Summary
Sensory tissues exposed to the environment, such as skin, olfactory epithelia and taste buds, continuously renew; therefore, peripheral neurons must have mechanisms to maintain appropriate innervation patterns. Although somatosensory neurons regenerate after injury, little is known about how these neurons cope with normal target-organ changes. To elucidate neuronal plasticity in healthy skin, we analyzed the structure of Merkel-cell afferents, which are gentle touch receptors, during skin remodeling that accompanies mouse hair-follicle regeneration. The number of Merkel cells is reduced by 90% and axonal arbors are simplified during active hair growth. These structures rebound within just days. Computational modeling predicts that Merkel-cell changes are probabilistic but myelinated branch stability depends on Merkel-cell inputs. Electrophysiology and behavior demonstrate that tactile responsiveness is less reliable during active growth than in resting skin. These results reveal that somatosensory neurons display structural plasticity at the cost of impairment in the reliability of encoding gentle touch.
Keywords: tactile, somatosensory, neuronal plasticity, Merkel cell, anagen, hair cycle
Graphical Abstract
Introduction
Sensory stimuli are encoded by the peripheral nervous system, which displays a remarkable ability to regenerate (Chen et al., 2007). Several sensory tissues continuously renew, which is proposed to play a role in maintenance and sensory optimization (Feng et al., 2014; Mouret et al., 2009). In skin, tactile afferents innervate discrete sensory structures such as touch domes or hair follicles, which regenerate throughout an animal’s lifetime (Plikus and Chuong, 2008; Schneider et al., 2009). Although the structural plasticity of somatosensory neurons that innervate skin has been studied in the context of injury and disease, little is known about their plasticity in healthy tissue (Cheng et al., 2010; Rajan et al., 2003) Thus, hair growth provides an opportunity to study how somatosensory afferents respond to normal target-organ remodeling.
Mouse hair growth is synchronized, resulting in periods of massive cellular turnover and skin structural changes (Muller-Rover et al., 2001). Two reports indicate that innervation density increases during hair growth (Botchkarev et al., 1997; Peters et al., 2001). Similarly, Merkel cells, which are epidermal components of gentle touch receptors, have been reported to be more abundant during follicle growth (Moll et al., 1996; Nakafusa et al., 2006). These studies suggest that cutaneous neurons and Merkel cells engage plasticity mechanisms during hair-follicle regeneration; however, the dynamics and physiological consequences of neuronal plasticity in touch receptors are unknown.
To address this gap, we analyzed the structure and function of Merkel-cell afferents over the mouse hair cycle. Merkel cells and their afferents produce slowly adapting type I (SAI) responses, which encode pressure and object features (Johnson et al., 2000; Maksimovic et al., 2014; Woo et al., 2014). We report that Merkel-cell afferents undergo rapid remodeling during healthy skin renewal, which is accompanied by transient impairment in gentle-touch responses.
Results
The hair cycle stimulates touch-receptor remodeling
To assess neuronal plasticity in healthy skin, we analyzed peripheral end organs of Merkel-cell afferents in the mouse hindlimb throughout complete hair cycles, which are governed by molecular crosstalk between follicle stem cells and neighboring cells (Alonso and Fuchs, 2006 Jahoda and Christiano, 2011; Plikus and Chuong, 2008). The hair cycle comprises three stages: telogen, a resting phase when stem cells are quiescent; anagen, the growth phase when follicles nearly triple in length; and catagen, a regression phase (Fuchs, 2009; Schneider et al., 2009). In pigmented mice, skin color darkens during anagen, which facilitates hair-cycle staging (Figure 1A; Muller-Rover et al., 2001).
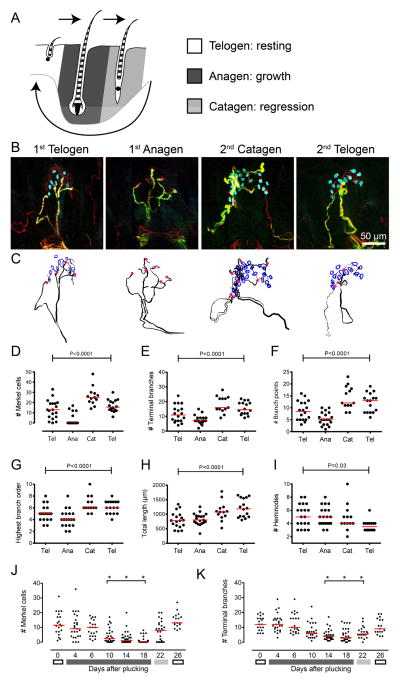
Figure 1. Touch receptors show plasticity during the hair cycle.
(A) Diagram of the mouse hair cycle. (B) Representative axial projections of touch domes in Atoh1/nGFP mouse hindlimb during a spontaneous hair cycle: first telogen (P28), late anagen (stages IV–VI; P39), catagen (stages III–XIII; P40) and second telogen (P66). K8: cyan, NFH: red, MBP: green. Scale applies to all panels. (C) Neurolucida tracing of projections from B. Merkel cells are outlined in blue. Magenta dots mark heminodes. (D–I) Quantitative morphometry showing Merkel-cell number (D), number of terminal branches (E), number of branch points (F), highest branching order (G), total afferent length (H) and number of heminodes, identified as endpoints of MBP staining (I). P values are results from one-way ANOVAs (N=13–19 touch domes from 3–4 animals per group). (J–K) Quantification of Merkel cells (J) and terminal neurites in NFH+ afferents during an induced hair cycle (K; N=17–31 touch domes from 3–4 mice per group; P<0.0001 for both measures, one-way ANOVA). Asterisks denote groups that significantly differed from Day 0 (P68–70) by Tukey’s post hoc comparisons. Grayscale bars indicate hair-cycle stage as in A. For D–K, each point represents a single touch dome and red lines denote median values. See also Videos 1–4 for 3D reconstructions and Figures S1–S3.
Whole-mount immunohistochemistry was used to visualize touch-receptor endings in their entirety (Figure 1B). Specimens were stained for markers of mature Merkel cells (Keratin 8; K8; Kim and Holbrook, 1995), myelinated afferents (Neurofilament H; NFH) and myelin (myelin basic protein; MBP; Lesniak et al., 2014). Merkel-cell afferents were identified based on morphology and juxtaposition to guard hair follicles (Iggo and Muir, 1969), which were recognized by their NFH-positive lanceolate endings (Bai et al., 2015; Li et al., 2011). Merkel-cell afferents, which branch to contact Merkel cells, can be distinguished from other NFH-positive afferents based on two additional anatomical features. First, their myelin end-points lie just beneath the epidermal-dermal border (Lesniak et al., 2014). Second, their unmyelinated neurites penetrate the epidermis. In telogen, 94% of afferents that met these criteria innervated Merkel cells (N=97 and Lesniak et al., 2014).
We first asked whether tactile afferents undergo plasticity during the spontaneous hair cycle (P28–P70 in hindlimb skin; Figure S1). Three-dimensional reconstructions were used to trace Merkel-cell afferents from the dermal nerve plexus to the epidermis (Figure 1C and Videos 1–4). Morphometric quantification revealed that Merkel cells and terminal neurites were unexpectedly dynamic over the hair cycle (Figure 1D–E). Surprisingly, two thirds of touch domes lacked K8-positive Merkel cells in late anagen. Anagen afferents also displayed few terminal branches (Figure 1E) and, in many cases, lacked branching neurites that normally extend from myelin endpoints. In these arbors, NFH immunoreactivity was often diffuse, resembling swollen neuronal terminals observed during Wallerian degeneration (Figure S2; Dubovy and Aldskogius, 1996; Hsieh et al., 2000). Merkel cells and terminal branches rebounded in catagen. These results reveal that Merkel-cell afferents are capable of dramatic structural plasticity during the spontaneous hair cycle.
To assess the extent of neuronal remodeling, we next analyzed arbor complexity. The number of branch points and highest branch order, a measure of nested branching, fluctuated in a pattern that mirrored Merkel-cell numbers (Figure 1F–1G). Unexpectedly, branching parameters only correlated with Merkel-cell number in catagen (Figure S3; P=0.002–0.01; Pearson’s correlation). Total afferent length progressively increased over the hair cycle (Figure 1H), whereas number of heminodes, which are sites of spike initiation at myelin end-points (Figure 1I; Lesniak et al., 2014), decreased from anagen to second telogen (Figure 1I). Together, these data suggest unmyelinated branches become more complex as Merkel-cell numbers rebound whereas myelinated branches refine over the hair cycle.
To analyze temporal dynamics of end-organ remodeling, we used a controlled model of hair cycling. Anagen was induced by plucking hair from 2-cm2 patches of hindlimb skin (Plikus et al., 2008) and specimens were analyzed at 2–4 day intervals over a complete cycle (Figure S1; Plikus et al., 2008). The number of Merkel cells per touch dome progressively decreased from telogen (day 0) through late anagen (days 10–18), with 63% of touch domes lacking Merkel cells at day 18 (Figure 1J). Remaining Merkel-cell clusters were also smaller in late anagen (day 18: 3.6±2.7 Merkel cells, mean±SD, N=10) compared with telogen (day 0: 12.6±7.7, N=20; P=0.001, Student’s two-tailed t test), resulting in a 90% reduction of Merkel cells overall. Merkel cells recovered in catagen (day 22; Figure 1J), only four days after the nadir. Terminal neurites decreased in tandem with Merkel cells through anagen, and then arbors returned to resting-stage structures within eight days (day 26; Figure 1K). Thus, Merkel cell-neurite complexes show rapid structural changes during hair growth.
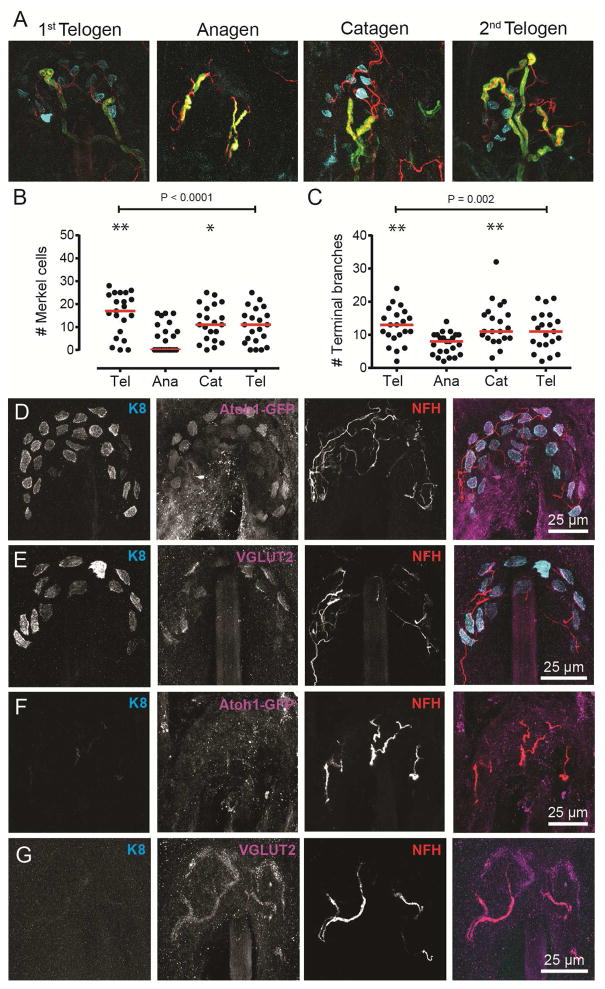
To determine whether remodeling is a general feature of touch-dome afferents, we reconstructed touch domes at a second body site (Figure 2A). Back skin was chosen to match previous studies (Moll et al., 1996; Nakafusa et al., 2006). As in hindlimb skin, back-skin afferents had fewest Merkel cells and terminal branches in late anagen, with 52% of anagen touch domes lacking K8-positive Merkel cells (N=23; Figure 2B–C). We also tested alternate Merkel-cell markers in anagen, catagen and telogen. We found that 77% of K8-positive Merkel cells expressed the early marker Atoh1-GFP (N=391 from 5 mice; Figure 2D; Wright et al., 2015). VGLUT2 immunoreactivity, which is found in mature Merkel cells and touch-dome afferents (Haeberle et al., 2004), co-localized with K8 in 96% of Merkel cells (N=489 from 5 mice; Figure 2E). Importantly, touch domes that lacked K8-positive cells showed no Atoh1-GFP expression (Figure 2F; N=19 touch domes) or VGLUT2-positive epidermal cells (Figure 2G; N=11 touch domes). Thus, neither regional differences in end-organ plasticity nor selective loss of K8 explains the changes in Merkel-cell numbers we observed.
Figure 2. Afferent remodeling is observed across skin regions and Merkel-cell markers.
(A) Axial projections of touch domes in back skin during a spontaneous hair cycle: first telogen (P23), anagen (stages IV–VI; P35), catagen (stages I–II; P44) and second telogen (P70). K8: cyan, NFH: red, MBP: green. (B–C) Quantitative morphometry of Merkel cells (B) and terminal branches (C). P values are results from one-way ANOVAs (N=21–23 touch domes from 2–3 animals group). Red lines: medians. Asterisks indicate groups significantly different from anagen based on Tukey’s post hoc comparisons (*P=0.03, **P<0.003). (D–G) Axial projections of touch domes containing (D, E) or lacking (F, G) K8+ Merkel cells stained with antibodies against GFP (Atoh1A1GFP/A1GFP mice; D, F) or VGLUT2 (E, G) in hindlimb skin during a spontaneous hair cycle. K8: cyan, NFH: red, Atoh1-GFP or VGLUT2: magenta. Images are representative of 43–46 touch domes from five mice per marker. See also Figure S4.
We wondered whether neuronal sprouting outside of touch domes might explain the abundance of anagen afferents lacking Merkel cells. Indeed, the density of myelinated afferents terminating near the epidermis increased in anagen compared with catagen (Figure S4). Thus, newly sprouted myelinated branches could account for some low-complexity arbors in late anagen; however, sprouting cannot explain the progressive decline in Merkel cells and neurites between telogen and late anagen (Figure 1J).
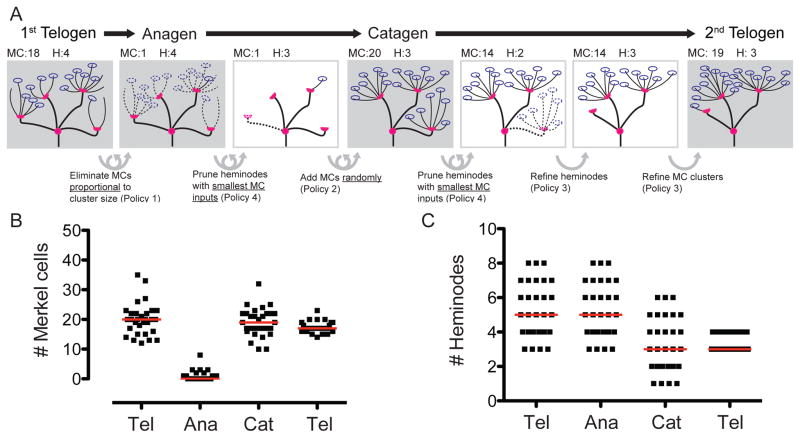
To identify principles that specify how arbors change over the hair cycle, we built a computational model to evaluate policies of Merkel-cell and heminode dynamics (Figure 3). Thirty end organs were auto-generated by clustering Merkel cells at heminodes, whose numbers were probabilistically drawn from first-telogen distributions (Figure 1D, I). Each end organ then went through a complete hair cycle, with iterations of remodeling following four policies: 1) the probability of losing a Merkel cell from a cluster is proportional to cluster size, 2) the probability of adding a Merkel cell is random across heminodes; 3) myelinated branches refine to achieve a range of 3–4 heminodes, 4) heminodes that lack Merkel cells are most likely to be pruned (Figure 3A). These simple rules produced distributions of Merkel cells (Figure 3B) and heminodes (Figure 3C) in anagen, catagen and telogen that agreed with experimental observations (compare with Figure 1D, I). Thus, we propose that Merkel-cell loss and addition is probabilistic among heminodes, but that stability of heminodes, and thus myelinated branches, depends on Merkel-cell inputs.
Figure 3. Computational simulations reveal principles of end-organ remodeling.
(A) Schematic of a simulation. Blue ovals represent Merkel cells; magenta half-circles denote heminodes; dashed lines depict eliminated structures; looped arrows indicate iterative steps. Hair-cycle stages, Merkel-cell (MC) and heminode (H) counts are indicated above and remodeling policies are summarized below. (B,C) Each square represents a single auto-generated afferent that progressed through simulation. Predicted distributions of Merkel-cell numbers (B) and heminodes (C) at the indicated hair-cycle stages. Red lines denote medians.
Touch-receptor reliability is compromised during skin remodeling
We next assessed the functional consequences of afferent plasticity. To determine whether force-sensing machinery was intact in anagen, we assessed expression of a transgenic Piezo2-GFP reporter (Figure S4; Woo et al., 2014). Piezo2, a mechanosensitive ion channel critical for gentle touch (Ranade et al., 2014), was observed in all touch-dome afferents, including those that lacked terminal neurites, which suggests that anagen afferents can transduce mechanical stimuli.
To measure touch-evoked firing of Merkel-cell afferents over the spontaneous hair cycle, we used an ex vivo skin-nerve preparation to record from Aβ low-threshold mechanoreceptors (LTMRs) whose end organs were visualized by FM1-43 labeling (Maksimovic et al., 2014; Wellnitz et al., 2010). Merkel-cell afferents produce SAI responses, which are characterized by receptive fields limited to touch domes, high frequency dynamic firing, low frequency sustained firing with irregular interspike intervals and a lack of response to guard-hair movement (Iggo and Muir, 1969). In an unbiased survey of Aβ LTMRs, we observed canonical SAI responses in 5% (1/20) of anagen afferents compared with 26% (5/19) in telogen (Table S1). These results indicate that SAI responses occur less frequently in anagen, when Merkel-cell afferents show reduced arbor complexity.
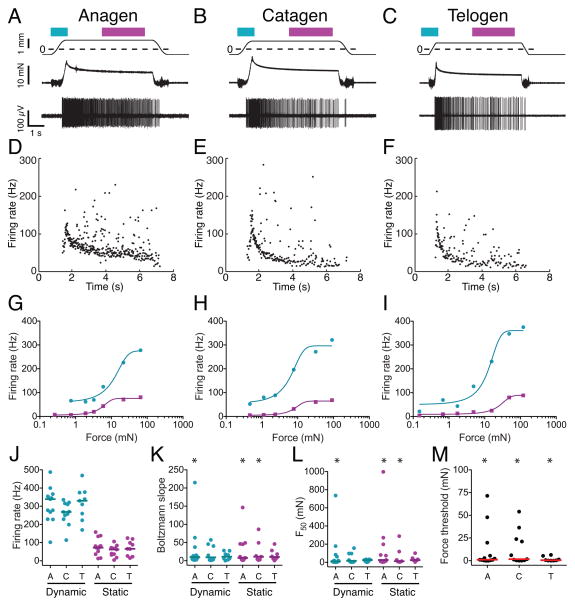
We next used targeted recordings to analyze SAI responses in spontaneous anagen (Figure 4A, D), catagen (Figure 4B, E) and telogen (Figure 4C, F). The sensitivity of each afferent’s response was estimated by fitting force-firing rate curves during dynamic and static stimulus phases with Boltzmann relations (Figure 4G–I). Boltzmann slope was used as a measure of mechanical sensitivity, with lower values signifying steeper curves and, thus, higher sensitivity. Mechanical set-points were estimated from the mid-points of force-firing curves (F50), as well as force threshold, defined as the lowest stimulus amplitude that elicited reliable firing. Maximal firing rates did not differ across hair-cycle stages (Figure 4J).
Figure 4. SAI response properties are less reliable during the hair cycle.
(A–C) Representative traces show SAI responses in spontaneous anagen (A), catagen (B) and telogen (C). Top traces: Displacements, with dashed ‘0’ lines indicating point of probe contact with skin. Bars denote stimulus phases (teal: dynamic; magenta: static). Middle traces: Forces. Lower trace: Action-potential trains. (D–F) Instantaneous firing frequency versus time for the traces above. (G–I) Stimulus-response curves of the units in A–C. (J–M) Aggregate data in anagen (A; N=13 units), catagen (C; N=11 units) and telogen (T; N=9 units). (J) Maximal firing rates. (K) Boltzmann slopes. (L) Mid-points of Boltzmann fits (F50). (M) Force thresholds. Asterisks mark non-normal distributions (P≤0.05, Kolmogorov-Smirnov test). Lines denote medians. See also Table S2.
When stimulus-response parameters of Merkel-cell afferents were compared, we found that median values were indistinguishable across hair-cycle stages (P>0.05; Kruskal-Wallis tests); however, fit parameters followed distinct distributions (Figure 4K, L and Table S2). Boltzmann slopes and F50 values were normally distributed in telogen but followed log normal distributions in anagen. Catagen represented an intermediate phase, with parameters following normal distributions for dynamic firing but not for static firing. These different distributions precluded direct comparison of variance between populations; however, anagen data displayed more pronounced rightward skew than telogen populations (Table S2). Overall, 29% of afferents in anagen and catagen displayed markedly lower sensitivities (Figure 4K) and high mechanical set-points compared with telogen afferents (Figure 4L, M). Together, these data suggest that in the midst of afferent remodeling, Merkel-cell afferents are mechanosensitive but their ability to encode gentle touch is compromised.
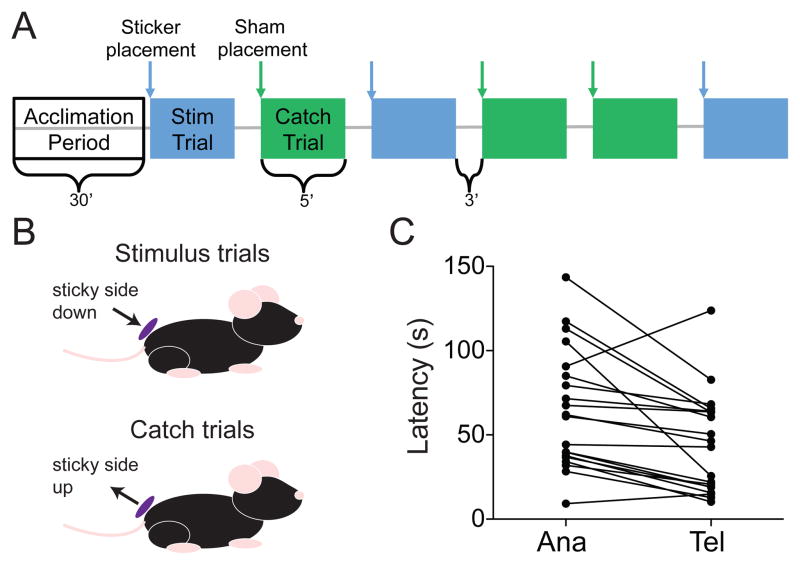
We wondered whether these physiological differences might have behavioral consequences. To evaluate the contribution of SA afferents in hairy skin, we modified a tape response assay (Bouet et al., 2009; Ranade et al., 2014) by applying a small sticker to the lower back after hair removal (Figure 5A–B). This paradigm delivers sustained pressure, whereas stroking or air puff represents a dynamic stimulus that engages rapidly adapting receptors (Bai et al., 2015; Garrison et al., 2012). Mice had fewer detections, defined as touching the sticker with either the nose or paw, in anagen (N=109/117 trials in 20 mice) compared with telogen (N=114/115 trials in 20 mice; P=0.036; two-tailed Fisher’s exact test). Moreover, detection latencies were significantly longer in anagen than in telogen (Figure 5C; P=0.0014; Wilcoxon matched-pairs signed rank test). Thus, we conclude that behavioral responsiveness to tactile pressure is reduced in anagen.
Figure 5. Behavioral touch responses are impaired during anagen.
(A) Behavioral testing paradigm. The order of stimulus (blue) and catch trials (green) was randomized. (B) Diagram of sticker placement. (C) Latency to sticker detection at each hair-cycle stage. Dots represent the mean latency for 3–6 trials per mouse. Lines connect latencies from individual mice (P=0.001; Wilcoxon matched-pairs signed rank test).
Discussion
This study reveals the remarkable plasticity of the peripheral nervous system in healthy skin. Our results demonstrate that Merkel-cell afferents are capable of dramatic remodeling over a few days. Although the turnover of sensory epithelia is established in tastebuds and the olfactory system, the mechanisms that preserve proper innervation in these tissues are not well defined (Castillo et al., 2014; Ma et al., 2014; Tsai and Barnea, 2014). We found that the skin’s nervous system undergoes renewal as a part of normal epithelial remodeling, which highlights the utility of the mouse hair cycle as a system for studying pathways that govern intrinsic neuronal remodeling programs in the absence of injury or pathophysiology.
Plasticity in Merkel-cell afferents
Structural analysis of tactile afferents demonstrated that both myelinated and unmyelinated branches remodel over the hair cycle. Interestingly, these two domains in the peripheral arbor follow different trajectories: unmyelinated neurites fluctuate in tandem with Merkel-cell numbers, whereas myelinated branches refine progressively. Future studies are needed to determine whether common mechanims govern these processes.
Computational simulations indicate that simple principles of plasticity can account for the distributions of Merkel cell and heminode numbers observed in the spontaneous hair cycle. In particular, the model suggests that Merkel cells are stochastically incorporated into the arbor but that heminodes with low Merkel-cell numbers are pruned. The correlation between Merkel-cell number and heminodes during catagen supports the validity of these principles, as end organs with more Merkel cells also have more heminodes. The reduction of Merkel cells during anagen coincided with the loss of neurites in touch-dome afferents, raising the possibility that these cell types are interdependent. It is unlikely that loss of Merkel cells drives neuronal remodeling, as touch-dome innervation does not require intact Merkel cells (Maricich et al., 2009; Reed-Geaghan et al., 2016). By contrast, intact innervation and neural-derived signals are needed to maintain full complements of touch-dome Merkel cells (Nurse et al., 1984; Xiao et al., 2015). Our observation that innervation and Merkel cells change in concert over a few days extend this model by suggesting a tight temporal dependence of touch-dome Merkel cells on intact innervation. Note, however, that the Merkel cell’s neural dependency differs across tissues (Mills et al., 1989; Ebara et al., 2002).
Lineage-tracing studies report that Merkel cells are replenished slowly from epidermal progenitors, with a life-span of at least 7 weeks (Doucet et al., 2013; Wright et al., 2015). Nonetheless, most Merkel cells are newly specified over the course of two hair cycles (Xiao et al., 2015). Our observation that touch domes had few Merkel cells in late anagen but normal Merkel-cell complements in catagen indicates that Merkel cells might undergo rapid turnover. Alternatively, it is possible that Merkel cells are not replaced but display coordinated changes in Merkel-specific protein expression over the hair cycle. Additional studies are needed to distinguish between these mechanisms.
Our finding that Merkel-cell numbers transiently decrease in anagen contrasts with previous reports that keratin 20 (K20)-positive Merkel cells in back skin are most abundant in anagen (Moll et al., 1996; Nakafusa et al., 2006). This apparent discrepancy is unlikely to be caused by use of different Merkel-cell markers because K20 and K8 co-localize in 99% of mouse Merkel cells (Wright et al., 2015). Moreover, we confirmed the loss of Merkel-cell-specific protein expression with two additional markers. Other methodological differences might account for this disparity. First, a study of plucking-induced hair cycles quantified Merkel cells in histological sections, which inherently undersamples rare cell types (Moll et al., 1996). Second, an analysis of Merkel-cell numbers in rat back skin by whole-mount immunohistochemistry found that dendritic Merkel cells peak in mid-anagen and progressively decrease through late anagen (Nakafusa et al., 2006). This progressive decrease is consisent with our observations in the mouse; however, the peak in Merkel cells in mid-anagen might reflect a species difference.
The surprising degree of afferent plasticity during hair growth raises the question of whether hair cycle-stimulated remodeling is required for afferent maintenance. Interestingly, Hairless mice, whose follicles do not cycle, have innervated Merkel cells, indicating that the hair cycle itself is not needed to maintain Merkel-cell end organs (Xiao et al., 2015). Thus, we propose that neuronal plasticity is a normal process that is synchronized by hair growth.
Functional consequences of afferent plasticity
Electrophysiological recordings demonstrated that touch reception is maintained during anagen and catagen, but that mechanical sensitivity is compromised in many afferents during these stages. Such changes might reflect differences in terminal architecture or alterations in ion channels that govern neuronal excitability. Changes in skin geometry might also alter tactile sensitivity; however, the impact of tissue properties can be minimized if neurons encode compressive stress rather than strain (Wang et al., 2016; Ge and Khalsa, 2002). Given that most touch-dome afferents produced typical SAI responses, it is also possible that tactile sensitivity is largely preserved through homeostatic changes in excitability that offset differences in end-organ structure and skin geometry.
Because recent studies have shown that touch-dome afferents that lack mechanosensitive Merkel cells produce intermediately adapting (IA) firing patterns (Maksimovic et al., 2014; Woo et al., 2014), we expected to observe IA responses in late anagen. Instead, almost all touch-dome units had SA firing patterns. We speculate that these responses are produced by intact Merkel-cell afferents. Alternatively, touch-dome afferents might produce sustained firing when Merkel cells are lost postnatally (Kinkelin et al., 1999). We favor the former model because optogenetic silencing of Merkel cells attenuates sustained SAI firing (Baumbauer et al., 2015; Maksimovic et al., 2014), which demonstrates that Merkel-cell activity is required.
Our behavioral studies show that mice in anagen have impaired tactile responsiveness to sustained pressure. Future studies will delineate whether morphological and functional changes extend to other afferent subtypes. This surprising result also suggests that evolutionarily, the benefits of afferent remodeling offset a transient loss in tactile sensitivity.
Hair-cycle signaling pathways
Molecular signals from follicular and dermal cells could be driving the hair cycle-associated changes observed in neurons and Merkel cells. Several pathways that are required for hair cycling are also involved in neuronal outgrowth and Merkel-cell maintenance, including bone morphogenetic proteins (Plikus et al., 2008, Kobielak et al., 2007, Bhattacherjee et al., 2013), sonic hedgehog (Oro and Higgins, 2003, Xiao et al., 2015), and fibroblast growth factor 5 (Scarlato et al., 2001; Suzuki et al., 2000, Hebert et al., 1994). The results of this study sets the stage for assessing the role of these hair-cycle signaling pathways in control of cutaneous innervation.
Somatosensory neurons undergo degeneration and sprouting after injury and in skin diseases (Kinkelin et al., 2000; Taneda et al., 2011). It is possible that mechanisms of healthy neuronal remodeling and pathological neurite clearance are shared. Axon remodeling in the context of injury is a multi-faceted, active process governed by cues from the soma (Barnett et al., 2016; Cashman and Hoke, 2015; Gerdts et al., 2015). Moreover, neurotrophins are upregulated in skin during injury-induced neuronal sprouting (Constantinou et al., 1994; Jankowski and Koerber, 2010; Quarta et al., 2014). Maintenance programs could piggyback on these injury pathways. Delineating and harnessing these processes at a molecular level could allow for interventions to improve touch-receptor recovery after injury and maintenance of touch receptors during aging.
EXPERIMENTAL PROCEDURES
Detailed methods are included in Supplemental Experimental Procedures. Statistical tests were selected based on number of groups to be compared and normality of data, and are indicated in the text and figure legends.
Animal studies complied with the Institutional Animal Care and Use Committee of Columbia University. Specimens were collected from mice during either the first spontaneous hair cycle or a plucking-induced hair cycle. Hair-cycle stages were verified histologically as described (Muller-Rover et al., 2001). Late anagen was defined as anagen IV-VI. C57BL/6 mice were used except where indicated. Electrophysiology, quantitative immunohistochemisty, and 3D reconstuctions were performed as previously described (Maksimovic et al., 2014, Lesniak et al., 2014).
Tactile sensitivity was tested using a modified version of the tape response test (Bouet et al., 2009; Ranade et al., 2014). Two cohorts (N=10 mice each) were tested on their ability to detect a sticker placed on depilated skin in anagen and second telogen.
Computational simulations were programmed in Python. Transitions between hair-cycle stages were modeled as separate simulations.
Supplementary Material
Acknowledgments
We thank Dr. Seung Woo for assistance with Piezo2-gfp mice, Ms. Emily Chang for help with hair-cycle induction and Ms. Milda Stanislauskas for histology. Ms. Blair Jenkins contributed to early project stages. Drs. David Owens, Angela Christiano, Diana Bautista and members of the Lumpkin, Gerling and Christiano laboratories provided helpful discussions. This research was supported by NINDS R01NS073119 (to EAL & GJG). RCC was supported by NICHD 2T32HD007430-18 and NINDS 5T32NS064928-07. Core facilities were supported by the Columbia University EpiCURE Center (NIAMS P30AR044535) and the Herbert Irving Comprehensive Cancer Center (NCI P30CA013696).
Footnotes
Author Contributions
Conceptualization: KLM, GJG, EAL; Software: RLO, GJG; Validation: RCC; Formal analysis: KLM, RCC, YB, RLO, GJG, EAL; Investigation: KLM, RCC, YB; Writing – original draft: KLM, RCC; Writing – review & editing: KLM, RCC, YB, RLO, GJG, EAL; Visualization: KLM, RCC, EAL; Supervision: EAL; Project Administration: EAL; Funding acquisition: GJG, EAL.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso L, Fuchs E. The hair cycle. J Cell Sci. 2006;119:391–393. doi: 10.1242/jcs.02793. [DOI] [PubMed] [Google Scholar]
- Bai L, Lehnert BP, Liu J, Neubarth NL, Dickendesher TL, Nwe PH, Cassidy C, Woodbury CJ, Ginty DD. Genetic Identification of an Expansive Mechanoreceptor Sensitive to Skin Stroking. Cell. 2015;163:1783–1795. doi: 10.1016/j.cell.2015.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett MH, Mathey E, Kiernan MC, Pollard JD. Axonal damage in central and peripheral nervous system inflammatory demyelinating diseases: common and divergent pathways of tissue damage. Curr Opin Neurol. 2016;29:213–221. doi: 10.1097/WCO.0000000000000334. [DOI] [PubMed] [Google Scholar]
- Baumbauer KM, DeBerry JJ, Adelman PC, Miller RH, Hachisuka J, Lee KH, Ross SE, Koerber HR, Davis BM, Albers KM. Keratinocytes can modulate and directly initiate nociceptive responses. Elife. 2015;4:e09674. doi: 10.7554/eLife.09674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacherjee A, Rumi MA, Staecker H, Smith PG. Bone morphogenetic protein 4 mediates estrogen-regulated sensory axon plasticity in the adult female reproductive tract. J Neurosci. 2013;33:1050–1061a. doi: 10.1523/JNEUROSCI.1704-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev VA, Eichmuller S, Johansson O, Paus R. Hair cycle-dependent plasticity of skin and hair follicle innervation in normal murine skin. J Comp Neurol. 1997;386:379–395. doi: 10.1002/(sici)1096-9861(19970929)386:3<379::aid-cne4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Bouet V, Boulouard M, Toutain J, Divoux D, Bernaudin M, Schumann-Bard P, Freret T. The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice. Nat Protoc. 2009;4:1560–1564. doi: 10.1038/nprot.2009.125. [DOI] [PubMed] [Google Scholar]
- Cashman CR, Hoke A. Mechanisms of distal axonal degeneration in peripheral neuropathies. Neurosci Lett. 2015;596:33–50. doi: 10.1016/j.neulet.2015.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo D, Seidel K, Salcedo E, Ahn C, de Sauvage FJ, Klein OD, Barlow LA. Induction of ectopic taste buds by SHH reveals the competency and plasticity of adult lingual epithelium. Development. 2014;141:2993–3002. doi: 10.1242/dev.107631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- Cheng C, Guo GF, Martinez JA, Singh V, Zochodne DW. Dynamic plasticity of axons within a cutaneous milieu. J Neurosci. 2010;30:14735–14744. doi: 10.1523/JNEUROSCI.2919-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou J, Reynolds ML, Woolf CJ, Safieh-Garabedian B, Fitzgerald M. Nerve growth factor levels in developing rat skin: upregulation following skin wounding. Neuroreport. 1994;5:2281–2284. doi: 10.1097/00001756-199411000-00019. [DOI] [PubMed] [Google Scholar]
- Doucet YS, Woo SH, Ruiz ME, Owens DM. The Touch Dome Defines an Epidermal Niche Specialized for Mechanosensory Signaling. Cell Rep. 2013 doi: 10.1016/j.celrep.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovy P, Aldskogius H. Degeneration and regeneration of cutaneous sensory nerve formations. Microsc Res Tech. 1996;34:362–375. doi: 10.1002/(SICI)1097-0029(19960701)34:4<362::AID-JEMT7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Ebara S, Kumamoto K, Matsuura T, Mazurkiewicz JE, Rice FL. Similarities and differences in the innervation of mystacial vibrissal follicle-sinus complexes in the rat and cat: a confocal microscopic study. J Comp Neurol. 2002;449:103–119. doi: 10.1002/cne.10277. [DOI] [PubMed] [Google Scholar]
- Feng P, Huang L, Wang H. Taste bud homeostasis in health, disease, and aging. Chem Senses. 2014;39:3–16. doi: 10.1093/chemse/bjt059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009;137:811–819. doi: 10.1016/j.cell.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison SR, Dietrich A, Stucky CL. TRPC1 contributes to light-touch sensation and mechanical responses in low-threshold cutaneous sensory neurons. J Neurophysiol. 2012;107:913–922. doi: 10.1152/jn.00658.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W, Khalsa PS. Encoding of compressive stress during indentation by slowly adapting type I mechanoreceptors in rat hairy skin. J Neurophysiol. 2002;87:1686–1693. doi: 10.1152/jn.00414.2001. [DOI] [PubMed] [Google Scholar]
- Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, Milbrandt J. SARM1 activation triggers axon degeneration locally via NAD(+) destruction. Science. 2015;348:453–457. doi: 10.1126/science.1258366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeberle H, Fujiwara M, Chuang J, Medina MM, Panditrao MV, Bechstedt S, Howard J, Lumpkin EA. Molecular profiling reveals synaptic release machinery in Merkel cells. Proc Natl Acad Sci U S A. 2004;101:14503–14508. doi: 10.1073/pnas.0406308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert JM, Rosenquist T, Gotz J, Martin GR. FGF5 as a regulator of the hair growth cycle: evidence from targeted and spontaneous mutations. Cell. 1994;78:1017–1025. doi: 10.1016/0092-8674(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Hsieh ST, Chiang HY, Lin WM. Pathology of nerve terminal degeneration in the skin. J Neuropathol Exp Neurol. 2000;59:297–307. doi: 10.1093/jnen/59.4.297. [DOI] [PubMed] [Google Scholar]
- Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969;200:763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahoda CA, Christiano AM. Niche crosstalk: intercellular signals at the hair follicle. Cell. 2011;146:678–681. doi: 10.1016/j.cell.2011.08.020. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Koerber HR. Neurotrophic Factors and Nociceptor Sensitization. In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton, FL: 2010. [Google Scholar]
- Johnson KO, Yoshioka T, Vega-Bermudez F. Tactile functions of mechanoreceptive afferents innervating the hand. J Clin Neurophysiol. 2000;17:539–558. doi: 10.1097/00004691-200011000-00002. [DOI] [PubMed] [Google Scholar]
- Kim DK, Holbrook KA. The appearance, density, and distribution of Merkel cells in human embryonic and fetal skin: their relation to sweat gland and hair follicle development. J Invest Dermatol. 1995;104:411–416. doi: 10.1111/1523-1747.ep12665903. [DOI] [PubMed] [Google Scholar]
- Kinkelin I, Motzing S, Koltenzenburg M, Brocker EB. Increase in NGF content and nerve fiber sprouting in human allergic contact eczema. Cell Tissue Res. 2000;302:31–37. doi: 10.1007/s004410000202. [DOI] [PubMed] [Google Scholar]
- Kinkelin I, Stucky CL, Koltzenburg M. Postnatal loss of Merkel cells, but not of slowly adapting mechanoreceptors in mice lacking the neurotrophin receptor p75. Eur J Neurosci. 1999;11:3963–3969. doi: 10.1046/j.1460-9568.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci U S A. 2007;104:10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniak DR, Marshall KL, Wellnitz SA, Jenkins BA, Baba Y, Rasband MN, Gerling GJ, Lumpkin EA. Computation identifies structural features that govern neuronal firing properties in slowly adapting touch receptors. Elife. 2014;3:e01488. doi: 10.7554/eLife.01488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Wu Y, Qiu Q, Scheerer H, Moran A, Yu CR. A developmental switch of axon targeting in the continuously regenerating mouse olfactory system. Science. 2014;344:194–197. doi: 10.1126/science.1248805. [DOI] [PubMed] [Google Scholar]
- Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo SH, Ranade S, Patapoutian A, et al. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature. 2014;509:617–621. doi: 10.1038/nature13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricich SM, Wellnitz SA, Nelson AM, Lesniak DR, Gerling GJ, Lumpkin EA, Zoghbi HY. Merkel cells are essential for light-touch responses. Science. 2009;324:1580–1582. doi: 10.1126/science.1172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills LR, Nurse CA, Diamond J. The neural dependency of Merkel cell development in the rat: the touch domes and foot pads contrasted. Dev Biol. 1989;136:61–74. doi: 10.1016/0012-1606(89)90130-9. [DOI] [PubMed] [Google Scholar]
- Moll I, Paus R, Moll R. Merkel cells in mouse skin: intermediate filament pattern, localization, and hair cycle-dependent density. J Invest Dermatol. 1996;106:281–286. doi: 10.1111/1523-1747.ep12340714. [DOI] [PubMed] [Google Scholar]
- Mouret A, Lepousez G, Gras J, Gabellec MM, Lledo PM. Turnover of newborn olfactory bulb neurons optimizes olfaction. J Neurosci. 2009;29:12302–12314. doi: 10.1523/JNEUROSCI.3383-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- Nakafusa J, Narisawa Y, Shinogi T, Taira K, Tanaka T, Inoue T, Misago N. Changes in the number of Merkel cells with the hair cycle in hair discs on rat back skin. Br J Dermatol. 2006;155:883–889. doi: 10.1111/j.1365-2133.2006.07441.x. [DOI] [PubMed] [Google Scholar]
- Nurse CA, Macintyre L, Diamond J. Reinnervation of the rat touch dome restores the Merkel cell population reduced after denervation. Neuroscience. 1984;13:563–571. doi: 10.1016/0306-4522(84)90249-5. [DOI] [PubMed] [Google Scholar]
- Oro AE, Higgins K. Hair cycle regulation of Hedgehog signal reception. Dev Biol. 2003;255:238–248. doi: 10.1016/s0012-1606(02)00042-8. [DOI] [PubMed] [Google Scholar]
- Peters EM, Botchkarev VA, Botchkareva NV, Tobin DJ, Paus R. Hair-cycle-associated remodeling of the peptidergic innervation of murine skin, and hair growth modulation by neuropeptides. J Invest Dermatol. 2001;116:236–245. doi: 10.1046/j.1523-1747.2001.01232.x. [DOI] [PubMed] [Google Scholar]
- Plikus MV, Chuong CM. Complex hair cycle domain patterns and regenerative hair waves in living rodents. J Invest Dermatol. 2008;128:1071–1080. doi: 10.1038/sj.jid.5701180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, Chuong CM. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta S, Baeumer BE, Scherbakov N, Andratsch M, Rose-John S, Dechant G, Bandtlow CE, Kress M. Peripheral nerve regeneration and NGF-dependent neurite outgrowth of adult sensory neurons converge on STAT3 phosphorylation downstream of neuropoietic cytokine receptor gp130. J Neurosci. 2014;34:13222–13233. doi: 10.1523/JNEUROSCI.1209-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan B, Polydefkis M, Hauer P, Griffin JW, McArthur JC. Epidermal reinnervation after intracutaneous axotomy in man. J Comp Neurol. 2003;457:24–36. doi: 10.1002/cne.10460. [DOI] [PubMed] [Google Scholar]
- Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Begay V, Coste B, Mainquist J, et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature. 2014;516:121–125. doi: 10.1038/nature13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Wright MC, See LA, Adelman PC, Lee KH, Koerber HR, Maricich SM. Merkel Cell-Driven BDNF Signaling Specifies SAI Neuron Molecular and Electrophysiological Phenotypes. J Neurosci. 2016;36:4362–4376. doi: 10.1523/JNEUROSCI.3781-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlato M, Xu T, Bannerman P, Beesley J, Reddy UR, Rostami A, Scherer SS, Pleasure D. Axon-Schwann cell interactions regulate the expression of fibroblast growth factor-5 (FGF-5) J Neurosci Res. 2001;66:16–22. doi: 10.1002/jnr.1193. [DOI] [PubMed] [Google Scholar]
- Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19:R132–142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Ota Y, Ozawa K, Imamura T. Dual-mode regulation of hair growth cycle by two Fgf-5 gene products. J Invest Dermatol. 2000;114:456–463. doi: 10.1046/j.1523-1747.2000.00912.x. [DOI] [PubMed] [Google Scholar]
- Taneda K, Tominaga M, Negi O, Tengara S, Kamo A, Ogawa H, Takamori K. Evaluation of epidermal nerve density and opioid receptor levels in psoriatic itch. Br J Dermatol. 2011;165:277–284. doi: 10.1111/j.1365-2133.2011.10347.x. [DOI] [PubMed] [Google Scholar]
- Tsai L, Barnea G. A critical period defined by axon-targeting mechanisms in the murine olfactory bulb. Science. 2014;344:197–200. doi: 10.1126/science.1248806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Baba Y, Lumpkin EA, Gerling GJ. Computational modeling indicates that surface pressure can be reliably conveyed to tactile receptors even amidst changes in skin mechanics. J Neurophysiol. 2016 doi: 10.1152/jn.00624.2015. jn 00624 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellnitz SA, Lesniak DR, Gerling GJ, Lumpkin EA. The regularity of sustained firing reveals two populations of slowly adapting touch receptors in mouse hairy skin. J Neurophysiol. 2010;103:3378–3388. doi: 10.1152/jn.00810.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 2014;509:622–626. doi: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MC, Reed-Geaghan EG, Bolock AM, Fujiyama T, Hoshino M, Maricich SM. Unipotent, Atoh1+ progenitors maintain the Merkel cell population in embryonic and adult mice. J Cell Biol. 2015;208:367–379. doi: 10.1083/jcb.201407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Thoresen DT, Williams JS, Wang C, Perna J, Petrova R, Brownell I. Neural Hedgehog signaling maintains stem cell renewal in the sensory touch dome epithelium. Proc Natl Acad Sci U S A. 2015;112:7195–7200. doi: 10.1073/pnas.1504177112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.