Abstract
Over the years, Newcastle disease (ND) has defied all available control measures. The disease has remained at the forefront of infectious diseases afflicting poultry production after avian influenza. Despite the continuous global use of million doses of ND vaccine annually, the causative pathogen, avian paramyxovirus type 1 also known as Newcastle disease virus (NDV) has continued to evolve causing, even more, a threat not only to the unvaccinated but the vaccinated flocks inclusive. The disease has been well studied in the developed countries where the virus is found in circulation. However, limited information exists on the epizootiology and circulating genotypes of the virus in developing countries where the majority of the flocks are raised on the extensive management system. Identification of virulent NDV in apparently healthy free-range ducks in this system calls for concern and pragmatic approach to investigate factor(s) that favour the virus inhabiting the ducks without clinical manifestation of the disease. Recently, novel genotypes (XIV, XVII, and XVIII) with peculiarity to West and Central African countries have been discovered and due to lack or poor surveillance system possibility of hitherto unreported genotypes are likely. This review elucidates and discusses available literature on the diversity of the circulating NDV genotypes across the West Africa countries and the epizootiology (molecular) of the disease in Nigeria with the view of identifying gaps in knowledge that can assist in the development of effective vaccines and control strategies to combat the peril of the disease.
Keywords: Epizootiology, Newcastle disease, Genotypes, Nigeria, West Africa
Introduction
The first outbreak of Newcastle disease (ND) in poultry can be traced back to 1926. This occurred at two diverse geographical points on the world map, Newcastle-upon-Tyne, England [26] and the Island of Java, Indonesia [55]. However, there are strong suggestions that ND outbreaks might have occurred earlier than reported by Doyle and Kraneveld as contained in the paper written by Macpherson [60] who drew attention to the poem titled “Call nan Cearc” (The loss of the hens) written by John Campbell on his conviction that the outbreak described in the poem which occurred in 1898 in Western Isles of Scotland was caused by ND. This review will toe the line of reports of Doyle and Kraneveld since the description in the poem could resemble that of “fowl plague” (avian influenza) which was ravaging poultry around that time in Italy [87]. The disease has continuously decimated and threatened the growth of poultry production, particularly in developing countries [9].
ND is a highly infectious disease with great economic losses, making it one of the “notifiable diseases” on the World Organization for Animal Health (OIE) list (http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.03.14_NEWCASTLE_DIS.pdf). Designation of pathogens as “notifiable diseases” requires immediate and prompt reporting to the relevant authorities to curtail the spread. Outbreaks of ND occur across the globe on a daily basis, especially in developing countries where the disease is enzootic with only a few countries reporting to the OIE. In a 12 year study (January 2000–December 2011) conducted to examine the frequency of reporting of ND outbreaks in 54 African countries, only 40.7 % was discovered to have always reported to OIE [38]. According to OIE (www.oie.int/wahis_2/public/wahid.php/), outbreaks of ND have been reported at one time or the other in some countries from Africa, South America, Middle East, Europe and Asia from 2005 to 2015.
From the time of the first reported outbreaks in 1926, four panzootics of ND have been documented [9, 45] and the possibility of another panzootic is imminent [67]. Based on clinical signs in infected birds, five pathotypes of NDV isolates have been identified, namely: neurotropic velogenic, viscerotropic velogenic, mesogenic, lentogenic and asymptomatic. Pathogenicity for chickens varies significantly, ranging from asymptomatic (no apparent disease) to severe. In severe cases, it manifest as respiratory and/or neurological disease culminating in 100 % mortality of infected flocks in the case of the velogenic strains. Assessment of economic impact due to ND is not only limited to high mortality recorded from the outbreaks and the cost of control measures but also on the trade restrictions placed on the localities where outbreaks have been reported [8, 9, 37].
Poultry production in Nigeria
The poultry industry in Nigeria is the most capitalized among the Agricultural sectors in the country and contribute the largest to the economy next to the oil industry [3, 27]. About 65–75 % of the populace depends on agriculture and agro-based businesses for their income [http://www.cenbank.org/OUT/PUBLICATIONS/REPORTS/RSD/2009/CBN Annual report for the year ended 31st December 2007–Executive summary. Pdf; 13] with poultry identified as a major source of national income that provides about 9–10 % of the nation’s gross domestic product (GDP) worth $250 million [13]. In Nigeria, an increase in poultry production was actually noticed in the early 1980s. At this time, subsidy was introduced on day-old chicks (DOC) and feed ingredients by the government. Recently, the government supported small scale poultry farmers with DOC and other incentives at highly subsidized rates to boost poultry production through the Agricultural Transformation Agenda. These initiatives have encouraged several people, including civil servants, housewives, and artisans to embrace poultry production by keeping birds as part or full-time commercial ventures. As practiced in most developing economies, poultry production systems are generally categorized into two major groups, namely; subsistent (free-range) and commercial [4]. Based on biosecurity practices, FAO further categorizes poultry production systems into 4 sectors viz; sector 1 with high, sector 2 with high to moderate, sector 3 and 4 with low biosecurity. In Nigeria, sector 4 (free-range poultry) accounts for over 80 % of the poultry population [3]. Different species of poultry, including layer, cockerels, guinea fowls, ducks, turkeys, and pigeons are raised by the operators of the various systems. In the 1990s, Japanese quail (Coturnix coturnix japonica) was introduced into Nigeria by National Veterinary Research Institute, Vom to diversify the poultry subsector and provide alternatives to poultry consumers in terms of meat and eggs [41]. Consequently, Japanese quail products have received general acceptance and patronage from poultry consumers across Nigeria due to its nutritional, economic and perceived medicinal values [16]. However, these production systems, especially the subsistence are faced with myriads of challenges amongst which disease is topmost. Notable among the diseases afflicting the industry are Infectious bursal disease (Gumboro), Chicken anaemia virus, Infectious Laryngotracheitis, Fowl pox, Salmonellosis, Chronic respiratory disease, Marek’s Disease, ND, Egg drop syndrome, Infectious bronchitis, Avian Influenza and so on [3, 31, 32, 50, 76, 81, 84]. The sustainability of this subsector is being threatened as a result of incessant outbreaks of ND in unvaccinated flocks and sporadically in vaccinated flocks [5, 102].
The first report of ND in Nigeria was in 1952 [44], thereafter several cases have been reported in commercial, rural scavenging, captive and free-living wild birds making it enzootic across the entire country [5, 28, 31, 40, 48, 70, 72, 74, 82]. Reports have shown that ND was ranked first among other diseases affecting the poultry industry [3, 39]. Economic and financial losses as a result of incessant ND outbreaks in Nigeria are not being regularly quantified. An estimated 78,526 outbreaks of the disease were reported in 2008 across Nigeria with an estimated financial burden of 8.9 billion naira for local chickens alone [29]. This report is an underestimation of financial implications of annual incidence of ND across the country in terms of the magnitude of unreported outbreaks occurring in remote areas of the country.
Biosecurity and vaccination are veritable tools that are used in combating the disease. Various types of vaccines are in use in the country for the control of ND menace. Notable among the vaccines are live attenuated Lasota and Hitchner B1 strains which are commonly used in the commercial poultry. In addition, inactivated oil-emulsion vaccines are often used by some farmers, which come as a single package or in combination with other poultry agents. The vaccination regimen (Table 1) is routinely undertaken in commercial poultry. However, vaccination in free-range poultry system is barely performed due to the large dosage presentation of conventional ND vaccines, and maintenance of cold chain. On the other hand, thermostable NDV-I2 and V4 vaccine strains have been successfully introduced into Nigeria and used in combating ND threat for free-range chickens with good protection level [68, 78]. The free-range chickens have been implicated in harbouring velogenic strains of the virus [5, 28, 62] which have been considered a threat to the commercial poultry.
Table 1.
Current vaccination schedule for Newcastle Disease (ND) in Nigeria
| S/N | ND vaccine | Age of vaccination | Route of inoculation |
|---|---|---|---|
| 1. | Live attenuated Hitchner B1 | Day old at the hatchery | Intra-ocular through spraying |
| 2. | Live attenuated La Sota | 1–2 weeks | Oral through drinking water |
| 3. | Live attenuated Komarov | 7–10 weeks | Intramuscular through injection |
| 4. | Inactivated oil-emulsion NDV | 16–18 weeks | Intramuscular through injection |
Definition of Newcastle disease
In general terms, infection of birds with any strain of NDV may be referred to as ND but because ND is a “notifiable disease” to the OIE the appropriate definition must, therefore, be followed. According to the definition by OIE (http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.03.14_NEWCASTLE_DIS.pdf), ND is an infection of birds caused by APMV-1 that fulfils any of the following criteria for virulence (a) Exhibition of intracerebral pathogenicity index (ICPI) value of 0.7 or greater in day-old chicks or (b) Presence of at least three basic amino acids (arginine or lysine) at between residue positions 113 and 116 of the C-terminus of the F2 protein and phenylalanine at position 117, which is the N-terminus of the F1 protein. In cases where the pattern of amino acid residues observed contradicts what was stated above, characterization of the virus by intra-cerebral pathogenicity index (ICPI) test would be performed. Identification of the amino acid residue positions starts from the N-terminus of the amino acid sequence deduced from the nucleotide sequence of the F0 gene with 113-116 corresponding to residues −4 to −1 from the cleavage site [9].
Molecular determinant of virulence
The molecular basis for pathogenicity can be deduced from the presence of multiple basic amino acids at the cleavage site of the fusion protein or ICPI index of 0.7 or greater than in day-old chickens [9].
Newcastle disease virus
NDV is classified into the genus Avulavirus, subfamily Paramyxovirinae and family Paramyxoviridae (http://www.ictvonline.org/virusTaxonomy.asp). Based on serological tests and complete genome sequence, APMVs have been officially classified into 12 serotypes designated as APMV-1 to 12 with NDV categorized into APMV-1 [7, 10, 65, 106]. The virus genome has a negative-sense, non-segmented, single-stranded RNA with a genome length of approximately 15,200 nucleotides (nt). Isolates of early 1930–1960s have a genome size of 15,186 nt while those of late 1960s have 15,192 nt and 15,198 nt for those of Class I isolates from waterfowls [21, 63]. The genome codes for six genes encoding seven proteins, namely: nucleocapsid protein (N), phosphoprotein (P), matrix protein (M), fusion protein (F), hemagglutinin-neuraminidase protein (HN), large polymerase protein (L), and an additional protein V that is expressed by RNA editing of P mRNA [56]. The HN and F proteins are two spike projections on the envelope of the virus and the F-gene in addition to the HN and L genes have been identified as a major determinant of virulence [23, 47, 86]. The genome size is a multiple of six nt, referred to as the ‘rule of six’ that makes efficient replication occur [21, 24, 54]. Though, the virus exists as a single serotype antigenic and genetic diversities have been identified among the isolates across the globe [93].
Full genome sequences of APMV-1 strains of the differently identified genotypes circulating across the globe have been published [17, 20, 34, 52, 59, 107]. In West Africa, however, only four complete genome sequences of the virus circulating in this sub-region are available in the sequence repositories (GenBank, DDBJ, and EMBL) as of 30th May, 2016. In addition, complete genome sequence of APMV-1 representing genotypes XIV and XVII from Nigeria has just been published [97, 98]. The paucity of information on the circulating genotypes in the sub-region is a limitation in the development of effective control measures.
Newcastle disease outbreaks in Nigeria
In general, all avian species are susceptible to ND infection, but the chickens are the most affected in terms of severity of the disease [10]. In chickens, 100 % mortality has been recorded with an infection involving velogenic strains [37]. Ducks and quails are resistant to the disease, but recent studies have shown their susceptibility even though the morbidity and mortality are lower than chickens [58, 80]. In Nigeria, ND outbreaks have been reported in free-range local and exotic chickens, guinea-fowls, wild and captive birds, quail, dove, mallard duck, ostrich, turkey, vulture, eagle, sparrows, crows, parrot [6, 39, 40, 48, 72, 78, 83] (Shittu et al. 2016 unpublished data).
Epizootiology of ND in Nigeria
Newcastle disease is a global disease with a presence in six out of the seven continents [66]. In Nigeria, the disease has been detected in all the agro-ecological zones of the country (NVRI unpublished data, 2006). Over the years, epizootiology of ND in Nigeria has been based mostly on conventional methods viz: serological [6, 69, 71, 75, 83, 85, 92] and virus isolation with biological characterization [5, 28, 40, 48, 62, 70, 74, 96]. Recently, however, attempts were made by using molecular techniques at defining the genotypes and molecular epidemiology of the circulating virus in West and Central Africa including Nigeria [19, 99, 101–103, 108].
Using serological methods [haemagglutination-inhibition (HI) or Enzyme-linked immune sorbent assay (ELISA)], antibodies to NDV have been detected in different species of poultry across the country [11, 12, 30, 61, 75, 89]. Over the decades, seroprevalence studies conducted in different locations in Nigeria on birds kept on the extensive production system showed diverse ranges from 38 to 74.3 % [72]. In a more recent seroprevalence study, the figures have not considerably changed (Table 2). There is no doubt to the fact that ND is enzootic in Nigeria, deductions made from serological survey data, therefore, needs to be interpreted with caution. Most of these studies were conducted in or near urban areas. Commercial backyard poultry in these areas raised on semi-intensive system are mostly vaccinated against ND. These birds are sometimes allowed to commingle with the free-range scavenging birds. In another scenario, “spent” hens from commercial poultry which are regularly vaccinated are procured and raised along with the free-range birds. These scenarios make the deductions of the true prevalence from these studies biased due to the confounding factors associated. This may explain the differences in seroprevalence from the same epidemiological units (Table 2). It is, therefore, envisaged that future survey for NDV should be aimed more at virological and genetic characterization rather than serological which provide limited information especially in terms of pathotypes and genotypes.
Table 2.
Seroprevalence study of ND in local chickens and live bird markets Nigeria
| Location | Year | Prevalence % | References |
|---|---|---|---|
| Ogun | 2006 | 100 (180/180) | [85] |
| Plateau | 2009 | 51.9 (627/1208) | [69] |
| Jigawa | NA | 38.8 (72/250) | [109] |
| Bauchi | NA | 56.3 (169/300) | [71] |
| Nassarawa | 2011 | 28.1 (289/1030) | [92] |
| Nassarawa | 2011–2012 | 28.7 (359/1250) | [43] |
| FCT (Abuja) | NA | 17 (34/200) | [2] |
| FCT (Abuja) | NA | 57 (228/400) | [14] |
| Zamafara | NA | 32.5 (164/504) | [49] |
As shown in Table 3, retrospective studies of clinical case report covering 9–10 years conducted in some northern states of Nigeria identified ND as most often diagnosed disease with a prevalence of 36.7 % [15] and 52.2 % [91] in Borno; 29.1 % [110], 32.3 % [90] and 33.2 % [73] in Kaduna; 14.1 % [79] in Kwara and 55.5 % [57] in Gombe. Also, an isolation rate of 21.0 % was reported in a limited epizootiological study involving apparently healthy birds from four different species in two live bird markets (LBM) in Southwestern Nigeria [96]. In a 3 year prospective study conducted in southeastern Nigeria involving a clinical and laboratory-based test, ND outbreaks were observed to peak during the dry harmattan period (November to February) with another marginal peak recorded during the height of the rainy season (June to July) [77]. In other reports, high prevalence of ND outbreaks was also observed mostly in the dry harmattan period [57, 64, 69, 73, 77, 90, 110] in which stress and cold associated with the weather are said to be responsible for this occurrence [64, 77]. In addition, movements of birds across different parts of the country are witnessed around this time in preparation for the festive period (Christmas and Easter). Likewise, the role of LBM in the perpetuation of the virus cannot be ignored as recently reported [96] which may explain the sporadic outbreaks experienced outside the peak period. Prior to the onset of the raining season in Nigeria, some farmers sell off their poultry and the proceeds are used to procure seeds for the farming season. In some instance, live birds are bought from LBM or poultry vendors in exchange as a gift. Such birds when not immediately needed are introduced into pre-existing flock if any, or left to scavenge in the neighbourhood. These birds might be incubating the virus and shed into the environment. The introduction of seemingly healthy, but inadvertently infected new bird to an existing flock has been reported to result in an outbreak of ND and highly pathogenic avian influenza (NVRI unpublished data, 2006).
Table 3.
Retrospective study of ND in Nigeria
| Location | Year | Specific rate (%) | References |
|---|---|---|---|
| Maiduguri, Borno state | 2004–2012 | 36.7 (851/2317) | [15] |
| Maiduguri, Borno state | 2000–2009 | 52.2 (2427/4647) | [91] |
| Zaria, Kaduna state | 1990–1999 | 32.3 (812/2513) | [90] |
| Zaria, Kaduna state | 2003–2012 | 29.1 (868/2983) | [110] |
| Mando, Kaduna state | 1996–2005 | 33.2 (1050/3164) | [73] |
| Ilorin, Kwara state | 2000–2009 | 14.1 (517/3655) | [79] |
| Gombe state | 2004–2013 | 55.5 (5531/9970) | [57] |
Also, reports of isolation of virulent NDV from apparently healthy birds have been documented [18, 22, 28, 62, 99]. In all of these reports, free-range local chickens and ducks were involved. The perpetuation of velogenic strain in apparently healthy birds, therefore, poses a threat to commercial poultry. Future work should be designed to identify the natural genetic resistance gene possessed by these local indigenous birds that enable it to harbour the virus with subclinical infection. Identification of such gene(s) may help to develop resistant chickens through reverse genetics that can help to combat Newcastle disease in both free-range scavenging and exotic commercial chickens.
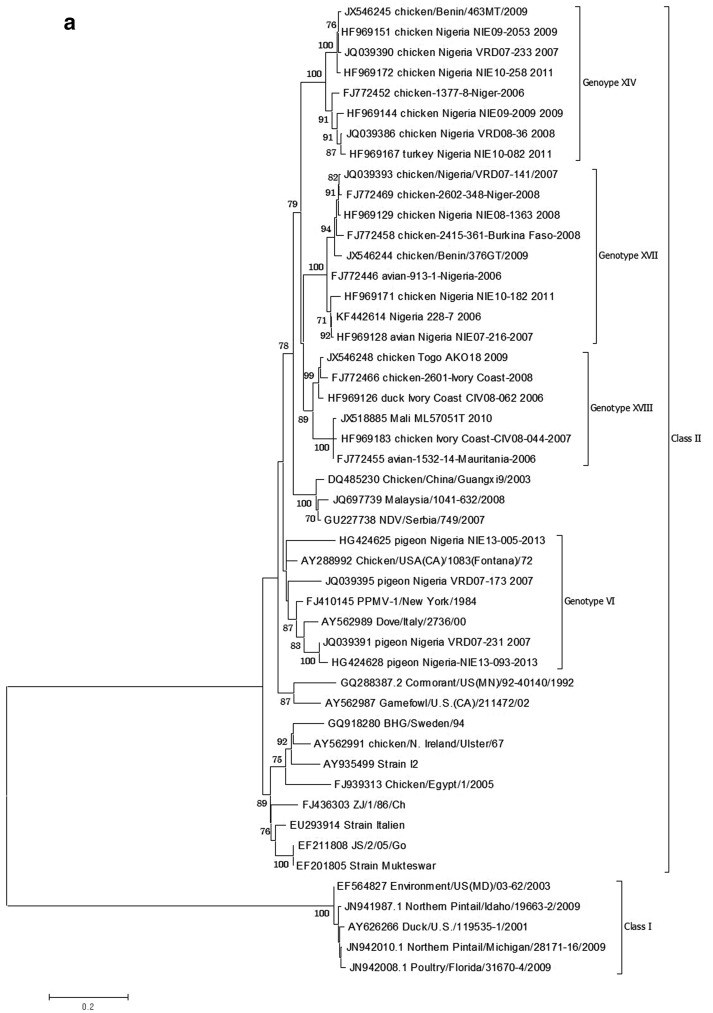
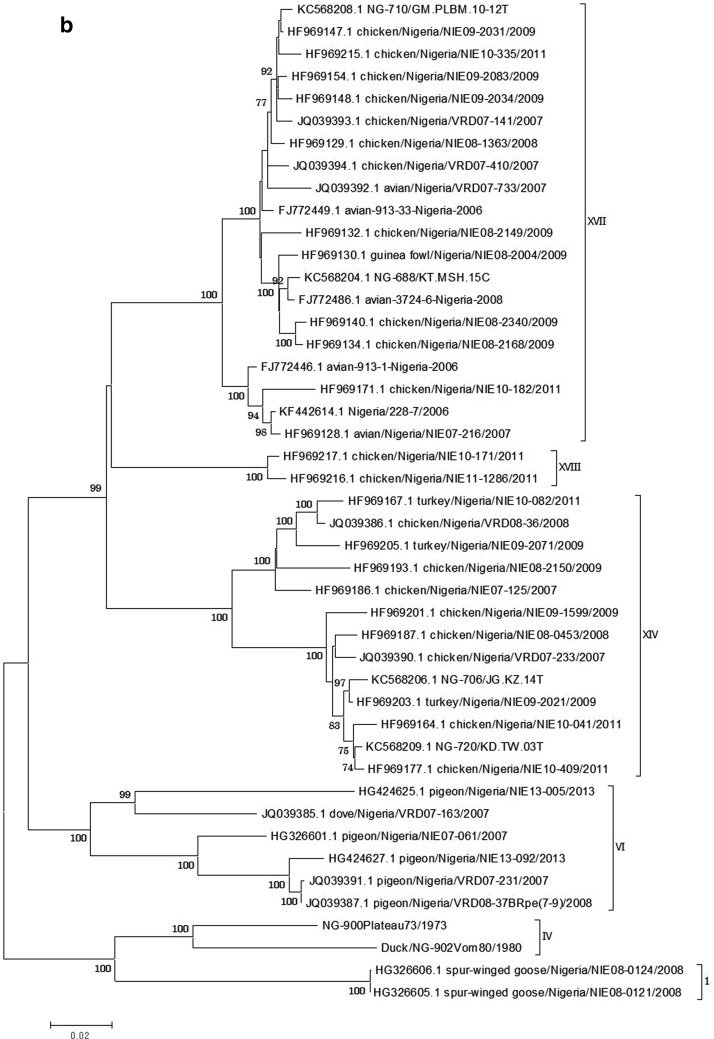
Unlike other NDV genotypes circulating in other parts of the World, little is known about the genotypes circulating in West Africa. Three new genotypes XIV, XVII and XVIII and two sub-genotypes (Fig. 1a) each has been described and are responsible for the current ND outbreaks being experienced across the sub-region. These genotypes and sub-genotypes are in circulation and have not been detected elsewhere outside the sub-region, making them indigenous [19, 95, 99, 101–103, 108]. All NDV genotypes that have been reported to be circulating in Nigeria are shown in Fig. 1b. Genotype IV was discovered in uncharacterized archival samples in our laboratory dating back to 1980 and 1973 (Shittu et al. 2016 unpublished data). Though, this genotype has not been detected from any of our current surveillance (active and passive) activities. The existence of the genotype has been reported to have ceased to be in circulation across the globe since 1989 [66]. Genotype VI viruses are characterized by pigeon paramyxoviruses (PPMV) which are variants of NDV. They have been reported to be in circulation from 1970 to 1980 and are responsible for the third panzootic [10]. The virus has been reported in different West and Central African countries, including Nigeria with sub-genotypes VIg, h, and i being in circulation [100, 108]. Genotypes I and II were discovered in free-range and commercial poultry respectively, with close relatedness to NDV-I2 and LaSota vaccines [76] (Shittu et al. 2016 unpublished data).
Fig. 1.
NDV isolates Maximum Likelihood Phylogenetic tree of the complete fusion gene (1662nt). a Phylogenetic tree of Nigerian isolates and other published West African NDV sequences. b Phylogenetic tree of NDV genotypes reported in Nigeria with the exception of genotype II (No complete fusion gene available in the GenBank from Nigeria). Sequences from the GenBank are indicated by their accession numbers. The two genotype IV sequences are yet to be deposited in the sequence repository. The evolutionary history was inferred by using the Maximum Likelihood method based on the Kimura 2-parameter model [105]. The tree with the highest log likelihood (−10,045.3536) is shown. Codon positions included were 1st + 2nd + 3rd + Noncoding. All positions containing gaps and missing data were eliminated. At the nodes, only bootstrap values greater than 70 % are shown. Scale bar represents number of substitutions per site
Globally, genotype VII has been reported to be responsible for outbreaks in other countries of the world and current panzootic [66] with no report yet in West Africa. Although, the presence of the genotype has been established elsewhere in Africa, namely; Egypt [88], Libya [51], Sudan [42], South Africa [1], Mozambique [35], and Ethiopia [33].
Challenges and way forward
Prompt diagnosis is the hallmark of disease prevention and control. Unfortunately, as observed in the literature reviewed, most prevalence reports on ND diagnosis in Nigeria have been based on clinical signs and post mortem lesions [57, 91]. The diagnoses are rarely confirmed using laboratory-based diagnostic methods (virus isolation and pathotyping) knowing well that NDV infection is not pathognomonic; this will present unreliable results as revealed by our laboratory data (Shittu et al. 2016 unpublished data). Enzootic status of ND can be upheld based on the continuous circulation of velogenic strains of different genotypes reported to be in circulation in both apparently healthy, wild bird and sick/dead birds in live bird markets, free-range and commercial poultry [19, 98, 99, 102, 108]. As of 30th May 2016, only 8 out of the 18 countries constituting the West Africa sub-region have sequences of NDV in the sequence repositories. In contrast to thousands of available sequences of genotype VII, which have been identified as responsible for the fourth ND panzootic [67]. Having more sequences will aid in understanding the evolutionary dynamics of the circulating strains which will help in the design of effective control of the disease. It is interesting to note that, no reports of genotype VII have been documented in West Africa in spite of the transborder movements of poultry and poultry products which take place legally and illegally across the regions and continents. In addition to migration of wild birds which have been identified as possible sources of introduction of avian influenza [36]. The none detection of this genotype could be as a result of poor surveillance system which exists in Nigeria as well as the sub-region as a whole. Initially, due to the genetic relatedness of the currently circulating genotypes indigenous to West Africa, earlier studies on NDV genotyping in West Africa, grouped the viruses to lineage 5 and 7 (genotype XIV and VII) [19, 94, 99]. The classification was later streamlined to genotypes XIV, XVII, and XVII with the existence of the unified nomenclature for the classification of NDV as proposed by Diel et al. [25] and expanded by Snoeck et al. [101]. Movements of poultry and poultry products within and outside states and the sub-region have assisted in the spread of the disease. This is evident from the genetic relatedness shared among the isolates from different locations across the country. Solomon et al. [103] reported sequence similarities of 99.3–100 % among NDV isolates from within and outside Nigeria. Surveillance activities are to help in early detection and the establishment of effective preventive and control strategy. It is obvious that surveillance for infectious diseases has been lacking which has favoured the spread of these genotypes across many countries in the sub-region largely due to trade and movement of poultry and poultry products.
Concluding remarks
Since the identification of novel genotypes of ND from some West and Central Africa poultry population [19, 99] information on the circulating genotypes has increased. Though this information is still limited, it however provides insight into the diversity of ND across the sub-region. For effective control strategy to be implemented, identifying the various genotypes in circulation is the first step in achieving the desired goals. The current vaccination program in Nigeria and the sub-region at large needs to be reviewed to accommodate the current circulating genotypes. Though, few studies have been carried out to assess the protection level of the currently circulating NDV genotypes (XIV and XVII) [94, 104] with the exception of genotype XVIII against Lasota and V4 vaccine strains. The vaccination regimen in Nigeria includes the use of Komarov (Table 1) for the initially primed chicks at 7–10 weeks of age. The protection level of this vaccination schedule should be assessed with the circulating strains. In addition, the clamour for a genotype-matched vaccine has been in the news lately with several successes recorded using genotype VII [46, 53, 111]. It may therefore be imperative to develop a genotype-matched vaccine against the predominant genotype in West Africa to ameliorate the vaccination program.
Acknowledgments
This work was part of the PhD study of I.S. The authors acknowledge the support and co-operation of the management of the National Veterinary Research Institute, Vom, Nigeria. We appreciate the comments and critical reading of the manuscript by Dr. Claudio L. Afonso.
References
- 1.Abolnik C, Horner RF, Bisschop SPR, Parker ME, Romito M, Viljoen GJ. A phylogenetic study of South African Newcastle disease virus strains isolated between 1990 and 2002 suggests epidemiological origins in the Far East. Arch Virol. 2004;149:603–619. doi: 10.1007/s00705-003-0218-2. [DOI] [PubMed] [Google Scholar]
- 2.Abraham-Oyiguh J, Sulaiman LK, Meseko CA, Ismail S, Suleiman I, Ahmed SJ, et al. Prevalence of Newcastle disease antibodies in local chicken in federal capital territory, Abuja, Nigeria. Int Sch Res Not. 2014;2014:1–3. doi: 10.1155/2014/796148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adene D, Oguntade A. The structure and importance of the commercial and village based poultry industry in Nigeria. Nigerian Poultry Sector Report. Rome; 2006. http://www.fao.org/docs/eims/upload//214281/ReviewNigeria.
- 4.Adeyemo AA, Onikoyi MP. Prospects and challenges of large scale commercial poultry production in Nigeria. Agric J. 2012;7:388–393. [Google Scholar]
- 5.Adu FD, Oyejide O, Ikede BO. Characterization of Nigerian strains of Newcastle disease virus. Avian Dis. 1985;29:829–831. doi: 10.2307/1590674. [DOI] [PubMed] [Google Scholar]
- 6.Adu FD, Edo U, Sokale B. Newcastle disease: the immunological status of Nigerian chickens. Trop Vet. 1986;24:149–152. [Google Scholar]
- 7.Afonso CL, Amarasinghe GK, Bányai K, Bào Y, Basler CF, Bavari S, et al. Taxonomy of the order Mononegavirales: update 2016. Arch Virol. 2016;161:2351–2360. doi: 10.1007/s00705-016-2880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldous EW, Alexander DJ. Newcastle disease in pheasants (Phasianus colchicus): a review. Vet J. 2008;175:181–185. doi: 10.1016/j.tvjl.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander DJ. Newcastle disease. Br Poult Sci. 2001;42:5–22. doi: 10.1080/713655022. [DOI] [PubMed] [Google Scholar]
- 10.Alexander DJ. Newcastle disease. In: Saif YM, Barnes HJ, Fadly AM, Glisson JR, McDougald LR, Swayne DE, editors. Diseases of poultry. 11. Ames: Iowa State University Press; 2003. pp. 64–87. [Google Scholar]
- 11.Ambali AG, Mamman AS, Abubakar MB. Sero-prevalence study of Newcastle disease in feral and domestic pigeons in semi-arid zone of Borno State, Nigeria. Sokoto J Vet Sci. 2002;4:30–32. [Google Scholar]
- 12.Ameji ON, Sa`idu L, Abdu PA. Newcastle disease antibodies in apparently healthy wild birds in Kogi State, Nigeria. Res J Vet Sci. 2015;8:52–60. doi: 10.3923/rjvs.2015.52.60. [DOI] [Google Scholar]
- 13.Anzaku S, Jarlath U, Abdu P. Participatory epidemiological investigation of Newcastle disease in local chickens in the Federal Capital Territory, Nigeria U UJ editor. Int J Livest Res. 2014;2:1. doi: 10.5455/ijlr.20140629095805. [DOI] [Google Scholar]
- 14.Anzaku SA, Umoh JU, Abdu PA, Kabir J, Bala A. Serological study of Newcastle disease in local chickens in the federal capital territory, Abuja, Nigeria. Sci J Vet Adv. 2014;3:101–103. [Google Scholar]
- 15.Balami AG, Ndahi J, Zaifada A, Mustapha M, Jarafu D, Asogwa N, et al. A retrospective study of poultry diseases diagnosed in Maiduguri, NorthEast, Nigeria. Poult Fish Wildl Sci. 2014;2:113. doi: 10.4172/2375-446X.1000113. [DOI] [Google Scholar]
- 16.Balarabe RM, Ejiofor C. The prospects and limitations of Japanese quail (Coturnix coturnix japonica) production in Nigeria: a review. Int J Multidiscip Curr Res. 2015;3:920–926. [Google Scholar]
- 17.Briand FF-X, Henry A, Brown P, Massin P, Jestin V. Complete genome sequence of a Newcastle disease virus strain belonging to a recently identified genotype. Genome Announc. 2013;1:2009–2010. doi: 10.1128/genomeA.00100-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byarugaba DK, Mugimba KK, Omony JB, Okitwi M, Wanyana A, Otim MO, et al. High pathogenicity and low genetic evolution of avian paramyxovirus type I (Newcastle disease virus) isolated from live bird markets in Uganda. Virol J. 2014;11:173. doi: 10.1186/1743-422X-11-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cattoli G, Fusaro A, Monne I, Molia S, Le Menach A, Maregeya B, et al. Emergence of a new genetic lineage of Newcastle disease virus in West and Central Africa—implications for diagnosis and control. Vet Microbiol. 2010;142:168–176. doi: 10.1016/j.vetmic.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 20.Chellappa MM, Dey S, Gaikwad S, Kataria JM, Vakharia VN. Complete genome sequence of newcastle disease virus mesogenic vaccine strain R2B from India. J Virol. 2012;86:13814–13815. doi: 10.1128/JVI.02552-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czeglédi A, Ujvári D, Somogyi E, Wehmann E, Werner O, Lomniczi B. Third genome size category of avian paramyxovirus serotype 1 (Newcastle disease virus) and evolutionary implications. Virus Res. 2006;120:36–48. doi: 10.1016/j.virusres.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 22.de Almeida RS, Hammoumi S, Gil P, Briand F-X, Molia S, Gaidet N, et al. New Avian paramyxoviruses type I strains identified in Africa provide new outcomes for phylogeny reconstruction and genotype classification. PLoS One. 2013;8:e76413. doi: 10.1371/journal.pone.0076413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Leeuw OS. Virulence of Newcastle disease virus is determined by the cleavage site of the fusion protein and by both the stem region and globular head of the haemagglutinin-neuraminidase protein. J Gen Virol. 2005;86:1759–1769. doi: 10.1099/vir.0.80822-0. [DOI] [PubMed] [Google Scholar]
- 24.de Leeuw O, Peeters B. Complete nucleotide sequence of Newcastle disease virus: evidence for the existence of a new genus within the subfamily Paramyxovirinae. J Gen Virol. 1999;80:131–136. doi: 10.1099/0022-1317-80-1-131. [DOI] [PubMed] [Google Scholar]
- 25.Diel DG, da Silva LHA, Liu H, Wang Z, Miller PJ, Afonso CL. Genetic diversity of avian paramyxovirus type 1: proposal for a unified nomenclature and classification system of Newcastle disease virus genotypes. Infect Genet Evol. 2012;12:1770–1779. doi: 10.1016/j.meegid.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Doyle T. A hitherto unrecorded disease of fowls due to a filter-passing virus. J Comp Pathol Ther. 1927;40:144–169. [Google Scholar]
- 27.Ducatez MF, Olinger CM, Owoade AA, Tarnagda Z, Tahita MC, Sow A, et al. Molecular and antigenic evolution and geographical spread of H5N1 highly pathogenic avian influenza viruses in western Africa. J Gen Virol. 2007;88:2297–2306. doi: 10.1099/vir.0.82939-0. [DOI] [PubMed] [Google Scholar]
- 28.Echeonwu GON, Iroegbu CU, Emeruwa AC. Recovery of velogenic Newcastle disease virus from dead and healthy free-roaming birds in Nigeria. Avian Pathol. 1993;22:383–387. doi: 10.1080/03079459308418928. [DOI] [PubMed] [Google Scholar]
- 29.Fadiga M, Jost C, Ihedioha J. Financial costs of disease burden, morbidity and mortality from priority livestock diseases in Nigeria. Disease burden and cost–benefit analysis of targeted interventions. ILRI Res. Rep. Nairobi, Kenya; 2013. p. 1–84. https://cgspace.cgiar.org/bitstream/handle/10568/33418/ResearchReport_33.pdf?sequence=2.
- 30.Fagbohun OA, Oluwayelu DO, Owoade AA, Olayemi FO. Survey for antibodies to Newcastle disease virus in Cattle Egrets, Pigeons and Nigerian Laughing Doves. Afr J Biomed Res. 2000;3:193–194. [Google Scholar]
- 31.Fatumbi O, Adene D. Susceptibility of the Nigerian local chickens to a fulminating Newcastle disease outbreak. Niger Vet J. 1979;8:30–32. [Google Scholar]
- 32.Fatunmbi OO. Infectious sinusitis in turkeys at Ibadan, Nigeria. Avian Dis. 1984;28:734–736. doi: 10.2307/1590242. [DOI] [PubMed] [Google Scholar]
- 33.Fentie T, Heidari A, Aiello R, Kassa T, Capua I, Cattoli G, et al. Molecular characterization of Newcastle disease viruses isolated from rural chicken in northwest Ethiopia reveals the circulation of three distinct genotypes in the country. Trop Anim Health Prod. 2014;46:299–304. doi: 10.1007/s11250-013-0487-z. [DOI] [PubMed] [Google Scholar]
- 34.Fernandes CC, Varani AM, Lemos EGM, de Miranda VFO, Silva KR, Fernando FS, et al. Molecular and phylogenetic characterization based on the complete genome of a virulent pathotype of Newcastle disease virus isolated in the 1970s in Brazil. Infect Genet Evol. 2014;26:160–167. doi: 10.1016/j.meegid.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Fringe R, Bosman A, Ebersohn K, Bisschop S, Abolnik C, Venter E. Molecular characterisation of Newcastle disease virus isolates from different geographical regions in Mozambique in 2005. Onderstepoort J Vet Res. 2012;79:E1–E7. doi: 10.4102/ojvr.v79i1.409. [DOI] [PubMed] [Google Scholar]
- 36.Fusaro A, Nelson MI, Joannis T, Bertolotti L, Monne I, Salviato A, et al. Evolutionary Dynamics of Multiple Sublineages of H5N1 Influenza Viruses in Nigeria from 2006 to 2008. J Virol. 2010;84:3239–3247. doi: 10.1128/JVI.02385-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganar K, Das M, Sinha S, Kumar S. Newcastle disease virus: current status and our understanding. Virus Res. 2014;184:71–81. doi: 10.1016/j.virusres.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gardner E, Alders R. livestock risks and opportunities: Newcastle disease and Avian influenza in Africa. Planet@Risk [Internet]. Davos: Global Risk Forum GRF Davos; 2014. p. 208–11. http://vet.tufts.edu/wp-content/uploads/Gardner-MCM-case-study-pub-article.pdf.
- 39.Geidam YA, Ayi VK, Umar II, Sunday J, Musa D, Goni B, et al. Participatory disease surveillance in the detection of trans-boundary animal diseases (TADS) in Borno State of arid north-eastern Nigeria. Bull Anim Heal Prod Africa. 2013;61:231–239. [Google Scholar]
- 40.Haruna ES, Shamaki D, Echeonwu GO, Majiyagbe KA, Shuaibu Y, Du DR. A natural outbreak of Newcastle disease in guinea-fowl (Numida meleagris galeata) in Nigeria. Rev Sci Tech. 1993;12:887–893. doi: 10.20506/rst.12.3.731. [DOI] [PubMed] [Google Scholar]
- 41.Haruna ES, Musa U, Lombin LH, Tat PB, Shamaki D, Okewole P, et al. Introduction of quail production in Nigeria. Niger Vet J. 1997;18:104–107. [Google Scholar]
- 42.Hassan W, Khair SAM, Mochotlhoane B, Abolnik C. Newcastle disease outbreaks in the Sudan from 2003 to 2006 were caused by viruses of genotype 5d. Virus Genes. 2010;40:106–110. doi: 10.1007/s11262-009-0424-4. [DOI] [PubMed] [Google Scholar]
- 43.Hassan DI, Yusuf ND, Musa-Azara IS, Ari MM, Ogah D, Alaga A, et al. Prevalence of newcastle disease in village chickens reared in Lafia, Nasarawa State, Nigeria. Egypt Poult Sci. 2013;33:135–142. [Google Scholar]
- 44.Hill HD, Davis OS, Wilde JE. Newcastle disease in Nigeria. Br Vet J. 1953;109:381–385. [Google Scholar]
- 45.Hines NL, Miller CL. Avian paramyxovirus serotype-1: a review of disease distribution, clinical symptoms, and laboratory diagnostics. Vet Med Int. 2012;2012:1–17. doi: 10.1155/2012/708216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu S, Ma H, Wu Y, Liu W, Wang X, Liu Y, et al. A vaccine candidate of attenuated genotype VII Newcastle disease virus generated by reverse genetics. Vaccine. 2009;27:904–910. doi: 10.1016/j.vaccine.2008.11.091. [DOI] [PubMed] [Google Scholar]
- 47.Huang Z, Panda A, Elankumaran S, Govindarajan D, Rockemann DD, Samal SK. The hemagglutinin-neuraminidase protein of Newcastle disease virus determines tropism and virulence. J Virol. 2004;78:4176–4184. doi: 10.1128/JVI.78.8.4176-4184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ibu J, Okoye J, Adulugba E, Chah K, Shoyinka S, Salihu E, et al. Prevalence of Newcastle disease virus in wild and captive birds in Central Nigeria. Int J Poult Sci. 2009;8:574–578. doi: 10.3923/ijps.2009.574.578. [DOI] [Google Scholar]
- 49.Jibril AH, Umoh JU, Kabir J, Saidu L, Magaji AA, Bello MB, et al. Newcastle disease in local chickens of live bird markets and households in Zamfara State, Nigeria. ISRN Epidemiol. 2014;2014:1–4. doi: 10.1155/2014/513961. [DOI] [Google Scholar]
- 50.Joannis T, Lombin LH, De Benedictis P, Cattoli G, Capua I. Confirmation of H5N1 avian influenza in Africa. Vet Rec. 2006;158:309–310. doi: 10.1136/vr.158.9.309-b. [DOI] [PubMed] [Google Scholar]
- 51.Kammon A, Heidari A, Dayhum A, Eldaghayes I, Sharif M, Monne I, et al. Characterization of avian influenza and Newcastle disease viruses from poultry in Libya. Avian Dis. 2015;59:422–430. doi: 10.1637/11068-032215-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 52.Kim S-H, Nayak S, Paldurai A, Nayak B, Samuel A, Aplogan GL, et al. Complete genome sequence of a novel Newcastle disease virus strain isolated from a chicken in West Africa. J Virol. 2012;86:11394–11395. doi: 10.1128/JVI.01922-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim S-H, Wanasen N, Paldurai A, Xiao S, Collins PL, Samal SK. Newcastle disease virus fusion protein is the major contributor to protective immunity of genotype-matched vaccine. PLoS One. 2013;8:e74022. doi: 10.1371/journal.pone.0074022. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Kolakofsky D, Pelet T, Garcin D, Hausmann S, Curran J, Roux L. Paramyxovirus RNA synthesis and the requirement for hexamer genome length: the rule of six revisited. J Virol. 1998;72:891–899. doi: 10.1128/jvi.72.2.891-899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kraneveld FC. A poultry disease in the Dutch East Indies. Ned Indisch Bl voor Diergeneeskd. 1926;38:448–450. [Google Scholar]
- 56.Lamb RA, Parks GD. Paramyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields virol. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1450–1496. [Google Scholar]
- 57.Lawal J, Jajere S, Mustapha M, Bello A, Wakil Y, Geidam Y, et al. Prevalence of Newcastle disease in Gombe, Northeastern Nigeria: a ten-year retrospective study (2004–2013) Br Microbiol Res J. 2015;6:367–375. doi: 10.9734/BMRJ/2015/15955. [DOI] [Google Scholar]
- 58.Liu H, Wang Z, Wang Y, Sun C, Zheng D, Wu Y. Characterization of Newcastle disease virus isolated from waterfowl in China. Avian Dis. 2008;52:150–155. doi: 10.1637/8030-061507-Reg. [DOI] [PubMed] [Google Scholar]
- 59.Liu H, Chen F, Zhao Y, Zheng D, Li J, Xu T, et al. Genomic characterization of the first class I Newcastle disease virus isolated from the mainland of China. Virus Genes. 2010;40:365–371. doi: 10.1007/s11262-010-0452-0. [DOI] [PubMed] [Google Scholar]
- 60.Macpherson LW. Some observations on the epizootiology of newcastle disease. Can J Comp Med Vet Sci. 1956;20:155–168. [PMC free article] [PubMed] [Google Scholar]
- 61.Mai HM, Ogunsola OD, Obasi OL. Serological survey of the Newcastle disease and infectious bursal disease in local ducks and local guinea fowls in Jos, Plateau State, Nigeria. Rev Elev Med Vet Pays Trop. 2004;57:41–44. [Google Scholar]
- 62.Majiyagbe K, Nawathe D. Isolation of virulent Newcastle disease virus from apparently normal ducks in Nigeria. Vet Rec. 1981;108:190. doi: 10.1136/vr.108.9.190. [DOI] [PubMed] [Google Scholar]
- 63.Maminiaina OF, Gil P, Briand F-X, Albina E, Keita D, Andriamanivo HR, et al. Newcastle disease virus in madagascar: identification of an original genotype possibly deriving from a died out ancestor of genotype IV. PLoS One. 2010;5:e13987. doi: 10.1371/journal.pone.0013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manchang T, Abdu P, Saidu L. Epidemiology and clinicopathologic manifestations of Newcastle disease. Pathol Infect. 2004;57:35–39. [Google Scholar]
- 65.Miller PJ, Afonso CL, Spackman E, Scott MA, Pedersen JC, Senne DA, et al. Evidence for a New avian paramyxovirus serotype 10 detected in rockhopper penguins from the Falkland Islands. J Virol. 2010;84:11496–11504. doi: 10.1128/JVI.00822-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller PJ, Decanini EL, Afonso CL. Newcastle disease: evolution of genotypes and the related diagnostic challenges. Infect Genet Evol. 2010;10:26–35. doi: 10.1016/j.meegid.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 67.Miller PJ, Haddas R, Simanov L, Lublin A, Rehmani SF, Wajid A, et al. Identification of new sub-genotypes of virulent Newcastle disease virus with potential panzootic features. Infect Genet Evol. 2015;29:216–229. doi: 10.1016/j.meegid.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 68.Musa U, Abdu P, Mera U, Emmenna P, Ahmed M. Vaccination with newcastle disease vaccines strain I2 and Lasota in commercial and local chickens in Plateau State Nigeria. Niger Vet J. 2010; 31.
- 69.Musa U, Abdu PA, Dafwang II, Umoh JU, Saidu L, Mera UM, et al. Seroprevalence, seasonal occurrence and clinical manifestation of Newcastle disease in rural household chickens in Plateau State, Nigeria. Int J Poult Sci. 2009;8:200–204. doi: 10.3923/ijps.2009.200.204. [DOI] [Google Scholar]
- 70.Nawathe DR, Majiyagbe KA, Ayoola SO. Characterization of Newcastle disease virus isolates from Nigeria. Bull Int Epizoot. 1975;83:11–12. [Google Scholar]
- 71.Nwankiti O, Ejekwolu A, Ibrahim I, Ndako J, Echeonwu G. Detection of serum antibody levels against Newcastle Disease in local chickens in Bauchi metropolis, Bauchi State, Nigeria. Afr J Clin Exp Microbiol. 2010;11:95–101. [Google Scholar]
- 72.Nwanta JA, Abdu PA, Ezema W. Epidemiology, challenges and prospects for control of Newcastle disease in village poultry in Nigeria. Worlds Poult Sci J. 2008;64:119–127. doi: 10.1017/S0043933907001766. [DOI] [Google Scholar]
- 73.Nwanta JA, Egege SC, Alli-Balogun JK, Ezema W. Evaluation of prevalence and seasonality of Newcastle disease in chicken in Kaduna, Nigeria. Worlds Poult Sci J. 2008;64:416–423. doi: 10.1017/S0043933908000147. [DOI] [Google Scholar]
- 74.Ojeh CK, Okoro HO. Isolation and characterisation of Newcastle disease virus strain in a feral dove (Stigmatopelia senegalensis) in Nigeria. Trop Anim Health Prod. 1992;24:211–215. doi: 10.1007/BF02356749. [DOI] [PubMed] [Google Scholar]
- 75.Okoh AEJ. Newcastle disease in Falcons. J Wildl Dis. 1979;15:479–480. doi: 10.7589/0090-3558-15.3.479. [DOI] [PubMed] [Google Scholar]
- 76.Okoye JO, Uzoukwu M. An outbreak of infectious bursal disease among chickens between 16 and 20 weeks old. Avian Dis. 1981;25:1034–1038. doi: 10.2307/1590079. [DOI] [PubMed] [Google Scholar]
- 77.Okwor EC, Eze DC. The annual prevalence of Newcastle disease in commercial chickens reared in South Eastern Savannah Zone of Nigeria. Res J Poult Sci. 2010;3:23–26. doi: 10.3923/rjpscience.2010.23.26. [DOI] [Google Scholar]
- 78.Olabode AO, Ndako JA, Echeonwu GO, Nwankiti OO, Chukwuedo AA. Use of cracked maize as a carrier for NDV4 vaccine in experimental vaccination of chickens. Virol J. 2010;7:67. doi: 10.1186/1743-422X-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Olabode HO, Damina MS, Ahmed AS, Moses GD, Wungak Y. Retrospective study of newcastle disease in commercial poultry farms in Ilorin, Kwara State-Nigeria. Vom J Vet Sci. 2012;9:66–70. [Google Scholar]
- 80.Oladele SB, Enoch I, Ibu OJ. Clinico-pathological features of Newcastle disease in Japanese quails (Coturnix coturnix japonica) infected with Newcastle disease virus Kudu 113 strain. Int J Poult Sci. 2008;7:165–168. doi: 10.3923/ijps.2008.165.168. [DOI] [Google Scholar]
- 81.Oluwayelu DO, Emikpe BO, Fagbohun OA, Ohore OG. Prevalence of antibodies to three avian viral diseases in guineafowls in Ibadan, Nigeria. Bull Anim Heal Prod Africa. 2005;53:274–276. [Google Scholar]
- 82.Onunkwo O, Momoh MA. Characterization of a Newcastle disease virus isolated from a parrot (Psittacus erythracus) in Nigeria. J Wildl Dis. 1981;17:463–465. doi: 10.7589/0090-3558-17.3.463. [DOI] [PubMed] [Google Scholar]
- 83.Oranusi NA, Onyekaba CO. Serological study of Newcastle in poultry farm in Niger Delta Nigeria. Bull Anim Heal Prod Africa. 1986;34:290–292. [Google Scholar]
- 84.Owoade AA, Ducatez MF, Muller CP. Seroprevalence of avian influenza virus, infectious bronchitis virus, reovirus, avian pneumovirus, infectious laryngotracheitis virus, and avian leukosis virus in Nigerian poultry. Avian Dis. 2006;50:222–227. doi: 10.1637/7412-071505R.1. [DOI] [PubMed] [Google Scholar]
- 85.Oyekunle MA, Talabi AO, Okeowo AO. Serological status for Newcastle disease virus in unvaccinated indigenous chickens in Yewa division of Ogun State, Nigeria. Int J Poult Sci. 2006;5:1119–1122. doi: 10.3923/ijps.2006.1119.1122. [DOI] [Google Scholar]
- 86.Panda A, Huang Z, Elankumaran S, Rockemann DD, Samal SK. Role of fusion protein cleavage site in the virulence of Newcastle disease virus. Microb Pathog. 2004;36:1–10. doi: 10.1016/j.micpath.2003.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Perroncito CE. Typhoid epizootic in gallinaceous birds [it. Epizoozia tifoide nei gallinacei. Torino] Ann Accad Agric. 1878;21:87–126. [Google Scholar]
- 88.Radwan MM, Darwish SF, El-Sabagh IM, El-Sanousi AA, Shalaby MA. Isolation and molecular characterization of Newcastle disease virus genotypes II and VIId in Egypt between 2011 and 2012. Virus Genes. 2013;47:311–316. doi: 10.1007/s11262-013-0950-y. [DOI] [PubMed] [Google Scholar]
- 89.Sa’idu L, Tekdek LB, Abdu PA. Prevalence of Newcastle disease antibodies in domestic and semi-domestic birds in Zaria Nigeria. Vet Arh. 2004;74:309–317. [Google Scholar]
- 90.Sa’idu L, Abdu PA, Tekdek LB, Umoh JU, et al. Retrospective study of Newcastle disease cases in Zaria, Nigeria. Niger Vet J. 2006;27:53–62. [Google Scholar]
- 91.Sadiq MA, Nwanta JA, Okolocha EC, Tijjani AN. Retrospective (2000–2009) study of Newcastle disease (ND) cases in Avian species in Maiduguri, Borno State, North Eastern Nigeria. Int J Poult Sci. 2011;10:76–81. doi: 10.3923/ijps.2011.76.81. [DOI] [Google Scholar]
- 92.Salihu AE, Chukwuedo AA, Echeonwu GON, Ibu JO, Chukwuekezie JO, Ndako J, et al. Seroprevalence of newcastle disease virus infection in rural household birds in Lafia, Akwanga and Keffi Metropolis, Nasarawa State Nigeria. Int J Agric Sci. 2012;2:109–112. [Google Scholar]
- 93.Samal SK. Newcastle disease and related avian paramyxoviruses. In: Samal SK, editor. Biology of paramyxoviruses. Norfolk: Caister Academic Press; 2011. pp. 69–114. [Google Scholar]
- 94.Samuel A, Nayak B, Paldurai A, Xiao S, Aplogan GL, Awoume KA, et al. Phylogenetic and pathotypic characterization of Newcastle Disease viruses circulating in West Africa and efficacy of a current vaccine. J Clin Microbiol. 2013;51:771–781. doi: 10.1128/JCM.02750-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Servan de Almeida R, Maminiaina OF, Gil P, Hammoumi S, Molia S, Chevalier V, et al. Africa, a reservoir of new virulent strains of Newcastle disease virus? Vaccine. 2009;27:3127–3129. doi: 10.1016/j.vaccine.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 96.Shittu I, Joannis TM, Odaibo GN, Olaleye DO. Epizootiology of Newcastle disease in two live bird markets in Ibadan, South Western Nigeria. Bull Anim Heal Prod Africa. 2015;63:249–255. [Google Scholar]
- 97.Shittu I, Sharma P, Joannis TM, Volkening JD, Odaibo GN, Olaleye DO, et al. Complete genome sequence of a genotype XVII Newcastle disease virus, isolated from an apparently healthy domestic duck in Nigeria. Genome Announc. 2016;4:e01716-15. doi: 10.1128/genomeA.01716-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shittu I, Sharma P, Volkening JD, Solomon P, Sulaiman LK, Joannis TM, et al. Identification and complete genome sequence analysis of a genotype XIV Newcastle disease Virus from Nigeria. Genome Announc. 2016;4:e01581-15. doi: 10.1128/genomeA.01581-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Snoeck CJ, Ducatez MF, Owoade AA, Faleke OO, Alkali BR, Tahita MC, et al. Newcastle disease virus in West Africa: new virulent strains identified in non-commercial farms. Arch Virol. 2009;154:47–54. doi: 10.1007/s00705-008-0269-5. [DOI] [PubMed] [Google Scholar]
- 100.Snoeck CJ, Adeyanju AT, Owoade AA, Couacy-Hymann E, Alkali BR, Ottosson U, et al. Genetic diversity of Newcastle disease virus in wild birds and pigeons in West Africa. Appl Environ Microbiol. 2013;79:7867–7874. doi: 10.1128/AEM.02716-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Snoeck CJ, Owoade A, Couacy-Hymann E, Alkali BR, Okwen MP, Adeyanju AT, et al. High genetic diversity of Newcastle disease virus in poultry in West and Central Africa: cocirculation of genotype XIV and newly defined genotypes XVII and XVIII. J Clin Microbiol. 2013;51:2250–2260. doi: 10.1128/JCM.00684-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Solomon P, Abolnik C, Joannis TM, Bisschop S. Virulent Newcastle disease virus in Nigeria: identification of a new clade of sub-lineage 5f from livebird markets. Virus Genes. 2012;44:98–103. doi: 10.1007/s11262-011-0678-5. [DOI] [PubMed] [Google Scholar]
- 103.Solomon P, Bisschop S, Joannis TM, Shittu I, Meseko C, Sulaiman L, et al. Phylogenetic analysis of Newcastle disease viruses isolated from asymptomatic guinea fowls (Numida meleagris) and Muscovy ducks (Cariana moscata) in Nigeria. Trop Anim Health Prod. 2012;45:53–57. doi: 10.1007/s11250-012-0173-6. [DOI] [PubMed] [Google Scholar]
- 104.Susta L, Jones MEB, Cattoli G, Cardenas-Garcia S, Miller PJ, Brown CC, et al. Pathologic characterization of genotypes XIV and XVII Newcastle disease viruses and efficacy of classical vaccination on specific pathogen-free birds. Vet Pathol. 2015;52:120–131. doi: 10.1177/0300985814521247. [DOI] [PubMed] [Google Scholar]
- 105.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Terregino C, Aldous EW, Heidari A, Fuller CM, De Nardi R, Manvell RJ, et al. Antigenic and genetic analyses of isolate APMV/wigeon/Italy/3920-1/2005 indicate that it represents a new avian paramyxovirus (APMV-12) Arch Virol. 2013;158:2233–2243. doi: 10.1007/s00705-013-1735-2. [DOI] [PubMed] [Google Scholar]
- 107.Uthrakumar A, Vijayarani K, Kumanan K, Bhuvaneswari S, Kuchipudi SV, Elankumaran S. Complete genome sequence of a velogenic Newcastle disease virus isolated from an apparently healthy village chicken in South India. Genome Announc. 2014;2:e00597-14. doi: 10.1128/genomeA.00597-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Van Borm S, Obishakin E, Joannis T, Lambrecht B, van den Berg T. Further evidence for the widespread co-circulation of lineages 4b and 7 velogenic Newcastle disease viruses in rural Nigeria. Avian Pathol. 2012;41:377–382. doi: 10.1080/03079457.2012.696311. [DOI] [PubMed] [Google Scholar]
- 109.Wakawa AM, Abdu PA, Umoh JU, Lawal S, Miko RB. Serological evidence of mixed infections with avian influenza and Newcastle disease in village chickens in Jigawa State, Nigeria. Vet Arh. 2009;79:151–155. [Google Scholar]
- 110.Wakawa AM, Waziri MI, Aliyu HB, Talba AM, Sa’idu L, Abdu PA. Retrospective study of some viral poultry diseases diagnosed in Nigeria. Int J Basic Appl Virol. 2014;3:16–21. [Google Scholar]
- 111.Xiao S, Nayak B, Samuel A, Paldurai A, Kanabagattebasavarajappa M, Prajitno TY, et al. Generation by reverse genetics of an effective, stable, live-attenuated newcastle disease virus vaccine based on a currently circulating, highly virulent Indonesian strain. PLoS One. 2012;7:e52751. doi: 10.1371/journal.pone.0052751. [DOI] [PMC free article] [PubMed] [Google Scholar]