Abstract
Objective
Antibiotic use, particularly type and duration, is a crucial modifiable risk factor for Clostridium difficile. Cardiac surgery is of particular interest because prophylactic antibiotics are recommended for 48 hours or less (vs ≤24 hours for noncardiac surgery), with increasing vancomycin use. We aimed to study associations between antibiotic prophylaxis (duration/vancomycin use) and C difficile among patients undergoing coronary artery bypass grafting.
Methods
We extracted data on coronary artery bypass grafting procedures from the national Premier Perspective claims database (2006–2013, n = 154,200, 233 hospitals). Multilevel multivariable logistic regressions measured associations between (1) duration (<2 days, “standard” vs ≥2 days, “extended”) and (2) type of antibiotic used (“cephalosporin,” “cephalosporin + vancomycin,” “vancomycin”) and C difficile as outcome.
Results
Overall C difficile prevalence was 0.21% (n = 329). Most patients (59.7%) received a cephalosporin only; in 33.1% vancomycin was added, whereas 7.2% received vancomycin only. Extended prophylaxis was used in 20.9%. In adjusted analyses, extended prophylaxis (vs standard) was associated with significantly increased C difficile risk (odds ratio, 1.43; confidence interval, 1.07–1.92), whereas no significant associations existed for vancomycin use as adjuvant or primary prophylactic compared with the use of cephalosporins (odds ratio, 1.21; confidence interval, 0.92–1.60, and odds ratio, 1.39; confidence interval, 0.94–2.05, respectively). Substantial inter-hospital variation exists in the percentage of extended antibiotic prophylaxis (interquartile range, 2.5–35.7), use of adjuvant vancomycin (interquartile range, 4.2–61.1), and vancomycin alone (interquartile range, 2.3–10.4).
Conclusions
Although extended use of antibiotic prophylaxis was associated with increased C difficile risk after coronary artery bypass grafting, vancomycin use was not. The observed hospital variation in antibiotic prophylaxis practices suggests great potential for efforts aimed at standardizing practices that subsequently could reduce C difficile risk.
Keywords: Clostridium difficile, coronary artery bypass graft, antibiotic prophylaxis, vancomycin
Graphical Abstract
There is substantial hospital variation in antibiotic pro-phylaxis more than 48 hours in patients undergoing CABG.
Clostridium difficile infections are an increasing problem in hospitalized patients, currently affecting more than 300,000 hospitalizations annually in the United States.1 The development of C difficile compromises patient safety2 and increases length of hospital stay and costs of hospitalization, with overall cost estimates of C difficile of up to $3.2 billion per year.3–5 Antibiotic use is regarded as a crucial modifiable risk factor for C difficile, with studies demonstrating increased risks associated with the extended use of antibiotics, number of antibiotics used, and use of cephalosporins, clindamycin, and fluoroquinolones in particular.6–8 Because prophylactic antibiotics are routinely administered to patients undergoing surgery, characteristics of this practice provide an important target to explore in efforts to reduce C difficile. Of particular interest are patients undergoing cardiac surgery because they differ from those undergoing general surgery regarding, for example, the use of cardiopulmonary bypass, hypothermia, the length of surgery, or indwelling chest catheters after surgery.5,9 Therefore, several guidelines (eg, Society of Thoracic Surgeons) recommend 48 hours or less for antibiotic prophylaxis compared with 24 hours or less for noncardiac surgery.10–13 A cephalosporin (usually cefazolin) is recommended as the antibiotic of choice; vancomycin is recommended as an adjuvant or the primary prophylactic antibiotic in a high-risk case for staphylococcal infection or β-lactam/penicillin allergy, respectively.14 However, concerns have been raised on the effect of both the longer duration of prophylaxis and the increasing use of vancomycin on the risk for resistant organisms and C difficile.15,16 Moreover, C difficile has recently been found to be one of the main infections after cardiac surgery and is increasingly recognized as an important contributor to morbidity and mortality in this patient group.5,17
By using a nationwide claims-based database, we therefore sought to study the association between characteristics of antibiotic prophylaxis and C difficile in patients undergoing coronary artery bypass grafting (CABG) surgery with or without valve repair/replacement. The specific characteristics of interest were the extended use of prophylactic antibiotics (≥2 days after surgery) and the use of vancomycin as an adjuvant to cephalosporins or as the primary prophylactic antibiotic. We hypothesized both characteristics to be associated with an increased risk for C difficile in patients undergoing CABG.
MATERIALS AND METHODS
Data Source and Study Design
For this retrospective study, we used data from the Premier Perspective database18 (Premier Inc, Charlotte, NC) containing surgical hospital discharges from January 2006 to December 2013. This database contains complete billing information on a patient’s hospitalization. Apart from International Classification of Diseases-9th revision Clinical Modification (ICD-9 CM) codes and Current Procedural Terminology codes, this claims-based dataset provides standardized billing items. Up to 30 ICD-9 CM codes can be recorded for each hospitalization. Before data are incorporated in the Premier database, the vendor performs a rigorous data validation and quality assurance process. This process involves a 7-step integrity analysis, followed by approximately 150 sampling and statistical validity and integrity assurance crosschecks on all hospital-supplied data. For standardized codes, such as ICD-9 and Current Procedural Terminology codes, the codes are ascertained to be valid for the time period the patient record is reported. The dataset is increasingly used by a variety of study groups addressing clinical questions.19,20 These data meet the requirements of de-identification as defined by the Health Insurance Portability and Accountability Act and were exempt from consent requirements of the Mount Sinai Medical Center Institutional Review Board (project HS#: 14-00,647).
Study Sample
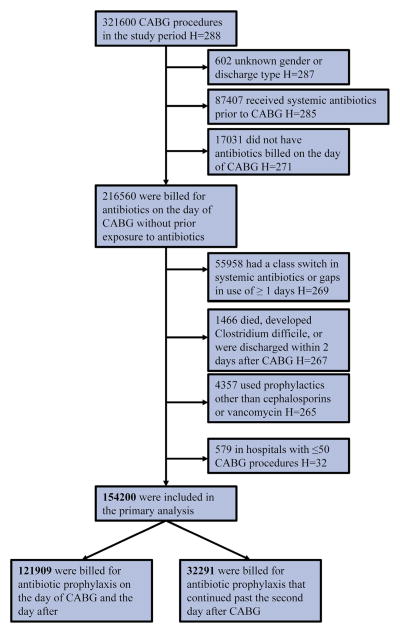
The cohort contained CABG procedures (with or without valve repair/replacement) performed from 2006 to 2013 indicated by ICD-9 CM code 36.1 (including subcodes). Exclusion criteria were based on previous studies.8,16 We excluded cases with unknown gender or discharge type (n = 602), systemic antibiotic use before surgery (n = 87,407), no billing for antibiotic use on the day of surgery (17,031), a switch in antibiotic class or gaps in antibiotic use of 1 or more days (to distinguish between treatment and prophylaxis, n = 55,958), and patients who died, developed C difficile, or were discharged within 2 days after surgery (n = 1466), the minimum plausible induction time for C difficile to be associated with the studied exposure.21 Finally, we also excluded patients who were billed for prophylactic antibiotics other than cephalosporins or vancomycin (n = 4357). In particular, the exclusion of patients who died within 2 days was applied to keep our cohort strategy similar to a comparable study16 that studied the association between adjuvant vancomycin and C difficile risk in patients undergoing CABG. The main rationale is that these patients were not at risk of developing C difficile because 2 days is the minimum plausible induction time. To have a sufficient sample size per cluster, we also included only hospitals with more than 50 CABG procedures.22
Study Variables
The main exposures of interest were the extended use of prophylactic antibiotics and the use of vancomycin as an adjuvant to cephalosporins or the primary prophylactic antibiotic. Prophylactic antibiotic use was categorized into parenteral use of a cephalosporin only, a cephalosporin and vancomycin, or vancomycin only. Use was defined as billing for prophylaxis on the day of surgery or both the day of surgery and the day after (standard) versus billing for prophylaxis up to day 2 after surgery or later (extended).
Patient demographics included age, gender, and race (white, black, other). Healthcare-related variables included transfer from another hospital, type of insurance (commercial, Medicaid, Medicare, uninsured, other), hospital teaching status, and the mean annual number of CABG surgeries per hospital. Procedure-related variables included the day of procedure (as a measure of preoperative stay), the number of grafts involved (1-≤4), concomitant valve repair/replacement, use of an internal thoracic artery graft, previous CABG surgery, emergency admission, and year of procedure. Variables related to comorbidity burden were Quan and colleagues’23 update of the Deyo adaptation of the Charlson comorbidities and index, and the use of antacid medications (proton pump inhibitors and H2 receptor antagonists) because these medications have been associated with increased C difficile risk.24
The main outcome, C difficile 48 hours or more postoperatively, was defined as the presence of all of the following criteria16: (1) having an ICD-9 code 008.45, (2) billing for a stool study for C difficile, and (3) billing for C difficile therapy, that is, intravenous or oral metronidazole or oral vancomycin 2 days or more after surgery.
Statistical Analysis
The univariable association between extended antibiotic use and study variables was assessed using chi-square tests and t tests for categoric and continuous variables, respectively. To demonstrate potential inter-hospital variability, the percentage (y-axis) of (1) extended antibiotic use, (2) adjuvant vancomycin use, and (3) just vancomycin use by hospital (x-axis) was illustrated on separate graphs.
A multilevel multivariable logistic regression was then performed to measure the association among extended antibiotic use, vancomycin use, and C difficile. It included a random intercept term that varies at the level of each hospital and accounts for correlation of patients within hospitals. The model was adjusted using all variables found significant at the P less than .15 level from the univariable tests and had at least 10 cases within each category. Adjusted odds ratio (OR), 95% confidence interval (CI), and P value are reported and to be used together as a measure of overall significance. Hospital variation in C difficile was measured using the intraclass correlation coefficient of the final model. Model discrimination was evaluated using the C-statistic.
Similar to others, we chose to use a conservative measure of C difficile defined by 3 criteria.16 However, numerous studies often have relied only on ICD-9 coding because a high sensitivity (71%) and specificity (99%) for C difficile have been demonstrated.25,26 Therefore, we assessed sensitivity to the definition of C difficile diagnosis by repeating the model with C difficile defined as just the presence of ICD-9 code 008.45, irrespective of a stool sample or antibiotic therapy indication.
RESULTS
The final study sample consisted of 154,200 CABG surgeries performed in 233 hospitals (Figure 1).
FIGURE 1.
Patient flow chart.* *Patients may belong to more than 1 exclusion criteria in each step. “H” stands for the number of hospitals; because exclusions are on the patient level, the number of hospitals mentioned in each step does not reflect the number of hospitals excluded. In any of the exclusion steps, no complete hospitals (ie, all CABG cases in those hospitals) are excluded. CABG, Coronary artery bypass grafting.
Characteristics
Overall, 92,013 (59.7%; range, 0–100) of patients received a cephalosporin only as prophylaxis; in 51,109 patients (33.1%; range, 0–100), vancomycin was added, and in 11,078 patients (7.2%; range, 0–82.6), vancomycin was used as the primary prophylactic. Extended prophylaxis was used in 32,291 (20.9%; range, 0–95.4) cases.
Table 1 provides a breakdown of the characteristics of antibiotic prophylaxis, patient demographics, healthcare-related variables, and procedure-related variables by standard versus extended antibiotic prophylaxis. Although statistically significant, there were no major differences between the standard group and the extended antibiotic prophylaxis group. Most notable (comparing standard vs extended antibiotic prophylaxis), mean age differed only slightly (65.4 vs 65.7 years), as did the mean Deyo–Charlson comorbidity index (0.96 vs 0.93) (both P <.01). Appendix E1 provides more insight into the distribution of C difficile by the study variables. Appendix E2 provides the separate Deyo–Charlson comorbidities by standard versus extended antibiotic prophylaxis.
TABLE 1.
Patient demographics, healthcare related, procedure related, and comorbidity variables by standard versus extended use of antibiotic prophylaxis
| Characteristics | Antibiotic prophylaxis
|
P value† | |||
|---|---|---|---|---|---|
| Standard
|
Extended
|
||||
| N* | %* | N* | %* | ||
| Antibiotic prophylaxis group | |||||
| Cephalosporin only | 71,934 | 59.0 | 20,079 | 62.2 | <.01 |
| Cephalosporin with Vancomycin | 40,357 | 33.1 | 10,752 | 33.3 | |
| Vancomycin only | 9618 | 7.9 | 1460 | 4.5 | |
| Patient demographics | |||||
| Age continuous* | 65.4 | 10.6 | 65.7 | 10.8 | <.01 |
| Age category | |||||
| <45 y | 3449 | 2.8 | 924 | 2.9 | <.01 |
| 45–54 y | 16,155 | 13.3 | 4112 | 12.7 | |
| 55–64 y | 35,541 | 29.2 | 9232 | 28.6 | |
| 65–74 y | 40,217 | 33.0 | 10,633 | 32.9 | |
| >75 y | 26,547 | 21.8 | 7390 | 22.9 | |
| Gender | |||||
| Female | 30,681 | 25.2 | 8027 | 24.9 | .25 |
| Male | 91,228 | 74.8 | 24,264 | 75.1 | |
| Race | |||||
| White | 92,037 | 75.5 | 22,330 | 69.2 | <.01 |
| Black | 7471 | 6.1 | 1782 | 5.5 | |
| Other | 22,401 | 18.4 | 8179 | 25.3 | |
| Healthcare related | |||||
| Transfer from other hospital | 16,878 | 13.8 | 4750 | 14.7 | <.01 |
| Insurance type | |||||
| Commercial | 42,685 | 35.0 | 10,959 | 33.9 | <.01 |
| Medicaid | 5831 | 4.8 | 1739 | 5.4 | |
| Medicare | 65,238 | 53.5 | 17,497 | 54.2 | |
| Uninsured/other | 8155 | 6.7 | 2096 | 6.5 | |
| Hospital teaching status | |||||
| Nonteaching | 54,998 | 45.1 | 15,334 | 47.5 | <.01 |
| Teaching | 66,911 | 54.9 | 16,957 | 52.5 | |
| Mean annual no. of CABGs per hospital* | 211 | 144 | 184 | 123 | <.01 |
| Procedure related | |||||
| Mean day of procedure | 1.6 | 2.2 | 1.6 | 2.2 | .97 |
| 1 | 23,435 | 19.22 | 6519 | 20.19 | <.01 |
| 2 | 42,651 | 34.99 | 11,456 | 35.48 | .10 |
| 3 | 33,978 | 27.87 | 8861 | 27.44 | .12 |
| ≥4 | 14,958 | 12.27 | 3997 | 12.38 | .60 |
| Concomitant valve repair/replacement | 16,892 | 13.86 | 4872 | 15.09 | <.01 |
| ITA graft | 106,835 | 87.64 | 27,762 | 85.97 | <.01 |
| Previous CABG surgery | 290 | 0.2 | 68 | 0.2 | .36 |
| Emergency admission | 26,399 | 21.7 | 7448 | 23.1 | <.01 |
| Year of procedure | |||||
| 2006 | 16,108 | 13.2 | 4284 | 13.3 | <.01 |
| 2007 | 16,362 | 13.4 | 4022 | 12.5 | |
| 2008 | 14,308 | 11.7 | 3553 | 11.0 | |
| 2009 | 16,119 | 13.2 | 3171 | 9.8 | |
| 2010 | 16,244 | 13.3 | 3594 | 11.1 | |
| 2011 | 15,186 | 12.5 | 3996 | 12.4 | |
| 2011 | 14,677 | 12.0 | 4794 | 14.8 | |
| 2013 | 12,905 | 10.6 | 4877 | 15.1 | |
| Comorbidity burden | |||||
| Mean Deyo–Charlson comorbidity index* | 0.96 | 1.33 | 0.93 | 1.30 | <.01 |
| Use of antacid medications | 116,377 | 95.5 | 31,095 | 96.3 | <.01 |
CABG, Coronary artery bypass grafting; ITA, internal thoracic artery.
Continuous variable mean and standard deviation instead of N and %, respectively.
Chi-square test for categoric variables, t test for continuous variables.
APPENDIX E1.
Patient demographics, healthcare related, procedure related, and comorbidity variables by yes or no for Clostridium difficile
| Characteristics |
C difficile
|
P value† | |||
|---|---|---|---|---|---|
| No
|
Yes
|
||||
| N* | %* | N* | %* | ||
| Antibiotic prophylaxis | |||||
| Duration | |||||
| Extended | 122,124 | 79.1 | 234 | 69.9 | <.01 |
| Standard | 32,320 | 20.9 | 101 | 30.1 | |
| Type of antibiotic | |||||
| Cephalosporin only | 92,146 | 59.7 | 168 | 50.1 | <.01 |
| Cephalosporin with vancomycin | 51,187 | 33.1 | 130 | 38.8 | |
| Vancomycin only | 11,111 | 7.2 | 37 | 11.0 | |
| Patient demographics | |||||
| Age continuous* | 65.4 | 10.7 | 70.2 | 10.8 | <.01 |
| Age category | |||||
| <45 y | 4394 | 2.8 | 7 | 2.1 | <.01 |
| 45–54 y | 20,324 | 13.2 | 24 | 7.2 | |
| 55–64 y | 44,879 | 29.1 | 60 | 17.9 | |
| 65–74 y | 50,942 | 33.0 | 111 | 33.1 | |
| >75 y | 33,905 | 22.0 | 133 | 39.7 | |
| Gender | |||||
| Female | 38,736 | 25.1 | 125 | 37.3 | <.01 |
| Male | 115,708 | 74.9 | 210 | 62.7 | |
| Race | |||||
| White | 114,474 | 74.1 | 251 | 74.9 | .88 |
| Black | 9258 | 6.0 | 18 | 5.4 | |
| Other | 30,712 | 19.9 | 66 | 19.7 | |
| Healthcare related | |||||
| Transfer from other hospital | 21,615 | 14.0 | 70 | 20.9 | <.01 |
| Insurance type | |||||
| Commercial | 53,784 | 34.8 | 59 | 17.6 | <.01 |
| Medicaid | 7589 | 4.9 | 21 | 6.3 | |
| Medicare | 82,795 | 53.6 | 243 | 72.5 | |
| Uninsured/other | 10,276 | 6.7 | 12 | 3.6 | |
| Hospital teaching status | |||||
| Nonteaching | 70,640 | 45.7 | 160 | 47.8 | .46 |
| Teaching | 83,804 | 54.3 | 175 | 52.2 | |
| Mean annual no. of CABGs per hospital* | 205 | 141 | 188 | 141 | .26 |
| Procedure related | |||||
| Mean day of procedure | 1.6 | 2.2 | 1.9 | 2.4 | <.01 |
| No. of grafts | |||||
| 1 | 30,002 | 19.4 | 73 | 21.8 | .27 |
| 2 | 54,189 | 35.1 | 108 | 32.2 | .28 |
| 3 | 42,914 | 27.8 | 88 | 26.3 | .54 |
| ≥4 | 18,979 | 12.3 | 51 | 15.2 | .10 |
| Concomitant valve repair/replacement | 21,738 | 14.1 | 94 | 28.1 | <.01 |
| ITA graft | 134,815 | 87.3 | 272 | 81.2 | <.01 |
| Previous CABG surgery | 358 | 0.2 | 0 | 0.0 | .38 |
| Emergency admission | 33,938 | 22.0 | 82 | 24.5 | .27 |
| Year of procedure | |||||
| 2006 | 20,406 | 13.2 | 49 | 14.6 | .24 |
| 2007 | 20,376 | 13.2 | 50 | 14.9 | |
| 2008 | 17,839 | 11.6 | 39 | 11.6 | |
| 2009 | 19,262 | 12.5 | 51 | 15.2 | |
| 2010 | 19,864 | 12.9 | 30 | 9.0 | |
| 2011 | 19,261 | 12.5 | 33 | 9.9 | |
| 2011 | 19,562 | 12.7 | 42 | 12.5 | |
| 2013 | 17,874 | 11.6 | 41 | 12.2 | |
| Comorbidity burden | |||||
| Mean Deyo–Charlson comorbidity index* | 0.95 | 1.3 | 2.02 | 1.9 | <.01 |
| Use of antacid medications 147,661 | 95.6 324 | 96.7 | .32 | ||
CABG, Coronary artery bypass grafting; ITA, internal thoracic artery.
Continuous variable mean and standard deviation instead of N and %, respectively.
Chi-square test for categoric variables, t test for continuous variables.
APPENDIX E2.
Deyo–Charlson comorbidities by standard versus extended use of antibiotic prophylaxis
| Comorbidities | Antibiotic prophylaxis
|
P value* | |||
|---|---|---|---|---|---|
| Standard
|
Extended
|
||||
| N | % | N | % | ||
| Deyo–Charlson comorbidity grouping | |||||
| Myocardial infarction | 46,017 | 37.6 | 12,416 | 38.3 | .02 |
| Congestive heart failure | 23,970 | 19.6 | 6622 | 20.4 | <.01 |
| Peripheral vascular disease | 18,933 | 15.5 | 4648 | 14.3 | <.01 |
| Cerebrovascular disease | 11,696 | 9.6 | 3033 | 9.4 | .27 |
| Dementia | 359 | 0.3 | 89 | 0.3 | .57 |
| Chronic pulmonary disease | 30,157 | 24.6 | 7226 | 22.3 | <.01 |
| Rheumatologic disease | 2331 | 1.9 | 619 | 1.9 | .96 |
| Peptic ulcer disease | 1800 | 1.5 | 451 | 1.4 | .28 |
| Mild liver disease | 1844 | 1.5 | 492 | 1.5 | .89 |
| Moderate/severe liver disease | 202 | 0.2 | 55 | 0.2 | .86 |
| Diabetes | 44,951 | 36.7 | 12,272 | 37.9 | <.01 |
| Diabetes with chronic complications | 6857 | 5.6 | 1591 | 4.9 | <.01 |
| Hemiplegia or paraplegia | 589 | 0.5 | 185 | 0.6 | .04 |
| Renal disease | 15,156 | 12.4 | 3677 | 11.3 | <.01 |
| Any malignancy | 2983 | 2.4 | 758 | 2.3 | .30 |
| Metastatic solid tumor | 316 | 0.3 | 63 | 0.2 | .04 |
Chi-square test.
Univariable and Multivariable Clostridium difficile Risk
Overall, C difficile was observed in 329 patients (0.21%) with great hospital-level variation (range, 0–2; interquartile range [IQR], 0.1–0.3). Those who received extended antibiotic prophylaxis (compared with standard prophylaxis) had a higher C difficile prevalence: 0.30% versus 0.19%, respectively (P <.01) (Table 2). When looking at type of antibiotic prophylaxis, higher C difficile risks were observed for adjuvant vancomycin (0.25%) and vancomycin only (0.33%) compared with the use of cephalosporins (0.18%) (P<.01).
TABLE 2.
Extended antibiotic prophylaxis and type of antibiotic by Clostridium difficile occurrence
| Characteristics |
C difficile
|
P value* | ||
|---|---|---|---|---|
| No
|
Yes
|
|||
| N | N | % | ||
| Duration of antibiotic prophylaxis | ||||
| Extended | 32,193 | 98 | 0.30 | <.01 |
| Standard | 121,678 | 231 | 0.19 | |
| Type of antibiotic prophylaxis | ||||
| Cephalosporin only | 91,848 | 165 | 0.18 | <.01 |
| Cephalosporin with Vancomycin | 50,982 | 127 | 0.25 | |
| Vancomycin only | 11,041 | 37 | 0.33 | |
Chi-square test.
After adjustment for relevant covariates (Table 3), the association with increased C difficile risk remained: extended prophylaxis (vs standard) (OR, 1.43; CI, 1.07–1.92). However, there was no significant association between C difficile and vancomycin use as adjuvant or alone (compared with the use of cephalosporins) (OR, 1.21; CI 0.92–1.60, and OR, 1.39; CI, 0.94–2.05, respectively).
TABLE 3.
Adjusted* association between antibiotic prophylaxis measures and Clostridium difficile
| Definition of Clostridium difficile
|
||
|---|---|---|
| Restrictive
|
Less restrictive (sensitivity analysis)
|
|
| OR* (95% CI) | OR* (95% CI) | |
| Extended antibiotic prophylaxis | ||
| No | Reference | Reference |
| Yes | 1.43 (1.07–1.92) | 1.39 (1.08–1.82) |
| Type of antibiotic prophylaxis | ||
| Cephalosporin only | Reference | Reference |
| Cephalosporin with Vancomycin | 1.21 (0.92–1.60) | 1.16 (0.90–1.48) |
| Vancomycin only | 1.39 (0.94–2.05) | 1.37 (0.96–1.95) |
CI, Confidence interval; OR, odds ratio.
Adjusted for age, race, transfer from another hospital, insurance type, hospital teaching status, mean annual number of CABG surgeries per hospital, preoperative hospital stay, number of grafts, concomitant valve repair/replacement, internal thoracic artery graft, emergency admission, year of procedure, Deyo–Charlson comorbidities, use of antacid medications; all P<.05.
The results were comparable when using a less-restrictive definition of C difficile (n = 398, overall prevalence 0.26%). The model’s c-statistic was 0.76 for the main model and 0.75 for the sensitivity analysis. The intraclass correlation coefficient of the final model was 15.1% depicting the proportion of variation between hospitals that explains the outcome of C difficile.
To assess the potential effect of missing data on our multivariable results, we also studied the C difficile rates in 2 excluded groups: (1) those with unknown gender or discharge type (n = 602; no C difficile cases) and (2) those who were not billed for antibiotics on the day of surgery (n = 17,031; C difficile rate 0.56%). Because the latter is higher than the C difficile rate found in the study cohort (0.21%), the exclusion might have led to a bias of our effect estimates toward the null, meaning that our results could be an underestimation of the actual effect.
Hospital Variation in Antibiotic Prophylaxis Characteristics
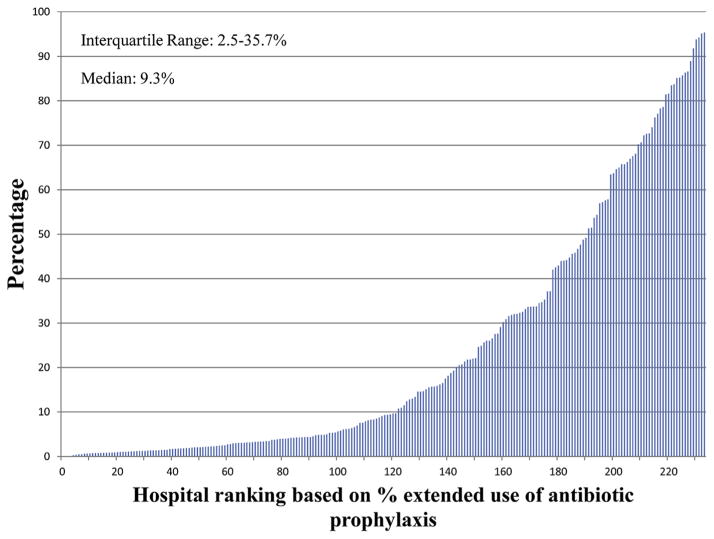
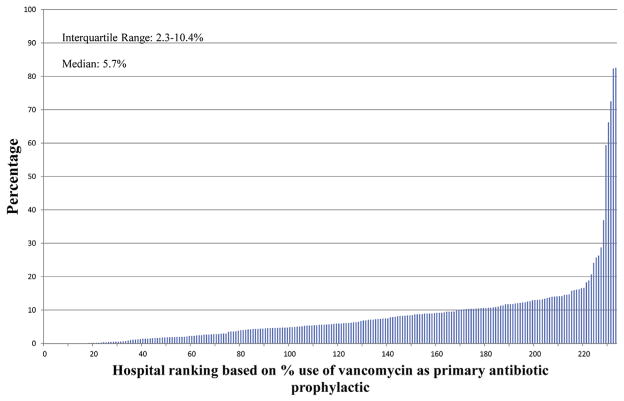
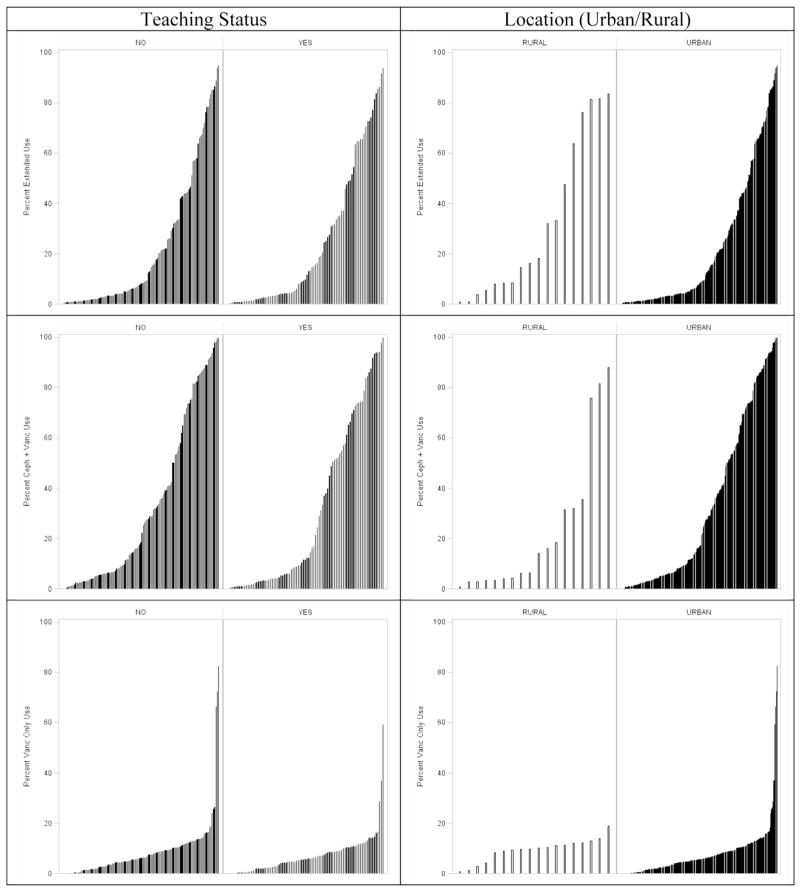
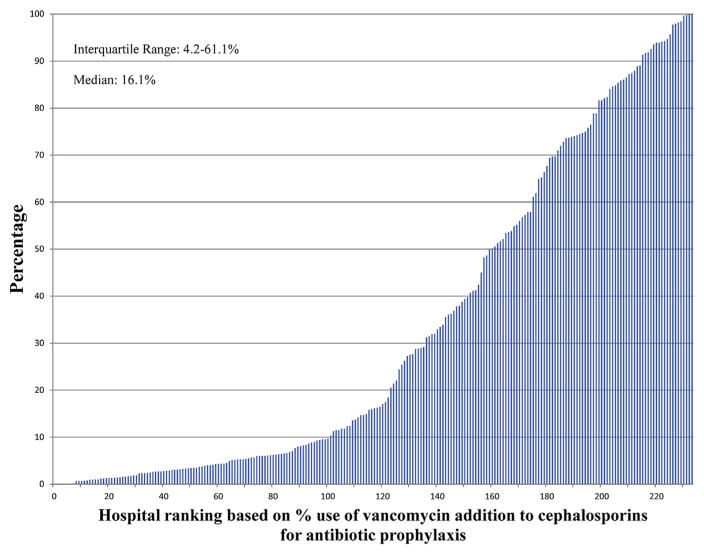
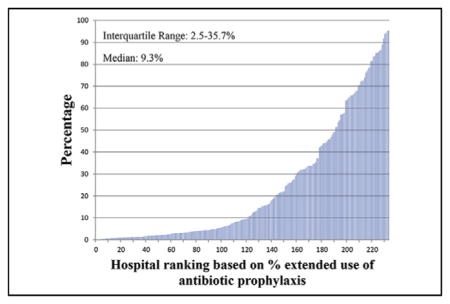
Figures 2–4 show the extent of inter-hospital variation of the percentage of extended antibiotic prophylaxis use (IQR, 2.5–35.7), the percentage of adjuvant vancomycin (IQR, 4.2–61.1), and the percentage of vancomycin use as the primary prophylactic (IQR, 2.3–10.4), respectively. Appendix E3 provides a breakdown for these figures by hospital teaching status and hospital location (urban vs rural), showing no apparent differences between hospital types.
FIGURE 2.
Percentage of CABG surgeries in which there is extended antibiotic prophylaxis, by hospital.
FIGURE 4.
Percentage of CABG surgeries in which vancomycin is used as primary antibiotic prophylactic, by hospital.
APPENDIX E3.
Hospital variation in characteristics of antibiotic prophylaxis by hospital teaching status and location (urban vs rural).
DISCUSSION
In this large population-based study using data from 154,200 patients who underwent CABG surgery in 233 hospitals, we found extended antibiotic prophylaxis to be associated with increased risks for C difficile. On the basis of C difficile definition, this increased risk ranged from 43% to 39%. Conversely, the use of vancomycin, as the primary prophylactic or added to a cephalosporin, was not associated with an increased C difficile risk. We further demonstrated substantial inter-hospital variation in antibiotic prophylaxis characteristics, which suggests great potential for efforts aimed at standardizing these practices, which subsequently could reduce C difficile risks.
Several recent studies have emphasized the importance of C difficile and its link to antibiotic prophylaxis in patients undergoing cardiac surgery.2,17,27 Traditionally, the prevention of surgical site infections is considered the main objective of antibiotic prophylaxis. However, a recent study shifted the focus to other infections after cardiac surgery. By using prospective and detailed data from more than 5000 patients, Gelijns and colleagues17 demonstrated the majority (79%) of infections after cardiac surgery to be pneumonia, bloodstream infections, or C difficile. Adding more priority to this topic are the current developments at the Centers for Medicare & Medicaid Services (CMS). As of October 2008, the CMS does not reimburse care related to several so-called hospital-acquired conditions, including some infections. Although C difficile infections have been considered, they have not yet been included in this list.28 However, as regular reevaluations have been announced, C difficile infections might be included in the future, potentially resulting in a considerable financial burden for hospitals.
In this study, we focused on 2 characteristics that are perhaps the most important modifiable risk factors for C difficile6–8: the extended use of prophylactic antibiotics and the type of antibiotic used for prophylaxis, in our study the use of vancomycin alone or as an adjuvant. Currently, extended use has a different definition in cardiac surgery than in noncardiac surgery: more than 48 hours versus more than 24 hours postoperatively, respectively. Two recent meta-analyses have focused on the question of optimal duration in light of surgical site infections after cardiac surgery. However, the trials that were included in these meta-analyses mostly did not report on adverse events such as resistant bacteria or C difficile.9,29 Moreover, analysis of recent data has shown a similar postoperative infection risk for less than 24 hours compared with less than 48 hours of antibiotic prophylaxis.17 This underlines the need for additional studies, particularly those that assess effect on surgical site infections and adverse events.
Data on vancomycin as a risk factor for C difficile are sparse, as are data on a potential mechanism of action. Although oral vancomycin is excreted in the stool and can serve as treatment of C difficile, intravenous administration does not. Moreover, intravenous vancomycin has been linked to ecologic disturbances of the intestinal microflora predisposing C difficile overgrowth.21,30,31 Only 1 previous study has assessed the role of vancomycin addition to cephalosporins in C difficile after cardiac surgery.16 The C difficile prevalence in this respective cohort of patients undergoing CABG was 0.4% (n = 256). In line with our findings, the authors did not find an association between adjuvant vancomycin and C difficile risk. Moreover, they used a propensity score analysis to adjust for confounding, whereas we have used a multilevel multivariable logistic regression approach. The fact that both strategies demonstrate no association of vancomycin use (while using similar definitions) with increased C difficile risk adds to the robustness of this finding. Important future work could be aimed at (hospital-specific) factors affecting the choice of specific antibiotics as prophylaxis. Although guidelines specifically mention adjuvant vancomycin in case of high risk for staphylococcal infection, or vancomycin as primary prophylactic antibiotic in β-lactam/penicillin–allergic patients, additional data on the rationale for antibiotic choice seem imperative.10–12,14 Moreover, guidelines, however complete, may not always be applicable to every situation. White and colleagues27 have recently described their institutional transition from cephalosporin-based antimicrobial prophylaxis for cardiac surgery to flucloxacillin (or teicoplanin) and gentamicin after several apparent prophylaxis failures, continued institutional presence of methicillin-resistant staphylococcus aureus, and ongoing problems with C difficile. They found their practice change to have decreased C difficile without changing the incidence of wound infections or renal complications.27
Inter-hospital variation in antibiotic prophylaxis practices has been described for several procedures, including cardiac surgery.17,32 The significant variation demonstrated in the current study suggests great potential for efforts such as Surgical Care Improvement Project (SCIP), a collaboration between the CMS and the Centers for Disease Control and Prevention to improve and standardize surgical care by a set of measures eventually reducing surgical complications, including surgical site infections.33 A number of studies have assessed the impact of SCIP on outcomes, including surgical infections in mostly noncardiac surgery, however, with mixed results.34,35 Although several SCIP measures have been retired recently (January 2015), continuing evaluation of practice standardization and outcomes and implementation barriers of SCIP measures will be crucial in its success.
Study Limitations
The main limitation of our study is the lack of detailed clinical information in the billing dataset used; data are collected for the purpose of billing, not research. This limitation particularly pertains to the indications for the billed antibiotics for which we cannot distinguish between prophylaxis and treatment in case of extended use or if there were any particular reasons to use vancomycin; this might have resulted in indication bias. In addition, what is captured in billing databases does not necessarily reflect what is actually administered to the patient because there might be a mismatch between the two. We have tried to account for this by limiting our dataset to patients with antibiotic use starting on the day of surgery and excluding combinations of antibiotic use (except for vancomycin combined with cephalosporins). Moreover, we expect the effect of the mismatch to be minimal because our study objective focused on daily use without going into details on dosages. Another limitation, partly referring to the lack of clinical information, pertains to the definition of C. difficile from billing data for the inpatient period only. The latter seems particularly important because a substantial proportion (45%) of infections after cardiac surgery (including C difficile) occur after hospital discharge.17 Although the effect of this limitation theoretically could go in both directions, we believe, on the basis of the described effects of antibiotic use on C difficile,6–8 that it results in an underestimation of the effect of extended antibiotic prophylaxis on C difficile. Several other mechanisms also emphasize the conservative measure of our effect estimates. The excluded group of patients with no billing for antibiotics on the day of surgery (n = 17,031) (Figure 1) had a higher C difficile rate than our study cohort (0.56% vs 0.21%, respectively); the same was true for the group of patients using prophylactic antibiotics other than those used in the study (n = 4357; C difficile rate 2.6%). Moreover, we used a restrictive C difficile definition as previously described16 and found the same results when using a more inclusive ICD-9 only definition, illustrating the robustness of our findings. Adding to this, but also illustrating the heterogeneity in populations, our C difficile prevalence estimates are comparable to some27 but not other studies demonstrating higher prevalences.5,17 Given the limitations pertaining to the use of billing data to answer clinical questions, we believe that our study provides some useful insights. Important next steps are additional retrospective studies looking into the effect of antibiotic prophylaxis on C difficile risk in other procedures and using other data sources. Furthermore, well-designed prospective studies would shed additional light on this important clinical question, which would also benefit from a (multicenter) clinical registry in which all variables of interest geared toward this question (eg, exact timing and duration of prophylaxis) are captured. A study geared toward prophylaxis patterns and the incidence of all postoperative infections would be needed to consider actual changes in antibiotic guidelines. The objective would be to weigh the risk of particularly C difficile and resistant organisms against infections (eg, mediastinitis) that might be positively affected by a longer duration of prophylaxis or the use of vancomycin. Theoretically, reducing postoperative mediastinitis with a longer duration of antibiotic prophylaxis at the expense of more C difficile may be completely justified.
CONCLUSIONS
With the use of billing data from 154,200 patients who underwent CABG, we found extended antibiotic prophylaxis to be associated with increased risks for C difficile, whereas the use of vancomycin was not. Substantial inter-hospital variation in antibiotic prophylaxis characteristics suggests great potential for efforts aimed at standardizing these practices, which could subsequently reduce C difficile risks. Current patient safety and financial stakes combined with possible future reimbursement penalties demand prioritization of this issue and effective strategies to decrease C difficile.
FIGURE 3.
Percentage of CABG surgeries in which vancomycin is added to cephalosporins for antibiotic prophylaxis, by hospital.
Central Message.
Although extended use of antibiotic prophylaxis was associated with increased C difficile risk after CABG surgery, vancomycin use was not.
Perspective.
In patients undergoing CABG, extended antibiotic prophylaxis was associated with increased C difficile risks with substantial inter-hospital variation in antibiotic prophylaxis characteristics. Current patient safety and financial stakes combined with possible future reimbursement penalties demand prioritization of this issue and effective strategies to decrease C difficile.
Acknowledgments
M.M., A.M., and A.C.G. are partially funded by Cardiothoracic Surgical Trials Network (http://www.ctsurgerynet.org/). Institutional Review Board approval was obtained (project HS#: 14-00647).
Abbreviations and Acronyms
- CABG
coronary artery bypass grafting
- CI
confidence interval
- CMS
Centers for Medicare & Medicaid Services
- ICD-9 [CM]
International Classification of Diseases-9th revision [Clinical Modification]
- IQR
interquartile range
- OR
odds ratio
- SCIP
Surgical Care Improvement Project
Footnotes
Supplemental material is available online.
Conflict of Interest Statement
Authors have nothing to disclose with regard to commercial support.
References
- 1.Lucado J, Gould C, Elixhauser A Agency for Healthcare Research and Quality. [Accessed August 18, 2014];Statistical Brief #124 Clostridium difficile infections (CDI) in hospital stays. 2009 Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb124.pdf. [PubMed]
- 2.Crabtree T, Aitchison D, Meyers BF, Tymkew H, Smith JR, Guthrie TJ, et al. Clostridium difficile in cardiac surgery: risk factors and impact on postoperative outcome. Ann Thorac Surg. 2007;83:1396–402. doi: 10.1016/j.athoracsur.2006.10.067. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien JA, Lahue BJ, Caro JJ, Davidson DM. The emerging infectious challenge of clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28:1219–27. doi: 10.1086/522676. [DOI] [PubMed] [Google Scholar]
- 4.Gabriel L, Beriot-Mathiot A. Hospitalization stay and costs attributable to Clostridium difficile infection: a critical review. J Hosp Infect. 2014;88:12–21. doi: 10.1016/j.jhin.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Keshavamurthy S, Koch CG, Fraser TG, Gordon SM, Houghtaling PL, Soltesz EG, et al. Clostridium difficile infection after cardiac surgery: Prevalence, morbidity, mortality, and resource utilization. J Thorac Cardiovasc Surg. 2014;148:3157–65. doi: 10.1016/j.jtcvs.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Owens RC, Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S19–31. doi: 10.1086/521859. [DOI] [PubMed] [Google Scholar]
- 7.Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother. 2014;69:881–91. doi: 10.1093/jac/dkt477. [DOI] [PubMed] [Google Scholar]
- 8.Calvert JK, Holt SK, Mossanen M, James AC, Wright JL, Porter MP, et al. Use and outcomes of extended antibiotic prophylaxis in urological cancer surgery. J Urol. 2014;192:425–9. doi: 10.1016/j.juro.2014.02.096. [DOI] [PubMed] [Google Scholar]
- 9.Mertz D, Johnstone J, Loeb M. Does duration of perioperative antibiotic prophylaxis matter in cardiac surgery? A systematic review and meta-analysis. Ann Surg. 2011;254:48–54. doi: 10.1097/SLA.0b013e318214b7e4. [DOI] [PubMed] [Google Scholar]
- 10.The Joint Commission. Specifications Manual for National Hospital Inpatient Quality Measures; Discharges 01-01-14 through 12-31-14. [Accessed August 19, 2014];Surgical Care Improvement Project National Hospital Inpatient Quality Measures. Available at: http://www.jointcommission.org/specifications_manual_for_national_hospital_inpatient_quality_measures.aspx.
- 11.Antimicrobial prophylaxis for surgery. Treat Guidel Med Letter. 2012;10:73–8. quiz 79–80. [PubMed] [Google Scholar]
- 12.Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt) 2013;14:73–156. doi: 10.1089/sur.2013.9999. [DOI] [PubMed] [Google Scholar]
- 13.Edwards FH, Engelman RM, Houck P, Shahian DM, Bridges CR Society of Thoracic Surgeons. The Society of Thoracic Surgeons practice guideline series: antibiotic prophylaxis in cardiac surgery, part I: duration. Ann Thorac Surg. 2006;81:397–404. doi: 10.1016/j.athoracsur.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 14.Engelman R, Shahian D, Shemin R, Guy TS, Bratzler D, Edwards F, et al. The Society of Thoracic Surgeons practice guideline series: Antibiotic prophylaxis in cardiac surgery, part II: Antibiotic choice. Ann Thorac Surg. 2007;83:1569–76. doi: 10.1016/j.athoracsur.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 15.Levine DP. Vancomycin: a history. Clin Infect Dis. 2006;42(Suppl 1):S5–12. doi: 10.1086/491709. [DOI] [PubMed] [Google Scholar]
- 16.Bateman BT, Rassen JA, Schneeweiss S, Bykov K, Franklin JM, Gagne JJ, et al. Adjuvant vancomycin for antibiotic prophylaxis and risk of Clostridium difficile infection after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2013;146:472–8. doi: 10.1016/j.jtcvs.2013.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelijns AC, Moskowitz AJ, Acker MA, Argenziano M, Geller NL, Puskas JD, et al. Management practices and major infections after cardiac surgery. J Am Coll Cardiol. 2014;64:372–81. doi: 10.1016/j.jacc.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Premier Inc. [Accessed May 25, 2015];Premier Perspective Database. Available at: https://www.premierinc.com/transforming-healthcare/healthcare-performance-improvement/premier-research-services.
- 19.Dharmarajan K, Strait KM, Lagu T, Lindenauer PK, Tinetti ME, Lynn J, et al. Acute decompensated heart failure is routinely treated as a cardiopulmonary syndrome. PLoS One. 2013;8:e78222. doi: 10.1371/journal.pone.0078222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safavi KC, Dharmarajan K, Kim N, Strait KM, Li SX, Chen SI, et al. Variation exists in rates of admission to intensive care units for heart failure patients across hospitals in the United States. Circulation. 2013;127:923–9. doi: 10.1161/CIRCULATIONAHA.112.001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubberke ER, Reske KA, Yan Y, Olsen MA, McDonald LC, Fraser VJ. Clostridium difficile–associated disease in a setting of endemicity: identification of novel risk factors. Clin Infect Dis. 2007;45:1543–9. doi: 10.1086/523582. [DOI] [PubMed] [Google Scholar]
- 22.Moineddin R, Matheson FI, Glazier RH. A simulation study of sample size for multilevel logistic regression models. BMC Med Res Methodol. 2007;7:34. doi: 10.1186/1471-2288-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 24.Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107:1001–10. doi: 10.1038/ajg.2012.179. [DOI] [PubMed] [Google Scholar]
- 25.Scheurer DB, Hicks LS, Cook EF, Schnipper JL. Accuracy of ICD-9 coding for Clostridium difficile infections: a retrospective cohort. Epidemiol Infect. 2007;135:1010–3. doi: 10.1017/S0950268806007655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins CE, Ayturk MD, Flahive JM, Emhoff TA, Anderson FA, Jr, Santry HP. Epidemiology and outcomes of community-acquired Clostridium difficile infections in Medicare beneficiaries. J Am Coll Surg. 2014;218:1141–7. e1. doi: 10.1016/j.jamcollsurg.2014.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White RW, West R, Howard P, Sandoe J. Antimicrobial regime for cardiac surgery: the safety and effectiveness of short-course flucloxacillin (or teicoplanin) and gentamicin-based prophylaxis. J Cardiovasc Surg. 2013;28:512–6. doi: 10.1111/jocs.12155. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Medicare & Medicaid Services. [Accessed August 19, 2014];Press release: CMS proposes to expand quality program for hospital inpatient services in FY 2009. Available at: http://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-releases/2008-Press-releases-items/2008-04-14.html.
- 29.Lador A, Nasir H, Mansur N, Sharoni E, Biderman P, Leibovici L, et al. Antibiotic prophylaxis in cardiac surgery: systematic review and meta-analysis. J Antimicrob Chemother. 2012;67:541–50. doi: 10.1093/jac/dkr470. [DOI] [PubMed] [Google Scholar]
- 30.Rafii F, Sutherland JB, Cerniglia CE. Effects of treatment with antimicrobial agents on the human colonic microflora. Ther Clin Risk Manag. 2008;4:1343–58. doi: 10.2147/tcrm.s4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis. 2011;53:42–8. doi: 10.1093/cid/cir301. [DOI] [PubMed] [Google Scholar]
- 32.Beckmann A, Doebler K, Schaefer E, Koetting J, Gastmeier P, Graf K. Sternal surgical site infection prevention - is there any room for improvement? Eur J Cardiothorac Surg. 2011;40:347–51. doi: 10.1016/j.ejcts.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 33.Bratzler DW, Hunt DR. The surgical infection prevention and surgical care improvement projects: national initiatives to improve outcomes for patients having surgery. Clin Infect Dis. 2006;43:322–30. doi: 10.1086/505220. [DOI] [PubMed] [Google Scholar]
- 34.Rasouli MR, Jaberi MM, Hozack WJ, Parvizi J, Rothman RH. Surgical care improvement project (SCIP): has its mission succeeded? J Arthroplasty. 2013;28:1072–5. doi: 10.1016/j.arth.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Stulberg JJ, Delaney CP, Neuhauser DV, Aron DC, Fu P, Koroukian SM. Adherence to surgical care improvement project measures and the association with postoperative infections. JAMA. 2010;303:2479–85. doi: 10.1001/jama.2010.841. [DOI] [PubMed] [Google Scholar]