneous coronary intervention (PCI) of non–left anterior descending vessels. HCR is increasingly used to treat multivessel coronary artery disease that includes stenoses in the proximal left anterior descending artery and at least 1 other vessel, but its effectiveness has not been rigorously evaluated.
OBJECTIVES
This National Institutes of Health–funded, multicenter, observational study was conducted to explore the characteristics and outcomes of patients undergoing clinically indicated HCR and multivessel PCI for hybrid-eligible coronary artery disease, to inform the design of a confirmatory comparative effectiveness trial.
METHODS
Over 18 months, 200 HCR and 98 multivessel PCI patients were enrolled at 11 sites. The primary outcome was major adverse cardiac and cerebrovascular events (MACCE) (i.e., death, stroke, myocardial infarction, repeat revascularization) within 12 months post-intervention. Cox proportional hazards models were used to model time to first MACCE event. Propensity scores were used to balance the groups.
RESULTS
Mean age was 64.2 ± 11.5 years, 25.5% of patients were female, 38.6% were diabetic, and 4.7% had previous stroke. Thirty-eight percent had 3-vessel coronary artery disease, and the mean SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) score was 19.7 ± 9.6. Adjusted for baseline risk, MACCE rates were similar between groups within 12 months post-intervention (hazard ratio [HR]: 1.063; p = 0.80) and during a median 17.6 months of follow-up (HR: 0.868; p = 0.53).
CONCLUSIONS
These observational data from this first multicenter study of HCR suggest that there is no significant difference in MACCE rates over 12 months between patients treated with multivessel PCI or HCR, an emerging modality. A randomized trial with long-term outcomes is needed to definitively compare the effectiveness of these 2 revascularization strategies. (Hybrid Revascularization Observational Study; NCT01121263)
Keywords: coronary artery bypass, coronary vessels, drug-eluting stents, follow-up studies, percutaneous coronary intervention
The tradeoffs in the benefits and risks associated with surgical and percutaneous coronary revascularization strategies pose challenges to physicians and patients alike when selecting the preferred intervention. The durability of surgical arterial grafts, weighed against the decreased invasiveness of percutaneous coronary revascularization, and the risks associated with both procedures have been the focus of important comparative effectiveness trials over the past 2 decades. More recent trials have sought optimal approaches for subgroups of patients on the basis of coronary anatomy or comorbidities. The recently reported 5-year outcomes from the SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) trial, for example, demonstrated that coronary artery bypass graft (CABG) was superior to percutaneous coronary intervention (PCI) in patients with complex left main coronary artery (LMCA) or 3-vessel coronary artery disease (CAD) (1). Moreover, the FREEDOM (Comparison of Two Treatments for Multivessel Coronary Artery Disease in Individuals With Diabetes) trial showed that patients with diabetes mellitus treated with CABG had longer survival and fewer major adverse cardiac and cerebrovascular events (MACCE) than those treated with multivessel PCI, particularly patients with left anterior descending (LAD) artery disease, of whom more than 90% received a surgical left internal mammary artery (LIMA) graft (2). The benefits of CABG over PCI in these subpopulations have also been supported by the results of large registry studies (3). However, a trend toward a higher incidence of stroke was observed in the CABG arm of the SYNTAX trial, and a statistically significant increase in the incidence of stroke was observed in the CABG arm of the FREEDOM trial. The long-term patency of saphenous vein grafts has been questioned, with 1-year failure rates up to 46%, whereas later-generation everolimus and zotarolimus drug-eluting stents (DES) have 1-year restenosis rates <5% (4). The optimal revascularization strategy would combine a minimally invasive procedure that reduces perioperative risk, while maximizing durability and survival.
Hybrid coronary revascularization (HCR), combining minimally invasive CABG to the LAD coronary artery and percutaneous intervention (PCI with DES) of non-LAD vessels, offers potential advantages beyond CABG or PCI alone, and, as such, could have a major impact on health outcomes and the health care system. The ability to deliver a new therapy for CAD that provides durability, without the trauma and prolonged recovery time associated with conventional CABG, would be a major advance in cardiovascular medicine. The interdisciplinary HCR approach has been steadily growing in cardiac centers across the United States and has the potential to disseminate widely to become the third major coronary artery revascularization alternative for patients with multivessel CAD. However, the known efficacy and safety of this novel approach rests upon data obtained through predominantly small, single-center observational studies (5–9).
The overall objectives of this observational study were to explore the characteristics and outcomes of a contemporary patient population undergoing clinically indicated HCR in order to inform the design and feasibility of a subsequent comparative effectiveness trial. MACCE rates in patients undergoing clinically indicated HCR or multivessel PCI were therefore assessed, as were management practices for both revascularization procedures among participating institutions.
METHODS
This prospective cohort study was conducted at 11 clinical centers in the United States and was funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health. In-person assessments were conducted at an initial visit, and patient follow-up was collected by telephone at 6, 12, 18, and 21 months after the initial revascularization or until August 31, 2012, whichever came first. Study data were transmitted from the clinical sites to a secure server at the Data Coordinating Center using a web-based, Health Insurance Portability and Accountability Act–compliant electronic data-capture system. All clinical centers and the Data Coordinating Center obtained institutional review board approval, and all patients provided informed consent. The study is registered at the National Institutes of Health ClinicalTrials.gov website with identifier NCT01121263.
STUDY POPULATION
Two patient populations were enrolled in this study: 1) patients who underwent HCR with surgical LIMA grafting to the LAD combined with PCI to non-LAD vessels (hybrid group); and 2) patients who met anatomic and clinical eligibility criteria and underwent multivessel PCI with DES (PCI group). The selection of HCR or PCI for revascularization was at the discretion of the clinical site cardiologist and surgeon. Anatomic and clinical eligibility criteria that were developed for a proposed subsequent randomized comparative effectiveness trial of HCR and PCI were used in this observational study to identify the subgroup of patients undergoing PCI at the clinical sites who would have been eligible to participate in a randomized trial. Accordingly, patients who underwent clinically indicated multivessel PCI were required to meet the pre-specified anatomic and clinical eligibility for enrollment in the PCI group; however, any patient who underwent clinically indicated HCR at a participating clinical site was deemed eligible for enrollment in the hybrid group. Angiographic inclusion criteria for PCI group eligibility included the following: 1) a proximal LAD lesion of at least 70% with a vessel suitable for LIMA to LAD revascularization, in addition to at least 1 non-LAD lesion in the right coronary artery (RCA) and/or left circumflex coronary artery distributions of at least 70%, amenable to PCI with DES; and 2) importantly, agreement regarding anatomic suitability for an HCR procedure by both a cardiothoracic surgeon and an interventional cardiologist at the site. Patients with complex lesions at a LAD-diagonal bifurcation were included in the HCR group when the diagonal vessel was large enough to warrant revascularization. Clinical eligibility for enrollment in the PCI group included the ability to tolerate dual antiplatelet therapy for at least 12 months. Selected exclusion criteria for the PCI group were as follow: previous stent placement within 1 month prior to enrollment for bare-metal stents, and within 6 months prior to enrollment for DES; evidence of in-stent restenosis; left main disease (≥50% stenosis); presence of fresh coronary thrombus; previous cardiac surgery; previous ST-segment elevation myocardial infarction within 30 days prior to intervention; ejection fraction <30%; acute decompensated heart failure within 30 days prior to intervention; hemodynamic instability at screening; creatinine clearance ≤50 ml/min; and body mass index >40 kg/m2. Complete anatomic and clinical eligibility criteria for the PCI group of this observational study are detailed in the Online Appendix. Enrollment was completed over a 12-month period, with planned follow-up for a minimum of 18 and a maximum of 21 months following the procedure. Figure 1 illustrates the study flow.
FIGURE 1. Study Flowchart.
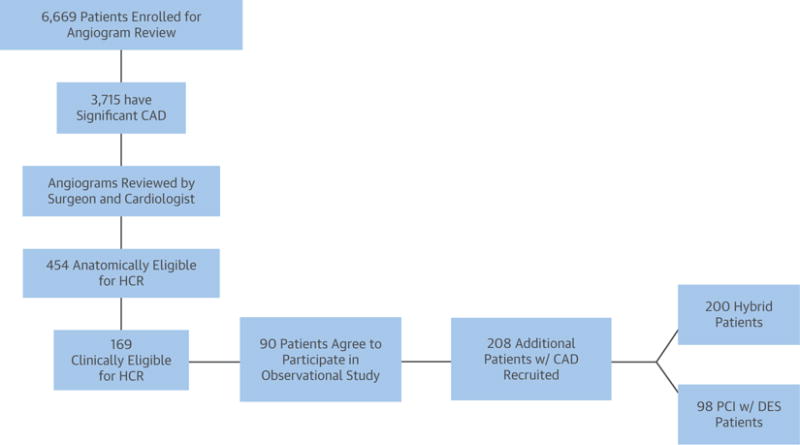
Ninety of the patients from the angiogram screening cohort were combined with 208 patients with hybrid coronary revascularization (HCR)-eligible coronary artery disease (CAD) treated with either HCR or multivessel percutaneous coronary intervention (PCI) to compose the 298 patients who consented to be enrolled in this observational clinical study. Of those 298 enrolled patients, 200 were treated with HCR and 98 were treated with multivessel PCI at the discretion of local cardiologists and surgeons. DES = drug-eluting stent(s).
Interventions
For the purposes of this study, HCR was defined as a planned surgical revascularization of the LAD combined with percutaneous revascularization of at least 1 non-LAD target. The planned revascularization targets were identified prior to the initial revascularization procedure, and staged procedures were expected to be completed within 6 weeks after the first revascularization intervention. Timing strategy (surgery followed by PCI, PCI followed by surgery, or simultaneous PCI and surgery) was left to the discretion of the treating clinicians. All percutaneous interventions were performed using standard techniques with commercially available DES, selected at the discretion of the operator. PCI staging was also at the discretion of the operator.
OUTCOME MEASURES
The primary outcome was the incidence of MACCE—defined as death, stroke, myocardial infarction (MI), or repeat revascularization—at 12 months following the initial procedure. Secondary outcomes included the following: incidence of MACCE at 18 months and 21 months, or through the end of study follow-up (whichever came first); the incidence of the individual components of MACCE at 12, 18, and 21 months, or through the end of study follow-up; and the incidence of serious non-MACCE events over the same time period.
STATISTICAL ANALYSIS
Demographic and cardiovascular history data are displayed as mean ± SD for continuous variables and as proportions for categorical variables. To account for differences in baseline characteristics between patients selected for HCR as compared to multivessel PCI alone, a propensity score model was computed using logistic regression in which the dependent variable was treatment received (i.e., HCR or PCI). Predictor variables in the propensity score model included the following: demographics; medical history (history of stroke, MI, peripheral artery disease, cerebrovascular disease, and chronic obstructive pulmonary disease); baseline creatinine level; risk of CAD; SYNTAX score; Canadian Cardiovascular Society Classification; and procedure status (elective, urgent, emergent, or emergent salvage). Cox proportional hazards regression weighted by the inverse of the propensity scores was used to compare events for the HCR and the PCI groups. Events within the first 12 months from the index procedure and through the end of study follow-up were modeled separately. For individual components of MACCE, incidence was calculated as unadjusted rates per person-year by treatment. Kaplan-Meier curves were calculated from the weighted Cox proportional hazards models.
A sensitivity analysis using clinical site as an instrumental variable was performed to test the robustness of results obtained by the Cox proportional hazards models weighted with propensity scores and to address potential biases associated with unmeasured confounding variables. Clinical site was dichotomized in the models on the basis of high versus low utilization of HCR. Baseline characteristics of the 2 groups were compared to verify that the instrumental variable produced comparable groups. The results of the instrumental variables analysis were consistent with those presented here using propensity scores. All analyses were conducted using SAS (version 9.2, SAS Institute, Cary, North Carolina).
RESULTS
CHARACTERISTICS OF THE PATIENT POPULATION
During a 3-month window, 6,669 consecutive patients who had coronary angiography across the 11 participating clinical sites were screened for this observational study; 3,715 of the patients were found to have significant CAD, and 454 (12.2%) of those were deemed by a local cardiologist and cardiac surgeon to have coronary anatomy eligible for HCR. Reasons for anatomic ineligibility included presence of single-vessel disease in 1,232 patients (33.2%), previous CABG in 949 (25.5%), absence of LAD disease in 830 (22.3%), nongraftable LAD in 235 (6.3%), and other in 664 (17.9%). Of the patients deemed anatomically eligible for HCR, 169 were also determined to be clinically eligible for HCR on the basis of the preliminary (exploratory) eligibility criteria proposed for the future randomized trial. As pre-specified in the protocol, the HCR-eligible patients from the angiogram screening cohort who consented to participate in the long-term outcomes part of the study and underwent either HCR or multivessel PCI (n = 90) were combined with additional patients identified at the sites between May 2010 and November 2011 (after the angiographic screening period) who underwent HCR or were found to be HCR-eligible prior to undergoing multivessel PCI. Ninety of the patients from the angiogram screening cohort were therefore combined with 208 patients with HCR-eligible CAD treated with either HCR or multivessel PCI to compose the 298 patients who consented to be enrolled in this observational clinical study. Of those 298 enrolled patients, 200 were treated with HCR and 98 were treated with multivessel PCI at the discretion of local cardiologists and surgeons (Figure 1). There was substantial heterogeneity in the selected revascularization paradigm for hybrid-eligible patients across participating sites; the ratio of HCR to PCI varied from 0% to 95%. Five of the 11 sites performed a higher percentage of HCR than PCI, whereas 5 performed more PCI (1 site had an even distribution between the therapeutic interventions). Overall, the mean age of enrolled patients was 64.2 ± 11.5 years; 25.5% were female; 38.6% were diabetic; and 4.7% had previous stroke. HCR patients had higher incidence of previous MI, history of peripheral arterial disease, previous cardiovascular interventions, and previous neurological events. Unadjusted and propensity score-adjusted patient demographics, baseline characteristics, and medical history are presented by group in Table 1.
TABLE 1.
Patient Demographics, Baseline Characteristics, and Medical History
| Unadjusted
|
Weighted by Propensity Score
|
|||
|---|---|---|---|---|
| HCR (n = 200) |
PCI With DES (n = 98) |
HCR (n = 183)* |
PCI With DES (n = 89)* |
|
| Demographics | ||||
| Age, yrs | 64.4 ± 11.8 | 63.9 ± 10.8 | 64.3 ± 12.1 | 64.5 ± 10.5 |
| Male | 152 (76.0) | 70 (71.4) | 75.2 | 74.4 |
| Race | ||||
| White | 161 (83.0) | 83 (85.6) | 85.0 | 84.2 |
| Black | 28 (14.4) | 11 (11.3) | 12.3 | 13.3 |
| Asian | 4 (2.1) | 3 (3.1) | 2.1 | 2.6 |
| Pacific Islander | 1 (0.5) | 0 (0) | 0.6 | 0 |
|
Baseline characteristics | ||||
| BMI, kg/m2 | 29.1 ± 5.3 | 29.7 ± 5.0 | 29.3 ± 5.3 | 29.1 ± 5.3 |
| Creatinine | 1.2 ± 1.5 | 1.0 ± 0.6 | 1.0 ± 0.6 | 1.1 ± 1.0 |
|
Cardiovascular disease history | ||||
| Myocardial infarction | 74 (37.0) | 23 (23.5) | 32.0 | 38.0 |
| Peripheral arterial disease | 25 (12.5) | 7 (7.1) | 9.5 | 9.9 |
| Diabetes | 79 (39.5) | 36 (36.7) | 39.1 | 41.2 |
|
Cardiovascular procedure history | ||||
| CABG | 4 (2.0) | 0 (0.0) | 1.7 | 0.0 |
| PCI | 49 (24.5) | 19 (19.4) | 23.7 | 20.0 |
|
Cerebrovascular history | ||||
| Stroke | 12 (6.0) | 2 (2.0) | 3.1 | 3.3 |
| TIA | 7 (3.5) | 0 (0.0) | 4.1 | 0.0 |
|
Lung disease | ||||
| None | 182 (91.0) | 86 (87.8) | 91.0 | 93.9 |
| Mild/moderate | 15 (7.5) | 9 (9.2) | 7.4 | 4.9 |
| Severe | 3 (1.5) | 3 (3.1) | 1.6 | 1.2 |
Values are mean ± SD, n (%), or %.
Number of patients included in the propensity score analysis.
BMI = body mass index; CABG = coronary artery bypass graft; DES = drug-eluting stent(s); HCR = hybrid coronary revascularization; PCI = percutaneous coronary intervention; TIA = transient ischemic attack.
Baseline coronary angiography revealed that 59% of the 298 patients enrolled in the study had 2-vessel CAD and 38% had 3-vessel CAD. The mean SYNTAX score was 19.7 ± 9.6. Sixty-two percent of patients had proximal LAD disease, and 52% had mid- and/or distal LAD disease. Non-LAD disease in the overall cohort was located in the circumflex (50%), ramus (9%), and RCA (63%). LMCA stenosis was almost 3-fold more common in the HCR group than in the PCI group (7 patients were enrolled in the PCI group despite having LMCA stenosis that was an exploratory exclusion criterion for the PCI group). Of patients undergoing HCR, 18% had significant (≥50%) LMCA disease and 70% had proximal LAD disease, whereas 47% of the PCI patients had proximal LAD and 71% had mid- and/or distal LAD disease. Adjusted for the propensity to undergo HCR rather than PCI, the mean SYNTAX scores were low in both the HCR and PCI groups (18.4 ± 9.0 and 17.2 ± 9.6, respectively), and the mean STS (Society of Thoracic Surgeons) score predicted risk of 30-day mortality in the HCR group was 1.8 ± 2.6%. Baseline cardiovascular anatomy and characteristics, unadjusted and adjusted for the propensity scores, are shown in Table 2. Completeness of revascularization was defined for each patient as the percentage of planned vessels actually revascularized. The mean of this quotient was reported for both the HCR and PCI groups, expressed as a percentage.
TABLE 2.
Baseline Cardiovascular Anatomy and Characteristics
| Unadjusted
|
Weighted by Propensity Score
|
|||
|---|---|---|---|---|
| HCR (n = 200) |
PCI With DES (n = 98) |
HCR (n = 183)* |
PCI With DES (n = 89)* |
|
| CAD | ||||
| Single-vessel | 4 (2.0) | 3 (3.1) | (2.1) | (3.9) |
| Double-vessel | 116 (58.0) | 61 (62.2) | (61.5) | (50.8) |
| Triple-vessel | 80 (40.0) | 34 (34.7) | (36.4) | (45.3) |
|
Diseased vessels | ||||
| LMCA | 35 (17.5) | 7 (7.1) | (18.7) | (6.8) |
| Proximal LAD | 140 (70.0) | 46 (46.9) | (69.9) | (52.0) |
| Mid/distal LAD | 85 (42.5) | 70 (71.4) | (43.3) | (72.0) |
| Circumflex distribution | 104 (52.0) | 46 (46.9) | (51.0) | (50.6) |
| Ramus | 17 (8.5) | 9 (9.2) | (9.1) | (8.2) |
| RCA distribution | 125 (62.5) | 63 (64.3) | (61.1) | (67.7) |
|
| ||||
| SYNTAX score | 21.5 ± 9.5 | 15.8 ± 8.5 | 18.4 ± 9.0 | 17.2 ± 9.6 |
|
| ||||
| STS score | 1.8 ± 2.5 | — | 1.8 ± 2.6 | — |
|
| ||||
| CAD presentation | ||||
| No Sxs, no angina | 44 (22.0) | 14 (14.3) | (21.5) | (15.3) |
| Sxs unlikely to be ischemic | 3 (1.5) | 0 (0.0) | (1.7) | (0.0) |
| Stable angina | 55 (27.5) | 31 (31.6) | (28.3) | (35.2) |
| Unstable angina | 53 (26.5) | 33 (33.7) | (27.3) | (24.0) |
| Non-STEMI | 32 (16.0) | 18 (18.4) | (14.9) | (24.9) |
| STEMI | 13 (6.5) | 2 (2.0) | (6.4) | (0.6) |
Values are mean ± SD or n (%).
Number of patients included in the propensity score analysis. Dashes indicate that data were unavailable.
CAD = coronary artery disease; LAD = left anterior descending; LMCA = left main coronary artery; RCA = right coronary artery; STEMI = ST-segment elevation myocardial infarction; SYNTAX = Synergy Between PCI With Taxus and Cardiac Surgery; STS = Society of Thoracic Surgeons; Sxs = symptom; other abbreviations as in Table 1.
PROCEDURAL CHARACTERISTICS
Surgical approaches to left internal thoracic artery (LITA)-LAD grafting varied between centers according to surgeon preference. Robotic minimally invasive direct coronary artery bypass (robotic ITA harvest and left microthoracotomy for anastomosis) was used in 108 of 200 HCR procedures, whereas robotic totally endoscopic coronary artery bypass surgery was performed in 42 of 200 HCR procedures. Minimally invasive direct coronary artery bypass (small left thoracotomy with direct ITA harvest and anastomosis) was used for LITA-LAD grafting in 38 of 200 HCR procedures, whereas planned sternotomy was used in 12 of 200 HCR cases. In total, cardiopulmonary bypass was used in 16 of 200 HCR procedures, typically as part of a totally endoscopic coronary artery bypass procedure. The majority of the 200 HCR procedures (76%) were performed in 2 stages, and 12% were performed by simultaneous surgical and percutaneous revascularization procedures. Four percent of HCR procedures were completed in more than 2 stages. Sixteen patients (8%) initially assigned to the HCR group received only the surgical revascularization, at the discretion of the patients and their cardiologists. Of the 98 multivessel PCI patients, 64% underwent revascularization in a single-staged procedure and 31% in 2-staged procedures, whereas 3% underwent a 3-staged procedure (Table 3). Two patients enrolled in the PCI group on the basis of an initial treatment plan to perform multivessel PCI underwent surgical revascularization of the LAD. One patient had an elective totally endoscopic coronary artery bypass surgery, followed approximately a month later by an elective PCI of the first diagonal. The other patient underwent an elective sternotomy LAD revascularization, followed by an emergent PCI of the LMCA 5 days post-operatively.
TABLE 3.
Procedure and Procedure Staging
| HCR (n = 200) |
PCI With DES (n = 98) |
|
|---|---|---|
| Surgical approach to LITA-LAD grafting | ||
| Robotic MIDCAB (robotic ITA harvest with direct anastomosis) | 108 (54) | |
| Robotic TECAB (robot used for ITA harvest and anastomosis) | 42 (21) | |
| MIDCAB (small left thoracotomy with direct ITA harvest and anastomosis) | 38 (19) | |
| Sternotomy (planned) | 12 (6) | |
| Cardiopulmonary bypass used | 32 (16) | |
|
| ||
| Hybrid procedures: staging of surgery and initial PCI | ||
| Surgery followed by PCI | 110 (55.0) | 2 (2.0) |
| PCI followed by surgery | 43 (21.5) | 0 (0.0) |
| Simultaneous surgery and PCI | 24 (12.0) | 0 (0.0) |
| Surgery only | 16 (8.0) | 0 (0.0) |
| Surgery and PCI completed on same day (order unknown) | 7 (3.5) | 0 (0.0) |
|
| ||
| PCI-only procedure staging | ||
| Single PCI procedure | 0 (0.0) | 63 (64.3) |
| 2 PCI procedures | 0 (0.0) | 30 (30.6) |
| 3 PCI procedures | 0 (0.0) | 3 (3.1) |
Values are n (%).
ITA = internal thoracic artery; LITA = left internal thoracic artery; MIDCAB = minimally invasive direct coronary artery bypass; TECAB = totally endoscopic coronary artery bypass; other abbreviations as in Table 1.
OUTCOMES
Among 98 patients with hybrid-eligible coronary anatomy who had multivessel PCI in this observational study, the mean completeness of revascularization was 87.7%. Ninety-six of the 98 patients (98.0%) had revascularization of the LAD by PCI and 2 (2.0%) had surgical revascularization of the LAD prior to PCI. An average of 90.8% of planned vessels in the left circumflex territory and 92.9% of planned branches of the RCA were revascularized in the PCI group.
Among 200 patients who underwent planned HCR, the mean completeness of revascularization was 75.2%. All had surgical grafting of the LAD, whereas an average of 79.8% of planned vessels in the left circumflex territory and 87.5% of planned branches of the RCA were revascularized by planned PCI in the HCR group.
A total of 46 MACCE events were reported in 35 of the 298 patients during the follow-up after the revascularization procedure; the median duration of follow-up was 17.6 months. The propensity score-adjusted event-free survival at 12 months was similar between the 2 groups (adjusted hazard ratio [HR]: 1.063; adjusted 95% confidence interval [CI]: 0.666 to 1.697). Incidence of MACCE and its individual components that occurred during the first 12 months after the initial procedure and through the end of study follow-up are shown in Table 4. In total, there were 3 deaths, 5 MI, 5 strokes, and 15 late revascularization procedures reported in the patients assigned to HCR, whereas 2 deaths, 4 MI, no strokes, and 12 late revascularization procedures were reported in the PCI group.
TABLE 4.
Incidence of MACCE at 30 Days, 12 Months, and Through End of Study
| HCR (n = 200) |
PCI With DES (n = 98) |
HR (95% CI) | |||
|---|---|---|---|---|---|
| n | Incidence Rate Per Person-Year | n | Incidence Rate Per Person-Year | ||
| MACCE incidence at 30 days | |||||
| Any MACCE | 6 | 0.393 | 2 | 0.264 | 2.658 (0.839–8.421) |
| Death | 1 | 0.064 | 0 | 0.000 | |
| Myocardial infarction | 3 | 0.195 | 1 | 0.131 | |
| Stroke | 0 | 0.000 | 0 | 0.000 | |
| Revascularization | 4 | 0.260 | 1 | 0.131 | |
|
MACCE incidence at 12 months | |||||
| Any MACCE | 23 | 0.143 | 10 | 0.119 | 1.063 (0.666–1.697) |
| Death | 3 | 0.017 | 1 | 0.011 | |
| Myocardial infarction | 4 | 0.024 | 3 | 0.034 | |
| Stroke | 5 | 0.030 | 0 | 0.000 | |
| Revascularization | 14 | 0.085 | 8 | 0.094 | |
|
MACCE incidence through end of study | |||||
| Any MACCE | 23 | 0.103 | 12 | 0.103 | 0.868 (0.556–1.355) |
| Death | 3 | 0.012 | 2 | 0.016 | |
| Myocardial infarction | 4 | 0.017 | 3 | 0.024 | |
| Stroke | 5 | 0.021 | 0 | 0.000 | |
| Revascularization | 14 | 0.061 | 10 | 0.084 | |
CI = confidence interval; HR = hazard ratio; MACCE = major adverse cardiac and cerebrovascular events; other abbreviations as in Table 1.
At 12 months of follow-up, there were 5 strokes in the HCR group and none in the PCI group, which remained unchanged at the end of study follow-up. All 5 strokes occurred at least 2.5 months after the LIMA-LAD grafting (range 2.6 to 6.9 months), and none occurred within 30 days of the PCI portion of the HCR procedure.
Fourteen HCR and 10 PCI patients had unplanned repeat revascularization through the end of study follow-up (median 17.6 months). Among 14 HCR patients who had repeat revascularization, 1 had conversion to sternotomy and CABG at the index procedure and no further repeat revascularization thereafter. Of the 13 remaining HCR patients who had repeat revascularization with PCI, 5 had PCI to the LAD or LIMA-LAD anastomosis, whereas 6 had PCI to address restenosis of coronary arteries that were stented as part of the combined HCR procedure, 1 had a previously untreated vessel stented, and 1 lacked adequate data in follow-up to determine which vessel was stented. Of 10 PCI patients who underwent repeat revascularization during follow-up, 1 had multivessel CABG 14 months after the index multivessel PCI procedure. The other 9 PCI patients had repeat PCI for restenosis of vessels stented as part of the index procedure.
LMCA stenosis was defined as a clinical exclusion criterion for HCR eligibility in this study; thus, we intended to exclude patients with LMCA stenosis from the multivessel PCI cohort. There were no significant differences in outcomes for HCR patients with LMCA stenoses compared with those without LMCA stenoses. Similarly, PCI patients with LMCA stenoses had outcomes comparable to those of PCI patients without LMCA stenoses.
The propensity-score adjusted Kaplan-Meier MACCE-free survival curve (Central Illustration) illustrates a trend toward increased early risk with HCR compared with PCI over the first 6 months following the initial intervention, as well as a trend toward reduction in late risk between 15 months and the end of study follow-up (adjusted HR: 0.868; adjusted 95% CI: 0.556 to 1.355).
CENTRAL ILLUSTRATION. Multicenter HCR Study: MACCE-Free Survival at End of Study Follow-Up.
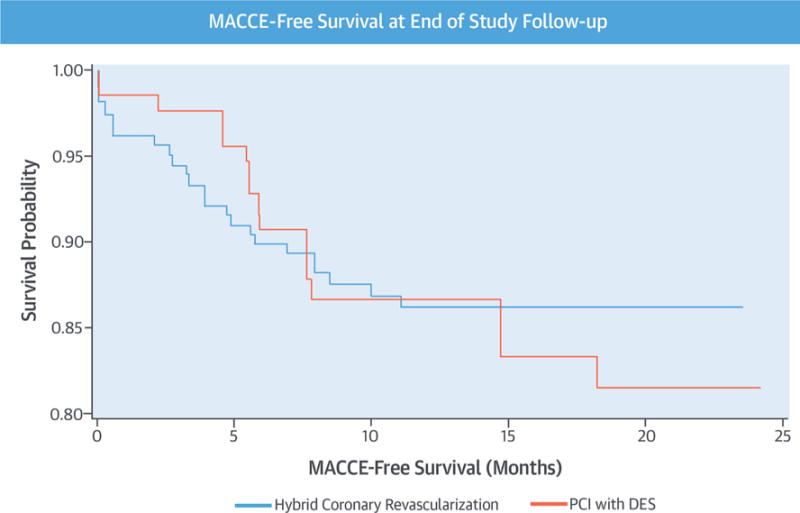
In this first multicenter observational study of hybrid coronary revascularization (HCR) and multivessel percutaneous coronary intervention (PCI) for patients with hybrid-eligible coronary anatomy, risk-adjusted major adverse cardiovascular and cerebrovascular events (MACCE) rates were similar between groups through 12 months of follow-up. During longer follow-up, at 18 months, MACCE-free survival curves for HCR versus PCI began to diverge, with increasing MACCE in the multivessel PCI group. DES = drug-eluting stent(s).
DISCUSSION
HCR seeks to optimize outcomes of revascularization by combining the most beneficial attributes of surgical coronary revascularization and percutaneous intervention. The rationale for HCR stems from a number of compelling observations: the LAD is the most important of the 3 coronary branches, supplying 50% to 60% of the ventricular mass and twice the mass of either the circumflex or right coronary distributions; LIMA to LAD bypass has been shown to be more effective than PCI with respect to event-free survival, relief of angina, and long-term patency (10,11); the LIMA to LAD bypass graft contributes the majority of the survival advantage provided by CABG, whereas the value of additional arterial grafts to non-LAD targets is relatively smaller (12,13); and the early restenosis rate of non-LAD vessels after PCI with DES appears to be significantly less than the early occlusion rate of saphenous vein grafts, but the clinical impact of this difference has not yet been defined (4,14,15).
The published HCR experience is limited and, at most, hypothesis-generating. Over a 10-year period, the collective published work reflects the outcomes of approximately 500 patients from a number of small, single-center series (11–15). Nonetheless, these uncontrolled studies suggest that HCR may provide a higher degree of durability, symptom relief, and survival than 3-vessel stenting, afford a stroke rate comparable to PCI and lower than standard CABG, presumably by avoiding manipulation of the ascending aorta, and offer a low infection rate, transfusion rate, and recovery time by avoiding a median sternotomy. Although no randomized trial comparing HCR to PCI has been conducted to date, early experience suggests that HCR has the potential to disseminate widely and become the third major interventional alternative for patients with multivessel CAD. Without convincing data from a randomized clinical trial, there is insufficient evidence to guide dissemination of this potentially important procedure to large patient populations. Moreover, there are potential disadvantages to the HCR approach, including (but not limited to) a small risk of adverse coronary events during the interval of time between phases of the combined procedure when these are staged, the cumulative sum of the typical periprocedural complications associated with both minimally invasive CABG and PCI, and the risks associated with dual antiplatelet therapy.
In this first multicenter observational study of HCR and multivessel PCI for patients with hybrid-eligible coronary anatomy, there was significant heterogeneity in management of patients with hybrid-eligible coronary anatomy across the 11 experienced study sites. This demonstrates the absence of consensus among experienced HCR and PCI operators due to lack of evidence regarding the relative effectiveness of these 2 alternative revascularization strategies in patients with hybrid-eligible CAD.
The vast majority of HCR procedures performed during the study were performed in stages, rather than simultaneously. Of staged procedures, the surgical LIMA to the LAD was performed prior to PCI of non-LAD target vessels in the majority of cases, indicating a preference of the heart teams for this approach. This approach offers the opportunity to verify patency of the LIMA graft prior to stenting non-LAD targets, enables the surgery to be performed without dual antiplatelet therapy (as would be required by a PCI-first staged approach), which may reduce the risk of perioperative bleeding complications, and provides flexibility for scheduling the multidisciplinary teams of operators. Among interventional cardiologists, there was a similarly strong preference for a single-stage approach to multivessel stenting. The staged approach with LIMA-LAD revascularization performed first also allows for routine imaging of the bypass graft during the PCI completion of the HCR.
Risk-adjusted MACCE rates were similar between HCR and PCI groups through 12 months of follow-up. These outcomes, albeit short-term, establish equipoise and support the hybrid investigators’ heterogeneous approach to selecting revascularization strategies for hybrid-eligible patients. Certainly, the early clinical outcomes in this trial do not provide clear guidance as to whether either therapy is superior.
Interestingly, by 18 months of follow-up, the MACCE-free survival curves for HCR versus PCI began to diverge, with decreasing event-free survival in the PCI-only arm relative to the HCR arm. The difference in outcomes did not reach statistical significance. This early “signal” may suggest, however, that longer follow-up of patients with hybrid-eligible CAD treated with HCR versus PCI might demonstrate continued divergence of these curves and a meaningful difference in outcomes between the 2 treatment strategies.
The rates of the individual components of MACCE, with the exception of stroke, were also similar between groups at 12 months and the end of study. The stroke rate was higher in the HCR group, yet interestingly, none occurred in temporal proximity to the surgical or percutaneous interventions; the earliest stroke occurred more than 2.5 months after the interventions. The HCR group had a higher prevalence of history of stroke than the PCI-only group did, and after propensity-score adjustment, the 2 groups were similar. There were many more LMCA stenoses in the HCR group than in the PCI group because LMCA disease was supposed to exclude participation from the study for the PCI group. However, the known association between LMCA stenosis and cerebrovascular disease cannot easily explain the difference in stroke outcomes between groups because all 5 strokes that occurred in the HCR group occurred in patients without LMCA stenosis.
One important goal of this observational trial was the refinement of the eligibility criteria for a future comparative effectiveness trial of HCR compared with multivessel PCI. The clinical outcomes observed in this study and the experience of the site heart teams in conducting this study informed the reevaluation and liberalization of several of the exploratory eligibility criteria that were originally developed for a proposed pivotal trial. Specifically, these investigators performed more HCR procedures on patients with LMCA stenoses than was originally expected, and their experience was favorable; surgeons believed that minimally invasive LIMA-LAD grafting was safe, and interventional cardiologists believed that stenting of the LMCA into the left circumflex was safe as well in the setting of a protective patent LIMA-LAD graft. Clinical outcomes for hybrid-eligible patients with LMCA stenosis were similar to outcomes for patients without LMCA stenosis in both the HCR and PCI groups. Furthermore, most participating sites performed ample LMCA PCI procedures in patients who were thought not to be surgical candidates.
STUDY LIMITATIONS
This study is limited by its observational and nonrandomized nature. While this allowed the description of the currently heterogeneous real-world approach to patients with hybrid-eligible coronary anatomy within our clinical network, it limits the generalizability of our findings. Longer follow-up would help allow a better understanding of the relative benefits of hybrid coronary revascularization and multivessel PCI in these low-SYNTAX score patients.
CONCLUSIONS
In this first multicenter observational study of HCR and multivessel PCI for patients with hybrid-eligible coronary anatomy, risk-adjusted MACCE rates were similar between groups through 12 months of follow-up. During longer follow-up, at 18 months, MACCE-free survival curves for HCR versus PCI began to diverge, with increasing MACCE in the multivessel PCI group. There is significant heterogeneity in current practices for management of patients with hybrid-eligible coronary anatomy due to the absence of comparative evidence. This multicenter study provides evidence to support equipoise and the need for a rigorous comparative effectiveness trial of these 2 alternative therapies.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS
In a multicenter observational study, HCR, combining minimally invasive CABG of the LAD artery with PCI of other coronary vessels, was associated with a composite rate of death, stroke, MI, and repeat revascularization during the first year following revascularization similar to that achieved with multivessel PCI.
TRANSLATIONAL OUTLOOK
Randomized trials are needed to compare the long-term clinical outcomes of these 2 revascularization strategies.
Acknowledgments
National Institutes of Health and National Heart, Lung, and Blood Institutes grant 1-RC1-HL100951 to Drs. Puskas and Ascheim funded this study. Dr. Halkos has served as a consultant to Intuitive Surgical and Medtronic. Dr. Sutter serves on the Speakers Bureau of Intuitive Surgical. Dr. Shapiro serves on the Speakers’ Bureau of Astra Zeneca; and serves as a consultant to Intuitive Surgical. Dr. Hoff serves as a peer trainer and consultant for Medtronic. Dr. Vassiliades is a full-time employee of Medtronic. All other authors have reported that they no relationships relevant to the contents of this paper to disclose. Friedrich-Wilhelm Mohr, MD, PhD, served as Guest Editor for this paper.
ABBREVIATIONS AND ACRONYMS
- CAD
coronary artery disease
- CI
confidence interval
- DES
drug-eluting stent(s)
- HCR
hybrid coronary revascularization
- HR
hazard ratio
- LAD
left anterior descending
- LIMA
left internal mammary artery
- LITA
left internal thoracic artery
- LMCA
left main coronary artery
- MACCE
major adverse cardiac and cerebrovascular event(s)
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- RCA
right coronary artery
Footnotes
APPENDIX For supplemental material, please see the online version of this paper.
References
- 1.Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomized, clinical SYNTAX trial. Lancet. 2013;381:629–38. doi: 10.1016/S0140-6736(13)60141-5. [DOI] [PubMed] [Google Scholar]
- 2.Farkouh ME, Domanski M, Sleeper LA, et al. for the FREEDOM Trial Investigators Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–84. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 3.Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;366:1467–76. doi: 10.1056/NEJMoa1110717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander JH, Hafley G, Harrington RA, et al. for the PREVENT IV Investigators Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–54. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 5.Halkos ME, Rab ST, Vassiliades TA, et al. Hybrid coronary revascularization versus off-pump coronary artery bypass for the treatment of left main coronary stenosis. Ann Thorac Surg. 2011;92:2155–60. doi: 10.1016/j.athoracsur.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Repossini A, Tespili M, Saino A, et al. Hybrid revascularization in multivessel coronary artery disease. Eur J Cardiothorac Surg. 2013;44:288–93. doi: 10.1093/ejcts/ezt016. [DOI] [PubMed] [Google Scholar]
- 7.Bonaros N, Schachner T, Wiedemann D, et al. Closed chest hybrid coronary revascularization for multivessel disease—current concepts and techniques from a two-center experience. Eur J Cardiothorac Surg. 2011;40:783–7. doi: 10.1016/j.ejcts.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 8.Adams C, Burns DJ, Chu MW, et al. Single-stage hybrid coronary revascularization with long-term follow-up. Eur J Cardiothorac Surg. 2014;45:438–42. doi: 10.1093/ejcts/ezt390. discussion 442–3. [DOI] [PubMed] [Google Scholar]
- 9.Harskamp RE, Brennan JM, Xian Y, et al. Practice patterns and clinical outcomes after hybrid coronary revascularization in the United States: an analysis from the Society of Thoracic Surgeons Adult Cardiac Database. Circulation. 2014;130:872–9. doi: 10.1161/CIRCULATIONAHA.114.009479. [DOI] [PubMed] [Google Scholar]
- 10.Diegeler A, Thiele H, Falk V, et al. Comparison of stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery. N Engl J Med. 2002;347:561–6. doi: 10.1056/NEJMoa013563. [DOI] [PubMed] [Google Scholar]
- 11.Loop FD. Internal-thoracic-artery grafts: biologically better coronary arteries. N Engl J Med. 1996;334:263–5. doi: 10.1056/NEJM199601253340411. [DOI] [PubMed] [Google Scholar]
- 12.Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1–6. doi: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- 13.Sergeant PT, Blackstone EH, Meyns BP. Does arterial revascularization decrease the risk of infarction after coronary artery bypass grafting? Ann Thorac Surg. 1998;66:1–10. doi: 10.1016/s0003-4975(98)00394-4. discussion 10–1. [DOI] [PubMed] [Google Scholar]
- 14.Moses JW, Leon MB, Popma JJ, et al. for the SIRIUS Investigators Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–23. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 15.Indolfi C, Pavia M, Angelillo IF. Drug-eluting stents versus bare metal stents in percutaneous coronary interventions (a meta-analysis) Am J Cardiol. 2005;95:1146–52. doi: 10.1016/j.amjcard.2005.01.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


