Abstract
AITL often shows systemic symptoms related to immune dysregulation and cytokine production. Biopsy harbors few malignant cells in abundant reactive background, diagnostically challenging in small biopsies. This study was to assess the value of flow cytometry (FC) and to determine the immunophenotypic alterations in 144 samples from 38 patients. FC detected an aberrant T-cell population in 97/144 samples that represented 0.5-90% of lymphocytes. Blood was involved in 11/16 patients. The most frequent immunophenotypic aberrancies included loss of CD3; altered T-cell receptor expression and aberrant CD10 expression. Altered CD3 expression was more frequently seen in PB and BM whereas aberrant CD10 expression was more common in LN. AITL cells often exhibit abnormal CD4+ immunophenotype with diminished or absent CD3 and variable CD10 expression. Multiparameter FC is an effective tool for supporting the diagnosis of AITL in any fluid and tissue specimens.
Keywords: AITL, angioimmunoblastic, T-cell lymphoma, flow cytometry, CD3, CD10, TCR, CHOP
Introduction
Angioimmunoblastic T-cell lymphoma (AITL) accounts for 15-20 % of peripheral T-cell lymphomas (PTCL) and 1-2% of all non-Hodgkin lymphomas [1]. Patients usually present with advanced stage disease and systemic symptoms related to immune dysregulation and cytokine production including autoimmune hemolytic anemia, thrombocytopenia, polyclonal hypergammaglobulinemia, hypereosinophilia, skin rash, body cavity effusions and arthralgias [2]. The prognosis of patients with AITL generally remains poor.
Histologically, the malignant cells of AITL are present in polymorphous inflammatory cell background composed of reactive lymphocytes, histiocytes, eosinophils, plasma cells, reactive B-cells and immunoblasts. Proliferation of high endothelial venules and follicular dendritic cells are characteristic features of AITL [1]. The diagnosis of AITL can be challenging in excisional biopsy specimens and a definitive diagnosis often requires an integrated approach incorporating comprehensive clinical, histopathologic, immunophenotypic and/or molecular data. The increasing trend towards using small-gauge needle core biopsy specimens to diagnose AITL further add to the diagnostic challenge and use additional ancillary testing, such as flow cytometry immunophenotyping (FCI) analysis, may be more greatly depended upon to accurately diagnose AITL.
Gene expression profiling studies of AITL cases have provided evidence that the lymphoma cells are of follicular helper T-cell (TFH) origin [3,4]. As such, AITL has been shown to express TFH-associated antigens including CD10, BCL6, CXCL13, ICOS, and PD1 by immunohistochemical and flow cytometry immunophenotyping (FCI) methods. [3,5-11] In particular, CD10 expression by FCI analysis has been suggested as a highly sensitive, but less specific marker when distinguishing AITL from other forms of PTCL. [7] In addition, circulating CD3-/CD4+ T-cells have been described in the setting of AITL [12] and more recently, Singh and colleagues compared AITL with other forms of leukemic CD4+ PTCL; they showed that CD3-/CD4+ circulating T-cells were present in the peripheral blood of all patients with AITL whereas circulating cells with this aberrant immunophenotype were observed in only 2.5 % of patients with other forms of peripheral T-cell lymphoma [13]. In the current study we assessed the utility of flow cytometry immunophenotyping (FCI) as an adjunct to aid in establishing the diagnosis of AITL. We also evaluated the spectrum of immunophenotypic alterations in AITL across different tissue types including bone marrow (BM), lymph node (LN), peripheral blood (PB), and fluid specimens in a given patient.
Materials and Methods
Patient selection
This study was approved by the Institutional Review Board of The University of Texas M.D. Anderson Cancer Center and performed in accordance with the Declaration of Helsinki. We searched the archives of our department for specimens from patients with AITL on which FCI analysis was performed. Every patient had at least one biopsy specimen with unequivocal involvement by AITL based on histologic evaluation and the results of ancillary studies according to the 2008 World Health Organization classification [1].
Histopathologic evaluation
We reviewed all available diagnostic samples and confirmed the diagnosis of AITL in all patients. Hematoxylin and eosin-stained formalin-fixed, paraffin-embedded tissue and/or BM trephine biopsy specimens, Wright-Giemsa stained BM aspirate smears, and Papanicolaou or Diff-Quick stained cytologic preparations from fine needle aspirate (FNA) specimens were used to evaluate morphologic features.
Flow cytometry immunophenotypic analysis
Flow cytometry immunophenotyping was performed on tissue biopsy PB and BM samples. Bone marrow aspirate and PB samples were incubated with monoclonal antibodies for 10 minutes at 4°C and erythrocytes were lysed with ammonium chloride (PharmLyseTM, BD Biosciences, San Diego, CA) at room temperature for 10 minutes using a standard lyse/wash technique. A total of 150 samples were assessed; 104 specimens were collected using eight-color FCI on the BD FACSCanto II cytometer (BD Biosciences, San Jose CA) using a comprehensive panel of antigens including several T-cell antigens including CD2, CD3, CD4, CD5, CD7, CD8, CD10, CD16, CD19, CD20, CD26, CD30, CD43, CD52, CD56, CD57, CD94, TCR αβ chain, and TCR γδ chain. The combination of antibody/laser-fluorochromes used is shown in Supplemental Table 1. Twenty-three samples were collected prior to 2009 and were analyzed using FACSCalibur cytometers (BD Biosciences, San Jose, CA); for these samples the analysis panel did not include CD10, CD30, CD57 or CD94. Twenty-three samples were assessed using a limited panel of T-cell antigens including CD3, CD4, CD5, CD8, and CD56 due to an initial concern for a B-cell lymphoma or myelodysplastic syndrome. The specific fluorescently labeled antibodies used in this study were purchased from BD Biosciences except for CD30, which was purchased from Beckman Coulter. A total of 100,000 events were acquired. Flow cytometric data were analyzed using FCS Express software (De Novo Software, Glendale CA). The intensity of antigen expression in aberrant T-cell populations was compared with background non-neoplastic T-cells and classified as “dim” or “bright”.
T-cell receptor (TCR) gene rearrangement analysis
DNA extracted from PB, BM and tissue samples was analyzed for T-cell receptor gamma (TCRG) and T-cell receptor beta (TCRB) chain genes and rearrangements by polymerase chain reaction (PCR), as described previously.[14] Briefly, we used a mixture of four family-specific multicolor fluorescently labeled consensus TCRG variable (V) and four unlabeled joining (J) segment primers for TCRG analysis and a mixture of unlabeled family-specific consensus TCRB variable (V) and multicolor fluorescently labeled joining (J) segment primers for TCRB analysis. For the TCRG analysis, the lower limit of detection (analytical sensitivity) of the assay for detection of a monoclonal T-cell population is between 0.01-10%, depending on the clonal diversity of the T-cells present and the V-gamma family involved. For the TCRB analysis, the lower limit of detection is about 2%.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 software. Fisher's exact test was used to assess categorical variables. A p<0.05 was considered statistically significant.
Results
Clinical presentation
The study included 38 patients, 20 (53%) women and 18 (47%) men, with a median age of 59 years at the time of diagnosis (range, 29-81 years). The clinical and laboratory features are summarized in Table 1.
Table 1. Summary of clinical and laboratory characteristics of patients with angioimmunoblastic lymphoma in this study (N=38).
| Characteristic | |
|---|---|
| Age, median (range) | 59 years (29-81) |
| Women (%) | 20 (53%) |
| Symptoms and signs | |
| Lymphadenopathy | 26 (68%) |
| B-type symptoms | 20/37 (54%) |
| Hepatosplenomegaly | 17 (45%) |
| Skin rash | 16 (42%) |
| Hypergammaglobulinemia | 14/34 (41%) |
| Anemia | 11 (29%) |
| Pruritus | 8 (21%) |
| Fatigue | 7/37 (19%) |
| Respiratory symptoms | 6/35 (17%) |
| Thrombocytopenia | 2/36 (6%) |
| Ann Arbor stage at diagnosis | |
| II | 2 (5%) |
| III | 12 (32%) |
| IV | 24 (63%) |
Flow cytometric immunophenotypic characterization of AITL
An aberrant T-cell population was detected by FCI analysis in 97 of 155 (63%) patient samples; 41 BM, 31 lymph node, 16 PB, 8 pleural fluid, and 1 CSF. Overall, AITL cells represented 0.5-90 % (median, 8%) of lymphocytes and 0.02-80% (median 2.3%) of all analyzed events. In all AITL cases the lymphoma cells were positive for CD4. CD3 was the most frequent aberrant marker including complete absence of surface CD3 (sCD3) in 53 (54%) samples, partial loss in 5 (5%) samples, decreased CD3 intensity of expression in 33 (34%) and 2 (2%) increased intensity of expression samples for a total of 88 (91%) samples that were aberrant. Altered sCD3 expression was coupled with loss of T-cell receptor (TCR) expression or decreased TCR alpha/beta expression in 56 of 80, (70%) cases. Other immunophenotypic aberrancies, in descending order of frequency, included the following: aberrant CD10 expression (58/85; 68%) in a subset or entire aberrant T-cell population; decreased (2; 4.9%) or increased (1; 1%) intensity of CD4 expression; loss of CD7 in 49 of 86 (56.9%) with complete absence in 37(43%) or partial absence in 12 (14%); decreased (3/86, 3.5%) or increased intensity (1/86, 1.2%) of CD7 expression;loss or decreased intensity of CD52 expression (7/74; 9.4%); loss of CD2 expression (1/85,1.2%), loss or decreased intensity of CD5 (2/89, 2.2%); and dual CD4+/CD8+ cells (1/97; 1%). Concurrent monotypic B-cells or plasma cells were present in 5 of 72 (6.9%) samples analyzed including 2 LN with monotypic plasma cells, 2 BM (from one patient) with monotypic B-cells, and 1 LN with aberrant mature B-cells that lacked of surface immunoglobulin expression. These results are summarized in Table 2. Detailed FCI data including other non-random immunophenotypic aberrancies for each patient are provided in Supplemental Table 2.
Table 2. Summary of immunophenotypic characterization of AITL by flow cytometry.
| Antigen/pattern | Bone marrow (%) | Peripheral blood (%) | Lymph node (%) | Body fluid (%) |
|---|---|---|---|---|
| Surface CD3 (N = 97) | N = 41 | N = 16 | N = 31 | N = 9 |
| Complete loss | 26 (63.4) | 13 (81.3) | 12 (38.7) | 2 (22.2) |
| Partial loss | 1 (6.3) | 4 (12.9) | ||
| ↓intensity | 12 (29.3) | 2 (12.5) | 15 (48.4) | 4 (44.4) |
| ↑Intensity | 2 (4.9) | |||
| TCRαβ (N = 80) | N = 39 | N = 12 | N = 22 | N = 8 |
| Complete loss | 25 (64.1) | 10 (83.3) | 11 (50.0) | 2 (25.0) |
| ↓intensity | 1 (2.6) | 2 (16.7) | 5 (22.7) | |
| CD10 (N = 85) | N = 36 | N = 14 | N = 27 | N = 8 |
| CD10+ | 5 (13.9 %) | 4 (28.6) | 11 (40.7) | 5 (62.5) |
| CD10+ (partial or subset) | 13 (36.1 %) | 5 (35.7) | 13 (48.1) | 2 (25) |
| CD7 (N = 86) | N = 39 | N = 14 | N = 24 | N = 9 |
| Complete loss | 16 (41.0) | 4 (28.6) | 9 (37.5) | 8 (88.9) |
| Partial loss | 9 (23.1) | 1 (7.1) | 1 (4.2) | 1 (11.1) |
| ↓intensity | 2 (5.1) | 1 (4.2) | ||
| ↑Intensity | 1 | |||
| CD4 (N = 97) | N = 41 | N = 16 | N = 31 | N = 9 |
| ↓intensity | 2 (4.9) | 4 (12.9) | 4 (44.4) | |
| ↑Intensity | 1 (3.2) | |||
| CD52 (N = 81) | N = 38 | N = 13 | N = 22 | N = 8 |
| Complete loss | 5 (13.2) | |||
| ↓intensity | 2 (9.1) | |||
| CD5 (N = 89) | N = 40 | N = 15 | N = 26 | N = 8 |
| Complete loss | 1 (2.5) | 1 (3.8) | ||
| CD2 (N = 85) | N = 40 | N = 14 | N = 23 | N = 8 |
| Complete loss | 1 (2.5) | |||
| CD8 (N = 97) | N = 41 | N = 16 | N = 31 | N = 9 |
| Partial expression (CD4+/CD8+) | 1 (2.4) |
A subset of specimens showed more than one immunophenotypic aberrancy in one (e.g., partial loss and decreased intensity) or more antigens.
Peripheral blood is commonly involved by AITL
Flow cytometry immunophenotypic analysis was performed on a total of 24 PB samples collected from 16 patients. FCI detected an aberrant T-cell population in 16 (66.7%) samples collected from 11 (68.8%) patients. The aberrant T-cells represented 0.5 to 80 % of lymphocytes (median, 8.5%) and 0.02 % to 67.5% (median 2.5%) of all events in PB.
Immunophenotypic features of AITL according to site of involvement
We compared the patterns of antigen expression in cases of AITL involving the PB and BM with cases involving LN. A complete or partial loss of CD3 expression was seen in 40 (70.2%) of 57 BM/PB samples whereas this pattern was observed in 16 of 31 (51.6%) LN. Complete loss of CD3 expression was seen in 39 (68.4%) of 57 BM/PB samples whereas only 12 (38.7%) of 31 cases of AITL involving LN showed this pattern (p=0.01). Conversely, CD10 expression (partial or complete) was seen in 27 (54%)of 50 BM/PB samples compared with 24 of 27 (88.9%) of LN specimens (p=0.04). As a group, there was no other significant immunophenotypic difference observed among these specimen types. These results are summarized in Table 2 and Fig. 1. We specifically compared sCD3 and CD10 expression in samples from 12 patients who had both LN and BM and/or PB involvement as determined by FCI. The pattern of expression for CD3 was consistent across BM/PB and LN specimens in 11/12 patients (92%) whereas aberrant CD10 expression was consistent across these tissue types in 8/10 patients (80%); in two patients CD10 expression was assessed only in one tissue type. Fig. 2 provides a FCI results from a representative case of AITL involving retroperitoneal LN and PB in the same patient.
Figure 1. Expression patterns of CD3 and CD10 by tissue type.
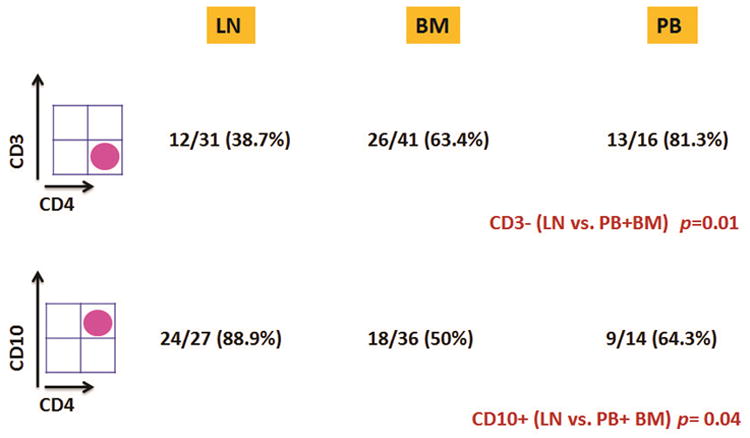
A CD3-/CD4+ immunophenotype is more common in AITL involving peripheral blood and bone marrow specimens compared with cases involving lymph nodes whereas CD10 expression is more commonly seen when AITL involves lymph node specimens.
Figure 2. Immunophenotypic features of AITL involving the retroperitoneal lymph nodes and peripheral blood in one representative patient.
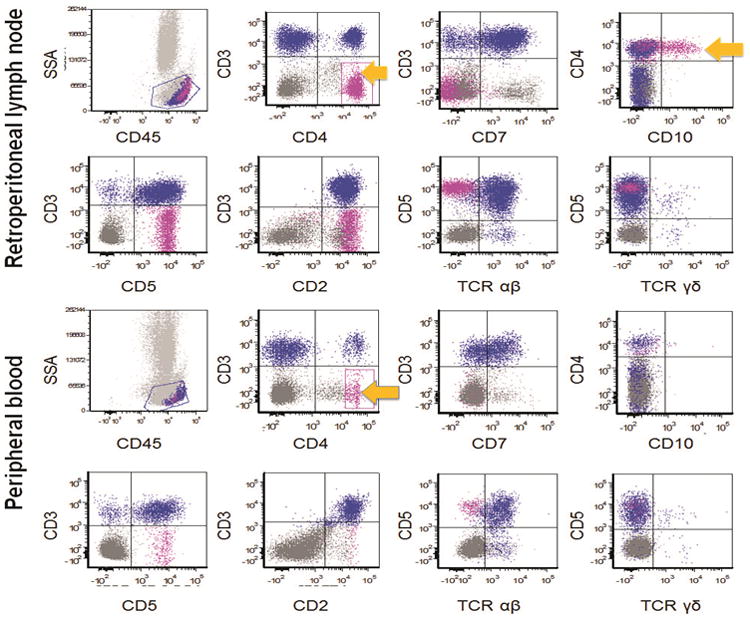
Flow cytometry was positive for an aberrant T-cell population in both the lymph node and peripheral blood collected from this patient. The aberrant T-cells (shown in pink and highlighted by the arrows) were positive for CD4 and showed loss of CD3 and CD7 in both samples. Of note CD10 was expressed by these cells in lymph node while they were virtually negative for CD10 expression in peripheral blood. SSA: side scatter analysis; TCR: T-cell receptor.
As noted above, multiple samples were obtained from several patients included in this study. Because the number of samples and the anatomic sites from which they were obtained was not equally represented among all patients we performed a second analysis taking into account only one specimen per site per patient and analyzed the above mentioned parameters. The samples in included in this analysis are marked with an asterisk in supplemental Table 2. A total of 58 specimens (24 LN, 17 BM, 11 PB, 5 pleural fluids and 1 CSF) from 34 patients were included in this analysis. Complete or partial loss of CD3 was observed in 52 of 58 cases (89.7%) and complete loss of CD3 expression was seen more commonly BM/PB specimens (18 of 28 cases, 64.3%) compared with LN specimens (10 of 24 cases, 41.7%) but the difference did not attain statistical significance. Conversely, CD10 expression (partial or complete) was seen in 11 (44%) of 25 BM/PB samples compared with 18 of 21 (85.7%) of LN specimens (p=0.0054).
Correlation of flow cytometry immunophenotypic analysis with morphologic findings
Morphologic assessment was performed on 126 of 150 specimens including 79 (62.7%) BM specimens, 35 (27.8%) LN (17 needle core biopsies, 11 excisional biopsies, and 7 fine needle aspiration specimens), 9 (7.1%) pleural fluid, 2 (1.6%) bronchoalveolar lavage, and 1 (0.8%) cerebrospinal fluid (CSF) specimen. Twenty-four PB specimens were not evaluated. Overall the results of morphologic assessment and FCI analysis were concordant in 97 (77%) specimens (63 positive and 34 negative). Eight cases were morphologically involved or suspicious for involvement by AITL but FCI was either uninformative (n=3) or failed to detect an aberrant cell population (n=5) (Fig. 3). These cases included 6 BM aspirate and two LN specimens. Of the six BM aspirates, one had an inadequate aspirate smear (dry tap), one was assessed using a panel geared towards detection of B-cell lymphoma with a limited number of T-cell antigens analyzed, two were older cases that were assessed using four-color flow cytometry and 2 were assessed using a comprehensive panel of T-cell antigens with eight-color flow cytometry. Both LN specimens were analyzed using the B-cell lymphoma panel. Conversely, in 17 samples, FCI detected an aberrant T-cell population but there was no morphological evidence of lymphoma. The aberrant T-cell population ranged from 0.1 to 20 % of total cells analyzed by FCI (median, 0.6%). These specimens included 15 BM, 1 LN FNA and 1 CSF. In an additional two samples that were positive by FCI, morphologic features were suspicious for involvement lymphoma and flow cytometry confirmed the diagnosis. (Fig.4) One BM biopsy specimen was inadequate for morphologic assessment and FCI did not detect an aberrant cell population in this case. These results are summarized in Table 3.
Figure 3. Lymph node involved by AITL with concordant flow cytometry and morphology results.
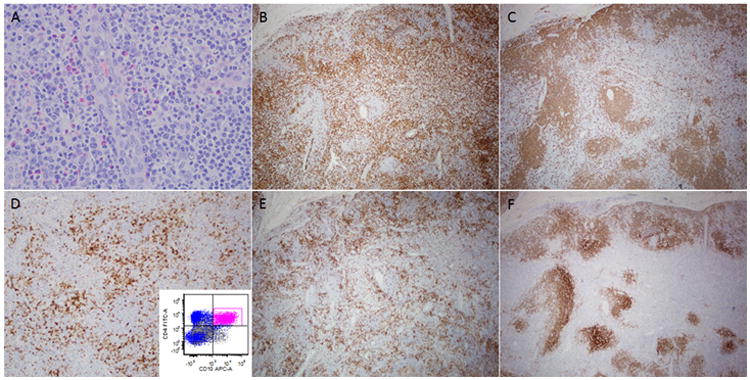
(A) The lymph node architecture was completely effaced by lymphoma. Atypical lymphoid cells with irregular nuclei and scant clear cytoplasm are present in the background of reactive eosinophils and plasma cells. A high endothelial venule is seen in the center of the field. The lymphoma has an interfollicular pattern of growth and is positive for CD3 (B), negative for CD20 (C), and shows aberrant expression of CD10 (D) also confirmed by flow cytometric analysis (inset). The neoplastic cells are positive for PD-1 (E). A CD21 stain highlights expanded and disrupted follicular dendritic meshworks (F). (A, hematoxylin and eosin; B-F, immunohistochemistry with hematoxylin counterstain)
Figure 4. Lymph node core biopsy suspicious of involvement by AITL.
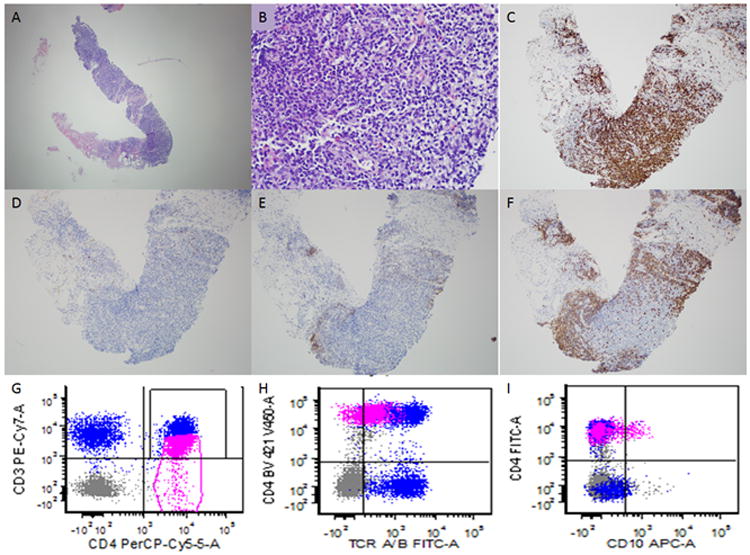
This patient had a previous history of AITL and had undergone chemotherapy. He presented with abdominal lymphadenopathy. A needle core biopsy was performed and minute fragments of tissue were present for evaluation (A). On higher power evaluation occasional atypical appearing lymphoid cells with clear cytoplasm are seen (B) which are present in association with occasional eosinophils. The majority of lymphoid cells are of T-cell lineage as demonstrated by CD3 (C). Rare CD10 positive cells are present (D). A CD21 immunohistochemical (E) stain is mostly negative and only highlights scattered follicular dendritic cells. The anti-CD20 antibody (F) highlights residual B-cell follicles. Although the findings were strongly suspicious for involvement by AITL, it was difficult to establish a definitive diagnosis due to the small nature of the biopsy. Flow cytometric immunophenotyping showed an aberrant T-cell population (shown in pink) with dim CD3 (G) and T-cell receptor alpha/beta expression (H) compared with background non-neoplastic T-cells. A subset of these cells showed aberrant CD10 expression. The flow cytometry results were helpful in establishing a definitive diagnosis. (A-B, hematoxylin and eosin; C-F, immunohistochemistry with hematoxylin counterstain)
Table 3.
Summary of the results of comparison of morphologic assessment with flow cytometric immunophenotyping.
| Flow cytometry + | Flow cytometry - | Flow cytometry uninformative | |
|---|---|---|---|
| Morphology + | 63 | 5 | 3 |
| Morphology - | 17 | 34 | |
| Morphology suspicious | 2 | 1 | |
| Morphology inadequate | 1 | ||
Peripheral blood specimens are not included.
Correlation of flow cytometry immunophenotypic analysis with T-cell receptor gene rearrangement studies
T-cell receptor (TCR) gene rearrangement studies were performed in 34 samples (28 BM, 3 LN, 2 PB and 1 pleural fluid) collected from 20 patients. The results of flow cytometry and TCR gene rearrangement studies were concordant (flow negative/TCR polyclonal or flow positive/TCR monoclonal) in 17 samples (50%). In 5 (15%) BM samples TCR studies showed monoclonal TCR beta (n=2) and/or gamma (n=4) rearrangements whereas FCI failed to detect an aberrant T-cell population. Conversely, in 2 BM samples FCI showed aberrant T-cells (0.3% and 0.5% of all cells) but no monoclonal TCR rearrangements were detected by PCR. In 10 (30%) samples (2 PB and 8 BM) PCR analysis showed only oligoclonal TCR beta (n=6) and/or gamma (n=9) gene rearrangements; seven of these samples were positive for AITL by FCI analysis.
Discussion
In this study we retrospectively reviewed the clinical, histopathologic and flow cytometry immunophenotypic (FCI) findings in 38 patients with a bona fide diagnosis of AITL. Our findings support the role of flow cytometry immunophenotyping in the assessment of patients with AITL and illustrate various immunophenotypic alterations that may be observed in cases of AITL. These results also show that the immunophenotype of the lymphoma cells varies according to site of involvement. Recognition of the immunophenotypic variability of AITL is very helpful in establishing a definitive diagnosis.
The malignant cells of AITL are often present in a polymorphous background of reactive cells including eosinophils, plasma cells, histiocytes and small non-neoplastic lymphocytes. The diagnosis of AITL can often be challenging, especially in the earlier phases of disease and with the use of small-gauge needle core biopsy specimens. In about 70-80 % of cases in this study group correlation of morphologic findings and FCI results was possible. The results show that morphologic assessment and FCI were concordant. Flow cytometry immunophenotypic analysis failed to detect an aberrant T-cell population in approximately 6% cases that were either morphologically positive or suspicious for involvement by AITL. Most (6/8) of these cases were either analyzed using a panel geared towards the detection of B-cell lymphoma or were older cases that were analyzed using four-color flow cytometry in which a limited number of T-cell-associated antigens were assessed. These results reinforce the need for using a comprehensive panel of T-cell markers in order to effectively identify the aberrant T-cell population in a background of many reactive T-cells in AITL. On the other hand, FCI detected an aberrant T-cell population, in relatively low quantity (median 0.6% of total events) in 13.5% of cases that were not morphologically involved by AITL; most of these cases were BM specimens with minimal involvement by AITL where the possibility of PB contamination should be considered.
In contrast to B-cell lymphomas in which one can reliably identify monotypic (or lack of) surface immunoglobulin light chain expression in neoplastic cell populations, the most helpful features in identifying neoplastic T-cells are altered patterns of expression in antigens that are normally present in non-neoplastic T-cells. The most common alteration we observed in this study was complete or partial loss or decreased intensity of expression of sCD3. This result is important because CD10 expression, although characteristic of AITL [5-7], is aberrantly expressed in 50-90 % AITL cases, depending on the site of involvement [10]. On the other hand, non-neoplastic T-cells may express CD10, including a subset of normal follicular helper T-cells [15], T-cells in lymph nodes with reactive follicular hyperplasia [16] and those undergoing apoptosis [17]. In this study, only 68 % of the cases showed CD10 expression whereas alterations of CD3 expression were seen in about 90% of the cases. This observation was even more pronounced in cases in which AITL involved the BM or PB. The less frequent expression of CD10 in PB and BM specimens may be due in part to the role of the microenvironment on AITL. In LNs, the presence of B-cells and follicular dendritic cells supports the expression of follicular helper T-cell-associated antigens including CD10, CXCL13, BCL6 and ICOS and the absence or altered microenvironment in the blood and BM may, in part, contribute to the less frequent expression of CD10 when compared with LN. An earlier study of AITL by Khokhar and colleagues [9] used immunohistochemistry and flow cytometry to assess BM specimens and showed that most of the neoplastic cells were negative for CXCL13 and approximately 75% of their cases were negative for CD10. To this effect, Attygalle and colleagues also have reported an association between the presence of follicular dendritic cells and expression of CD10 in the BM [18]. A similar phenomenon has been reported in cases of follicular lymphoma in which CD10 is downregulated in lymphoma cells when the neoplasm involves the interfollicular regions of LNs [19].
Another interesting observation in this study was that complete loss of CD3 expression by AITL cells was significantly more common in blood and BM specimens compared with LN specimens. The reason for this is not clear to us; however, the fact that CD3 expression patterns were consistent among different tissue types in patients that had both LN and BM/PB involvement mitigates the role of the microenvironment and suggests that AITL with complete loss of CD3 expression may be more likely to peripheralize compared with cases that retain, at least partially, CD3 expression.
Aberrant T-cells, albeit in small quantity, were very frequently detected in PB of patients with AITL in this study. Similarly, Singh and colleagues showed that an aberrant sCD3-/CD4+ cell population is a characteristic feature of AITL in patients with advanced-stage AITL [13]. These authors also showed that this aberrant immunophenotype is exceedingly rare in other leukemic T-cell lymphomas and that detection of cells with this immunophenotype in PB had a 94 % positive predictive value for the diagnosis of AITL. The sCD3-/CD4+ immunophenotype in AITL has been shown previously by other investigators as well [7,20,21].
In summary, we conclude that the diagnosis of AITL requires an integrated, multidisciplinary approach and can often be challenging, particularly in small needle biopsy specimens. In this study, we showed that FCI is an effective adjunct tool in the diagnosis of AITL and that immunophenotypic alterations can vary according to the site of involvement. Recognition of such variations will be helpful for establishing a more objective diagnosis of AITL.
In conclusion, our data show that the neoplastic cells in AITL commonly demonstrate an aberrant T-cell helper CD4+ immunophenotype with loss or decreased CD3 expression and variable expression of CD10. Circulating lymphoma cells are often identified in the blood. Multi-parameter FCI represents an effective and valuable approach in the diagnosis of this common variant of peripheral T-cell lymphoma and identification of AITL cells in blood, body fluids and fine needle aspiration specimens which will lead to timely and effective intervention. An illustrative summary of diagnostic and immunophenotypic aberrations in the context of different body site involvement by AITL is presented for the first time in this study.
Supplementary Material
Acknowledgments
S.L is the recipient of Molecular Diagnostic and Hematopathology Fellowship Awards. This study was supported by the National Cancer Institute/National Institutes of Health (R01CA138688 and 1RC1CA146299 to KHY). KHY is supported by The University of Texas MD Anderson Cancer Center Institutional Research and Development Fund, an Institutional Research Grant Award, an MD Anderson Cancer Center Lymphoma Specialized Programs on Research Excellence (SPORE) Research Development Program Award, an MD Anderson Cancer Center Myeloma SPORE Research Development Program Award, a Gundersen Lutheran Medical Foundation Award, and partially supported by the National Cancer Institute/National Institutes of Health (P50CA136411 and P50CA142509), and by MD Anderson's Cancer Center Support Grant CA016672.
List of abbreviations
- AITL
angioimmunoblastic T-cell lymphoma
- BM
Bone marrow
- CSF
cerebrospinal fluid
- FCI
flow cytometry immunophenotyping
- FNA
fine needle aspirate
- IL5
interleukin 5
- J
joining
- LN
lymph node
- PB
peripheral blood
- PCR
polymerase chain reaction
- PTCL
peripheral T-cell lymphoma
- TCR
T-cell receptor
- TFH
follicular helper T-cell
- Th2
T-helper 2
- V
variable
Footnotes
Potential Conflict of Interest: The authors declare no conflict of interest.
Authors' Contributions: Study design: SL, SAW, KHY; data collection and analysis and manuscript preparation: SL, SAW, JLJ, XL, ZYX-M, RNM, LJM, and KHY.
References
- 1.Dogan A, Gaulard P, Jaffe ES, Ralfkiaer E, Muller-Hermelink HK. Angioimmunoblastic T-cell Lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008. pp. 309–311. [Google Scholar]
- 2.Pircher A, Verdorfer I, Brunner A, Hopfinger G, Steurer M. Paraneoplastic phenomena and diagnostic challenges in angioimmunoblastic T-cell lymphoma (AITL): report of two cases and review of the literature. In Vivo. 2014;28:327–332. [PubMed] [Google Scholar]
- 3.de Leval L, Rickman DS, Thielen C, et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood. 2007;109:4952–4963. doi: 10.1182/blood-2006-10-055145. [DOI] [PubMed] [Google Scholar]
- 4.Piccaluga PP, Fuligni F, De Leo A, et al. Molecular profiling improves classification and prognostication of nodal peripheral T-cell lymphomas: results of a phase III diagnostic accuracy study. J Clin Oncol. 2013;31:3019–3025. doi: 10.1200/JCO.2012.42.5611. [DOI] [PubMed] [Google Scholar]
- 5.Lee PS, Lin CN, Chuang SS. Immunophenotyping of angioimmunoblastic T-cell lymphomas by multiparameter flow cytometry. Pathol Res Pract. 2003;199:539–545. doi: 10.1078/0344-0338-00459. [DOI] [PubMed] [Google Scholar]
- 6.Baseggio L, Berger F, Morel D, et al. Identification of circulating CD10 positive T cells in angioimmunoblastic T-cell lymphoma. Leukemia. 2006;20:296–303. doi: 10.1038/sj.leu.2404013. [DOI] [PubMed] [Google Scholar]
- 7.Stacchini A, Demurtas A, Aliberti S, et al. The usefulness of flow cytometric CD10 detection in the differential diagnosis of peripheral T-cell lymphomas. Am J Clin Pathol. 2007;128:854–864. doi: 10.1309/MC7QRGPTV0LRR98X. [DOI] [PubMed] [Google Scholar]
- 8.Zhang D, Saunders CJ, Zhao W, Davis M, Cunningham MT. The clonality of CD3+ CD10+ T cells in angioimmunoblastic T cell lymphoma, B cell lymphoma, and reactive lymphoid hyperplasia. Am J Hematol. 2009;84:606–608. doi: 10.1002/ajh.21483. [DOI] [PubMed] [Google Scholar]
- 9.Khokhar FA, Payne WD, Talwalkar SS, et al. Angioimmunoblastic T-cell lymphoma in bone marrow: a morphologic and immunophenotypic study. Hum Pathol. 2010;41:79–87. doi: 10.1016/j.humpath.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Baseggio L, Traverse-Glehen A, Berger F, et al. CD10 and ICOS expression by multiparametric flow cytometry in angioimmunoblastic T-cell lymphoma. Mod Pathol. 2011;24:993–1003. doi: 10.1038/modpathol.2011.53. [DOI] [PubMed] [Google Scholar]
- 11.Xerri L, Chetaille B, Serriari N, et al. Programmed death 1 is a marker of angioimmunoblastic T-cell lymphoma and B-cell small lymphocytic lymphoma/chronic lymphocytic leukemia. Hum Pathol. 2008;39:1050–1058. doi: 10.1016/j.humpath.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Serke S, van Lessen A, Hummel M, Szczepek A, Huhn D, Stein H. Circulating CD4+ T lymphocytes with intracellular but no surface CD3 antigen in five of seven patients consecutively diagnosed with angioimmunoblastic T-cell lymphoma. Cytometry. 2000;42:180–187. doi: 10.1002/1097-0320(20000615)42:3<180::aid-cyto4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Singh A, Schabath R, Ratei R, et al. Peripheral blood sCD3(-) CD4(+) T cells: a useful diagnostic tool in angioimmunoblastic T cell lymphoma. Hematol Oncol. 2014;32:16–21. doi: 10.1002/hon.2080. [DOI] [PubMed] [Google Scholar]
- 14.Vega F, Medeiros LJ, Bueso-Ramos C, et al. Hepatosplenic gamma/delta T-cell lymphoma in bone marrow. A sinusoidal neoplasm with blastic cytologic features. Am J Clin Pathol. 2001;116:410–419. doi: 10.1309/BM40-YM6J-9T3X-MH8H. [DOI] [PubMed] [Google Scholar]
- 15.Ame-Thomas P, Hoeller S, Artchounin C, et al. CD10 delineates a subset of human IL-4 producing follicular helper T cells involved in the survival of follicular lymphoma B cells. Blood. 2015;125:2381–2385. doi: 10.1182/blood-2015-02-625152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook JR, Craig FE, Swerdlow SH. Benign CD10-positive T cells in reactive lymphoid proliferations and B-cell lymphomas. Mod Pathol. 2003;16:879–885. doi: 10.1097/01.MP.0000084630.64243.D1. [DOI] [PubMed] [Google Scholar]
- 17.Cutrona G, Leanza N, Ulivi M, et al. Expression of CD10 by human T cells that undergo apoptosis both in vitro and in vivo. Blood. 1999;94:3067–3076. [PubMed] [Google Scholar]
- 18.Attygalle AD, Diss TC, Munson P, Isaacson PG, Du MQ, Dogan A. CD10 expression in extranodal dissemination of angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2004;28:54–61. doi: 10.1097/00000478-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Dogan A, Du MQ, Aiello A, et al. Follicular lymphomas contain a clonally linked but phenotypically distinct neoplastic B-cell population in the interfollicular zone. Blood. 1998;91:4708–4714. [PubMed] [Google Scholar]
- 20.Merchant SH, Amin MB, Viswanatha DS. Morphologic and immunophenotypic analysis of angioimmunoblastic T-cell lymphoma: Emphasis on phenotypic aberrancies for early diagnosis. Am J Clin Pathol. 2006;126:29–38. doi: 10.1309/28YP-0DEL-GKEJ-GRXG. [DOI] [PubMed] [Google Scholar]
- 21.Chen W, Kesler MV, Karandikar NJ, McKenna RW, Kroft SH. Flow cytometric features of angioimmunoblastic T-cell lymphoma. Cytometry B Clin Cytom. 2006;70:142–148. doi: 10.1002/cyto.b.20107. [DOI] [PubMed] [Google Scholar]
- 22.Lefevre G, Copin MC, Staumont-Salle D, et al. The lymphoid variant of hypereosinophilic syndrome: study of 21 patients with CD3-CD4+ aberrant T-cell phenotype. Medicine (Baltimore) 2014;93:255–266. doi: 10.1097/MD.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.d'Elbee JM, Parrens M, Mercie P, et al. Hypereosinophilic syndrome - lymphocytic variant transforming into peripheral T-cell lymphoma with severe oral manifestations. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:e185–190. doi: 10.1016/j.oooo.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 24.Kunimasa K, Arita M, Arai Y, et al. Peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) in a patient with hypereosinophilic syndrome showing multiple nodules on chest computed tomography. Intern Med. 2011;50:2417–2421. doi: 10.2169/internalmedicine.50.5936. [DOI] [PubMed] [Google Scholar]
- 25.Ravoet M, Sibille C, Gu C, et al. Molecular profiling of CD3-CD4+ T cells from patients with the lymphocytic variant of hypereosinophilic syndrome reveals targeting of growth control pathways. Blood. 2009;114:2969–2983. doi: 10.1182/blood-2008-08-175091. [DOI] [PubMed] [Google Scholar]
- 26.Roufosse F, Cogan E, Goldman M. Lymphocytic variant hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27:389–413. doi: 10.1016/j.iac.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Ravoet M, Sibille C, Roufosse F, et al. 6q- is an early and persistent chromosomal aberration in CD3-CD4+ T-cell clones associated with the lymphocytic variant of hypereosinophilic syndrome. Haematologica. 2005;90:753–765. [PubMed] [Google Scholar]
- 28.Roufosse F, de Leval L, van Krieken H, van Deuren M. Lymphocytic variant hypereosinophilic syndrome progressing to angioimmunoblastic T-cell lymphoma. Leuk Lymphoma. 2014:1–4. doi: 10.3109/10428194.2014.976823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


