Abstract
During formation of dental enamel maturation-stage ameloblasts express ion-transporting transmembrane proteins. The SLC4 family of ion-transporters regulates intra- and extracellular pH in eukaryotic cells by co-transporting HCO3− with Na+. Mutation in SLC4A4 (coding for the Na+ bicarbonate co-transporter NBCe1) induces developmental defects in human and murine enamel. We hypothesized that NBCe1 in dental epithelium is engaged in neutralizing protons released during crystal formation in the enamel space. We immunolocalized NBCe1 protein in mouse wild-type dental epithelium and examined the effect of NBCe1-null mutation on enamel formation in mice. Ameloblasts expressed gene transcripts for NBCe1 isoforms B/D/C/E. In wild-type mice weak to moderate immunostaining for NBCe1 with antibodies that recognize isoforms A/B/D/E and isoform C was seen in ameloblasts in secretory stage, no or very low staining in early maturation-stage but moderately to high staining in late maturation-stage. The papillary layer showed the opposite pattern and immunostained prominently at early maturation-stage but gradually showed less staining at mid- and late maturation-stage. In NBCe1−/− mice ameloblasts were disorganized, the enamel thin and severely hypomineralized. Enamel organs of CFTR−/− and AE2a,b−/− mice (believed to be pH regulators in ameloblasts) contained higher levels of NBCe1 protein than wild-type mice. Our data show that expression of NBCe1 in ameloblast and papillary layer cell depends on developmental stage and possibly responds to pH changes.
Keywords: pH regulation, mineralization, CFTR, AE2
INTRODUCTION
Enamel formation is a unique process of cell-regulated biomineralization that occurs in two stages: secretory phase characterized by the initiation of enamel crystals and their slow extension in length, and maturation-stage during which crystals rapidly expand in width and thickness (Smith 1998). The development of enamel requires tight control of pH at all stages of formation (Smith 1998; Lacruz et al. 2010a; Lee et al. 2013). The formation of hydroxyapatite crystals produces large quantities of protons that need to be buffered to sustain mineralization (Smith 1998). It is believed that in secretory stage, amelogenins play an important role in buffering pH but that in maturation stage after removal of amelogenins other mechanisms operate (Simmer and Fincham 1995; Smith 1998). A recent report proposed that maturation-stage ameloblasts secrete protons to keep the surface free from mineral (Josephsen et al. 2010). A majority of studies however is in favor for the concept that ameloblasts secrete bicarbonate to buffer protons released by mineral formation (Bronckers et al. 2011; Lacruz et al. 2010a; Paine et al. 2008; Smith 1998), either in response to or independent from extracellular acidification (Lyman and Waddell 1977).
Ameloblasts express a series of (mostly) transmembrane proteins typical for pH regulation and ion transport in epithelia (thyroid, non-acid secreting cell of the collecting ducts of kidney and pancreatic ducts). These proteins include several types of carbonic anhydrase (most abundantly types 2 and 6), the cystic fibrosis transmembrane conductance regulator (CFTR), the electrogenic sodium-bicarbonate cotransporter-e1 (NBCe1), the electroneutral sodium bicarbonate cotransporter 1 (NBCn1) and anion exchanger-2 (AE2) (Lacruz et al. 2012).
The electrogenic Na+: HCO3− co-transporter NBCe1, encoded by SLC4A4 is a member of the SLC4A (solute carrier bicarbonate transport) family (Bernardo et al. 2006; Parker and Boron 2013; Romero et al. 2013). SLC4A4 has three major variants, two N-terminally spliced (NBCe1-A and NBCe1-B), and one C-terminally spliced variant, (NBCe1-C) as well as several minor variants (D and E). Mutation of NBCe1 in human results in developmental defects in kidney, leading to acidosis (proximal renal tubular acidosis), and changes in eyes and teeth (Gawenis et al. 2007; Lacruz et al. 2012). NBCe1−/− mice have a severe and fatal phenotype, die before weaning (Gawenis et al. 2007; Lacruz et al. 2010b) and have “chalk white” enamel that easily fractures. The detection of transcripts of NBCe1-B and immunostaining of enamel organs of wild-type mice showed that NBCe1 operates locally in dental epithelium (Josephsen et al. 2010; Paine et al. 2008; Zheng et al. 2011).The enamel organ contains several cell types but which of these tissues synthesize which isoforms was not clear and antisera were not well-specified (Josephsen et al. 2010; Paine et al. 2008; Zheng et al. 2011). The function of NBCe1 in dental epithelium is also not yet clear, but NBCe1 could potentially be involved in pH regulation. The NBCe1 gene of murine ameloblast-like cells contains a pH-responsive element in its promoter region (Snead et al. 2011) and NBCe1 gene expression of mouse ameloblast-like cells increased when culture medium was acidified to pH 6.0 (Paine et al. 2008), a value reached in vivo in enamel below ruffle-ended ameloblasts during pH cycling (Sasaki et al. 1991)
The aims of the present study were (1) To record the expression of NBCe1 protein in enamel organ at all stages of enamel development; (2) To determine changes in structure of ameloblasts and enamel mineralization in teeth from NBCe1−/− mice and (3) To test whether ameloblasts in vivo respond to pH changes in situ by enhancing NBCe1 protein expression. For this we used two other mouse models in which pH regulation is disrupted which severely affects enamel formation: the AE2a,b−/− (Lyaruu et al. 2008) and CFTR−/− mice (Wright et al. 1996; Bronckers et al. 2010)
MATERIALS AND METHODS
Animals and tissues
Tissues were collected from wild-type mice 12–18 days old, 3–6 month old (adult) mice, hamsters (4–8 days old) and 9–12 weeks old rats (adult). After sacrifice complete upper and lower jaws were dissected as well as soft tissues as stomach, brain and kidney. Homozygous NBCe1−/− and heterozygous tissues were collected from NBCe1−/− mice at 14 days of age. The targeting procedure (described in Gawenis et al. 2007) replaced sequences that include part of exon 9 (amino acids 421–455, LNIQA….TDNMQ including 3’splice site) of the kidney variant and part of intron 9 with a neomycin resistance gen, removing the C-terminal portion of NBCe1 downstream from exon 9. Tissues and tissue sections from AE2a,b−/− and CFTR−/− mice were available from previous studies (Bronckers et al. 2010; Lyaruu et al. 2008). All animal handling complied with national and international regulations for Animal Care and permission was obtained from the Committee for Animal Care.
PCR
Fresh tissues were collected and mRNA extracted as reported (Bronckers et al. 2010). Table 1 presents the primer details to detect different transcripts for NBCe1.
Table 1.
Primer sequences for PCR variants of NBCe1/SLC4A4 in wild-type mice
| Gene variant | Gene Bank access number |
Position | primer sequence | Annealing temp °C |
Product size (bps) |
|---|---|---|---|---|---|
|
SLC4A4 isoforms B,C,E (kidney/pancreas variant) |
NM_001197147 | FW (58-79) | 5' CCCAGGAGGATGGAGGATGAAG 3' | 58 | 295 |
| REV (352-331) | 5' AGATGAATCGGATGCGTTCTGC 3' | ||||
|
SLC4A4 isoforms D and E (kidney/pancreas variant) |
NM_001197147 | FW (604-623) | 5' CCACAGCTGGTGGAGATGAT 3' | 63 | 182 |
| REV (785-768) | 5' GTCATGGCTGGGCTGCTT 3' | ||||
|
SLCA44 isoform C (brain variant) |
NM_001136260 | FW (3147-3167) | 5' GGATAGCGACAATGACGATGA 3' | 63 | 212 |
| REV (3358-3337) | 5' AAACAGGTTACAACGTGGTTTC 3' |
Antibodies
In rat NBCe1 consists of 1079–1094 amino acid residues and contains an N-terminal cytoplasmic domain of 428–468 amino acid residues long, a 521 amino acid residue long transdomain with as many as 14 transmembrane segments and a cytoplasmic C–terminus domain with 90–105 amino acid residues.
Two different polyclonal antibodies to NBCe1 were raised in rabbits using maltose-binding protein (MBP) fusion protein system (Bevensee et al. 2000).The first one (antiserum K1A) was raised against recombinant-produced peptide fragment coding for the C-terminal 46 amino acids of rat brain (DCPYSEKVPSIKIPMDITEQQPFLSDNKPLDRERSSTFLERHTSC) and reacts with the NBCe1-A/B/D/E isoforms. The rat NBCe1-A/B is 96% identical to NBCe1 from human pancreas and heart. The second antibody (antiserum B1B) was raised to the last 61 C-terminal amino acids present in and specific for the NBCe1-C isoform (EKDPQHSLNATHHADKIPFLESLGLPSPPRSPVKVVPQIRIELESEDNDYLWRNKGTETT L). In negative controls the primary antisera were replaced by non-immune rabbit sera. Kidney and brain were used as positive controls, and specificity of the antisera was tested by staining sections from jaws from NBCe1−/− mice.
Immunostaining
Paraffin sections were dewaxed in xylene, rehydrated in a descending series of ethanol, and rinsed in phosphate buffered saline and immunostained with peroxidase–technique using an Envision Kit (DakoCytomation, Denmark) with primary antibodies diluted 1:200–1:400. After washing, the peroxidase conjugates were visualized with 3,3′-Diaminobenzidine tetrahydrochloride (DAB) substrate (to produce a brown end-product; Envision Kit; DakoCytomation, Glostrup, Denmark) or with 3-Amino-9-ethylcarbazole (AEC) substrate (to produce a red end-product; Invitrogen) for 10 min at room temperature according to the manufacturer's instructions, and counterstained with hematoxylin or methyl green.
Western blotting
From freeze-dried mandibles from wild-type, CFTR−/− and AE2a,b−/− mice early maturation stage enamel organs were microdissected incisally from an imaginary reference line between M1 and M2 indicating the border between secretory and maturation stage. The apical half of the maturation stage enamel organ was removed by micodissection, dissolved under non-reducing condition in SDS loading buffer (from Nucleospin Triprep kit) and protein was measured using the BCA protein assay (Bio-Rad, Hercules, CA). Five to 10 µg of non-denatured protein were loaded on SDS PAGE in a 3–8% Tris acetate Nupage gel with Tris acetate as running buffer for 60 min at 150 V and electroblotted by an iBlot device (Invitrogen) on nitrocellulose membrane according to the manufacturer's instructions. Based on the prestained protein markers (Novex sharp protein standards; Invitrogen), blots were horizontally cut into two parts just above the 50-kDa prestained marker. The upper part, with protein was probed with either one of the rabbit NBCe1 antiserum (1:100). The lower part was probed with β-actin monoclonal mouse antibody (Sigma) with a dilution of 1:10,000. IRDye 800CW conjugated goat anti-rabbit IgG (H+L) highly cross-adsorbed (LI-COR; Product number: 926–32211) and IRDye 680CW conjugated goat anti-mouse IgG (H+L) highly-cross adsorbed (LI-COR; Product number: 926–32220) were applied as a second antibody for 90 minutes at room temperature (1:5000, LI-COR) prior to washing with phosphate buffered saline (PBS). Visualisation and quantification was carried out with the LI-COR OdysseyH scanner and software (LI-COR Biosciences). Red color (for actin) was detected at 680 nm wavelength and a green color (for NBCe1) was detected at 800 nm wavelength. For quantification we used Odyssey software. Values were normalized for actin and expressed as percentage of wild-type (100%).
Microcomputed tomography (microCT)
To determine the degree of mineral content, freeze-dried, MMA-embedded hemimaxillae from three 14-days old NBCe1−/− mice and three wild-type or heterozygous mice were scanned at a resolution of 8µm voxel using a MicroCT-40 high resolution scanner (Scanco Medical, AG, Bassersdorf, Switzerland). Mineral density was determined at sequential stages of development. Cross-sectional virtual images were collected from most developed (incisor tip) to the least developed (cervical area). The most incisal slice containing the most mineralized enamel was identified visually, and the mineral density [mg HA/ccm] measured at 3 sites in enamel, dentin and surrounding bone and values averaged per slice. Measurements were made at 100 micrometer intervals and slices at the same developmental stage from three mice per group averaged and plotted as function of stage (slice number). Independent t-test was used to compare the groups. Statistical significance was set at p <0.05 levels.
RESULTS
- Gene transcripts and NBCe1 protein in ameloblasts
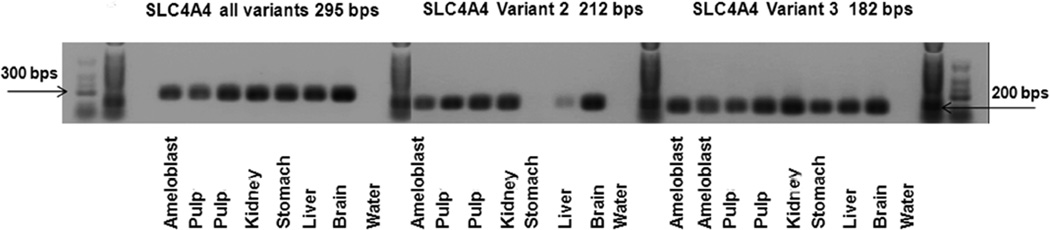
Transcripts for NBCe1-B/C/E (no further distinction, first primer set), isoform D/E (second primer set) and isoform C (third primer set) were detected in enamel organ of mouse incisors (Fig. 1).
Figure 1.
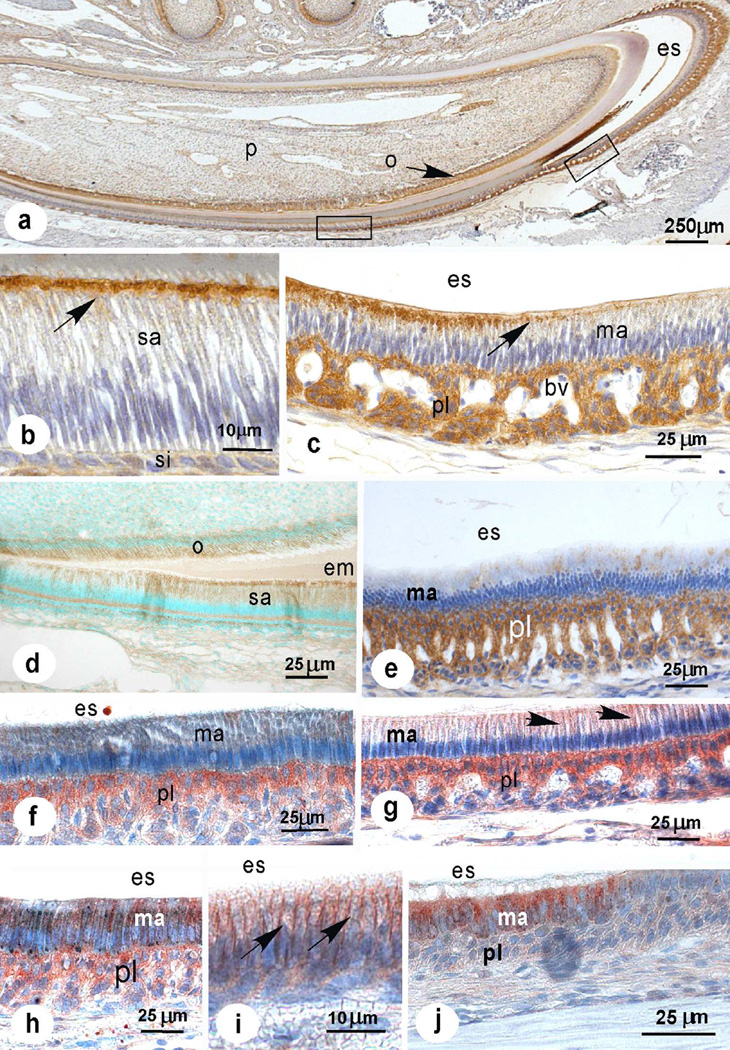
Incisors and developing molars gave positive immunostaining with both antisera in the dental epithelium of hamsters (Fig. 2a–c, 3h), mice (Fig. 2d–j) and rats (Fig 3a–c, g) depending on the stage of amelogenesis. In the cervical loop the first weak staining for NBCe1-A/B/D/E was seen in stratum intermedium, later appeared in early secretory ameloblasts and increased gradually (Fig. 2a, 2d). In secretory ameloblasts staining was diffuse intracellular and often confined to a sharp line at the base of the Tomes processes that disappeared during transitional stage (Fig. 2b–2d, Fig. 3a). The papillary layer stained strongly with both antisera (Fig. 2c, 2e–g, 3b) while the underlying ameloblast layer was almost negative in early maturation stage.
Figure 2.
Figure 3.
At mid-maturation-stage ameloblasts started to immunostain again and staining intensity increased to late maturation-stage while a gradual reduction of staining was noticed in the regressing papillary layer (Fig. 2g–j, 3c, e–g). Staining in maturation-stage ameloblasts was intracellular and in basolateral plasma membranes (Fig. 2i). In maturation-stage the positive-stained layer of ameloblasts contained occasionally small groups of unstained or weakly stained cells (possibly smooth-ended cells; Fig 3c, d) while the adjacent papillary layer remained immunopositive (Fig. 2c).
Both antisera gave a similar staining pattern, both in incisors and developing molars (Fig. 3h). Antiserum to NBCe1-A/B/D/E was more consistent than the anti-NBCe1C which gave more background. Both antisera also gave positive staining in nuclei of some cells in transitional, mid- and late maturation-stage, more frequently seen in antiserum to NBCe1-C (Fig. 3f). Extracellular enamel was also positive but when primary antiserum was replaced by non-immune sera it stained as well.
Specificity of the employed NBCe1 antisera was validated by staining incisors from NBCe1−/− mice. The strong intracellular staining and staining associated with the membranes was lost but a variable and weak overall staining remained (Fig. 3i), similar as obtained when primary antisera were replaced by non-immune sera. Also in tissues derived from NBCe1−/− mice the nuclei of some cells of the papillary layer reacted with the antisera, indicating that this staining was non-specific.
- Reduced enamel mineralization in NBCe1-null mice determined by microCT
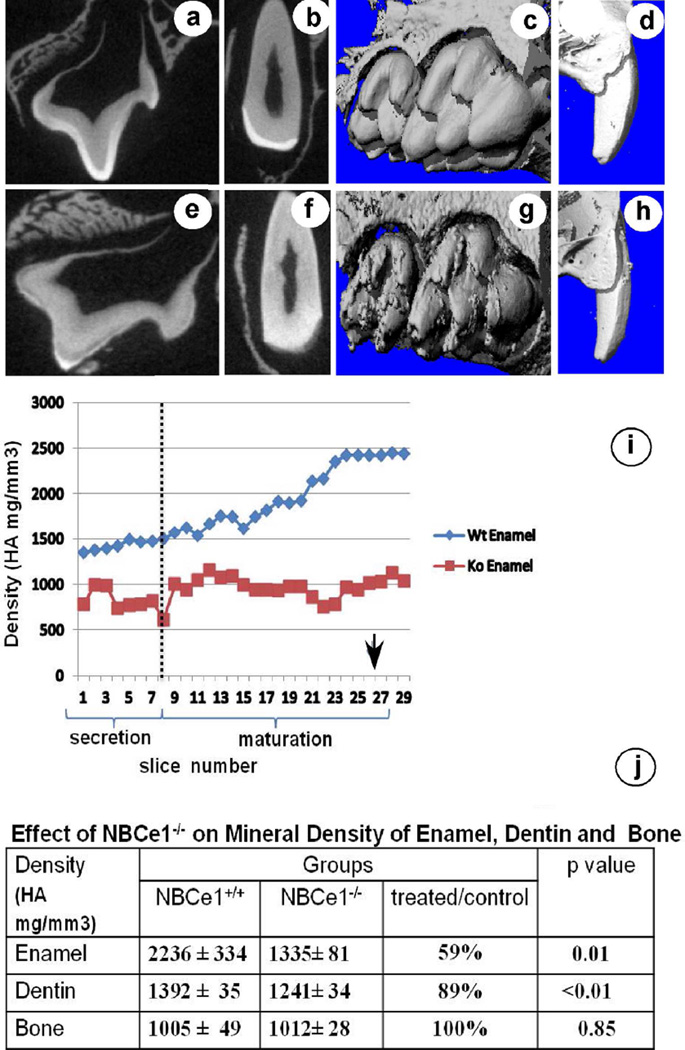
Three-dimensional reconstructions of microCT images showed that the surface of molar and incisor enamel was smooth and without defects in control mice (Fig 4a–d) but rough and irregular in NBCe1−/− mice (Fig. 4e–h).
Figure 4.
Mineral density in maxillary incisors of NBCe1−/− mice plotted as function of developmental stage was lower than in control enamel, and failed to increase in maturation-stage (Fig 4i) In NBCe1−/− mice the mean mineral density of enamel was 59% of control value and for dentin 89% while mineral density in bone was not changed (Fig. 4j).
Histological changes in enamel organ in NBCe1-null mice
In contrast to well-polarized ameloblasts formed in wild-type mice, ameloblasts of NBCe1−/− mice lost polarity, became short and round as reported (Lacruz et al. 2010b). The enamel matrix was not homogenous, irregular, and thin and an additional structureless enamel layer had deposited on top of normally structured layer. Cellular organization of odontoblasts and dentin layer was indistinguishable between wild-type and NBCe−/− mice.
Upregulation of NBCe1 protein in enamel organ of AE2a,b−/− and CFTR−/− mice
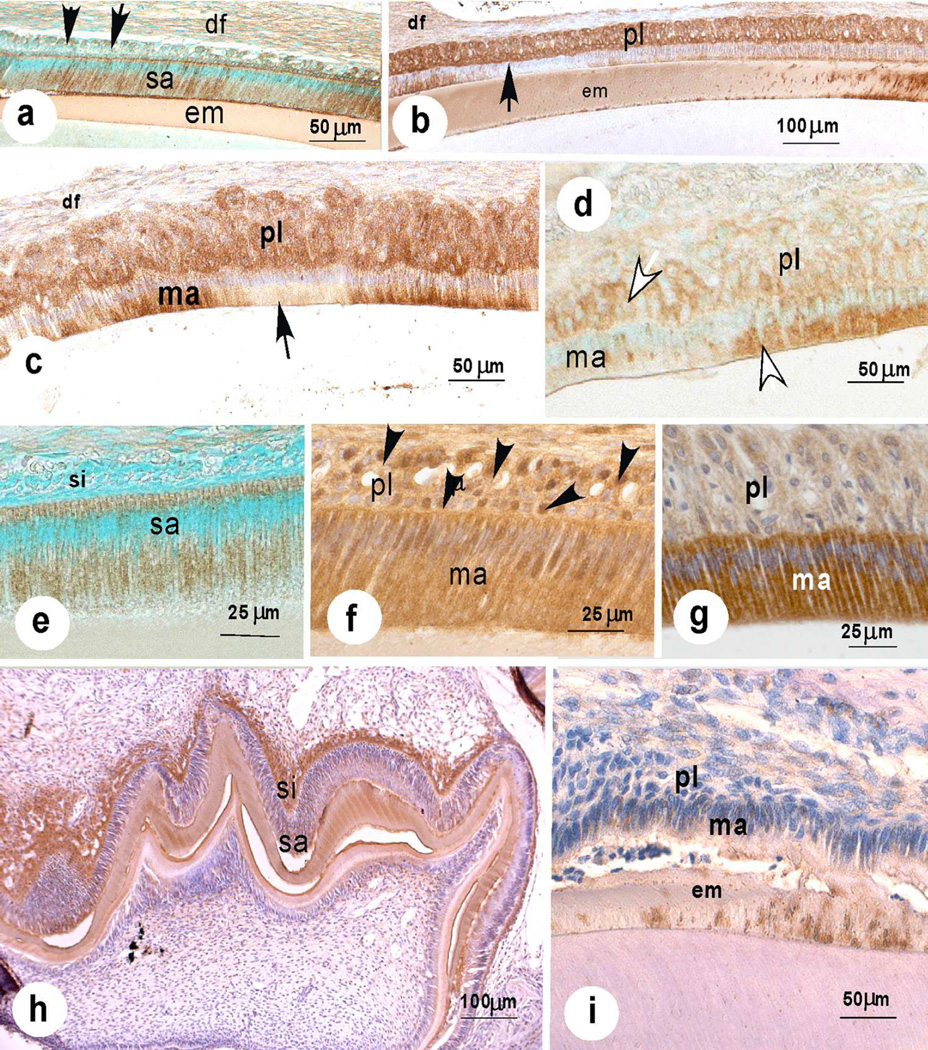
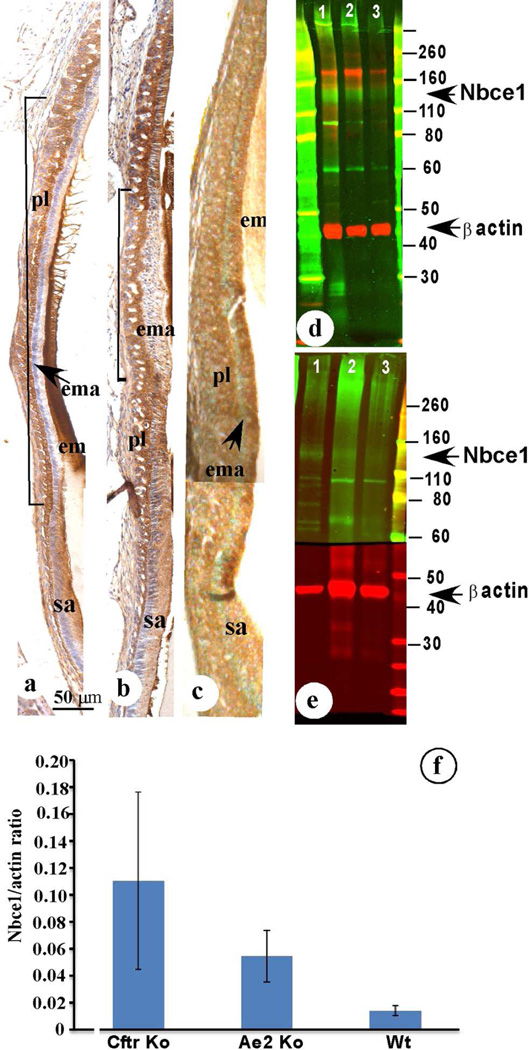
Western blotting of maturation-stage ameloblasts using anti-NBCe1-A/B/D/E (Fig. 5d green bands, K1A antibody), or anti-NBCe1-C (Fig. 5e green bands, antiserum B1B) showed a major broad band between 110 kD and 160kD, corresponding with the posttranslational modified 120 kD NBCe1.
Figure 5.
To investigate whether NBCe1 was involved in buffering of forming enamel and reacted to the higher acidity of the enamel matrix, due to proton release during mineral accretion we immunostained enamel organs from AE2a,b−/− and CFTR−/− mice with antisera to NBCe-1 to examine whether there was any attempt to compensate for defective pH regulation by enhancing NBCe1 production as seen in cholangiocytes in AE2a,b−/− mice (Uriarte et al. 2010). In enamel organs of AE2a,b−/− mice the ameloblasts stained more intense and early maturation-stage ameloblasts turned immunopositive sooner than in controls (Fig. 5a,b). This effect was more dramatic in enamel organs of CFTR−/− (Fig. 5c). On western blots of enamel organs NBCe1 protein was 4.3 fold higher in AE2a,b−/− mice (not significant) and 11.3 fold higher in CFTR−/− mice (p<0.05) in comparison with wild-type control mice (Fig. 5f)
Discussion
Intracellular immunostaining for NBCe1 was previously reported to be present in secretory ameloblasts of mouse and human incisors (Paine et al. 2008; Zheng et al. 2011), and in the rat in maturation-stage stage papillary layer but not in maturation-stage ameloblasts (Josephsen et al. 2010). We show here that the synthesis of NBCe1 protein depends on the developmental stage and changes with time and location. During the secretion stage ameloblasts stained weakly to moderately, virtually lost staining at early maturation-stage but restained at mid- and late maturation-stage. Gaps of negative immunostaining seen in small groups maturation-stage ameloblasts - also seen by immunostaining for AE2a,b (Bronckers et al. 2009; Lyaruu et al. 2008)-suggest that during maturation-stage some ameloblasts apparently cease production of NBCe1 and/or rapidly digest it to restart biosynthesis a short time later. These changes in production of NBCe1 likely reflect differences in activity between smooth-ended and ruffle-ended ameloblasts.
It has been reported that mouse ameloblasts and immortalized murine ameloblast-like cells (LS8) express NBCe1-B but not NBCe1-A or NBCe1-C transcripts (Lacruz et al. 2010b; Paine et al. 2008; Zheng et al. 2011). We report here that mouse ameloblasts express transcripts for NBCe1-B/D/E, NBCe1-D/E and NBCe1-C. Antiserum to NBCe1-A/B/D/E reacted strongly with dental epithelial cells indicating that NBCe1-isoform B and/or D/E isoforms are translated. Also the antiserum specific to NBCe1-C stained dental epithelium positive, essentially in a similar pattern. Enamel organ cells apparently produce various isoforms simultaneously. The differences in outcome between our present and previous data (Zheng et al. 2011) and data that ameloblasts do not express NBCe1-C transcripts (Lacruz et al. 2010b; Paine et al. 2008) may be explained by the use of different primer sets.
In a recent paper some of us (PdB, AB) reported expression of NBCe1-A in fetal human ameloblasts (Zheng et al. 2011). This however appeared to be incorrect by use of the wrong primer set for the human tissues as pointed out recently (Parker and Boron 2013).
NBCe1 is a bidirectionally operating electrogenic transmembrane ion-transporter which can cotransport 1 bicarbonate for 2 or 3 Na+, either in or out the cell depending on cell-type and the concentration gradient over the plasma membrane. The enhanced production of NBCe1 in maturation ameloblasts of CFTR−/− and AE2ab−/− mice (Fig. 5) suggests that these cells respond to changes in pH in the forming enamel by enhancing NBCe1 synthesis to compensate for the dysfunctioning of AE2a,b and CFTR. Whether the various NBCe1 isoforms have the same function at different stages of amelogenesis is not clear. The opposite immunostaining patterns for NBCe1 seen between maturation ameloblasts and papillary layer suggest both layers can cooperate with each other in dealing with pH and may pass small ions through the gap junctions between both cell types (Josephsen et al. 2010). The very high protein expression of NBCe1 in papillary layer and the fact that NBCe1 cannot compensate for absence of functional CFTR and AE2 suggests that NBCe1 primarily regulates intracellular pH in the papillary layer cells.
NBCe1 cotransports 1 Na+ with 2–3 bicarbonates which requires a Na+ gradient over the plasma membrane. Conceivably, the low intracellular Na+ that this cotransport needs is accomplished by the activity of Na+, K+-ATPase in plasma membranes of the papillary layer (Garant and Sasaki 1986; Josephsen et al. 2010; Wen et al. 2014,) continuously pumping out 3Na+ and importing 2 K+. In addition, other, yet unknown mechanisms must be operate in enamel organ cells to compensate for charge differences that result from increased negative charge when NBCe1 and Na+-K+ exchangers are functional.
The absence of functional NBCe1 in ameloblasts in NBCe1−/− mice does not rule out that also systemic changes as low bicarbonate levels in blood and acidosis due to disfunctioning of the kidney contribute to development of enamel defects.
In conclusion, ameloblasts express different isoforms of NBCe1 including B/D/E/, D/E and C. Ameloblasts and papillary layer cells need NBCe1 for normal functioning and to sustain mineral accretion likely by secreting buffer. Ameloblasts and papillary layer cells are complementary in expressing NBCe1 and likely closely cooperate to regulate pH.
Acknowledgments
This study was in part supported by NIH DE13508 (PDB, AB) and NIH DE019629 (MP). The authors thank Dr. H. de Jonge (Erasmus University, Rotterdam, Netherlands) and Dr. J. Bolscher (Dept Oral Biochemistry, ACTA, Amsterdam, the Netherlands) for advice. The authors would like to thank Dr. Gary E. Shull (Department of Molecular Genetics, Biochemistry and Microbiology, University of Cincinnati College of Medicine, Cincinnati, Ohio) for providing breeding pairs of the NBCe1+/− animals. Currently these mice are available from the Mutant Mice Regional Resource Centers (MMRRC) stock # 034263-JAX#
Reference List
- Bernardo AA, Bernardo CM, Espiritu DJ, Arruda JA. The sodium bicarbonate cotransporter: structure, function, and regulation. Semin Nephrol. 2006;26:352–360. doi: 10.1016/j.semnephrol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF. An electrogenic Na(+)-HCO(−)(3) cotransporter (NBC) with a novel COOH-terminus, cloned from rat brain. Am J Physiol Cell Physiol. 2000;278:C1200–C1211. doi: 10.1152/ajpcell.2000.278.6.C1200. [DOI] [PubMed] [Google Scholar]
- Bronckers A, Kalogeraki L, Jorna HJ, Wilke M, Bervoets TJ, Lyaruu DM, Zandieh-Doulabi B, DenBesten P, de JH. The cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in maturation stage ameloblasts, odontoblasts and bone cells. Bone. 2010;46:1188–1196. doi: 10.1016/j.bone.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronckers AL, Guo J, Zandieh-Doulabi B, Bervoets TJ, Lyaruu DM, Li X, Wangemann P, DenBesten P. Developmental expression of solute carrier family 26A member 4 (SLC26A4/pendrin) during amelogenesis in developing rodent teeth. Eur J Oral Sci. 2011;119(Suppl 1):185–192. doi: 10.1111/j.1600-0722.2011.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronckers AL, Lyaruu DM, Jansen ID, Medina JF, Kellokumpu S, Hoeben KA, Gawenis LR, Oude-Elferink RP, Everts V. Localization and function of the anion exchanger Ae2 in developing teeth and orofacial bone in rodents. J Exp Zool B Mol Dev Evol. 2009;312B:375–387. doi: 10.1002/jez.b.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garant PR, Sasaki S. Ultracytochemistry of ouabain sensitive, K+ dependent paranitrophenylphosphatase in rat enamel incisor organ. Anat Rec. 1986;216:1–9. doi: 10.1002/ar.1092160102. [DOI] [PubMed] [Google Scholar]
- Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/ J Biol Chem. 2007;282:9042–9052. doi: 10.1074/jbc.M607041200. [DOI] [PubMed] [Google Scholar]
- Josephsen K, Takano Y, Frische S, Praetorius J, Nielsen S, Aoba T, Fejerskov O. Ion transporters in secretory and cyclically modulating ameloblasts: a new hypothesis for cellular control of preeruptive enamel maturation. Am J Physiol Cell Physiol. 2010;299:C1299–C1307. doi: 10.1152/ajpcell.00218.2010. [DOI] [PubMed] [Google Scholar]
- Lacruz RS, Nanci A, Kurtz I, Wright JT, Paine ML. Regulation of pH during amelogenesis. Calcif Tissue Int. 2010a;86:91–103. doi: 10.1007/s00223-009-9326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz RS, Nanci A, White SN, Wen X, Wang H, Zalzal SF, Luong VQ, Schuetter VL, Conti PS, Kurtz I, Paine ML. The sodium bicarbonate cotransporter (NBCe1) is essential for normal development of mouse dentition. J Biol Chem. 2010b;285:24432–24438. doi: 10.1074/jbc.M110.115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz RS, Smith CE, Moffatt P, Chang EH, Bromage TG, Bringas P, Jr, Nanci A, Baniwal SK, Zabner J, Welsh MJ, Kurtz I, Paine ML. Requirements for ion and solute transport, and pH regulation during enamel maturation. J Cell Physiol. 2012;227:1776–1785. doi: 10.1002/jcp.22911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Boron WF, Parker MD. Substrate specificity of the electrogenic sodium/bicarbonate cotransporter NBCe1-A (SLC4A4, variant A) from humans and rabbits. Am J Physiol Renal Physiol. 2013;304:F883–F899. doi: 10.1152/ajprenal.00612.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyaruu DM, Bronckers AL, Mulder L, Mardones P, Medina JF, Kellokumpu S, Oude Elferink RP, Everts V. The anion exchanger Ae2 is required for enamel maturation in mouse teeth. Matrix Biol. 2008;27:119–127. doi: 10.1016/j.matbio.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyaruu DM, Medina JF, Sarvide S, Bervoets TJ, Everts V, Denbesten P, Smith CE, Bronckers AL. Barrier formation: potential molecular mechanism of enamel fluorosis. J Dent Res. 2014;93:96–102. doi: 10.1177/0022034513510944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman GE, Waddell WJ. pH gradients in the developing teeth of young mice from autoradiography of [14C]DMO. Am J Physiol. 1977;232:F364–F367. doi: 10.1152/ajprenal.1977.232.4.F364. [DOI] [PubMed] [Google Scholar]
- Paine ML, Snead ML, Wang HJ, Abuladze N, Pushkin A, Liu W, Kao LY, Wall SM, Kim YH, Kurtz I. Role of NBCe1 and AE2 in secretory ameloblasts. J Dent Res. 2008;87:391–395. doi: 10.1177/154405910808700415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MD, Boron WF. The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol Rev. 2013;93:803–959. doi: 10.1152/physrev.00023.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MF, Chen AP, Parker MD, Boron WF. The SLC4 family of bicarbonate (HCO3−) transporters. Mol Aspects Med. 2013;34:159–182. doi: 10.1016/j.mam.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Takagi T, Suzuki M. Cyclical changes in pH in bovine developing enamel as sequential bands. Arch Oral Biol. 1991;36:227–231. doi: 10.1016/0003-9969(91)90090-h. [DOI] [PubMed] [Google Scholar]
- Simmer JP, Fincham AG. Molecular mechanisms of dental enamel formation. Crit Rev Oral Biol Med. 1995;6:84–108. doi: 10.1177/10454411950060020701. [DOI] [PubMed] [Google Scholar]
- Smith CE. Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med. 1998;9:128–161. doi: 10.1177/10454411980090020101. [DOI] [PubMed] [Google Scholar]
- Snead CM, Smith SM, Sadeghein N, Lacruz RS, Hu P, Kurtz I, Paine ML. Identification of a pH-responsive DNA region upstream of the transcription start site of human NBCe1-B. Eur J Oral Sci. 2011;119(Suppl 1):136–141. doi: 10.1111/j.1600-0722.2011.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriarte I, Banales JM, Saez E, Arenas F, Oude Elferink RP, Prieto J, Medina JF. Bicarbonate secretion of mouse cholangiocytes involves Na(+)-HCO(3)(−) cotransport in addition to Na+-independent Cl−/HCO3− exchange. Hepatology. 2010;51:891–902. doi: 10.1002/hep.23403. [DOI] [PubMed] [Google Scholar]
- Wright JT, Hall KI, Grubb BR. Enamel mineral composition of normal and cystic fibrosis transgenic mice. Adv Dent Res. 1996;10:270–274. doi: 10.1177/08959374960100022501. [DOI] [PubMed] [Google Scholar]
- Wen X, Lacruz RS, Smith CE, Paine ML. Gene-expression profile and localization of Na+/K+-ATPase in rat enamel organ cells. Eur J Oral Sci. 2014;122:21–26. doi: 10.1111/eos.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Zhang Y, He P, Kim J, Schneider R, Bronckers AL, Lyaruu DM, DenBesten PK. NBCe1 in mouse and human ameloblasts may be indirectly regulated by fluoride. J Dent Res. 2011;90:782–787. doi: 10.1177/0022034511398273. [DOI] [PMC free article] [PubMed] [Google Scholar]