Abstract
Background
Obesity and metabolic syndrome lead to the development of metabolic heart disease (MHD) that is characterized by left ventricular hypertrophy (LVH), diastolic dysfunction, and increased mitochondrial ROS. Caloric restriction (CR) is a nutritional intervention that protects against obesity, diabetes, and cardiovascular disease. Healthy adipose tissue is cardioprotective via releasing adipokines such as adiponectin. We tested the hypothesis that CR can ameliorate MHD and it is associated with improved adipose tissue function as reflected by increased circulating levels of high molecular weight (HMW) adiponectin and AMP-activated protein kinase (AMPK) in db/db mice.
Methods
Genetically obese db/db and lean db/+ male mice were fed either ad libitum or subjected to 30% CR for 5 weeks. At the end of the study period, echocardiography was carried out to assess diastolic function. Blood, heart, and epididymal fat pads were harvested for mitochondrial study, ELISA, and Western blot analyses.
Results
CR reversed the development of LVH, prevented diastolic dysfunction, and decreased cardiac mitochondrial H2O2 in db/db (vs. ad lib) mice. These beneficial effects on the heart were associated with increased circulating level of HMW adiponectin. Furthermore, CR increased AMPK and eNOS activation in white adipose tissue of db/db mice, but not in the heart.
Conclusions
These findings indicate that even short-term CR protects the heart from MHD. Whether the beneficial effects of CR on the heart could be related to the improved adipose tissue function warrants future investigation.
Keywords: caloric restriction, metabolic heart disease, AMPK, adipose tissue
Background
Obesity and metabolic syndrome are associated with metabolic heart disease (MHD) (2). The key features of MHD are left ventricular hypertrophy (LVH), diastolic dysfunction, and compromised mitochondrial function with impaired energetics and increased ROS production (6, 13). Over time, MHD can lead to clinical heart failure with a preserved ejection fraction (HFpEF) which is associated with serious consequences for morbidity and mortality. Caloric restriction (CR) is an effective dietary intervention that protects against obesity-related comorbidities such as diabetes and cardiovascular disease (7). It is not known whether CR can protect the development of obesity-related MHD. However, in rodents, CR ameliorates diastolic dysfunction induced by abdominal aortic constriction, ischemic/reperfusion injury, and aging (7, 22). Accordingly, our first goal was to determine whether short-term (5 weeks) CR can ameliorate the development of MHD in genetically-obese db/db mice.
The mechanism by which CR exerts beneficial effects on the heart is not precisely known. Several possibilities have been suggested including reversal of insulin resistance, activation of myocardial AMPK and/or SIRT1 signaling, and increased circulating levels of the cardioprotecive adipokine adiponectin (8, 23). Recently, we observed that roux-en-y gastric bypass, an extreme form of CR, markedly increased adipose tissue AMPK activity and circulating levels of HMW adiponectin in obese patients (28). AMPK is a pivotal nutrient sensor with the ability to regulate whole-body metabolism (10); whereas adipose tissue exerts paracrine and endocrine actions on the heart by releasing a variety of adipokines. For example, circulating levels of adiponectin are decreased in obese humans and rodents, and a deficiency in adiponectin may contribute to the pathophysiology of several cardiovascular diseases including heart failure (19). Therefore, our second goal was to explore adipose tissue AMPK signaling and adiponectin production in db/db mice with CR intervention.
Methods
Animals and CR
10-week old male db/db and littermate lean db/+ mice were purchased from Jackson Laboratory (Bar Harbor, ME). Upon arrival, mice had ad libitum access to water and chow diet and were on a 12: 12 h light/dark cycle. All 24 mice (12 db/db and 12 db/+) were singly housed and food intake was recorded daily for 3-4 days. Next, mice were randomized into the following treatment groups for 5 weeks: control (ad libitum; D12450B, Research Diets, New Brunswick, NJ), or 30% CR (D03020702B, Research Diets). The CR diet is a 30% reduction in carbohydrate intake, and designed to limit total energy consumption while insuring adequate nutrition. The amount of food consumed by the db/db and the db/+ ad libitum groups was recorded daily, and 70% of that was given to the respective CR groups. Body weights were monitored weekly. At the whole-body level, CR prevented weight gain in db/db and caused weight loss in db/+ mice. At the end of the study, hearts were harvested for isolation of mitochondria and the remaining was snap-frozen in liquid nitrogen and stored at −80°C for further use. Epididymal fat pads were excised, snap-frozen, and stored at −80°C. All animal protocols were approved by the Boston University Institutional Animal Care and Use Committee.
Measurements in blood
Mice were fasted overnight and blood samples were collected from the chest cavity when the hearts were excised. Blood glucose was measured using an ACCU-CHEK Aviva glucometer (Roche, Indianapolis, IN). Commercial kits were used to measure plasma levels of insulin (ALPCO, Salem, NH), HMW & total adiponectin (ALPCO), resistin (R&D Systems, Minneapolis, MN), and visfatin (BioVision).
Western blot analyses
Total proteins were isolated from the heart that had been homogenized in RIPA buffer (Boston BioProducts, Ashland, MA) supplemented with protease (Roche) and phosphatase (Sigma-Aldrich, St. Louis, MO) inhibitors. Protein lysates from epididymal fat were prepared in lysis buffer (Cell Signaling, Beverly, MA). Protein concentrations were determined using the bicinchoninic acid assay (Thermo Scientific, Rockford, IL). Ten micrograms of protein were separated by gel electrophoresis, transferred to a PVDF membrane (Millipore, Billerica, MA), and then incubated with primary antibodies as described previously (29). Phospho (Thr172) and total AMPK, phospho (Ser1177) and total eNOS were purchased from Cell Signaling Technology. Antibody for GAPDH was from Santa Cruz Biotechnology (Santa Cruz, CA). Proteins were visualized by enhanced chemiluminescence (Thermo Scientific), and quantified with Scion Image Software (NIH).
Mitochondrial ROS production
Mitochondria from the hearts were isolated as previously described (16). Briefly, hearts were minced in cold relaxation buffer, homogenized in buffer containing 5 mM HEPES, 1 mM EDTA, 0.25 M sucrose with pH 7.4. The resulting lysates were centrifuged at 500 × g for 10 min, 4°C. The supernatant was centrifuged at 9,000 × g for 15 min, 4°C. The pellet (mitochondria) was re-suspended in 100 μL buffer containing 125 mM KCl, 10 mM HEPES, 5 mM MgCl2, 2 mM K2HPO4. Mitochondrial H2O2 production was measured using Amplex Ultra Red (Life Technologies, Grand Island, NY) as previously described (25).
Two-dimensional and M-mode echocardiography and Doppler echocardiography
LV dimensions and systolic function were measured in non-anesthetized mice using an Acuson Sequoia C-256 echocardiograph machine equipped with a 15 MHz linear transducer (model 15L8), as we have described previously (20). LV diastolic function was assessed by transmittal and tissue Doppler echocardiography in anesthetized mice as we have previously reported (21).
Statistics
Data are expressed as means ± SE. GraphPad Prism software (La Jolla, CA) was used for all analyses. The comparison was performed using Student’s t-test, one-way, or two-way ANOVA as appropriate. Minimal level of significance was set at p < 0.05.
Results
Effects of CR on body weight and circulating parameters
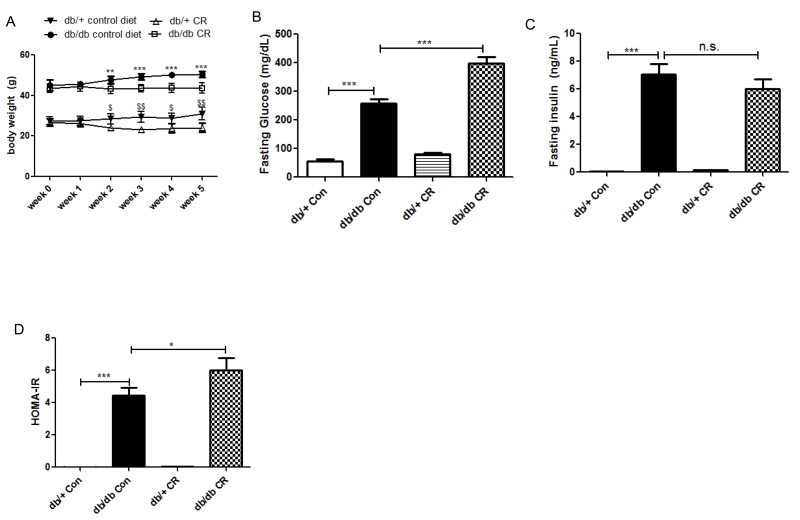
5 week, 30% CR prevented weight gain in db/db and caused weight loss in db/+ mice (Figure 1A). Compared to the db/+ group, the db/db mice had higher fasting plasma glucose, which increased further with CR (Figure 1B). Similarly, the fasting insulin level was higher in db/db vs. db/+ (Figure 1C).
Figure 1.
Effects of 5-week CR on body weight, fasting blood glucose and insulin in db/db and db/+ mice. *, p < 0.05, **, p < 0.01, ***, p < 0.0001 vs. db/db Con; $, p < 0.05, $$, p < 0.01 vs. db/+ CR. Data are means + S.E.M. (n = 6/group).
CR prevents LVH and diastolic dysfunction in db/db mice
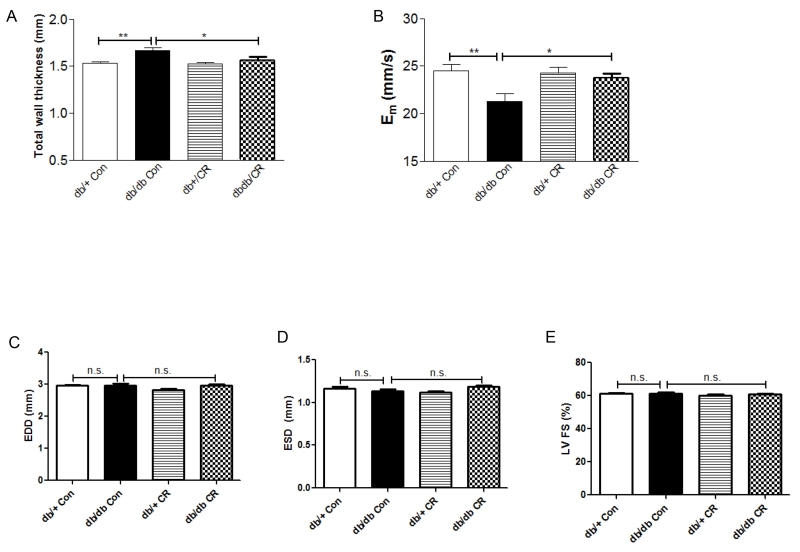
Left ventricle (LV) wall thickness was increased in db/db (vs. db/+) mice, and was reversed by 5-week CR (Figure 2A). Likewise, diastolic relaxation, as reflected by myocardial peak early diastolic velocity (Em), was decreased in db/db (vs. db/+) mice and corrected by CR (Figure 2B). LV end-diastolic dimension (EDD), systolic dimension (ESD), and fractional shortening (LVFS) were similar in db/db and db/+ ad lib mice, and were not affected by CR (Figure 2C-E).
Figure 2.
Measurements of LV wall thickness (A), parameters related to diastolic (B) and systolic function (C-E) in db/db and db/+ mice with CR. *, p < 0.05, **, p < 0.01 vs. db/db Con. Data are means + S.E.M. (n = 6/group).
Mitochondrial ROS production
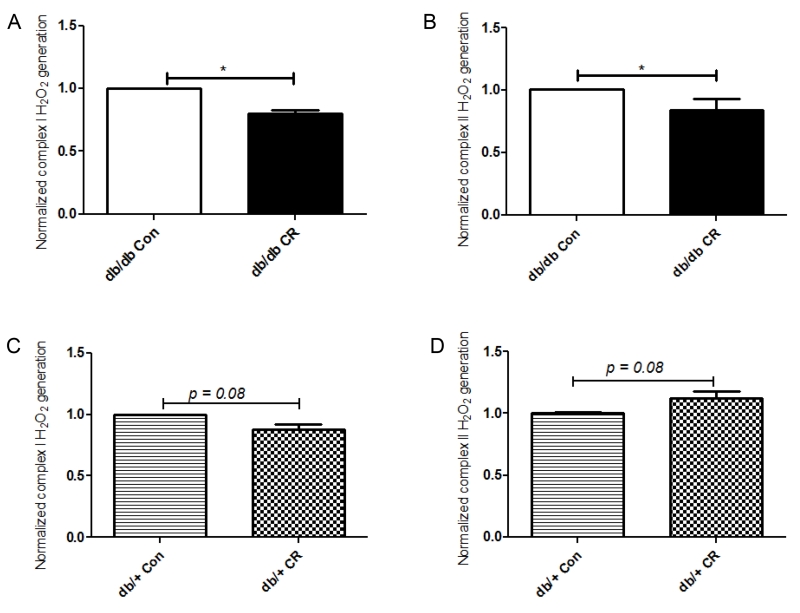
Complex I and II substrate-driven mitochondria H2O2 production was measured in isolated hearts. In db/db mice, CR (vs. ad lib) decreased the rate of H2O2 generation by both complex I and II substrates (Figures 3A,B). In contrast, CR had no effect on mitochondrial H2O2 production in db/+ mice (Figure 3C,D).
Figure 3.
Mitochondrial H2O2 production with a complex I substrate (A+C) and a complex II substrate (B+D) were measured in db/db and db/+ mice. The amounts of H2O2 produced in ad lib (Con) groups were set to 1 for both genotypes. *, p < 0.05 vs. db/db Con. Data are means + S.E.M. (n = 6/group).
Effect of CR on adipokine production
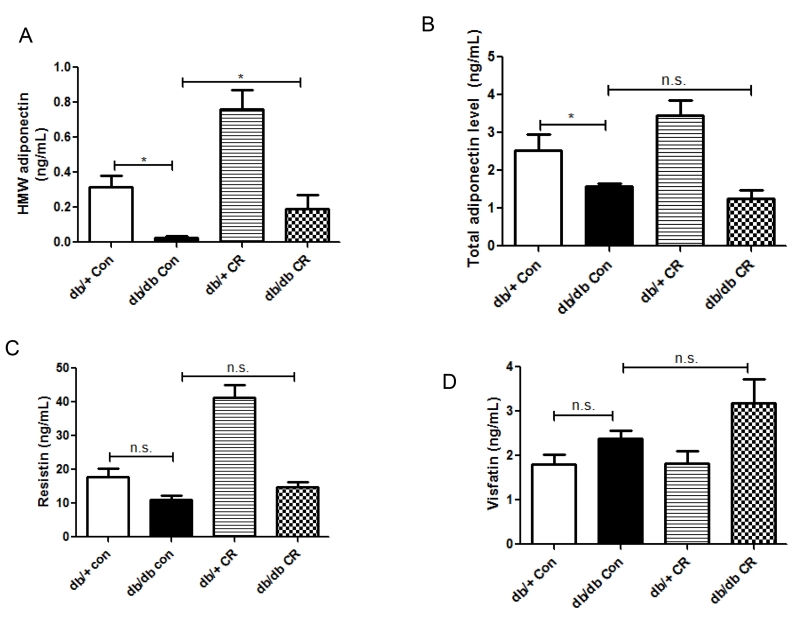
In epididymal fat, a visceral depot in rodents, the mRNA levels of adiponectin, resistin, visfatin, and omentin were not significantly different in db/db vs. db/+ mice on ad lib diet, nor were they affected by CR (data not shown). However, the plasma levels of total and HMW adiponectin were decreased in db/db (vs. db/+) mice (Figure 4A,B). CR increased HMW, but not total adiponectin production (Figure 4A,B). Plasma levels of resistin and visfatin were not different in db/db vs. db/+ mice, and were not affected by CR (Figure 4C,D).
Figure 4.
Effects of CR on the circulating levels of adipokines. *, p < 0.05 vs. db/db Con. Data are means + S.E.M. (n = 6/group).
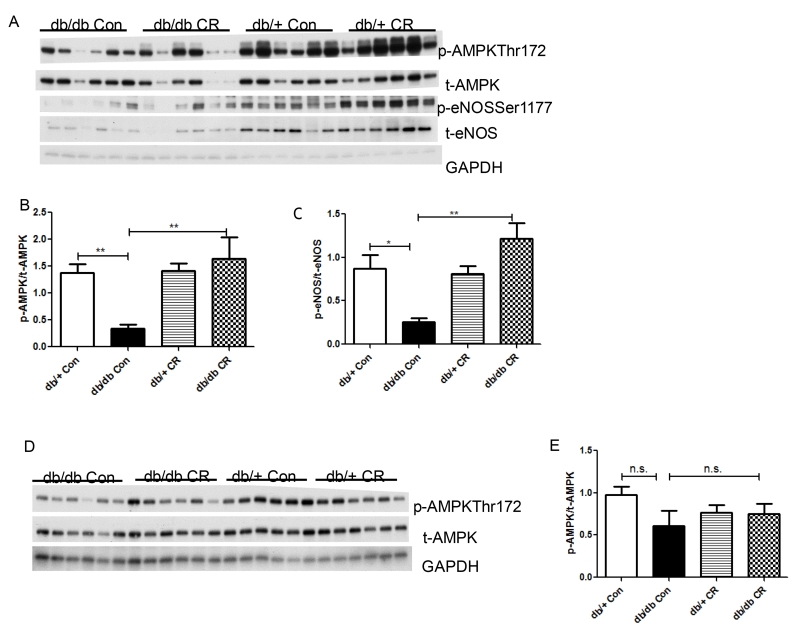
CR activates AMPK in white adipose tissue but not in the heart in db/db mice
AMPK phosphorylation at residue Thr172 was measured as an indicator of its activity (11). In epididymal fat, AMPK phosphorylation was decreased in db/db vs. db/+ mice, and was fully restored by CR (Figure 5A,B). Likewise, phosphorylation of an AMPK downstream target eNOS (Ser1177) was decreased in db/db (vs. db/+) on ad lib diet, and was restored by CR (Figure 5C). In contrast, cardiac AMPK phosphorylation was only slightly lower in db/db vs. db/+ on ad lib diet, and was not affected by CR (Figure 5D,E).
Figure 5.
Effects of CR on AMPK phosphorylation/activation in heart and epididymal fat. Relative phospho-protein levels were normalized against total AMPK and total eNOS, respectively. *, p < 0.05, **, p < 0.01 vs. db/db Con. Data are means + S.E.M. (n = 6/group).
Discussion
The main finding of this study is that in genetically obese db/db mice, CR for 5 weeks ameliorates the key features of MHD including LVH, diastolic dysfunction, and excess mitochondrial ROS. To the best of our knowledge, this is the first study to demonstrate the beneficial effects of short-term CR on obesity-related MHD in a mouse model. We also found that CR led to increased production of HMW adiponectin, which is associated with AMPK activation in white adipose tissue, but not in the heart.
CR prevents the development of MHD
The db/db mouse has been shown to develop LVH and both systolic and diastolic dysfunction (4). In this study, we found LVH and diastolic dysfunction in the db/db mice on ad libitum diet, whereas systolic function as reflected by ejection fraction, was normal. Differences in methodologies (1) and/or mice strains (27) could explain the discrepancy between our findings (normal systolic function) vs. others (systolic dysfunction). In support of our data, systolic function was preserved in diet-induced obese mice despite the presence of diastolic dysfunction (21, 25). Two limitations should be noted. First, the evaluation of LV diastolic function by tissue Doppler does not allow us to determine the relative effects of CR on relaxation versus a decrease in stiffness. Likewise, we were unable to confirm the echocardiographic assessment of LVH with a direct measurement of mass due to the need to snap freeze the tissue for optimal biochemical analysis.
Another important characteristic of MHD is mitochondrial dysfunction with impaired energetics and excess ROS (25, 26). Increased mitochondrial H2O2 production has been observed in obese db/db mice as well as mice with diet-induced obesity (5, 25), possibly as a consequence of increased fatty acid oxidation leading to electron leakage for the electron transport chain (5, 14). CR has been shown to decrease cardiac mitochondrial H2O2 generation in obese rats (18) and in lean aging mice (24). Our study extends these findings by demonstrating that CR also diminished cardiac mitochondrial H2O2 in db/db mice.
HMW Adiponectin
Adiponectin levels are known to decrease with obesity (15). Therefore, it was not surprising that plasma levels of adiponectin were lower in db/db (vs. db/+) mice. While CR had no effect on total adiponectin level in db/db mice, it increased the level of HMW adiponectin by 3 folds. Several isoforms of adiponectin are present in the plasma where the HMW form is considered most bioactive (15). Since adiponectin is cardioprotective, our data suggest that the beneficial effects of CR are mediated, in part, by increased HMW adiponectin. The mechanism responsible for the selective increase in HMW vs. total adiponectin is not clear. In a study with morbidly obese humans, we found that roux-en-y gastric bypass (an extreme form of CR) increased circulating HMW adiponectin 3 months post-operatively, although total adiponectin was not measured (28). In lean mice, 5 weeks of CR increased all three isoforms of adiponectin (23). Given that adiponectin is the most abundant adipokine secreted by white adipose tissue (17), our findings suggest that even short-term CR can improve adipose tissue health in db/db mice. In contrast to adiponectin, several other adipokines that are known to modulate cardiovascular function were unaffected by CR.
Role of AMPK
AMPK is a pivotal nutrient sensor with the ability to regulate whole-body metabolism (9) and it can be activated by adiponectin. Although cardiac AMPK has been shown to play a role in CR-mediated cardioprotection in several models (23), our data indicate that AMPK activity in the heart was only slightly decreased in db/db (vs. db/+) mice, and was not altered by CR. In obese humans, we demonstrated that adipose tissue AMPK activity is decreased in insulin resistant (vs. insulin sensitive) group (29); moreover, we found increased adipose tissue AMPK activity associated with higher circulating levels of HMW adiponectin following roux-en-y gastric bypass (28). In the present study, adipose tissue AMPK activity was markedly decreased in db/db (vs. db/+) mice, and was accompanied by a decrease in eNOS phosphorylation, a downstream target of AMPK. Conversely, CR increased both AMPK and eNOS phosphorylation/activation to levels comparable to non-obese db/+ mice. Whether adipose tissue AMPK can mediate a crosstalk with the heart warrants further investigation, as our conditioned media study shows that knocking down AMPK in adipocytes has an impact on cardiomyocyte function (Xu et al., unpublished).
Other effects of CR
Increased ROS production contributes to mitochondrial dysfunction. In the hearts of mice with diet-induced obesity, mitochondrial ROS production was elevated, causing oxidative post-translational modifications of mitochondrial proteins and leading to impaired mitochondrial oxidative respiration and ATP production (3, 26). Interestingly, overexpression of catalase targeted to the mitochondria prevented the obesity-induced LVH, diastolic dysfunction, and it also decreased mitochondrial ROS level (26). Our finding here that CR decreases cardiac mitochondrial ROS generation in db/db mice indicates that CR is associated with improved mitochondrial function of the heart. It further raises the possibility that the cardiac benefits of CR are due, at least in part, to decreased mitochondrial ROS production. CR-mediated decrease in mitochondrial ROS could be a direct consequence of decreased cardiac fatty acid oxidation.
While CR prevented weight gain in db/db mice, whole-body insulin resistance persisted. It is likely that a longer duration of CR is required to reverse insulin resistance in db/db mice (12). Furthermore, we cannot exclude the possibility that there was tissue-specific reversal of the insulin resistance.
To summarize, we show for the first time that in genetically-obese mice, short-term CR prevents the development of MHD. These beneficial effects occur despite whole-body insulin resistance, and are associated with improved adipose tissue health as reflected by elevated circulating HMW adiponectin and increased AMPK activation. These findings raise an intriguing possibility that interventions that target adipose tissue may be of value in the treatment of MHD.
Acknowledgments
Funding: This work is supported by BU CTSI KL2 award 1KL2TR1411-1 (XJX) and NIH grant HL-064750 (WSC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations
Competing interests: All authors have no conflict of interest to report.
Authors’ contributions: JX performed the study and wrote the manuscript. EB, FQ and DC performed the study and/or contributed to data analysis and interpretation. JX and WSC conceived the study, and participated in its design and critical review of the manuscript. All authors read and approved the final manuscript.
References
- 1.Aasum E, Belke DD, Severson DL, Riemersma RA, Cooper M, Andreassen M, Larsen TS. Cardiac function and metabolism in Type 2 diabetic mice after treatment with BM 17.0744, a novel PPAR-alpha activator. American journal of physiology Heart and circulatory physiology. 2002;283:H949–957. doi: 10.1152/ajpheart.00226.2001. [DOI] [PubMed] [Google Scholar]
- 2.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiological reviews. 2008;88:389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behring JB, Kumar V, Whelan SA, Chauhan P, Siwik DA, Costello CE, Colucci WS, Cohen RA, McComb ME, Bachschmid MM. Does reversible cysteine oxidation link the Western diet to cardiac dysfunction? FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014;28:1975–1987. doi: 10.1096/fj.13-233445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 5.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 6.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–671. doi: 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolinsky VW, Dyck JR. Calorie restriction and resveratrol in cardiovascular health and disease. Biochimica et biophysica acta. 2011;1812:1477–1489. doi: 10.1016/j.bbadis.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Dolinsky VW, Morton JS, Oka T, Robillard-Frayne I, Bagdan M, Lopaschuk GD, Des Rosiers C, Walsh K, Davidge ST, Dyck JR. Calorie restriction prevents hypertension and cardiac hypertrophy in the spontaneously hypertensive rat. Hypertension. 2010;56:412–421. doi: 10.1161/HYPERTENSIONAHA.110.154732. [DOI] [PubMed] [Google Scholar]
- 9.Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Current opinion in cell biology. 2015;33:1–7. doi: 10.1016/j.ceb.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature reviews Molecular cell biology. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. The Journal of biological chemistry. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 12.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E, Pennington CT. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. Jama. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huynh K, Bernardo BC, McMullen JR, Ritchie RH. Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacology & therapeutics. 2014;142:375–415. doi: 10.1016/j.pharmthera.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD. Mitochondrial proton and electron leaks. Essays in biochemistry. 2010;47:53–67. doi: 10.1042/bse0470053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. The Journal of clinical investigation. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liesa M, Luptak I, Qin F, Hyde BB, Sahin E, Siwik DA, Zhu Z, Pimentel DR, Xu XJ, Ruderman NB, Huffman KD, Doctrow SR, Richey L, Colucci WS, Shirihai OS. Mitochondrial transporter ATP binding cassette mitochondrial erythroid is a novel gene required for cardiac recovery after ischemia/reperfusion. Circulation. 2011;124:806–813. doi: 10.1161/CIRCULATIONAHA.110.003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochemical and biophysical research communications. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 18.Minamiyama Y, Bito Y, Takemura S, Takahashi Y, Kodai S, Mizuguchi S, Nishikawa Y, Suehiro S, Okada S. Calorie restriction improves cardiovascular risk factors via reduction of mitochondrial reactive oxygen species in type II diabetic rats. The Journal of pharmacology and experimental therapeutics. 2007;320:535–543. doi: 10.1124/jpet.106.110460. [DOI] [PubMed] [Google Scholar]
- 19.Ouchi N, Shibata R, Walsh K. Cardioprotection by adiponectin. Trends in cardiovascular medicine. 2006;16:141–146. doi: 10.1016/j.tcm.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin F, Lennon-Edwards S, Lancel S, Biolo A, Siwik DA, Pimentel DR, Dorn GW, Kang YJ, Colucci WS. Cardiac-specific overexpression of catalase identifies hydrogen peroxide-dependent and -independent phases of myocardial remodeling and prevents the progression to overt heart failure in G(alpha)q-overexpressing transgenic mice. Circulation Heart failure. 2010;3:306–313. doi: 10.1161/CIRCHEARTFAILURE.109.864785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin F, Siwik DA, Luptak I, Hou X, Wang L, Higuchi A, Weisbrod RM, Ouchi N, Tu VH, Calamaras TD, Miller EJ, Verbeuren TJ, Walsh K, Cohen RA, Colucci WS. The polyphenols resveratrol and S17834 prevent the structural and functional sequelae of diet-induced metabolic heart disease in mice. Circulation. 2012;125:1757–1764. S1751–1756. doi: 10.1161/CIRCULATIONAHA.111.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinmura K, Tamaki K, Bolli R. Short-term caloric restriction improves ischemic tolerance independent of opening of ATP-sensitive K+ channels in both young and aged hearts. Journal of molecular and cellular cardiology. 2005;39:285–296. doi: 10.1016/j.yjmcc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–2817. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- 24.Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mechanisms of ageing and development. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 25.Sverdlov AL, Elezaby A, Behring JB, Bachschmid MM, Luptak I, Tu VH, Siwik DA, Miller EJ, Liesa M, Shirihai OS, Pimentel DR, Cohen RA, Colucci WS. High fat, high sucrose diet causes cardiac mitochondrial dysfunction due in part to oxidative post-translational modification of mitochondrial complex II. Journal of molecular and cellular cardiology. 2015;78:165–173. doi: 10.1016/j.yjmcc.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sverdlov AL, Elezaby A, Qin F, Behring JB, Luptak I, Calamaras TD, Siwik DA, Miller EJ, Liesa M, Shirihai OS, Pimentel DR, Cohen RA, Bachschmid MM, Colucci WS. Mitochondrial Reactive Oxygen Species Mediate Cardiac Structural, Functional, and Mitochondrial Consequences of Diet-Induced Metabolic Heart Disease. Journal of the American Heart Association. 2016;5 doi: 10.1161/JAHA.115.002555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venardos K, De Jong KA, Elkamie M, Connor T, McGee SL. The PKD inhibitor CID755673 enhances cardiac function in diabetic db/db mice. PloS one. 2015;10:e0120934. doi: 10.1371/journal.pone.0120934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu XJ, Apovian C, Hess D, Carmine B, Ruderman N. Improved insulin sensitivity 3 months after RYGB surgery is associated with increased subcutaneous adipose tissue AMPK activity and decreased oxidative stress. Diabetes. 2015 doi: 10.2337/db14-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu XJ, Gauthier MS, Hess DT, Apovian CM, Cacicedo JM, Gokce N, Farb M, Valentine RJ, Ruderman NB. Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. Journal of lipid research. 2012;53:792–801. doi: 10.1194/jlr.P022905. [DOI] [PMC free article] [PubMed] [Google Scholar]