Abstract
Background
Hemorrhage is the leading cause of preventable trauma-related deaths. We have previously shown that treatment with Tubastatin A (Tub A), a histone deacetylase (HDAC) 6 inhibitor, can improve survival in a rodent model of septic shock. The aims of present study were to determine whether selective inhibition of HDAC 6 can promote survival in a model of hemorrhagic shock (HS).
Methods
Experiment I (survival study): Wistar-Kyoto rats were subjected to hemorrhagic shock (55% volume blood loss), followed by intraperitoneal injection of either Tub A (70 mg/kg) dissolved in dimethyl sulfoxide (DMSO), or DMSO only (vehicle group) (n=8/group). Survival was monitored for 24 hours. Experiment II (physiologic study): Rats were subjected to a sub-lethal HS (40% blood loss), followed by the same treatment with Tub A (treatment group) or DMSO only (vehicle group, n=5/group). All animals were sacrificed 6 hours after hemorrhage, and heart and liver tissues were harvested. Sham animals were not subjected to hemorrhage and treatment (sham group, n=5/group). Cardiac mitochondria were isolated to study the pyruvate dehydrogenase (PDH; an essential enzyme for ATP production) activity. Liver tissue lysates were analyzed for markers of apoptosis (cytochrome c, cleaved caspase-3), and inflammation (high mobility group box 1 (HMGB1) by Western blotting.
Results
Severe hemorrhagic shock (55% blood loss) was associated with 75% mortality, which was significantly improved by Tub A treatment (37.5% mortality in 24 hours; P=0.048). Tub A also significantly enhanced the cardiac PDH activity compared to the vehicle group, while suppressing the hepatic HMGB1 expression, cytochrome c release, and caspase-3 activation.
Conclusions
Our study has demonstrated for the first time that selective inhibition of HDAC6 can improve survival in a rodent model of HS. The potential mechanisms include enhanced PDH activity, decreased inflammatory drive, and attenuated cellular apoptosis.
Keywords: hemorrhagic shock, Tubastatin A, pyruvate dehydrogenase, high mobility group box 1, apoptosis
INTRODUCTION
Injuries are now the leading cause of death for individuals 46 years and younger (1), with hemorrhagic shock (HS) accounting for a major portion of morbidity and mortality among the trauma patients (2). Even the patients that survive the acute episode of blood loss often develop multiple organ dysfunction syndrome (MODS) due to tissue hypoxia and systemic inflammation (2, 3). Tissue hypoxia with resultant anaerobic metabolism has long been considered a leading etiology of post-shock MODS (4). However, efforts to simply increase the oxygen delivery are often unsuccessful due to impaired mitochondrial function, which leads to inefficient oxygen utilization (4, 5). Pyruvate dehydrogenase (PDH) is a key mitochondrial enzyme responsible for the conversion of pyruvate to acetyl-CoA and is therefore essential for adenosine triphosphate (ATP) production. During hypoxia, the activity of PDH is dramatically decreased (6, 7), which impairs the ability of pyruvate to enter the Krebs cycle to generate ATPs.
Current resuscitation therapies largely focus on restoring tissue perfusion but have largely failed to address the specific cellular dysfunction caused by shock. Acetylation is rapidly emerging as a key mechanism that regulates the expression of numerous genes (epigenetic modulation through activation of nuclear histone proteins), as well as functions of multiple cytoplasmic proteins involved in key cellular functions such as cell survival, repair/healing, metabolism, signaling, and proliferation (3, 8, 9). It has been reported that, at the molecular level, hemorrhage leads to an imbalance in acetylation of proteins (hypo-acetylation of proteins compared to the normal state) and that treatment with histone deacetylase (HDAC) inhibitors can promptly restore the balance (10). More recent findings indicate that treatment with HDAC inhibitors can improve survival in rodent models of hemorrhagic and septic shock (11) as well as lethal burns (12), and attenuate acute lung injury following lipopolysaccharide injection (13).
Histone acetyltransferase (HAT) and HDAC enzymes control the addition and removal of acetyl groups and maintain a dynamic balance of steady-state protein acetylation (14, 15). So far, 18 HDAC isoforms have been identified and are grouped into four classes (16, 17): the Zn2+ dependent hydrolases class I, II and IV, and NAD+-dependent class III sirtuins. The class II HDACs have been subdivided into class IIa (HDAC4, 5, 7 and 9) and IIb (HDAC6 and 10) based on domain organization (17). Class IIb HDACs (HDAC6 and HDAC10) are distinguished from the class IIa sub-family in possessing tandem deacetylase domains. HDAC6 is unique among the classical HDAC family in which it is a cytoplasmic enzyme that regulates many important biological processes, including cell migration, immune synapse formation, viral infection, and the degradation of misfolded proteins (18). It also regulates immune synapse formation, promote HSP90 chaperone function and inhibit Treg function (16, 19–21), and plays a protective role following nervous system injuries (22).
We have recently reported that inhibition of HDAC 6 improves survival and attenuates stress responses in a lethal septic model (23, 24). However, it is unclear whether the same strategy would be beneficial following HS. The present study was designed to test the hypothesis that treatment with Tubastatin A (Tub A) would reduce the mortality in a rat model of hemorrhagic shock.
MATERIALS AND METHODS
1. Animals
This study adhered to the principles stated in The Guide for the Care and Use of Laboratory Animals (7th ed., National Academies Press, 1996), and was approved by the Institutional Animal Care and Use Committee. Male Wistar Kyoto rats (227–311 grams) were purchased from Charles River Breeding Laboratories, Inc (Chicago, IL). Rats were allowed food and water ad libitum.
2. Surgical Procedure
On the day of experimentation, Tub A (70mg/kg, Calbiochem, San Diego, CA) solution was prepared by dissolving it in dimethylsulfoxide (DMSO, 1μl/gram of body weight) (Sigma-Aldrich, St. Louis, MO). Anesthesia was induced with 4% isoflurane (Abbott Laboratories, North Chicago, IL) mixed with air in an induction chamber, and maintained by delivering 0.8–1.5% isoflurane via the nose cone using a veterinary multi-channel anesthesia delivery system and vaporizer (Kent Scientific Corporation, Torrington, CT). Core body temperature was maintained with an automated heating pad. Bupivacaine (0.2mL of 0.25%, APP pharmaceuticals, LLC. Schaumburg, IL) was injected for local anesthesia, and using a micro cutdown technique femoral artery was cannulated with polyethylene 50 catheter (Clay Adams, Sparks, MD), which was used for blood withdrawal and hemodynamic monitoring (Ponemah Physiology Platform, Gould Instrument Systems, Valley View, OH).
3. Lethal Hemorrhagic Shock (HS) Protocol (Survival Study)
Total blood volume was calculated [estimated total blood volume (mL) = weight (g) ×0.06 (mL/g) + 0.77(25)] and 35% of it was withdrawn over 10 minutes, followed by another 20% over the next 30 minutes. After un-resuscitated shock for 30 minutes, blood samples were taken and animals were randomly assigned to receive either Tub A (70mg/kg ip; dissolved in DMSO, n=8) or vehicle (DMSO, n=8) without any additional resuscitation fluids. Following treatment, the catheter was removed, femoral artery was ligated, and the skin closed. The animals were recovered from anesthesia and survival was monitored for 24 hours.
4. Sub-lethal HS Protocol and Tissue Harvest (Physiologic Study)
A sub-lethal HS (40% blood volume) protocol was designed to ensure survival of all the rats until the end of the experiment to eliminate any survival bias in tissue analysis. After obtaining baseline arterial blood samples, 40% of the blood volume was removed over 10 minutes, followed by un-resuscitated shock for 30 minutes. Then the animals were given either Tub A as described above (treatment group, n=5/group), or vehicle (DMSO, 1μl/g, vehicle group, n=5/group), without any additional resuscitation fluids. All the animals were killed 6 hours after hemorrhage, and heart and liver tissues were harvested, rinsed with cold saline, frozen in liquid nitrogen, and stored at −80°C. Tissue samples were also obtained from normal rats (no hemorrhage and no treatment) to serve as the sham (n=5).
5. Preparation of Whole Cell Proteins, and Fractionation of Cytosolic and Mitochondrial Proteins
Heart and liver tissue (25 mg wet weight per sample, 5/group) was homogenized by using an ultrasonic homogenizer (Branson Ultrasonics Corp, Danbury, CT), and processed using the Whole Cell Extraction Kit (Millipore, Temecula, CA) according to the manufacturer’s instructions. The mitochondrial and cytosolic protein fractions were isolated using the Mitochondria/Cytosol Fractionation Kit (Abcam, Cambridge, MA). Briefly, same amount of the tissues were homogenized in 1 ml of Cytosol Extraction Buffer Mix containing DTT and Protease Inhibitors (Abcam, Cambridge, MA), by using Dounce tissue grinder on ice for around 40 passes. After incubation on ice for 10 minutes, homogenates were centrifuged at 700g for 10 min at 4°C. Supernatants were collected and centrifuged again at 13,000×g for 30 min at 4°C, the resulting supernatants corresponded to the cytosolic fraction. The pellets were taken as the mitochondrial fraction and resuspended in 0.1 ml PBS for analysis of PDH activity.
6. PDH Activity Assay
PDH activity was measured in the cardiac mitochondria according to manufacturer’s instruction (Abcam, Inc, Cambridge, MA). Intact PDH was solubilized by adding 1 volume of detergent to 19 volume of each sample, incubated on ice for 10 mins, then centrifuged for 10 mins at 4°C. The supernatant was then collected, and the PDH enzyme was immunocaptured within each well by PDH antibodies and subjected to functional activity and quantitative microplate assays (Abcam, Inc, Cambridge, MA). Absorbance of each well was measured at 450nm on a microplate reader (SpectraMax Plus, Molecular Devices, LLC) at room temperature using a kinetic program for 25 minutes. PDH activity was expressed as the initial rate of reaction, determined from the slopes of the curves generated.
7. Western blotting
Equal amounts of proteins were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) on 12% polyacrylamide gels and transferred onto nitrocellulose membranes (Bio-Rad Laboratories). The membranes were blocked in Tris-Buffered Saline-Tween 20 (TBS-T) containing 5% milk (Bio-Rad Laboratories) and then incubated with the primary antibody diluted in TBS-T containing 4% bovine serum albumin (Sigma-Aldrich) at 4°C overnight. Primary antibodies purchased from Cell Signaling Technology (Danvers, MA) were diluted as follows: high mobility group box 1 (HMGB1) (D3E5) rabbit monoclonal antibody (1:1000), cytochrome c antibody (1:1000) and cleaved caspase-3 (Asp175) antibody (1:1000). The primary antibody was detected by incubating membranes with anti-mouse or anti-rabbit secondary antibodies diluted 1:3000 in TBS-T with 5% milk at room temperature for 1 hour. Detection was performed using the Odyssey CLx Infrared Imaging System (Li-Cor, Inc, Lincoln. NE), and detected bands were quantitatively analyzed by using the Image Studio Lite Ver 4.0 (Li-Cor, Inc).
8. Statistical Analysis
All continuous variables are expressed as means ± standard error of the mean (SEM). Data were analyzed using GraphPad Prism 6.0 statistical software (GraphPad Software, Inc. La Jolla, CA). The independent samples unpaired t-test was used for comparisons between 2 groups. A paired sample t-test was used to compare two variables within a group. Survival differences were compared by Kaplan-Meier curve with log rank analysis. In all cases, statistical significance was defined as P<0.05.
RESULTS
1. Lethal Model: Physiological Data
There were no significant differences in body weight, estimated blood volume, cannulation time, lactate, hemoglobin, base excess, between the two groups of animals. However, at end of hemorrhage, all animals showed significantly elevated lactate, negative base excess, and decreased hemoglobin (table 1), consistent with severe hemorrhagic shock.
Table 1.
Selected laboratory Data
| Time points
|
|||
|---|---|---|---|
| Variables | Groups | Baseline | End of Hemorrhage |
| Weight (g) | Vehicle | 248.90 ± 7.77 | -- |
| Tubastatin A | 272.70± 8.91 | -- | |
| Total Blood Loss (ml) | Vehicle | 8.63 ± 0.26 | -- |
| Tubastatin A | 9.42 ± 0.29 | -- | |
| Cannulation Time (min) | Vehicle | 29.80 ± 3.17 | -- |
| Tubastatin A | 34.50 ± 1.15 | -- | |
| Hemoglobin (g/dL) | Vehicle | 12.38 ± 0.36 | 8.92 ± 0.32* |
| Tubastatin A | 12.10 ± 0.43 | 9.25 ± 0.24* | |
| Lactate (mmol/L) | Vehicle | 1.48 ± 0.20 | 6.82 ± 1.03* |
| Tubastatin A | 1.30 ± 0.08 | 5.78 ± 0.26* | |
| Base Excess (mmol/L) | Vehicle | −0.72 ± 1.36 | −9.17 ± 1.38* |
| Tubastatin A | 0.40 ± 0.24 | −5.25 ± 1.38* | |
| BUN (mg/dL) | Vehicle | 15.60 ± 0.93 | 19.00 ± 1.67* |
| Tubastatin A | 16.00 ± 1.16 | 19.75 ± 1.32* | |
Sham=no hemorrhage, no resuscitation; Vehicle = hemorrhage, treatment with 1ul/g DMSO ip; Tubastatin A = Tubastatin A 70 mg/kg in 1ul/g DMSO ip; Baseline = before start of hemorrhage; End of Shock= end of total blood loss (55%). Data presented as group means ± SEM.
P < 0.05 compared with baseline value of the same group.
2. Survival in the Lethal Model
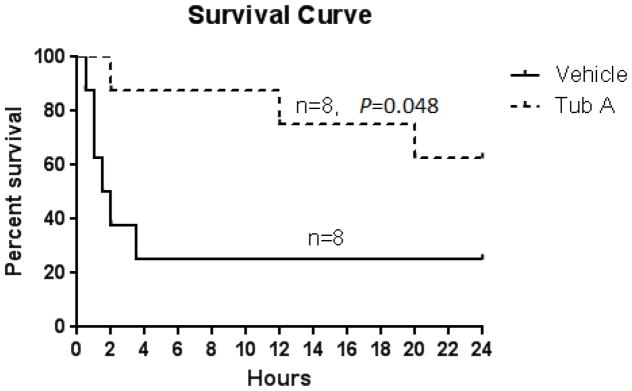
In this survival experiment, 6 out of 8 animals in vehicle group (75%), but only 3 out of 8 in the Tub A group (37.5%) died. Majority (75%) of animals from the vehicle group died within 3.5 hours after DMSO treatment, whereas Tub A treatment significantly prolonged the duration (average survival time > 24 hours; P=0.048 compared to the vehicle group, Figure 1).
Figure 1.
Tubastatin A promotes survival in a rat HS model. Animals were intraperitoneally administered 70mg/kg Tubastatin A in DMSO or vehicle DMSO only, at 30 minutes after hemorrhage (n=8/group). The Kaplan-Meier curve illustrates survival over the 24-hour period. Treatment with Tubastatin A significantly improved 24 hours survival compared to DMSO vehicle group (62.5% versus 25% survival, P=0.048). Tub A: Tubastatin A (70 mg/kg in DMSO).
3. Sub-lethal Model
All of animals in this model survived until tissue harvest, which eliminated any survival bias. There was no significant difference in body weight, total blood loss and cannulation time between different groups (P>0.05). Despite being sub-lethal, 40% blood loss still resulted in significant shock, lactic acidosis, and anemia (data not shown).
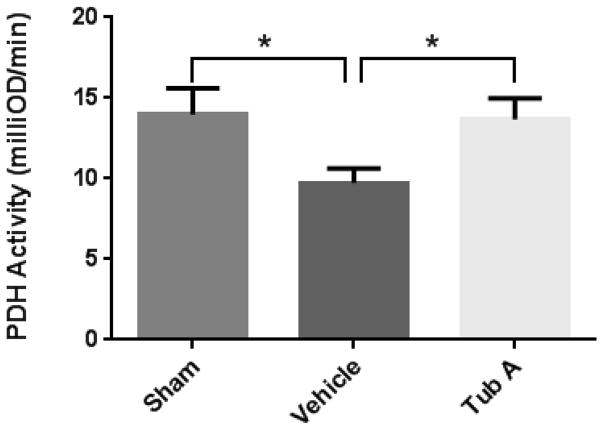
4. PDH activity
To investigate the effect of HS on mitochondrial metabolism, the activities of PDH in myocardial mitochondrial samples were studied. As shown in Figure 2, the 40% hemorrhage markedly decreased the PDH activity (9.73 ± 0.89 milliOD/min vs. 13.98 ± 1.63 milliOD/min for HS and sham groups, respectively; P=0.040). Tub A treatment significantly increased the PDH activity compared to the vehicle group (13.68 ± 1.33 milliOD/min vs. 9.73 ± 0.89 milliOD/min; P=0.041).
Figure 2.
Tubastatin A increases the activity of pyruvate dehydrogenase (PDH) in cardiac mitochondria. The values showed as change in milliOD over time (minute). Data presented as group mean values ± standard error of the mean (SEM). *P < 0.05 (sham vs. vehicle: P=0.040; Tub A vs. vehicle: P=0.041). Sham: no hemorrhage, no treatment; Vehicle: hemorrhage, treatment with DMSO; Tub A: Tubastatin A; PDH: pyruvate dehydrogenase.
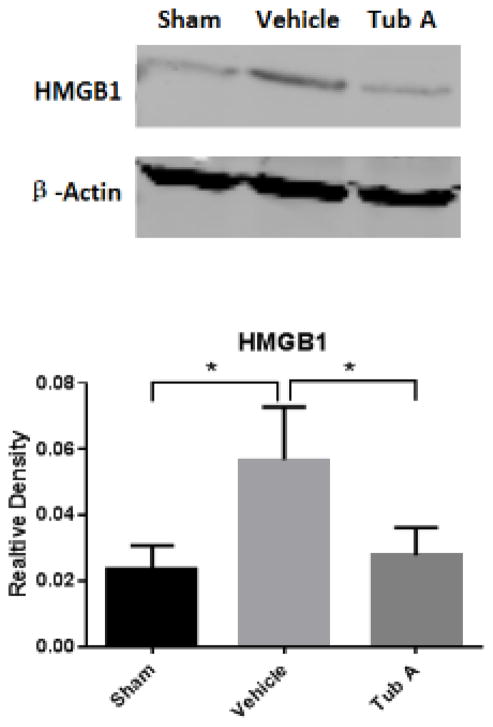
5. Expression of HMGB1 Protein
Western blot analysis confirmed that HS resulted in a sharp increase in the HMGB1 protein expression (P=0.043 compared to sham), which was attenuated by Tub A treatment (P=0.029, Figure 3).
Figure 3.
Tubastatin A attenuates HS-induced production of High Mobility Group Box 1 (HMGB1) protein in the liver. Protein bands quantified by densitometry were expressed as mean values ± SEM. *P < 0.05 compared to vehicle group (sham vs vehicle: P=0.043, Tub-A vs. vehicle: P=0.029); Tub A, Tubastatin A; Vehicle, DMSO treatment; Sham, no hemorrhage and no treatment.
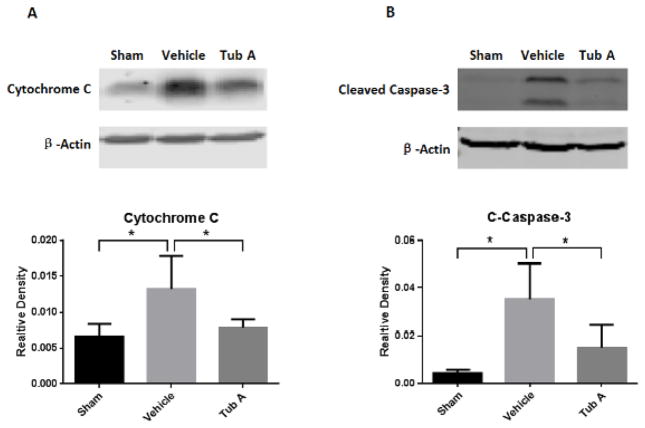
6. Analysis of Cytochrome C Release and Caspase-3 Activation
A unique 14 kDa band specific for cytochrome c was detected by Western blotting from the cytosolic fraction (Figure 4A). HS increased the cytochrome c release from the mitochondria compared to the sham animals (P=0.035), and post-shock administration of Tub A significantly decreased this release (P=0.031, Figure 4A). Two bands of 17 and 19 kDa from the cleavage of activated caspase-3 were observed in the vehicle treated animals (Figure 4B), which was also significantly attenuated by Tub-A treatment (P=0.030, Figure 4B).
Figure 4.
Tubastatin A decreases cytochrome c release (A) and caspase-3 activation (B) in the liver. Protein bands quantified by densitometry were expressed as mean values ± SEM. *P < 0.05 compared to vehicle group (P=0.035: sham vs. vehicle for cytochrome c, P=0.031: vehicle vs. Tub-A for cytochrome c, P=0.030: Tub-A vs. vehicle for activated caspase-3, P=0.020: sham vs. vehicle for activeated caspase-3); Tub A, Tubastatin A; Vehicle, DMSO treatment; Sham, no hemorrhage and no treatment.
DISCUSSION
Inhibition of HDAC is now being explored as a potential therapy for autoimmune diseases, cancers, and many neurodegenerative conditions (26–29). In the present study, we have demonstrated that administration of Tub A (HDAC6 inhibitor) can promote survival in a rodent model of hemorrhagic shock. Although the precise mechanisms are not clear, our data show that Tub A protects the cells against shock-induced damage.
It has been reported that compromised cellular energetics during hemorrhagic result not only from inadequate tissue perfusion but also due to impaired mitochondrial respiration and/or coupling (4, 5, 30). During shock, cells switch to anaerobic metabolism which is characterized by hyperlactataemia associated with an elevated lactate/pyruvate ratio, greater glucose utilization, and low energy production (31). Hypoxia blocks mitochondrial oxidative phosphorylation, and inhibits synthesis of ATP and reoxidation of NADH, leading to a decreased ATP/ADP ratio and an increased NADH/NAD ratio, as well as decreased PDH activity (31). Our current study shows that mitochondrial PDH activity decreased after HS, which is in accordance with the literature (7, 32–34). As PDH is a key mitochondrial enzyme responsible for the conversion of pyruvate to acetyl-CoA, low PDH activity could result in decreased ATP levels. Our data suggest that inhibition of HDAC6 could maintain the PDH activity during HS. Tub A treated animals showed significant higher PDH activity, which suggests that HDAC6 inhibition might either directly protect the mitochondria or potentially accelerate the recovery process through stimulation of mitochondrial biogenesis. It is not clear whether Tub A directly affects the PDH synthesis. Further experiments will have to be performed to investigate the precise underlying mechanisms.
In HS, the hypoperfusional state often triggers an exaggerated systemic inflammatory response which could lead to MODS and death (2). As a pro-inflammatory cytokine, HMGB1 protein plays a significant role in extracellular signaling associated with the inflammation (35–37). It functions as an alarmin (a danger-associated molecular pattern) in both infectious and non-infectious inflammatory conditions, such as autoimmune diseases, cancer, trauma, HS and ischemia perfusion injury (36, 38). The present study shows that HS increases the expression of HMGB1 in the liver, which is consistent with previous findings (36). Tub A treatment significantly attenuated the HMGB1 expression, suggesting that suppression of inflammatory response might be one of the possible mechanisms for promoting survival in this model.
In addition to inducing an inflammatory response, HMGB1 can also lead to an increase in cytochrome c release from the mitochondria into the cytosol, and the cleavage of procaspase-3 (39). Cytochrome c is a well conserved electron-transport protein and is part of the respiratory chain localized to mitochondrial intermembrane space (40). Upon apoptotic stimulation, cytochrome c released from mitochondria associates with procaspase-9/Apaf 1. This complex processes caspase-9 from inactive pro-enzyme to its active form (41), and further triggers caspase-3 activation, and eventually leads to apoptosis (42). We already know that HS can induce cellular apoptosis (43). In the present study, HS resulted in an increase in cytochrome c release and activated the caspase-3, while post-shock administration of Tub A suppressed these changes to protect the cells from apoptosis.
The current study was designed as a proof-of-concept experiment and as such it has certain limitations that must be acknowledged. Only PDH activity was tested for the mitochondrial metabolic analysis. Although it is an important component of the mitochondrial metabolic process, other key components of the ATP production chain should also be analyzed. Further studies are also needed to determine the exact mechanisms through which HDAC6 inhibitor regulates the PDH activity.
In summary, we present initial evidence that Tub A can improve survival in a rat model of HS. Our results indicate that treatment with Tub A could potentially: 1) make the cells more resistant to ischemia through an improvement in the PDH activity, 2) inhibit inflammation by reducing HMGB1 protein expression, and 3) decrease cellular apoptosis by suppressing expression of HMGB1 protein, diminishing release of cytochrome c and lessening the activation of caspase 3.
Acknowledgments
This research was funded by a grant from NIH RO1 GM084127 to HBA. Data presented at the 45th Annual Meeting of Western Trauma Association, March 1–6, 2015 in Telluride, Colorado.
Footnotes
Author Contribution: ZC and WH carried out the animal experiments and obtained the data. ZC, WH, YL, BL and YL performed the tissue analysis. ZC and YL wrote the manuscript, and IH, TD, YL and HBA performed critical revisions. HBA obtained the funding for this study.
References
- 1.Rhee P, Joseph B, Pandit V, Aziz H, Vercruysse G, Kulvatunyou N, Friese RS. Increasing trauma deaths in the United States. Annals of surgery. 2014;260(1):13–21. doi: 10.1097/SLA.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 2.Wilson M, Davis DP, Coimbra R. Diagnosis and monitoring of hemorrhagic shock during the initial resuscitation of multiple trauma patients: a review. The Journal of emergency medicine. 2003;24(4):413–22. doi: 10.1016/s0736-4679(03)00042-8. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Alam HB. Creating a pro-survival and anti-inflammatory phenotype by modulation of acetylation in models of hemorrhagic and septic shock. Advances in experimental medicine and biology. 2012;710:107–33. doi: 10.1007/978-1-4419-5638-5_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crouser ED. Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion. 2004;4(5–6):729–41. doi: 10.1016/j.mito.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Fink MP. Cytopathic hypoxia. Mitochondrial dysfunction as mechanism contributing to organ dysfunction in sepsis. Critical care clinics. 2001;17(1):219–37. doi: 10.1016/s0749-0704(05)70161-5. [DOI] [PubMed] [Google Scholar]
- 6.Andersen LW, Liu X, Peng TJ, Giberson TA, Khabbaz KR, Donnino MW. Pyruvate Dehydrogenase Activity and Quantity Decreases After Coronary Artery Bypass Grafting: A Prospective Observational Study. Shock. 2014 doi: 10.1097/SHK.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merante F, Mickle DA, Weisel RD, Li RK, Tumiati LC, Rao V, Williams WG, Robinson BH. Myocardial aerobic metabolism is impaired in a cell culture model of cyanotic heart disease. The American journal of physiology. 1998;275(5 Pt 2):H1673–81. doi: 10.1152/ajpheart.1998.275.5.H1673. [DOI] [PubMed] [Google Scholar]
- 8.Storey KB. Regulation of hypometabolism: insights into epigenetic controls. The Journal of experimental biology. 2015;218(Pt 1):150–9. doi: 10.1242/jeb.106369. [DOI] [PubMed] [Google Scholar]
- 9.Guan KL, Xiong Y. Regulation of intermediary metabolism by protein acetylation. Trends in biochemical sciences. 2011;36(2):108–16. doi: 10.1016/j.tibs.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzales E, Chen H, Munuve R, Mehrani T, Britten-Webb J, Nadel A, Alam HB, Wherry D, Burris D, Koustova E. Valproic acid prevents hemorrhage-associated lethality and affects the acetylation pattern of cardiac histones. Shock. 2006;25(4):395–401. doi: 10.1097/01.shk.0000209522.28120.c8. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Li Y, Chong W, Deperalta DK, Duan X, Liu B, Halaweish I, Zhou P, Alam HB. Creating a prosurvival phenotype through a histone deacetylase inhibitor in a lethal two-hit model. Shock. 2014;41(2):104–8. doi: 10.1097/SHK.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo HM, Hu S, Bai HY, Wang HB, Du MH, Lin ZL, Ma L, Wang H, Lv Y, Sheng ZY. Valproic acid treatment attenuates caspase-3 activation and improves survival after lethal burn injury in a rodent model. Journal of burn care & research: official publication of the American Burn Association. 2014;35(2):e93–8. doi: 10.1097/BCR.0b013e31828a8d32. [DOI] [PubMed] [Google Scholar]
- 13.Ji MH, Li GM, Jia M, Zhu SH, Gao DP, Fan YX, Wu J, Yang JJ. Valproic acid attenuates lipopolysaccharide-induced acute lung injury in mice. Inflammation. 2013;36(6):1453–9. doi: 10.1007/s10753-013-9686-z. [DOI] [PubMed] [Google Scholar]
- 14.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–5. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 15.Parbin S, Kar S, Shilpi A, Sengupta D, Deb M, Rath SK, Patra SK. Histone deacetylases: a saga of perturbed acetylation homeostasis in cancer. J Histochem Cytochem. 2014;62(1):11–33. doi: 10.1369/0022155413506582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shakespear MR, Halili MA, Irvine KM, Fairlie DP, Sweet MJ. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. 2011;32(7):335–43. doi: 10.1016/j.it.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Alam HB. Modulation of acetylation: creating a pro-survival and anti-inflammatory phenotype in lethal hemorrhagic and septic shock. Journal of biomedicine & biotechnology. 2011;2011:523481. doi: 10.1155/2011/523481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valenzuela-Fernandez A, Cabrero JR, Serrador JM, Sanchez-Madrid F. HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends in cell biology. 2008;18(6):291–7. doi: 10.1016/j.tcb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 19.de Zoeten EF, Wang L, Butler K, Beier UH, Akimova T, Sai H, Bradner JE, Mazitschek R, Kozikowski AP, Matthias P, et al. Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3(+) T-regulatory cells. Molecular and cellular biology. 2011;31(10):2066–78. doi: 10.1128/MCB.05155-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Molecular cell. 2005;18(5):601–7. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Serrador JM, Cabrero JR, Sancho D, Mittelbrunn M, Urzainqui A, Sanchez-Madrid F. HDAC6 deacetylase activity links the tubulin cytoskeleton with immune synapse organization. Immunity. 2004;20(4):417–28. doi: 10.1016/s1074-7613(04)00078-0. [DOI] [PubMed] [Google Scholar]
- 22.Rivieccio MA, Brochier C, Willis DE, Walker BA, D’Annibale MA, McLaughlin K, Siddiq A, Kozikowski AP, Jaffrey SR, Twiss JL, et al. HDAC6 is a target for protection and regeneration following injury in the nervous system. Proc Natl Acad Sci U S A. 2009;106(46):19599–604. doi: 10.1073/pnas.0907935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao T, Li Y, Bronson RT, Liu B, Velmahos GC, Alam HB. Selective histone deacetylase-6 inhibition attenuates stress responses and prevents immune organ atrophy in a lethal septic model. Surgery. 2014;156(2):235–42. doi: 10.1016/j.surg.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao T, Li Y, Liu B, Halaweish I, Mazitschek R, Alam HB. Selective inhibition of histone deacetylase 6 alters the composition of circulating blood cells in a lethal septic model. The Journal of surgical research. 2014;190(2):647–54. doi: 10.1016/j.jss.2014.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HB, Blaufox MD. Blood volume in the rat. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1985;26(1):72–6. [PubMed] [Google Scholar]
- 26.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nature reviews Cancer. 2006;6(1):38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, de Zoeten EF, Greene MI, Hancock WW. Immunomodulatory effects of deacetylase inhibitors: therapeutic targeting of FOXP3+ regulatory T cells. Nature reviews Drug discovery. 2009;8(12):969–81. doi: 10.1038/nrd3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dompierre JP, Godin JD, Charrin BC, Cordelieres FP, King SJ, Humbert S, Saudou F. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington’s disease by increasing tubulin acetylation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27(13):3571–83. doi: 10.1523/JNEUROSCI.0037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nature reviews Drug discovery. 2008;7(10):854–68. doi: 10.1038/nrd2681. [DOI] [PubMed] [Google Scholar]
- 30.Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 2014;5(1):66–72. doi: 10.4161/viru.26907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy B. Lactate and shock state: the metabolic view. Current opinion in critical care. 2006;12(4):315–21. doi: 10.1097/01.ccx.0000235208.77450.15. [DOI] [PubMed] [Google Scholar]
- 32.Patel TB, Olson MS. Regulation of pyruvate dehydrogenase complex in ischemic rat heart. The American journal of physiology. 1984;246(6 Pt 2):H858–64. doi: 10.1152/ajpheart.1984.246.6.H858. [DOI] [PubMed] [Google Scholar]
- 33.Bogaert YE, Sheu KF, Hof PR, Brown AM, Blass JP, Rosenthal RE, Fiskum G. Neuronal subclass-selective loss of pyruvate dehydrogenase immunoreactivity following canine cardiac arrest and resuscitation. Experimental neurology. 2000;161(1):115–26. doi: 10.1006/exnr.1999.7250. [DOI] [PubMed] [Google Scholar]
- 34.Lewandowski ED, Johnston DL. Reduced substrate oxidation in postischemic myocardium: 13C and 31P NMR analyses. The American journal of physiology. 1990;258(5 Pt 2):H1357–65. doi: 10.1152/ajpheart.1990.258.5.H1357. [DOI] [PubMed] [Google Scholar]
- 35.Campana L, Bosurgi L, Rovere-Querini P. HMGB1: a two-headed signal regulating tumor progression and immunity. Current opinion in immunology. 2008;20(5):518–23. doi: 10.1016/j.coi.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Molecular medicine (Cambridge, Mass) 2008;14(7–8):476–84. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 38.Kim JY, Park JS, Strassheim D, Douglas I, Diaz del Valle F, Asehnoune K, Mitra S, Kwak SH, Yamada S, Maruyama I, et al. HMGB1 contributes to the development of acute lung injury after hemorrhage. American journal of physiology Lung cellular and molecular physiology. 2005;288(5):L958–65. doi: 10.1152/ajplung.00359.2004. [DOI] [PubMed] [Google Scholar]
- 39.Gwak GY, Moon TG, Lee DH, Yoo BC. Glycyrrhizin attenuates HMGB1-induced hepatocyte apoptosis by inhibiting the p38-dependent mitochondrial pathway. World journal of gastroenterology: WJG. 2012;18(7):679–84. doi: 10.3748/wjg.v18.i7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schagger H. Respiratory chain supercomplexes of mitochondria and bacteria. Biochimica et biophysica acta. 2002;1555(1–3):154–9. doi: 10.1016/s0005-2728(02)00271-2. [DOI] [PubMed] [Google Scholar]
- 41.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91(4):479–89. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86(1):147–57. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 43.Van Way CW, 3rd, Dhar A, Morrison DC, Longorio MA, Maxfield DM. Cellular energetics in hemorrhagic shock: restoring adenosine triphosphate to the cells. The Journal of trauma. 2003;54(5 Suppl):S169–76. doi: 10.1097/01.TA.0000047226.36678.EE. [DOI] [PubMed] [Google Scholar]