Abstract
Background
Lung cancer is one of the leading causes of cancer mortalities worldwide, and non-small cell lung cancer (NSCLC) accounts for the majority of all lung cancer cases. Surgery remains one of the front-line treatment options for NSCLC, but events within the perioperative period were found to affect cancer prognosis, such as anesthesia procedures. Isoflurane, a commonly used volatile anesthetic, enhances the malignant potential of renal, prostate, and ovarian cancer cells, but its effects on NSCLC development have not been previously reported.
Material/Methods
CCK-8 and MTT cell proliferation assays were used to analyze NSCLC cell proliferation. Metastatic ability was examined by wound healing and transwell assays. We used Western blot analysis to study the mechanism of effect of Isoflurane in NSCLC development.
Results
We demonstrated that isoflurane promotes proliferation, migration and invasiveness of NSCLC cells, as well as upregulation of the Akt-mTOR signaling pathway in NSCLC cells. Pharmacological inhibition of Akt-mTOR signaling abolished the ability of isoflurane to promote proliferation, migration, and invasion of NSCLC cells, indicating that isoflurane promotes NSCLC cell malignancy by activating the Akt-mTOR signaling pathway.
Conclusions
Isoflurane promotes NSCLC proliferation, migration and invasion by activating the Akt-mTOR signaling pathway.
MeSH Keywords: Carcinoma, Non-Small-Cell Lung; Isoflurane; Proto-Oncogene Proteins c-akt; TOR Serine-Threonine Kinases
Background
Lung cancer is one of the leading causes of cancer mortalities worldwide in both males and females, with more than 1.5 million deaths annually [1]. Depending on histological characteristics, lung cancer can be categorized into two major types, small cell lung carcinoma (SCLC) and non-small cell lung carcinoma (NSCLC), of which NSCLC accounts for the vast majority of all lung cancer cases. Within the category of NSCLC, there are several subtypes of cancers with distinct cell origins and pathophysiologies, including lung adenocarcinoma, squamous cell carcinoma, and large cell lung cancer. Despite recent research progress in cancer treatment, the clinical prognosis of NSCLC remains still poor, primarily due to NSCLC cases often being diagnosed at advanced stages [2], leaving only limited effective treatment options available, and patients with advanced NSCLC commonly undergoing cancer metastasis and developing chemotherapy resistance [3]. This prompts the urgent need for identification of diagnostic markers, as well as elucidation of the molecular mechanisms responsible for metastasis and drug resistance.
Currently, surgery remains one of the front-line treatment options for NSCLC [4]. However, events within the perioperative period were found to affect cancer prognosis, including relapse, metastasis, and drug resistance for a variety of cancers [4]. Anesthesia has recently been linked with advancing cancer development through studies comparing regional vs. general anesthesia, with regional anesthesia being less pathogenic for various cancers [5–8]. Moreover, isoflurane, a widely used volatile anesthetic, has been found to enhance the malignant potential of renal [9], prostate [10] and ovarian cancer cells [11]. The mechanisms underlying the effects of isoflurane on cancer malignancy is under debate. Increased expression of insulin-like growth factor (IGF)-1 and vascular endothelial growth factor (VEGF) was suggested to cause enhanced cell cycle progression, cell proliferation and angiogenesis, which could contribute to accelerated cancer progression [11]. Other studies indicated that when the hypoxia-inducible factor (HIF) cellular signaling pathway is activated, it is responsible for isoflurane-induced cancer malignancies [9,10], as HIF signaling transcriptionally regulates various genes that have important roles in cancer activity, including cell growth, angiogenesis, glucose uptake, and metastasis [12].
To date, the effects of isoflurane on NSCLC development have not been previously evaluated. In our study, we are first to report that isoflurane treatment promotes proliferation, migration and invasion of NSCLC cells, and that pharmacological inhibition of Akt-mTOR signaling abolishes the ability of isoflurane to promote these processes of NSCLC cells, indicating that isoflurane promoted NSCLC cell malignancy via activating the Akt-mTOR signaling pathway. In addition, isoflurane treatment upregulates the Akt-mTOR signaling pathway in NSCLC cells.
Material and Methods
Reagents
MK2206 was obtained from Sigma (St. Louis, MO, USA). Antibodies against p-Akt, Akt, p-mTOR, mTOR, Cyclin D1, MMP2, MMP9 and β-actin were all purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell lines
NSCLC cell lines A549 and H1299 were cultured in 1640 Medium (Gibco-BRL, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco-BRL). The cells were maintained in a humidified 37°C incubator with 5% CO2.
Isoflurane gas exposure
Isoflurane gas exposure was performed as previously described [9].
CCK-8 assay
NSCLC cell proliferation was measured using the CCK-8 cell proliferation kit (Beyotime Biotechnology, Hainan, China) according to manufacturer’s instructions. Briefly, NSCLC cells were seeded into 96-well plates at 2×103 cells per well and cultured for 48 h after treatment. Ten μl CCK-8 (5 mg/ml) was added into the culture medium in each well. After 1 h incubation at 37°C, OD values were read with a microplate reader at the 450-nm wavelength.
MTT assay
NSCLC cells were plated into 96-well plates at a density of 3×103 cells/well. The cells were incubated with 20 μl MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) for 4 h at 37°C in the dark. DMSO (150 μl) was added to each well after 4-h incubation. Absorbance at 562 nm was measured with a microplate reader.
Wound healing assay
NSCLC cells were plated into 6-well plates and reached 80%-90% confluence. Artificial wounds were created on the confluent cell monolayer with 200-μL pipette tips, and the detached cells were washed twice with FBS. These cells were grown in complete 1640 medium, and migrating cells were examined under an inverted microscope with a digital camera (Nikon, Tokyo, Japan) and counted at 0 h and 24 h after culturing.
Transwell assay
Invasiveness of NSCLC cells was assessed using 8-mm pore polycarbonate transwell plates coated with Matrigel (BD Biosciences, San Jose, CA, USA). In brief, 2.5±104 cells in 1640 medium with 1% FBS were added to the upper chamber, and the lower chamber was filled with complete medium. After 12-h incubation, the non-migrating cells on the upper surface of the membrane were removed with cotton swabs. The invading cells on the lower surface of the membrane were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet dye. Six random fields per membrane were photographed and cells were quantified.
Western blot
Cells were harvested and lysed in RIPA buffer (Beyotime) containing protease inhibitor. The concentrations of total proteins were quantitated using a bicinchoninic acid (BCA) method (Beyotime). Proteins (20–40 μg) from each sample were loaded and separated by 8% SDS-polyacrylamide gel electrophoresis (PAGE), transferred onto nitrocellulose membranes (Millipore, Bedford, MA, USA), immunoblotted with specific primary antibodies, and incubated with corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies. All the immunoblots were visualized by enhanced chemiluminescence (Pierce, Rockford, IL, USA). β-actin was the internal control.
Statistical analysis
Statistical package 19.0 for Windows (SPSS, Chicago, IL, USA) was used for statistical analyses. The values were expressed as the mean ± standard deviation (mean ±SD). The data were analyzed by the paired-sample t-test or one-way analysis of variance, and p<0.05 was considered statistically significant. All experiments were performed in triplicate.
Results
Isoflurane promotes NSCLC cell proliferation
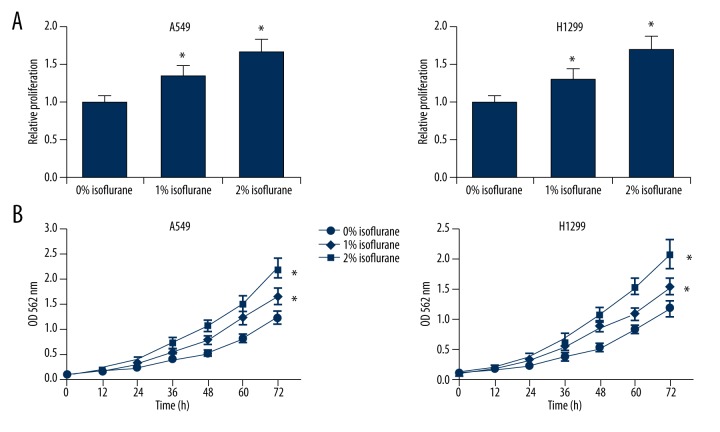
To investigate the effects of isoflurane on NSCLC malignancy, we first determined whether isoflurane could affect NSCLC cell proliferation. We treated two lines of NSCLC cells, A549 and H1299, with increasing concentrations of isoflurane (0%, 1% or 2%) and performed the CCK-8 cell proliferation assay. We found that isoflurane could significantly increase the proliferation ability of A549 and H1299 cells in a dose-dependent manner (Figure 1A). In addition, we performed MTT assays to monitor the time course of cell proliferation following isoflurane treatment, and we further confirmed that isoflurane could promote NSCLC cell proliferation in a dose-dependent manner (Figure 1B).
Figure 1.
Isoflurane promotes NSCLC cell proliferation. (A) CCK-8 proliferation assay of A549 and H1299 cells after isoflurane treatment (0%, 1% or 2%). (B) MTT assay of A549 and H1299 cells at the indicated time points after isoflurane treatment (0%, 1% or 2%). * p<0.05 compared with 0% isoflurane treatment.
Isoflurane promotes NSCLC cell migration and invasion
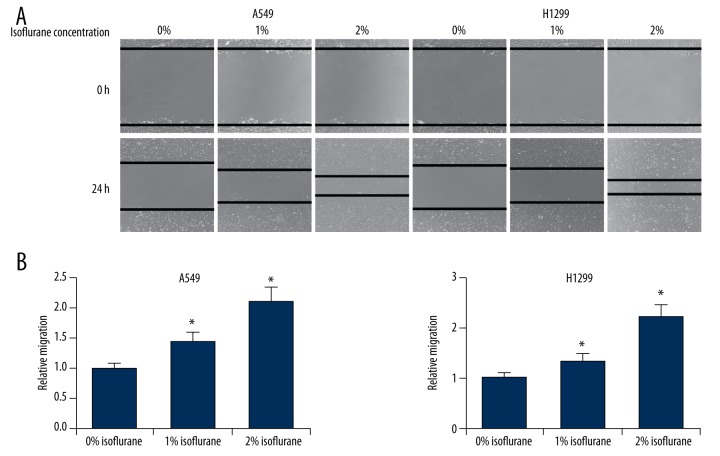
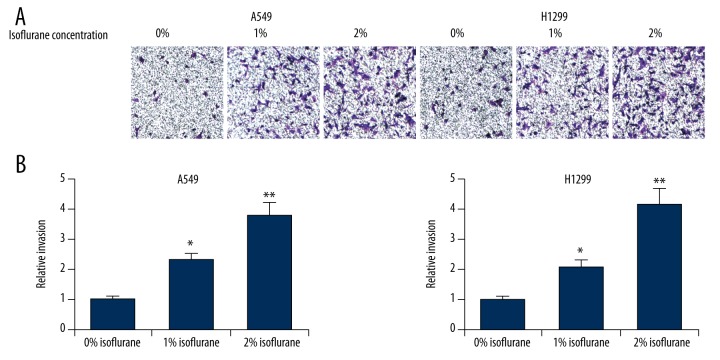
We next assessed the effects of isoflurane on NSCLC cell migration and invasion. We performed wound healing assays on A549 and H1299 cells treated with increasing concentrations of isoflurane (0%, 1% or 2%) and found that this treatment significantly increased migration speeds in both cell lines in a dose-dependent manner (Figure 2). Furthermore, we performed transwell invasion assays on isoflurane-treated A549 and H1299 cells, and observed that this treatment led to markedly increased numbers of invading cells (Figure 3). Taken together, these results indicated that isoflurane treatment promoted proliferation, migration, and invasiveness of NSCLC cells.
Figure 2.
Isoflurane promotes NSCLC cell migration. (A) Wound healing assay of A549 and H1299 cells after treatment of isoflurane (0%, 1% or 2%). (B) Quantification of the distance cell migrated. * p<0.05 compared with 0% isoflurane treatment.
Figure 3.
Isoflurane promotes NSCLC cell invasion. (A) Transwell invasion assay of A549 and H1299 cells after treatment with isoflurane (0%, 1% or 2%). (B) Quantification of the number of invading cells. * p<0.05, ** p<0.01, compared with 0% isoflurane treatment.
Isoflurane upregulates the Akt-mTOR signaling pathway in NSCLC cells
Isoflurane has been found to promote the malignancy renal and prostate cancer cells by activating the HIF signaling pathway, and this activation is mediated by Akt-mTOR signaling [9,10]. We therefore sought to determine whether isoflurane could activate the Akt-mTOR signaling pathway in NSCLC cells. We treated NSCLC cells with 2% isoflurane and examined both total and phosphorylated Akt levels by Western blot analysis. Significantly, in both A549 and H1299 cells, isoflurane was found to enhance phosphorylation of Akt (Figure 4). We also examined the levels of both total and phosphorylated mTOR in NSCLC cells treated with isoflurane, and observed that phosphorylation of mTOR was also significantly upregulated by isoflurane treatment (Figure 4). We examined the levels of a series of downstream targets of the Akt-mTOR signaling pathway (cyclin D1, MMP2 and MMP9), and found that the levels of these proteins were significantly elevated in response to isoflurane treatment in both A549 and H1299 cells (Figure 5). The results collectively demonstrated that isoflurane treatment upregulates the activity of Akt-mTOR signaling in NSCLC cells.
Figure 4.
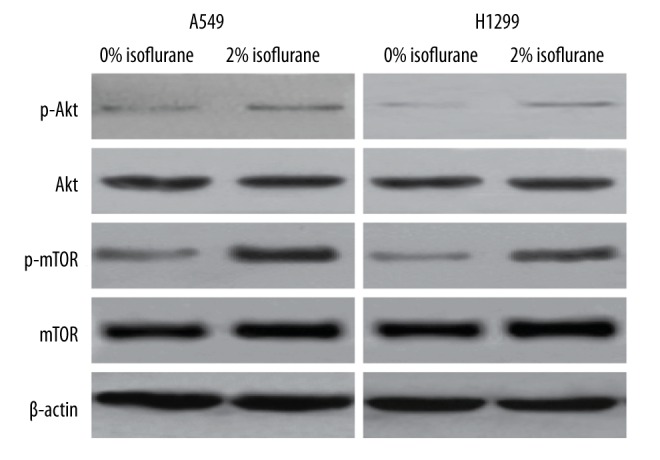
Isoflurane upregulates the Akt-mTOR signaling pathway in NSCLC cells. A549 and H1299 cells were treated with 0% or 2% isoflurane. The protein levels of phosphorylated Akt, total Akt, phosphorylated mTOR, and total mTOR were detected by Western blot analysis. β-actin was used as an internal control.
Figure 5.
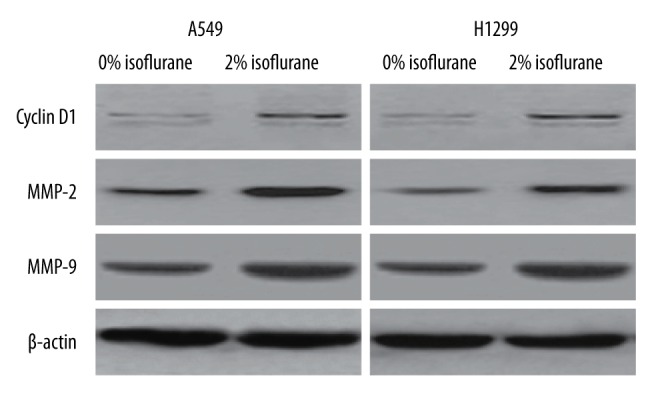
Isoflurane upregulates downstream target genes of Akt-mTOR signaling. A549 and H1299 cells were treated with 0% or 2% isoflurane. The protein levels of cyclin D1, MMP2 and MMP9 were detected by Western blot analysis. β-actin was used as an internal control.
Isoflurane promotes NSCLC cell malignancy via the Akt-mTOR signaling pathway
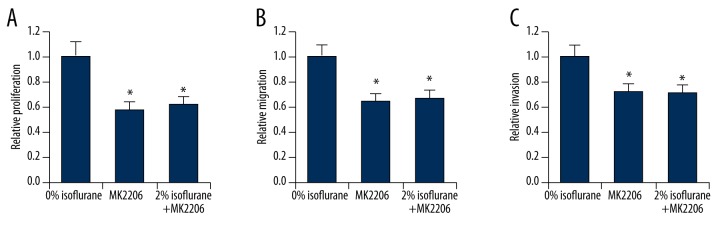
If isoflurane regulates NSCLC cell malignancy by activating the Akt-mTOR signaling pathway, isoflurane should not affect cell proliferation, migration and invasion in cells in which the Akt-mTOR signaling pathway is pharmacologically inhibited. To test this hypothesis, we treated A549 cells with a specific inhibitor of the Akt-mTOR signaling pathway, MK2206, and exposed those cells to 2% isoflurane. As expected, inhibition of Akt-mTOR signaling led to decreased levels of cell proliferation (Figure 6A), migration (Figure 6B), and invasion (Figure 6C), as determined by CCK-8 cell proliferation, wound healing, and transwell invasion assays, respectively. Significantly, in the presence of MK2206, isoflurane treatment could no longer induce elevated levels of cell proliferation (Figure 6A), migration (Figure 6B), or invasion (Figure 6C), suggesting that upregulation of NSCLC malignancy by isoflurane requires activation of Akt-mTOR signaling.
Figure 6.
Isoflurane promotes NSCLC cell malignancy via the Akt-mTOR signaling pathway. (A) CCK-8 proliferation assay, (B) Quantification of wound healing assay, and (C) Quantification of transwell invasion assay of A549 cells treated with MK2206, in the absence or presence of 2% isoflurane. * p<0.05 compared with 0% isoflurane treatment.
Discussion
We examined the potential impact of the anesthetic isoflurane on NSCLC cells, including their proliferation, migration, and invasion, and elucidated the effects of isoflurane on the Akt-mTOR signaling pathway in NSCLC cells. It is likely that isoflurane treatment activates downstream effectors and increases metastasis of NSCLC cells by enhancing the Akt-mTOR pathway. Treatment with isoflurane promoted the malignant functions of NSCLC cells, including proliferation, migration and invasion, consistent with several recent in vitro studies of other cancer types, including such as renal [9] and ovarian cancers [11]. Treatment with isoflurane alone or in combination with another anesthetic, propofol, was shown to modulate the malignancy of prostate cancer cells [10].
For many years, the effects of general anesthetics were considered reversible, particularly in the central nervous system. When anesthetics are cleared from their bioaction sites, the body returns to its normal state. Nevertheless, cellular signaling pathways and certain genes were shown by microarray data from human breast and brain tumor cell lines to be affected by anesthetics such as isoflurane [13].
The molecular mechanisms by which anesthetics modulate cancer cell malignancy are not well understood. In our study, we demonstrated that isoflurane treatment upregulates the Akt-mTOR signaling pathway in NSCLC cells. We also observed that pharmacologic inhibition of Akt-mTOR signaling abolished the ability of isoflurane to promote proliferation, migration and invasion of NSCLC cells. Our results strongly support the hypothesis that isoflurane promotes NSCLC cell malignancy via the Akt-mTOR signaling pathway. These results are consistent with previous studies with regard to the functions of the Akt signaling pathway in cancer development. The Akt pathway plays critical roles in regulation of apoptosis [14,15], which leads to expansion of cells and accumulation of genetic mutations [16,17], and therefore constitutes one of the key mechanisms in progression of cancers. The Akt signaling pathway was also demonstrated to play diverse roles in cancer development [18,19] through angiogenesis [20], cell growth [21], mesenchymal transition [22], and cell apoptosis and death [23]. In this context, regulation of proliferation and migration of NSCLC cells by isoflurane could be explained at least in part by modulation of the Akt-mTOR signaling pathway.
It is noteworthy that previous studies have suggested that isoflurane could induce upregulation of HIF-1α and its downstream effectors [9]. HIFs are a family of transcription factors that regulate certain genes that play roles essential in cancer development, including cell growth, angiogenesis, glucose uptake, and cell metastasis [12]. High levels of HIFs are found in most primary tumors and metastases, which indicates that they are detrimental to patient outcome [24,25]. Interestingly, inhibition of HIF-1 synthesis by blocking the upper Akt-mTOR pathway by an Akt inhibitor abolishes isoflurane-induced effects in prostate cancer cells [9], which is consistent with our conclusion that activity of the Akt-mTOR pathway is required for cancer cell malignancy to be affected by isoflurane.
There are several limitations of our study. We only examined the effect of one specific anesthetic in this study. Considering that many anesthetics and combinations are currently in use, future comparative studies of different anesthetics and their effects on NSCLC cells should be carried out. Although our experiments were designed to reflect the clinically relevant characteristics of cancer cells, it is not clear whether the conclusions of our study can be effectively extrapolated to an in vivo setting, since in vivo research is required for actual cancer environments and the effects of the anesthetics. We are actively working on these fronts and will address these limitations.
Conclusions
In this study we demonstrated that isoflurane promotes NSCLC proliferation, migration, and invasion by activating the Akt-mTOR signaling pathway. Isoflurane is widely used as an anesthetic for surgery, including tumor resection, so it is important to examine the roles of anesthetics during tumor treatment.
Footnotes
Conflict of interest
All authors declared no conflict of interest.
Source of support: Departmental sources
References
- 1.Cersosimo RJ. Lung cancer: A review. Am J Health Syst Pharm. 2002;59:611–42. doi: 10.1093/ajhp/59.7.611. [DOI] [PubMed] [Google Scholar]
- 2.Sakashita A, Sakashita M, Sound Tsao M. Genes and pathology of non-small cell lung carcinoma. Semin Oncol. 2014;41:28–39. doi: 10.1053/j.seminoncol.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–54. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 4.Tavare AN, Perry NJ, Benzonana LL, et al. Cancer recurrence after surgery: Direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130:1237–50. doi: 10.1002/ijc.26448. [DOI] [PubMed] [Google Scholar]
- 5.Biki B, Mascha E, Moriarty DC, et al. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: A retrospective analysis. Anesthesiology. 2008;109:180–87. doi: 10.1097/ALN.0b013e31817f5b73. [DOI] [PubMed] [Google Scholar]
- 6.Christopherson R, James KE, Tableman M, et al. Long-term survival after colon cancer surgery: A variation associated with choice of anesthesia. Anesth Analg. 2008;107:325–32. doi: 10.1213/ane.0b013e3181770f55. [DOI] [PubMed] [Google Scholar]
- 7.Exadaktylos AK, Buggy DJ, Moriarty DC, et al. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–64. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin L, Liu C, Tan H, et al. Anaesthetic technique may affect prognosis for ovarian serous adenocarcinoma: A retrospective analysis. Br J Anaesth. 2011;106:814–22. doi: 10.1093/bja/aer055. [DOI] [PubMed] [Google Scholar]
- 9.Benzonana LL, Perry NJ, Watts HR, et al. Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology. 2013;119:593–605. doi: 10.1097/ALN.0b013e31829e47fd. [DOI] [PubMed] [Google Scholar]
- 10.Huang H, Benzonana LL, Zhao H, et al. Prostate cancer cell malignancy via modulation of HIF-1alpha pathway with isoflurane and propofol alone and in combination. Br J Cancer. 2014;111:1338–49. doi: 10.1038/bjc.2014.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo X, Zhao H, Hennah L, et al. Impact of isoflurane on malignant capability of ovarian cancer in vitro. Br J Anaesth. 2015;114:831–39. doi: 10.1093/bja/aeu408. [DOI] [PubMed] [Google Scholar]
- 12.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 13.Huitink JM, Heimerikxs M, Nieuwland M, Loer SA, et al. Volatile anesthetics modulate gene expression in breast and brain tumor cells. Anesth Analg. 2010;111:1411–15. doi: 10.1213/ANE.0b013e3181fa3533. [DOI] [PubMed] [Google Scholar]
- 14.Hemmings BA. Akt signaling: Linking membrane events to life and death decisions. Science. 1997;275:628–30. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Tang N, Hadden TJ, et al. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813:1978–86. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Dai G, Zheng W, Ma X, Wang P. Multisite mutation of monomer survivin with enhanced effect on apoptosis regulation of breast cancer cells. Biomed Pharmacother. 2015;69:111–18. doi: 10.1016/j.biopha.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–44. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu X, Zhen Y, Yang H, et al. Loss of connective tissue growth factor as an unfavorable prognosis factor activates miR-18b by PI3K/AKT/C-Jun and C-Myc and promotes cell growth in nasopharyngeal carcinoma. Cell Death Dis. 2013;4:e634. doi: 10.1038/cddis.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie F, Bao X, Yu J, et al. Disruption and inactivation of the PP2A complex promotes the proliferation and angiogenesis of hemangioma endothelial cells through activating AKT and ERK. Oncotarget. 2015;6:25660–76. doi: 10.18632/oncotarget.4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou W, He MR, Jiao HL, et al. The tumor-suppressor gene LZTS1 suppresses colorectal cancer proliferation through inhibition of the AKT-mTOR signaling pathway. Cancer Lett. 2015;360:68–75. doi: 10.1016/j.canlet.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Zhou SL, Zhou ZJ, Hu ZQ, et al. CXCR2/CXCL5 axis contributes to epithelial-mesenchymal transition of HCC cells through activating PI3K/Akt/GSK-3beta/Snail signaling. Cancer Lett. 2015;358:124–35. doi: 10.1016/j.canlet.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 23.Frias A, Lambies G, Vinas-Castells R, et al. A switch in Akt isoforms is required for Notch-induced Snail1 expression and protection from cell death. Mol Cell Biol. 2015;36:923–40. doi: 10.1128/MCB.01074-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talks KL, Turley H, Gatter KC, et al. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157:411–21. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masoud GN, Li W. HIF-1alpha pathway: Role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378–89. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]