Abstract
Background
Omentin-1 is one of the adipokines associated with obesity, diabetes, and coronary heart disease development. We determined to investigate whether serum omentin-1 concentrations were correlated with the presence of atrial fibrillation (AF).
Material/Methods
Serum omentin-1 concentrations were examined in a cross-sectional population that included 220 patients with AF (70 with paroxysmal AF, 78 with persistent AF, and 72 with permanent AF) and 115 healthy controls.
Results
Reduced serum omentin-1 concentrations were found in AF patients compared to the controls. In addition, patients with permanent AF had lower serum omentin-1 concentrations compared to patients with persistent AF and patients with paroxysmal AF. Significantly decreased serum omentin-1 concentrations were observed in persistent AF patients compared to paroxysmal AF patients. Spearman correlation analysis suggested that serum omentin-1 concentrations were negatively correlated with left atrial diameter in AF patients.
Conclusions
Serum omentin-1 concentrations were correlated with the presence of AF and atrial remolding.
MeSH Keywords: Adipokines, Atrial Fibrillation, Inflammation Mediators, Obesity Hypoventilation Syndrome
Background
Atrial fibrillation (AF) is the most common cardiac arrhythmia in clinical practice, and contributes to a high prevalence of mortality and morbidity [1]. The exact mechanism underlying AF is complex and unclear. Factors such as aging, obesity, diabetes, hypertension, and cardiovascular diseases are considered to increase the risk of developing AF [2].
Omentin, a novel adipokine, is produced and secreted mainly by visceral adipose tissue. Omentin is codified by two genes named omentin-1 and omentin-2, and the former is the major circulating form [3]. Recombinant omentin-1 results in increased insulin-stimulated glucose uptake and Akt phosphorylation in human adipocytes [4]. Recently, omentin-1 has been shown to be correlated with obesity [5], hypertension [6], diabetes [7], and coronary artery disease (CAD) [8]. Omentin-1 is speculated to mediate the mechanism of AF.
The aim of this study was to determine the correlation between serum omentin-1 concentrations and the presence of AF and atrial remolding.
Material and Methods
Patients
A cross-sectional study was performed in a consecutive population of 220 patients who were diagnosed with AF. The criteria for a diagnosis of AF were in accordance with the guidelines established by American Heart Association [9]. Patients were excluded from the study if they had valvular heart disease, hyperthyroidism, acute coronary syndrome, previous cardiac surgery, or systemic disease. AF patients were divided into three groups according to American Heart Association guidelines [9]: paroxysmal AF (n=70), persistent AF (n=78), and permanent AF (n=72). Control participants were recruited from individuals presenting for routine checkup in our hospital. Patients with systemic disease were excluded from this study. The study plan was approved by the Research Ethics Committee of our hospital (NO. GDREC2012143H), and all patients provided informed consent.
Measurements
Anthropometric (height, weight, and blood pressure), clinical, and laboratory analysis were performed. Venous blood was collected after a minimum of 10 hours of fasting for further examination. An enzyme-linked immunosorbent assay kit (Cusabio Biotech Corporation, USA) was utilized to evaluate serum omentin-1 concentrations. Transthoracic echocardiography was performed by experienced echocardiologists on all patients to evaluate the characteristics of their left atrial diameter (LAD).
Statistical analysis
The data were exhibited as means ± standard errors (interquartile range). The unpaired t-test, chi-square tests, or the Mann-Whitney U test was utilized to determine the parameter differences between AF patients and control patients. Comparison of the characteristics between the three AF subgroups was performed by chi-square tests, one-way ANOVA, or the Kruskal-Wallis test. The correlation of serum omentin-1 concentrations with LAD were analyzed by Pearson correlation analysis. A value of p less than 0.05 was statistically significant.
Results
Baseline clinical characteristics
AF patients showed higher levels of SBP, DBP, LDL-C, and LAD, as well as statin, aspirin, and warfarin treatment, compared to healthy controls (Table 1).
Table 1.
Clinical and biochemical characteristics of AF patients and controls.
| The controls | AF patients | P value | |
|---|---|---|---|
| N | 115 | 220 | 0.244 |
| Age (years) | 58.43±9.55 | 59.75±10.03 | 0.770 |
| Gender (M/F) | 61/54 | 113/107 | 0.368 |
| BMI (Kg/m2) | 24.28±2.47 | 24.57±2.99 | <0.001 |
| SBP (mmHg) | 123.13±9.12 | 136.09±14.11 | <0.001 |
| DBP (mmHg) | 80.11±5.52 | 85.82±10.40 | 0.210 |
| TC (mmol/L) | 4.91±0.88 | 5.05±0.99 | 0.295 |
| TG (mmol/L) | 1.52±0.47 | 1.60±0.81 | 0.001 |
| LDL-C (mmol/L) | 3.19±0.45 | 3.43±0.74 | 0.126 |
| HDL-C (mmol/L) | 1.14±0.22 | 1.17±0.23 | 0.566 |
| Smoking, n (%) | 25 (21.74%) | 54 (24.55%) | <0.001 |
| LAD (mm) | 29.24±3.20 | 38.53±3.96 | <0.001 |
| Statin treatment, n (%) | 17 (14.78%) | 152 (70.45%) | <0.001 |
| aspirin treatment, n (%) | 21 (18.26%) | 115 (52.27%) | <0.001 |
| warfarin treatment, n (%) | – | 90 (40.91%) | |
| Omentin-1 (ng/mL) | 203.13 (168.39–245.07) | 156.49 (133.49–187.14) |
Serum omentin-1 concentrations in AF patients
AF patients showed significantly reduced serum omentin-1 concentrations compared to healthy controls (Table 1). The characteristics of AF subgroups are shown in Table 2. In AF subgroups, permanent AF patients had lower serum omentin-1 concentrations compared to paroxysmal AF and persistent AF patients (Figure 1). Furthermore, significant decreased serum omentin-1 concentrations were observed in persistent AF patients compared to paroxysmal AF patients (Figure 1).
Table 2.
Clinical and biochemical characteristics of AF subgroups.
| Paroxysmal AF | Persistent AF | Permanent AF | P value | |
|---|---|---|---|---|
| N | 70 | 78 | 72 | |
| Age (years) | 59.41±9.87 | 60.90±10.71 | 58.83±9.43 | 0.429 |
| Gender (M/F) | 33/37 | 41/37 | 39/33 | 0.680 |
| BMI (Kg/m2) | 24.41±2.89 | 24.74±3.14 | 24.55±2.96 | 0.799 |
| SBP (mmHg) | 138.21±12.57 | 133.21±13.24 a | 137.15±15.99 | 0.072 |
| DBP (mmHg) | 85.93±9.49 | 83.85±10.19 | 87.85±11.19 b | 0.062 |
| TC (mmol/L) | 5.02±0.97 | 4.96±0.97 | 5.17±1.04 | 0.407 |
| TG (mmol/L) | 1.53±0.65 | 1.49±0.76 | 1.80±0.97 ab | 0.036 |
| LDL-C (mmol/L) | 3.40±0.71 | 3.41±0.78 | 3.48±0.73 | 0.805 |
| HDL-C (mmol/L) | 1.16±0.23 | 1.12±0.20a | 1.11±0.21 b | 0.285 |
| Smoking, n (%) | 16 (22.86%) | 18 (23.08%) | 20 (27.78%) | 0.739 |
| LAD (mm) | 35.85±3.77 | 38.80±2.86a | 41.24±3.39ab | <0.001 |
| Statin treatment, n (%) | 40 (51.14%) | 50 (64.10%) | 62 (86.11)ab | <0.001 |
| Aspirin treatment, n (%) | 57 (81.43%) | 56 (71.79%) | 2 (2.78%)ab | <0.001 |
| Warfarin treatment, n (%) | 0 | 22 (28.21)a | 68 (94.44%)ab | <0.001 |
P<0.05 vs. paroxysmal AF;
P<0.05 vs. persistent AF.
Figure 1.
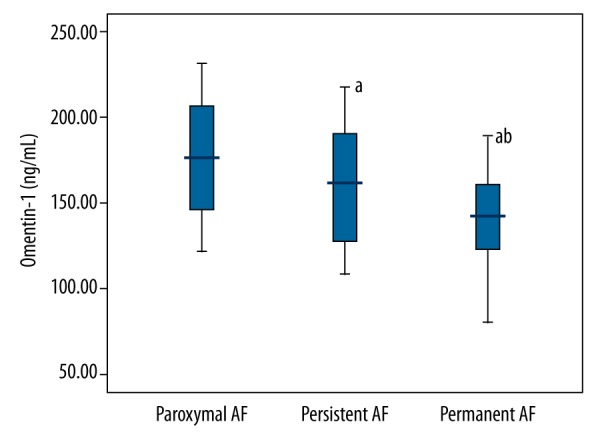
Serum omentin-1 concentrations in different AF patients.
The correlation of serum omentin-1 with AF
Simple and multiple logistic regressions both showed a significant association of serum omentin-1 with a decreased risk of developing AF (Table 3). The ROC curve of serum omentin-1 concentrations determining AF development are shown in Figure 2.
Table 3.
Logistic regression analysis for the presence of AF.
| Simple regression | Multiple regression | |||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Age (years) | 1.014 (0.991–1.038) | 0.244 | ||
| Gender (M/F) | 1.070 (0.681–1.680) | 0.770 | ||
| BMI (Kg/m2) | 1.038 (0.957–1.125) | 0.367 | ||
| SBP (mmHg) | 1.101 (1.073–1.130) | <0.001 | 1.126 (1.081–1.172) | <0.001 |
| DBP (mmHg) | 1.087 (1.052–1.123) | <0.001 | 0.961 (0.905–1.020) | 0.195 |
| TC (mmol/L) | 1.166 (0.917–1.482) | 0.210 | ||
| TG (mmol/L) | 1.191 (0.858–1.653) | 0.295 | ||
| LDL-C (mmol/L) | 1.794 (1.243–2.591) | 0.002 | ||
| HDL-C (mmol/L) | 0.453 (0.164–1.252) | 0.127 | ||
| Smoking, n (%) | 1.171 (0.683–2.008) | 0.566 | 0.999 (0.905–1.020) | 0.997 |
| Omentin-1 (ng/mL) | 0.972 (0.965–0.979) | <0.001 | 0.971 (0.963–0.979) | <0.001 |
Figure 2.
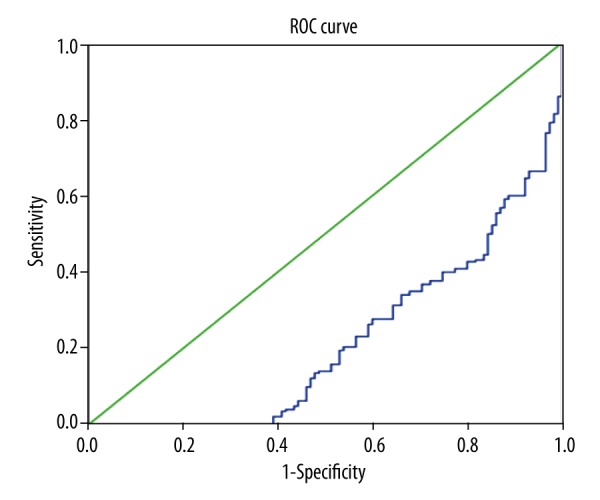
The ROC curve of serum omentin-1 concentrations determining AF development.
Serum omentin-1 concentrations with other characteristics
Pearson correlation analysis revealed a negative relationship of serum omentin-1 concentrations with BMI (r=−0.283, p<0.001), TG (r=−0.256, p<0.001), and LAD (r=−0.332, p<0.001).
Discussion
The present study indicated that AF patients had reduced serum omentin-1 concentrations compared to the healthy controls. Serum omentin-1 concentrations were negatively correlated with left atrial diameter in AF patients. In short, this is the first study that has demonstrated the association of serum omentin-1 and AF.
Adipokine is a cytokine produced from adipocytes. Recent studies have demonstrated the important role of adipokines in the mechanism of AF. Some adipokines, such as adiponectin [10] and resistin [11] were reported to be associated with the development of AF. Our results also showed a correlation between omentin-1, another adipokine, and AF development. These results point to an important role for adipose tissue and adipokine in the pathophysiology of AF. Nowadays, serum biomarkers are used in the diagnosis and prediction of cardiovascular disease [12,13]. Therefore, serum omentin-1 may be utilized as a new biomarker to predict and assess the risk of developing AF.
Obesity has been reported to be correlated with the development of AF. Large body size, assessed using body surface area in youth, and weight gain from age 20 to midlife, have both been independently correlated to AF development [14]. During a median time of 12.9 years’ follow-up, BMI was associated with an increased risk of 4.7% with each kilogram per square meter of developing AF [15]. In addition, overweight and obesity were associated with adjusted short-term increased risk of AF development [15]. Omentin-1 is a newly discovered adipokine associated with lipid metabolism and obesity. Plasma omentin-1 concentrations, as well as omentin-1 gene expression levels, were significantly decreased in obese and overweight subjects compared to lean subjects [5]. Furthermore, plasma omentin-1 concentrations were inversely correlated with BMI and waist circumference [5]. Circulating omentin-1 concentrations increased significantly after weight loss [16] and aerobic training [17]. These results point to the important role of omentin-1 in obesity. Therefore, omentin-1 may have a protective effect in AF development and persistence of AF partly by inhibiting obesity or regulating lipid metabolism.
Diabetes, hypertension, and CAD are clear risk factors for developing AF. Serum omentin-1 concentrations were markedly reduced in patients with type 2 diabetes [7]. Omentin inhibited platelet-derived growth factor BB-induced vascular smooth muscle cell migration by reducing oxidative stress [18]. This indicates that omentin-1 may serve as a target for treating hypertension by inhibiting vascular structural remodeling [18]. In addition, omentin treatment inhibited pulmonary arterial hypertension in rats by inhibiting vascular structural remodeling and abnormal contractile reactivity [6]. Furthermore, omentin treatment inhibited contractile dysfunction and insulin resistance in cardiomyocytes. Decreased serum omentin-1 concentrations were found in patients with CAD [8]. The aforementioned results indicate a close correlation between omentin-1 and diabetes, hypertension, and CAD.
Recent evidence has focused on the important role of inflammation in AF development. AF patients have shown elevated circulating inflammatory markers. Those inflammatory markers could predict AF development and AF recurrence [19]. Omentin-1 has been demonstrated to have an anti-inflammatory role. Omentin inhibited tumor necrosis factor (TNF)-induced vascular inflammation in human endothelial cells [20,21]. Furthermore, a negative correlation was found between serum omentin-1 and inflammatory mediators such as TNF-α, interleukin-6 (IL-6), and C-reactive protein [22,23]. Inflammation is a potential mechanism for AF. Omentin-1 is hypothesized to take part in the mechanism of AF by inhibiting inflammation.
The limitations of the present study should be considered. First, this is a cross-sectional study. Therefore, our findings should be validated by further longitudinal studies. Second, the sample of our study is relatively small.
Conclusions
In conclusion, serum omentin-1 concentrations were inversely correlated with the development of AF and atrial remolding.
Footnotes
Conflict of interests
All authors have no conflict of interests to declare.
Source of support: Guangdong Natural Science Foundation (No. S2013010016575)
References
- 1.Sabashnikov A, Weymann A, Haldar S, et al. Position of totally thoracoscopic surgical ablation in the treatment of atrialfibrillation: An alternative method of conduction testing. Med Sci Monit Basic Res. 2015;21:76–80. doi: 10.12659/MSMBR.894239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yalcin MU, Gurses KM, Kocyigit D, et al. Elevated M2-muscarinic and β1-adrenergic receptor autoantibody levels are associated with paroxysmal atrial fibrillation. Clin Res Cardiol. 2015;104:226–33. doi: 10.1007/s00392-014-0776-1. [DOI] [PubMed] [Google Scholar]
- 3.Schäffler A, Neumeier M, Herfarth H, et al. Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochim Biophys Acta. 2005;1732:96–102. doi: 10.1016/j.bbaexp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Yang RZ, Lee MJ, Hu H, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role inmodulating insulin action. Am J Physiol Endocrinol Metab. 2006;290:E1253–61. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 5.de Souza Batista CM, Yang RZ, Lee MJ, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–61. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 6.Kazama K, Okada M, Yamawaki H. A novel adipocytokine, omentin, inhibits monocrotaline-induced pulmonary arterial hypertension in rats. Biochem Biophys Res Commun. 2014;452:142–46. doi: 10.1016/j.bbrc.2014.08.070. [DOI] [PubMed] [Google Scholar]
- 7.Yan P, Liu D, Long M, et al. Changes of serum omentin levels and relationship between omentin and adiponectin concentrations in type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2011;119:257–63. doi: 10.1055/s-0030-1269912. [DOI] [PubMed] [Google Scholar]
- 8.Zhong X, Zhang HY, Tan H, et al. Association of serum omentin-1 levels with coronary artery disease. Acta Pharmacol Sin. 2011;32:873–78. doi: 10.1038/aps.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuster V, Rydén LE, Asinger RW, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: Executive summary. A Report of the American College of Cardiology/American Heart Association Task Force on PracticeGuidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation): Developed in Collaboration with the North American Society of Pacing and Electrophysiology. J Am Coll Cardiol. 2001;38:1231–66. doi: 10.1016/s0735-1097(01)01587-x. [DOI] [PubMed] [Google Scholar]
- 10.Choi BJ, Heo JH, Choi IS, et al. Hypoadiponectinemia in patients with paroxysmal atrial fibrillation. Korean Circ J. 2012;42:668–73. doi: 10.4070/kcj.2012.42.10.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Özcan KS, Güngör B, Altay S, et al. Increased level of resistin predicts development of atrial fibrillation. J Cardiol. 2014;63:308–12. doi: 10.1016/j.jjcc.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Demirtas S, Caliskan A, Karahan O, et al. Neutrophil gelatinase-associated lipocalin as a biomarker for acute kidney injury inpatients undergoing coronary artery bypass grafting. Exp Clin Cardiol. 2013;18:107–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Yavuz C, Guclu O, Demirtas S, et al. Can elevated prolidase activity predict the duration of ischemic exposure in different types of ischemia? Turk Gogus Kalp Dama. 2013;21:1000–4. [Google Scholar]
- 14.Rosengren A, Hauptman PJ, Lappas G, et al. Big men and atrial fibrillation: Effects of body size and weight gain on risk of atrial fibrillation in men. Eur Heart J. 2009;30:1113–20. doi: 10.1093/eurheartj/ehp076. [DOI] [PubMed] [Google Scholar]
- 15.Tedrow UB, Conen D, Ridker PM, et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women’s health study) J Am Coll Cardiol. 2010;55:2319–27. doi: 10.1016/j.jacc.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreno-Navarrete JM, Catalán V, Ortega F, et al. Circulating omentin concentration increases after weight loss. Nutr Metab (Lond) 2010;7:27. doi: 10.1186/1743-7075-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saremi A, Asghari M, Ghorbani A. Effects of aerobic training on serum omentin-1 and cardiometabolic risk factors in overweight and obese men. J Sports Sci. 2010;28:993–98. doi: 10.1080/02640414.2010.484070. [DOI] [PubMed] [Google Scholar]
- 18.Kazama K, Okada M, Yamawaki H. A novel adipocytokine, omentin, inhibits platelet-derived growth factor-BB-induced vascular smooth muscle cell migration through antioxidative mechanism. Am J Physiol Heart Circ Physiol. 2014;306:H1714–19. doi: 10.1152/ajpheart.00048.2014. [DOI] [PubMed] [Google Scholar]
- 19.Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–10. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 20.Yamawaki H, Kuramoto J, Kameshima S, et al. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem Biophys Res Commun. 2011;408:339–43. doi: 10.1016/j.bbrc.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 21.Kazama K, Usui T, Okada M, et al. Omentin plays an anti-inflammatory role through inhibition of TNF-α-induced superoxide production in vascular smooth muscle cells. Eur J Pharmacol. 2012;686:116–23. doi: 10.1016/j.ejphar.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 22.Pan HY, Guo L, Li Q. Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res Clin Pract. 2010;88:29–33. doi: 10.1016/j.diabres.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Moreno-Navarrete JM, Ortega F, Castro A, et al. Circulating omentin as a novel biomarker of endothelial dysfunction. Obesity (Silver Spring) 2011;19:1552–59. doi: 10.1038/oby.2010.351. [DOI] [PubMed] [Google Scholar]


